
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 17 July 2023
Sec. Heart Valve Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1171703
Background: Little research has been assessed atherosclerotic risk factors at various stages of calcific aortic valve disease. This study sought to determine risk factors of patients with aortic valve sclerosis (AVS) and mild to moderate aortic stenosis (AS).
Methods: The study included 1,007 patients diagnosed with AVS or mild to moderate AS according to echocardiographic criteria. Patients were identified as a rapid progression group if the annualized difference in peak aortic jet velocity (Vmax) between two echocardiographic examinations was >0.08 m/s/yr in AVS and >0.3 m/s/yr in AS, respectively. We used multivariable logistic regression analyses to assess the factors associated with rapid disease progression or progression to severe AS.
Results: Among 526 AVS patients, higher LDL-C level (odds ratio [OR] 1.22/per 25 mg/dl higher LDL-C, 95% confidence interval [CI] 1.05–1.43) was significantly associated with rapid disease progression. Compared to patients with LDL-C level <70 mg/dl, the adjusted OR for rapid progression were 1.32, 2.15, and 2.98 for those with LDL-C level of 70–95 mg/dl, 95–120 mg/dl, and ≥120 mg/dl, respectively. Among 481 mild to moderate AS patients, the baseline Vmax (OR 1.79/per 0.5 m/s higher Vmax, 95% CI 1.18–2.70) was associated with rapid progression. Compared to patients with Vmax 2.0–2.5 m/s, the adjusted OR for rapid progression were 2.47, 2.78, and 3.49 for those with Vmax of 2.5–3.0 m/s, 3.0–3.5 m/s, and 3.5–4.0 m/s, respectively. LDL-C and baseline Vmax values were independently associated with progression to severe AS.
Conclusion: Atherosclerotic risk factors such as LDL-C were significantly associated with the rapid progression in AVS and baseline Vmax was important in the stage of mild to moderate AS.
Calcific aortic valve (AoV) disease is a progressive condition from aortic valve sclerosis (AVS), mild leaflet thickening without valve obstruction, to severe aortic stenosis (AS) (1). The pathobiology of AVS and AS shares similarities with atherosclerosis involving lipid accumulation, inflammation, and calcification (2). The link between lipid, inflammation, and calcification in calcific AoV disease and the pathological similarities with atherosclerosis led to the hypothesis that statins might be beneficial in patients with AS. Some retrospective studies showed lipid-lowering therapies could prevent the progression to overt AS (3–5). However, prospective studies have demonstrated a failure to attenuate the progression of AS in statin-treated patients (6–8). The most plausible explanation for this inconsistent results is that whilst lipid deposition may play a pivotal role in the initiation phase, it has little effect in the advanced phase when fibrosis and calcification are the dominant pathological processes.
Hence, the independent contribution of atherosclerotic risk factors to disease progression at various stages of calcific AV disease remains unclear. In response, this study sought to determine the impact of contributing risk factors on the progression of patients with AVS and mild to moderate AS.
We retrospectively included 1,007 patients with AVS (irregular leaflet thickening, focally increased echogenicity) revealed by 2-dimensional echocardiography and [peak aortic jet velocity (Vmax), <2 m/s] by Doppler echocardiography, mild AS [aortic valve area (AVA), 1.5–2.0 cm2; Vmax, 2.0–3.0 m/s], or moderate AS (AVA, 1.0–1.5 cm2; Vmax, 3.0–4.0 m/s) and subsequently selected patients who had undergone ≥2 echocardiography examinations at ≥6 months apart during 2011–2020. Patients with other significant valvular diseases, left ventricular dysfunction [left ventricular ejection fraction (LVEF) < 50%], congenital heart diseases, cardiomyopathy, a permanent pacemaker, or a history of cardiac surgery were excluded. Flow diagram was presented in Figure 1.
The progression rates of AVS, mild AS, and moderate AS during a median follow-up period of 2.3 (interquartile range, 1.3–3.5) years were 0.01 (−0.10 to 0.08), 0.06 (0.00–0.16), and 0.17 (0.04–0.28) m/s/yr, respectively (Supplementary Figure S1). Patients were identified as a rapid progression group if the annualized difference in Vmax between two echocardiographic examinations was >0.08 m/s/yr (highest quartile) in AVS and >0.3 m/s/yr in AS, respectively.
The study protocol was approved by the institutional review board of a single center (KNUH IRB File No. 2022-02-010), and the need for informed consent was waived because of the retrospective nature of the study.
Clinical data, including the medical history and presence of risk factors, were obtained by a complete review of patient medical records. The presence of dyslipidemia was defined by a total cholesterol >200 mg/dl or use of lipid-lowering therapy; diabetes mellitus was defined by a fasting plasma glucose >126 mg/dl, plasma glucose level >200 mg/dl tested twice, or use of anti-diabetic medication; hypertension was defined by blood pressure ≥140/90 mmHg at office or use of anti-hypertensive medication; and coronary artery disease (CAD) was defined by previously documented myocardial infarction or coronary artery stenosis with a lumen diameter >50% on angiography.
Comprehensive transthoracic echocardiography was performed using commercially available equipment (Vivid E9 from GE Healthcare, Milwaukee, WI, USA or Acuson SC2000 from Siemens Medical Solutions, Mountain View, CA, USA). Standard M-mode, 2-dimensional, and color Doppler imaging were performed in parasternal, suprasternal, substernal, and apical views with positional adjustment of the patient. The first and last echocardiograms collected during the study period were used to evaluate echocardiographic changes. Anatomic measurements were performed according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging (9).
Continuous variables were tested for normality using the Shapiro–Wilk test. Results were expressed as mean ± standard deviation or median (25th–75th percentile) and compared with Student's t test or the Wilcoxon rank-sum test between patients with rapid versus slow progression in the AVS and mild to moderate AS groups. Categorical variables are presented as percentages and were compared with the Chi-square test or Fisher's exact test, as appropriate.
Multivariable logistic regression analyses were performed to assess the factors associated with rapid progression or progression to severe AS in AVS and mild to moderate AS patients, after adjusting for clinically relevant variables and variables with p < 0.20 in univariate analysis and carefully avoiding collinearity. The variables adjusted were age, sex, body mass index, smoking status, hypertension, diabetes, dyslipidemia, CAD, C-reactive protein (CRP) level, and LVEF.
p < 0.05 was considered statistically significant. Statistical analyses were performed using the R statistical software program (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria) and SPSS software version 25.0 (IBM Corp., Armonk, NY, USA).
Baseline characteristics are listed in Table 1. Among the 526 AVS patients (128 with rapid progression and 398 with slow progression), those with rapid progression were older (74 ± 8 vs. 69 ± 12 years, p = <0.001). In the rapid-progression group of AVS patients, the Vmax was 1.74 ± 0.15 m/s, the AVA was 1.83 ± 0.57 cm2, and the rate of progression was 0.19 (range, 0.13–0.32) m/s/yr. Co-morbidities and laboratory findings were comparable between the groups (all p > 0.08).
Among the 481 mild to moderate AS patients (62 with rapid progression and 419 with slow progression), there were significant differences between the rapid- and slow-progression groups in terms of male sex, creatinine, left ventricular end-systolic dimension (LVESD), LVEF, and E/e’. In the rapid-progression group of mild to moderate AS patients, the Vmax was 2.70 ± 0.52 m/s, the AVA was 1.53 ± 0.30 cm2, and the rate of progression was 0.46 (range, 0.35–0.73) m/s/yr.
In univariate analysis, age, body mass index, and LDL-C were significant (all p < 0.20). After adjustment for smoking, hypertension, diabetes, dyslipidemia, CAD and CRP level, LDL-C level (odds ratio [OR] 1.22/per 25 mg/dl higher LDL-C, 95% confidence interval [CI] 1.05–1.43) and age (OR 1.04/per 1 year higher age, 95% CI 1.02–1.07) were significantly associated with rapid disease progression in AVS patients (Table 2). The impact of LDL-C on AVS progression was attenuated by statin use, but showed consistent results regardless of statin (Supplementary Figure S2). Compared to patients with LDL-C < 70 mg/dl, the adjusted OR for rapid progression was 1.32 (95% CI 0.70–2.50) for those with LDL-C level 70–95 mg/dl, 2.15 (95% CI 1.17–3.97) for those with LDL-C level 95–120 mg/dl, and 2.98 (95% CI 1.62–5.48) for those with LDL-C level ≥120 mg/dl (Figure 2).
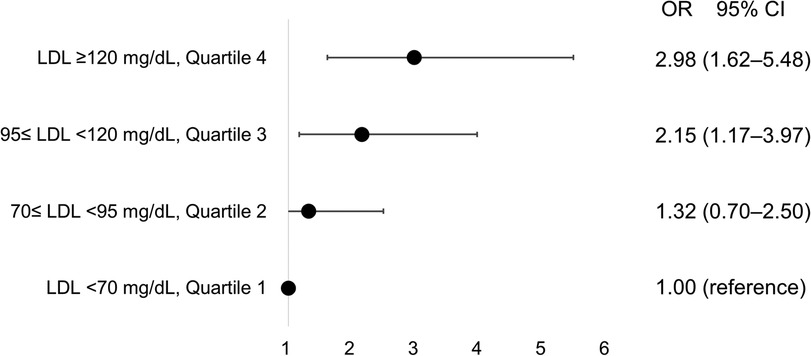
Figure 2. Incremental risk of progression in aortic valve sclerosis according to concentrations of low-density lipoprotein. CI, confidence interval; LDL, low-density lipoprotein; OR, odds ratio.
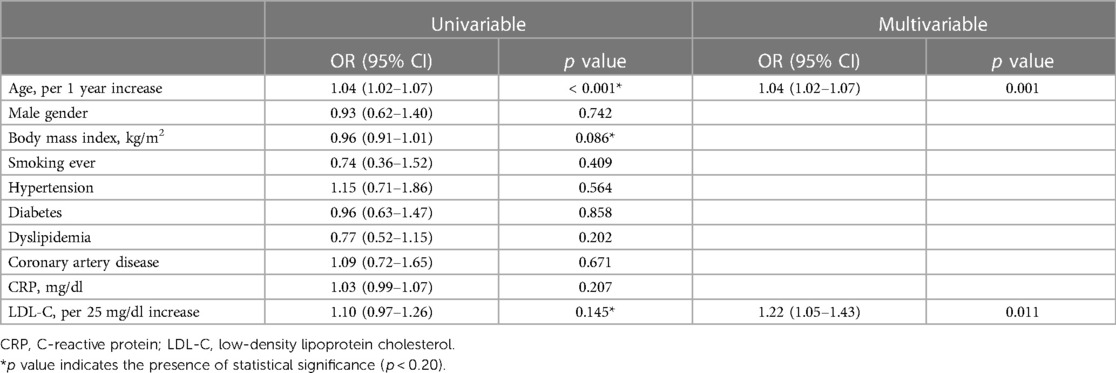
Table 2. Unadjusted and adjusted logistic regression analyses for rapid progression in patients with aortic valve sclerosis.
Atherosclerotic risk factors were not associated with rapid disease progression among mild to moderate AS patients; however, baseline Vmax (OR 1.79/per 0.5 m/s higher Vmax, 95% CI 1.18–2.70) and E/e′ (OR 1.08, 95% CI 1.01–1.15) were significantly associated with rapid disease progression in patients with mild to moderate AS (Table 3). Compared to patients with Vmax 2.0–2.5 m/s, the adjusted OR for rapid progression was 2.47 (95% CI 1.01–4.70) for those with Vmax 2.5–3.0 m/s, 2.78 (95% CI 1.23–6.47) for those with Vmax 3.0–3.5 m/s, and 3.49 (95% CI 1.39–9.17) for those with Vmax 3.5–4.0 m/s (Figure 3). Initial and follow-up AoV mean pressure gradient and AVA were also presented in Supplementary Figure S3.
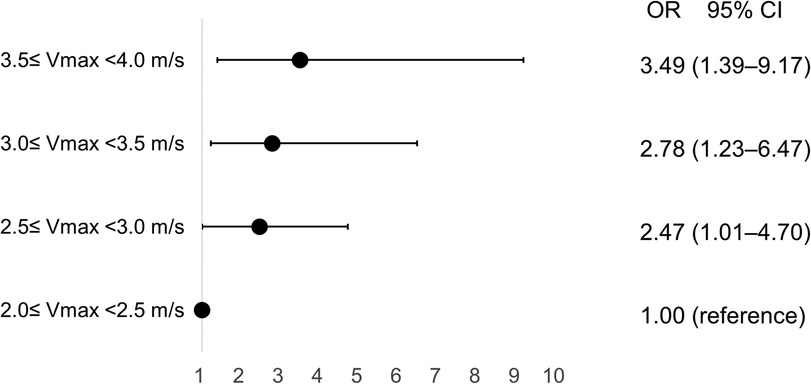
Figure 3. Incremental risk of progression in aortic valve stenosis according to baseline peak aortic jet velocity. CI, confidence interval; OR, odds ratio; Vmax, peak aortic jet velocity.
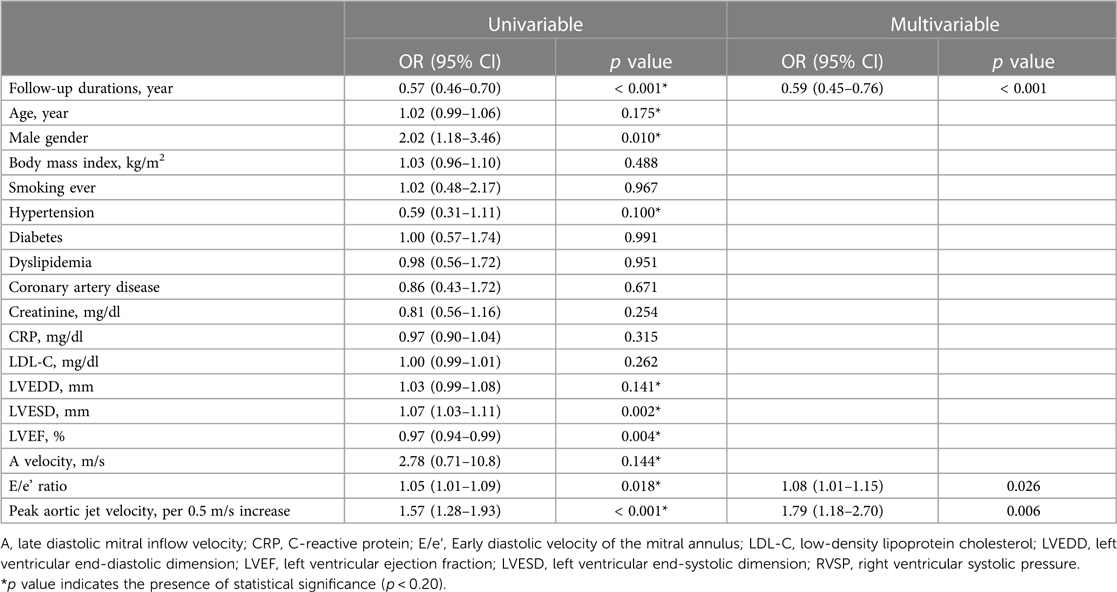
Table 3. Unadjusted and adjusted logistic regression analyses for rapid progression in patients with mild to moderate aortic stenosis.
During a median follow-up period of 2.3 years, no AVS patients progressed to severe AS, while 12 (3.0%) patients progressed from mild to severe AS and 31 (40%) patients progressed from moderate to severe AS (Supplementary Figure S4). Among all patients with calcific AV disease, LDL-C level (OR 1.23/per 25 mg/dl higher LDL-C, 95% CI 1.02–1.50), baseline Vmax (OR 6.38/per 0.5 m/s higher Vmax, 95% CI 4.12–9.89) were significantly associated with progression to severe AS (Table 4).
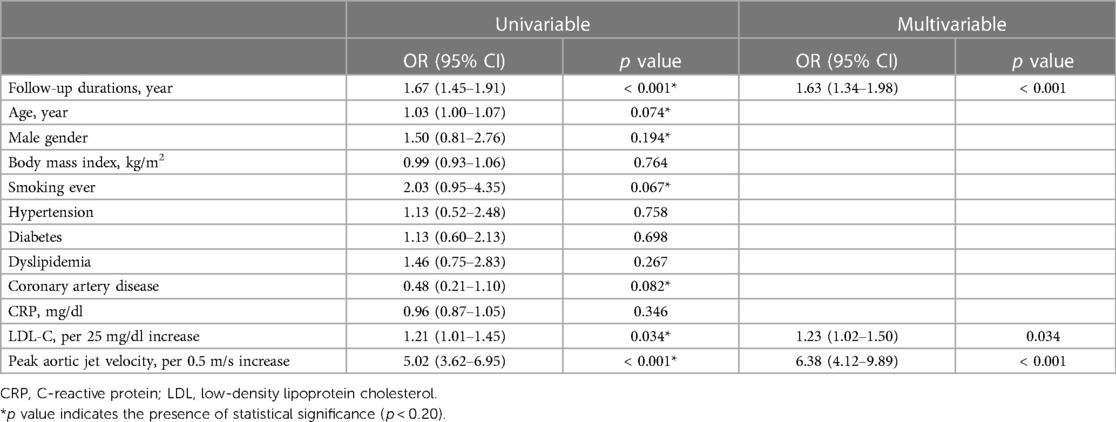
Table 4. Unadjusted and adjusted logistic regression analyses for progression to severe aortic stenosis in total patients with calcific aortic valve disease.
The main findings of this study are that: (1) Atherosclerotic risk factors were significantly associated with the rapid progression in AVS patients and LDL-C showed a markedly incremental risk of AVS progression; (2) baseline Vmax, not atherosclerotic risk factors, was associated with the rapid progression in patients with mild to moderate AS; (3) LDL-C and baseline Vmax were independently associated with progression to severe AS in patients with calcific AoV disease (Figure 4).
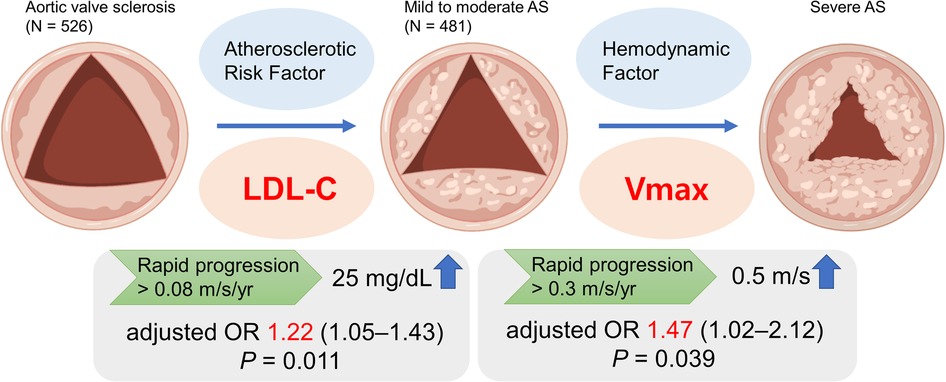
Figure 4. In a single center registry, low-density lipoprotein cholesterol (LDL-C) among atherosclerotic risk factors was associated with the rapid progression of aortic valve sclerosis (AVS) in multivariable analysis. In contrast, progression of mild to moderate aortic stenosis (AS) was associated with baseline peak aortic jet velocity (Vmax). AS, aortic stenosis; LDL-C, low-density lipoprotein cholesterol; Vmax, peak aortic jet velocity.
Some studies have reported the frequent coexistence of either AVS or AS in patients with underlying CAD (10). However, research to date has not been able to prove causality despite the frequent coexistence of these entities. Multicenter study showed that AVS was strongly associated with the presence and degree of CAD independently of clinical risk factors (11). A prospective study found a higher incidence of cardiovascular events and worse survival in AVS patient, but after adjustment such as CAD and CRP, no statistically significant differences were found (12). Other prospective study demonstrated a higher risk of myocardial infarction and cardiovascular mortality in subjects with AVS and no known CAD, after adjustment for traditional cardiac risk factors (13). The association between AVS and CAD warrants further research. The initial lesions in both AVS and CAD involve lipid deposition and focal sclerosis (2). The early phase of the disease, observed in patients with AVS, is characterized by prominent accumulation of LDL-C and lipoprotein(a) [Lp(a)] (14, 15). The current study demonstrates the incremental risk of LDL-C in the rapid progression of AVS. This result reveals LDL-C is important in the initiating step in the development of AVS. The extracellular lipid infiltration causes LDL oxidization, stimulates inflammatory process, and finally calcification (16). In this study, the association between LDL-C and the rapid progression of AVS is weak in univariable analysis (p > 0.10) and significant in multivariable anaylsis, therefore, it is difficult to reveal clearly that LDL-C affects AVS progression because of a confounder's effect. In addition, there is no significant difference on follow-up LDL-C between rapid and slow progression group in AVS patients, and this suggests the existence of other contributing factors. Well-controlled research about the impact of LDL-C on the progression of AVS is needed. Some patients with slow progression had a decline in transaortic velocity. First, it is possible that hemodynamic progression has not been established in AVS stages. Second, there is more difference in the measurement of Vmax in AVS than in AS. This is due to a suboptimal Doppler study with a nonparallel intercept angle. The principle that lower LDL-C is better in cardiovascular disease (17) may have more evidence for application in patients with AVS.
In this study, the role of atherosclerotic risk factors is not proven in the progression of mild to moderate AS. The study shows that higher baseline Vmax is associated with the rapid progression of AS. Upon mild valve obstruction, disease progression dictated neither by inflammation nor by lipid deposition, but rather by increasing hemodynamic severity (18). The stages of AS are characterized by fibrosis and accelerated calcification, leading to valvular dysfunction and changes in mechanical stress and flow (19). In addition to hemodynamic progression in the advanced stages of calcific AS, it has been speculated that hypertension and the increased stiffness of the aortic root that occurs with ageing may also cause abnormally high mechanical stress in the valve (20, 21). An unmet need exists to develop new pharmacological treatment strategies delaying calcific AS progression.
A gradual progression of calcific AV disease may ultimately to severe AS, which eventually leads to ventricular remodeling and hemodynamic compromise with a high morbidity and mortality if not treated (22). In this study, LDL-C and baseline Vmax are independently associated with progression to severe AS in total patients with calcific AV disease. Although the early stage of calcific AV disease is mainly mediated by lipid deposition and inflammation, the role of hemodynamic progression is more prominent in the later stage (23). In line, recent study has reported that Lp(a) is associated with new-onset AV calcium but not with AV calcium progression (24). Although statin attenuated the impact of LDL-C on AVS progression in subgroup analysis, it was not statistically significant. More research regarding the pathophysiology of calcific AoV disease continuum and novel targets holding potential for the progression is needed.
This study has several limitations. First, the retrospective nature of the study does not exclude other potential confounding variables not included in the analysis could have affected the results. Second, the study has a relatively short-term period to fully observe the progression of AVS. Third, we did not measure Lp(a), a biomarker for AS progression. However, we do not think the check for Lp(a) is routine in the current clinical practice. Fourth, only patients who underwent follow-up echocardiograhy were included in this study, therefore, selection bias might also affect the results. Fifth, this study did not show clinical events such as aortic valve intervention or mortality. However, observation of progression to severe AS is important due to its high morbidity. Finally, this study limits the participants to a single center and a single ethnicity. Hence, our findings should be expanded and further verified in well-controlled prospective studies.
In conclusion, atherosclerotic risk factors such as LDL-C were significantly associated with the rapid progression in AVS and baseline Vmax was important in the stage of mild to moderate AS. These findings provide insights for future research to identify novel therapeutic targets which alters the course of calcific AoV disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Kangwon National University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JS and DR designed the study and drafted the manuscript. Statistical analyses were performed by JS. JS, KK, KC, B-KL, B-RC, and DR critically revised the manucript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1171703/full#supplementary-material
Supplementary Figure S1.
The rate of progression according to the grade of calcific aortic valve disease. *p-value determined by one-way ANOVA Abbreviations: AS, aortic valve stenosis; AVS, aortic valve sclerosis.
Supplementary Figure S2.
Subgroup analysis for the impact of LDL-C on AVS progression according to statin use. Abbreviations: AVS, aortic valve sclerosis; LDL-C, low-density lipoprotein cholesterol.
Supplementary Figure S3.
Initial and follow-up AoV mean pressure gradient (A) and AVA (B). Abbreviations: AoV, aortic valve; AVA, aortic valve area.
Supplementary Figure S4.
The ratio of progression to severe aortic valve stenosis according to the grade of calcific aortic valve disease. *p-value determined by the chi-square test. Abbreviations: AS, aortic valve stenosis; AVS, aortic valve sclerosis.
1. Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: calcific aortic valve disease-2011 update. Circulation. (2011) 124(16):1783–91. doi: 10.1161/CIRCULATIONAHA.110.006767
2. Milin AC, Vorobiof G, Aksoy O, Ardehali R. Insights into aortic sclerosis and its relationship with coronary artery disease. J Am Heart Assoc. (2014) 3(5):e001111. doi: 10.1161/JAHA.114.001111
3. Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. (2002) 40(10):1723–30. doi: 10.1016/S0735-1097(02)02496-8
4. Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BPJC. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. (2001) 104(18):2205–9. doi: 10.1161/hc4301.098249
5. Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. (2004) 110(10):1291–5. doi: 10.1161/01.CIR.0000140723.15274.53
6. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, Investigators A. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. (2010) 121(2):306–14. doi: 10.1161/CIRCULATIONAHA.109.900027
7. Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. (2005) 352(23):2389–97. doi: 10.1056/NEJMoa043876
8. Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. (2008) 359(13):1343–56. doi: 10.1056/NEJMoa0804602
9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
10. Losi MA, Brevetti G, Schiano V, Barbati G, Parisi V, Contaldi C, et al. Aortic valve sclerosis in patients with peripheral and/or coronary arterial disease. Echocardiography. (2010) 27(6):608–12. doi: 10.1111/j.1540-8175.2009.01109.x
11. Rossi A, Gaibazzi N, Dandale R, Agricola E, Moreo A, Berlinghieri N, et al. Aortic valve sclerosis as a marker of coronary artery atherosclerosis; a multicenter study of a large population with a low prevalence of coronary artery disease. Int J Cardiol. (2014) 172(2):364–7. doi: 10.1016/j.ijcard.2014.01.024
12. Chandra HR, Goldstein JA, Choudhary N, O'Neill CS, George PB, Gangasani SR, et al. Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J Am Coll Cardiol. (2004) 43(2):169–75. doi: 10.1016/j.jacc.2003.08.036
13. Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. (2012) 5(6):619–25. doi: 10.1016/j.jcmg.2011.12.023
14. Kaiser Y, Singh SS, Zheng KH, Verbeek R, Kavousi M, Pinto SJ, et al. Lipoprotein(a) is robustly associated with aortic valve calcium. Heart. (2021) 107(17):1422–8. doi: 10.1136/heartjnl-2021-319044
15. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KDJC. Characterization of the early lesion of'degenerative'valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. (1994) 90(2):844–53. doi: 10.1161/01.CIR.90.2.844
16. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol. (1997) 29(3):630–4. doi: 10.1016/S0735-1097(96)00563-3
17. Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2020) 41(24):2313–30. doi: 10.1093/eurheartj/ehz962
18. Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. (2015) 66(5):561–77. doi: 10.1016/j.jacc.2015.05.066
19. Peeters F, Meex SJR, Dweck MR, Aikawa E, Crijns H, Schurgers LJ, et al. Calcific aortic valve stenosis: hard disease in the heart: a biomolecular approach towards diagnosis and treatment. Eur Heart J. (2018) 39(28):2618–24. doi: 10.1093/eurheartj/ehx653
20. Rahimi K, Mohseni H, Kiran A, Tran J, Nazarzadeh M, Rahimian F, et al. Elevated blood pressure and risk of aortic valve disease: a cohort analysis of 5.4 million UK adults. Eur Heart J. (2018) 39(39):3596–603. doi: 10.1093/eurheartj/ehy486
21. Robicsek F, Thubrikar MJ, Fokin AA. Cause of degenerative disease of the trileaflet aortic valve: review of subject and presentation of a new theory. Ann Thorac Surg. (2002) 73(4):1346–54. doi: 10.1016/S0003-4975(01)03001-6
22. Kraler S, Blaser MC, Aikawa E, Camici GG, Lüscher TF. Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J. (2022) 43(7):683–97. doi: 10.1093/eurheartj/ehab757
23. Alushi B, Curini L, Christopher MR, Grubitzch H, Landmesser U, Amedei A, et al. Calcific aortic valve disease-natural history and future therapeutic strategies. Front Pharmacol. (2020) 11:685. doi: 10.3389/fphar.2020.00685
Keywords: LDL cholesterol, aortic valve sclerosis, peak aortic jet velocity, aortic stenosis, echocardiogaphy
Citation: Seo JH, Kim KH, Chun KJ, Lee B-K, Cho B-R and Ryu DR (2023) Impact of low-density lipoprotein cholesterol on progression of aortic valve sclerosis and stenosis. Front. Cardiovasc. Med. 10:1171703. doi: 10.3389/fcvm.2023.1171703
Received: 22 February 2023; Accepted: 8 June 2023;
Published: 17 July 2023.
Edited by:
Marcello Rattazzi, University of Padua, ItalyReviewed by:
Tetsu Tanaka, University Hospital Bonn, Germany© 2023 Seo, Kim, Chun, Lee, Cho and Ryu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Ryeol Ryu cmRyMDIwM0BnbWFpbC5jb20=
Abbreviations AS, Aortic stenosis; AoV, Aortic valve; AVS, Aortic valve sclerosis; CAD, Coronary artery disease; LDL-C, Low-density lipoprotein cholesterol; Vmax, peak aortic jet velocity.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.