
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Cardiovasc. Med. , 12 April 2023
Sec. Atherosclerosis and Vascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1174902
This article is part of the Research Topic Lipids and Inflammation in Health and Disease, Volume II View all 11 articles
 Evgeny Bezsonov1,2,3,4*
Evgeny Bezsonov1,2,3,4* Mirza S. Baig5
Mirza S. Baig5 Michael Bukrinsky6
Michael Bukrinsky6 Veronika Myasoedova7
Veronika Myasoedova7 Alessio Ravani7
Alessio Ravani7 Vasily Sukhorukov2
Vasily Sukhorukov2 Dongwei Zhang8
Dongwei Zhang8 Victoria Khotina1,2
Victoria Khotina1,2 Alexander Orekhov9*
Alexander Orekhov9*
Editorial on the Research Topic
Lipids and inflammation in health and disease, volume II
The focus of this Research Topic is a continuation of the previous one and is devoted to the study of the role of lipids in inflammation in health and disease. Atherosclerosis is a classic example of how lipids involved in the normal functioning of cells and tissues can also induce inflammatory responses. Atherogenesis is far from being completely understood, and here we summarize the main pathological factors contributing to the development of this disease, focusing on the role of lipids.
Atherosclerosis is a disease of the intima of the arteries, leading to pathological thickening of the arterial wall with other secondary negative effects on the health of patients. The problem with this disease is that the exact causes of atherosclerosis are still not clarified, which limits the development of effective anti-atherosclerotic therapy. Thus, the identification of key molecular changes happening during the initiation and further progression of atherosclerosis, including the understanding of the role of lipids in this process, is a task of vital importance.
Atherosclerosis is an inflammatory disease associated with the infiltration of immune cells into the arterial wall (1, 2). Atherogenic factors (such as desialylated, oxidized, and electronegative LDL) (3, 4) are thought to be one of the main reasons for the accumulation of lipids in cells of the intima and macrophages, leading to the formation of so-called foam cells and further progression of atherosclerosis. There are gender differences in the development of atherosclerosis, including differences in lipid profiles (5).
Mitochondrial DNA mutations have been shown to be associated with atherosclerotic lesions, leading to the idea of a possible role of these mutations in the development of atherosclerosis (6). It should be noted that mitochondrial DNA mutations and mitochondrial dysfunction are associated not only with atherosclerosis (6), but also with a plethora of different diseases: non-alcoholic fatty liver disease, diabetes, polycystic ovarian syndrome, cancer, and various neurological diseases (7–9).
The components involved in the regulation of cholesterol metabolism (such as LDLR and PCSK9) can be considered potential targets for the development of drugs for the prevention of LDL-induced accumulation of cholesterol in cells (10, 11). It should also be noted also that PCSK9 may be associated with mitochondrial dysfunction (12), and even cancer in some cases (13).
In addition, atherosclerosis can be considered an autoimmune disease (14), and thus certain approaches used for the treatment of autoimmune and autoinflammatory diseases (15–17) may be potentially applicable to the cure of atherosclerosis.
Evidence is accumulating that non-coding RNAs are involved in the development of cardiovascular diseases including atherosclerosis (1), and, in addition to that, exosomes may also be involved in the progression of this disease (18).
Current pathological factors contributing to the development of atherosclerosis are shown in Figure 1.
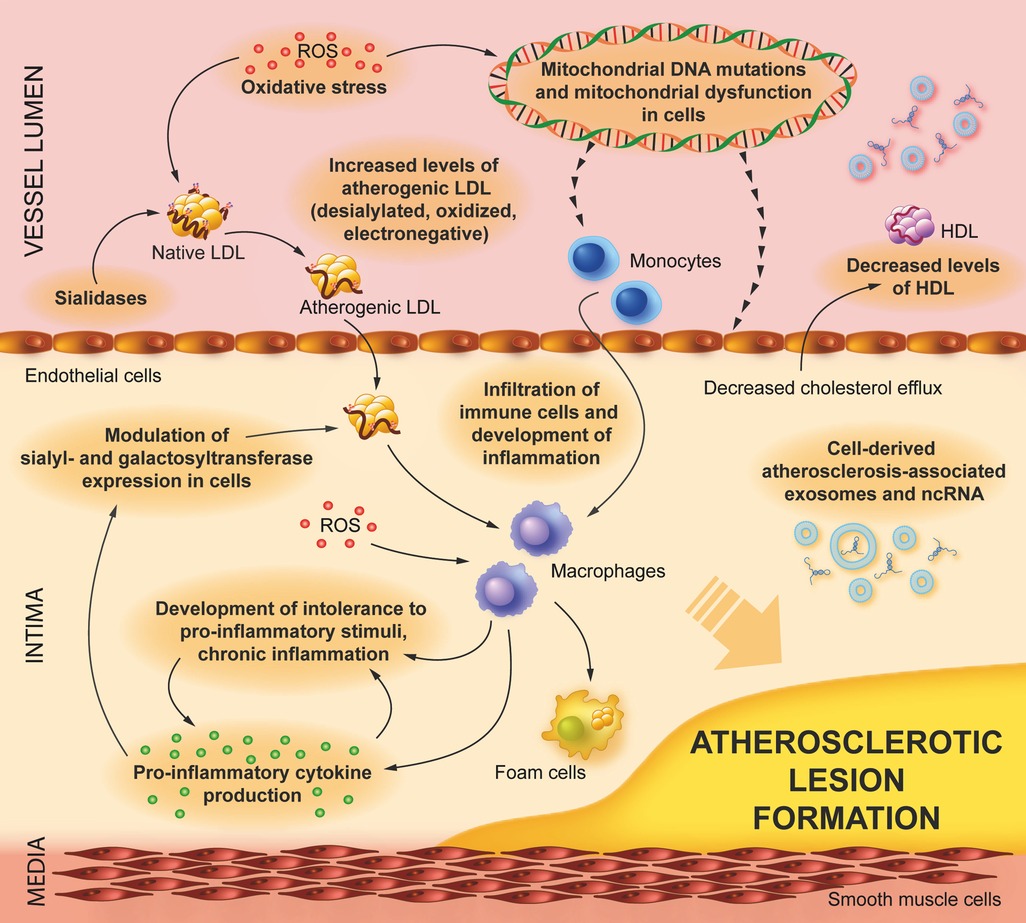
Figure 1. Pathological factors contributing to the development of atherosclerosis. Atherogenic LDL (desialylated, oxidized, and electronegative ones) induces lipid accumulation in endothelial cells and infiltrating macrophages, leading to foam cell formation, inflammation, and further progression of atherosclerosis. There are additional factors that contribute (or potentially contribute) to the development of atherogenesis, such as low levels of HDL, sialidase, and sialyl/galactosyltransferase activities leading to the generation of atherogenic LDL, intolerance to pro-inflammatory stimulation (including stimulation by atherogenic LDL), atherosclerosis-associated non-coding RNA (ncRNA), and exosomes.
This Research Topic collects 10 articles and reviews that are briefly discussed below.
A review by Poznyak et al. summarizes all known data on hypertension as a risk factor for atherosclerosis and risk assessment. The role of lipids in hypertension is discussed.
Data on oxidative stress as a primary factor linking lipids, inflammation, and atherosclerosis, in addition to therapeutic approaches to reduce oxidative stress were reviewed by Bale et al.
The reduction of plasma and liver lipid levels and atherosclerosis was discovered by Padalkar et al. in combined adenine-induced chronic kidney disease and diet-induced atherosclerosis in mice with a familial hypercholesterolemia mutation in the ldlr gene, leading to the conclusion that excessive adenine affects lipid metabolism.
The association of monomeric C-reactive protein (which is produced at sites of local inflammation) with carotid plaque number was found in patients with subclinical atherosclerosis in the study done by Melnikov et al.
The effects of lipoproteins, membranes, and extracellular vesicles on the structure and function of C-reactive protein were reviewed by Potempa et al.
Recent advances and future directions of research on the Von Willebrand factor (which may contribute to inflammation in atherosclerosis) with a focus on the diagnosis and treatment of cardiovascular diseases were reviewed by Kozlov et al.
The effect of Bie-Jia-Ruan-Mai-Tang (BJ, a traditional Chinese medicine formula) on proliferative diabetic retinopathy (the disease closely associated with inflammation) was investigated by Liu et al. using in vitro [human retinal capillary endothelial cells (HRCECs)] and in vivo (a diabetic mouse model) experiments.
The effects of forsythiasides [phenylethanol glycosides from the plant Forsythia suspensa (Thunb.) Vahl] on cardiovascular protection, anti-inflammation, antioxidation, and neuroprotection were reviewed by Yang et al.
The mechanism of action of monocyte locomotion inhibitory factor (MLIF) related to the reduction of ischemic stroke-induced inflammatory injury was investigated by Lv et al. with the conclusion about the anti-inflammatory effect of MLIF through the suppression of JNK/AP-1.
The attempt to identify molecular mechanisms of osteogenic differentiation of human valve interstitial cells (VIC) isolated from healthy donors or patients with calcific aortic valve disease associated with inflammation by RNA-seq transcriptomics and by shotgun proteomics was conducted by Semenova et al. with the finding of pro-osteogenic role of upregulated ZBTB16 gene.
The editors of Research Topic hope that the high standard of publications established in the first two volumes will be maintained in the third one.
EB and AO: writing and editing of the manuscript. MSB, MB, VM, AR, VS, VK, and DZ: editing of the manuscript. All authors contributed to the article and approved the submitted version.
The research was supported by the Russian Science Foundation, grant No. 22-15-00317.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Malekmohammad K, Bezsonov EE, Rafieian-Kopaei M. Role of lipid accumulation and inflammation in atherosclerosis: focus on molecular and cellular mechanisms. Front Cardiovasc Med. (2021) 8:707529. doi: 10.3389/fcvm.2021.707529
2. Poznyak AV, Bezsonov EE, Popkova TV, Starodubova AV, Orekhov AN. Immunity in atherosclerosis: focusing on T and B cells. IJMS. (2021) 22:8379. doi: 10.3390/ijms22168379
3. Mezentsev A, Bezsonov E, Kashirskikh D, Baig MS, Eid AH, Orekhov A. Proatherogenic sialidases and desialylated lipoproteins: 35 years of research and current state from bench to bedside. Biomedicines. (2021) 9:600. doi: 10.3390/biomedicines9060600
4. Mushenkova NV, Bezsonov EE, Orekhova VA, Popkova TV, Starodubova AV, Orekhov AN. Recognition of oxidized lipids by macrophages and its role in atherosclerosis development. Biomedicines. (2021) 9:915. doi: 10.3390/biomedicines9080915
5. Vakhtangadze T, Singh Tak R, Singh U, Baig MS, Bezsonov E. Gender differences in atherosclerotic vascular disease: from lipids to clinical outcomes. Front Cardiovasc Med. (2021) 8:707889. doi: 10.3389/fcvm.2021.707889
6. Bezsonov E, Sobenin I, Orekhov A. Immunopathology of atherosclerosis and related diseases: focus on molecular biology. IJMS. (2021) 22:4080. doi: 10.3390/ijms22084080
7. Dabravolski SA, Bezsonov EE, Baig MS, Popkova TV, Nedosugova LV, Starodubova AV, et al. Mitochondrial mutations and genetic factors determining NAFLD risk. IJMS. (2021) 22:4459. doi: 10.3390/ijms22094459
8. Dabravolski SA, Orekhova VA, Baig MS, Bezsonov EE, Starodubova AV, Popkova TV, et al. The role of mitochondrial mutations and chronic inflammation in diabetes. IJMS. (2021) 22:6733. doi: 10.3390/ijms22136733
9. Dabravolski SA, Nikiforov NG, Eid AH, Nedosugova LV, Starodubova AV, Popkova TV, et al. Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. IJMS. (2021) 22:3923. doi: 10.3390/ijms22083923
10. Puteri MU, Azmi NU, Kato M, Saputri FC. PCSK9 Promotes cardiovascular diseases: recent evidence about its association with platelet activation-induced myocardial infarction. Life. (2022) 12:190. doi: 10.3390/life12020190
11. Scharnagl H, März W. New lipid-lowering agents acting on LDL receptors. Curr Top Med Chem. (2005) 5(3):233–42. doi: 10.2174/1568026053544524
12. Ding Z, Liu S, Wang X, Mathur P, Dai Y, Theus S, et al. Cross-Talk between PCSK9 and damaged MtDNA in vascular smooth muscle cells: role in apoptosis. Antioxid Redox Signaling. (2016) 25:997–1008. doi: 10.1089/ars.2016.6631
13. Mahboobnia K, Pirro M, Marini E, Grignani F, Bezsonov EE, Jamialahmadi T, et al. PCSK9 And cancer: rethinking the link. Biomed Pharmacother. (2021) 140:111758. doi: 10.1016/j.biopha.2021.111758
14. Sima P, Vannucci L, Vetvicka V. Atherosclerosis as autoimmune disease. Ann Transl Med. (2018) 6(7):116. doi: 10.21037/atm.2018.02.02
15. El-Shebiny EM, Zahran ES, Shoeib SA, Habib ES. Bridging autoinflammatory and autoimmune diseases. Egypt J Intern Med. (2021) 33:11. doi: 10.1186/s43162-021-00040-5
16. Koushki K, Keshavarz Shahbaz S, Keshavarz M, Bezsonov EE, Sathyapalan T, Sahebkar A. Gold nanoparticles: multifaceted roles in the management of autoimmune disorders. Biomolecules. (2021) 11(9):1289. doi: 10.3390/biom11091289
17. Ghobadinezhad F, Ebrahimi N, Mozaffari F, Moradi N, Beiranvand S, Pournazari M, et al. The emerging role of regulatory cell-based therapy in autoimmune disease. Front Immunol. (2022) 13:1075813. doi: 10.3389/fimmu.2022.1075813
Keywords: lipids, inflammation, atherosclerosis, LDL, atherogenicity
Citation: Bezsonov E, Baig MS, Bukrinsky M, Myasoedova V, Ravani A, Sukhorukov V, Zhang D, Khotina V and Orekhov A (2023) Editorial: Lipids and inflammation in health and disease, volume II. Front. Cardiovasc. Med. 10:1174902. doi: 10.3389/fcvm.2023.1174902
Received: 27 February 2023; Accepted: 24 March 2023;
Published: 12 April 2023.
Edited and Reviewed by: Masanori Aikawa, Harvard Medical School, United States
© 2023 Bezsonov, Baig, Bukrinsky, Myasoedova, Ravani, Sukhorukov, Zhang, Khotina and Orekhov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Evgeny Bezsonov ZXZnZW55LmJlenNvbm92QGdtYWlsLmNvbQ== Alexander Orekhov YWxleGFuZGVybmlrb2xhZXZpY2hvcmVraG92QGdtYWlsLmNvbQ==
Specialty Section: This article was submitted to Atherosclerosis and Vascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.