- 1Tasly Pharmaceutical Group Co., Ltd., Tianjin, China
- 2The State Key Laboratory of Core Technology in Innovative Chinese Medicine, Tasly Academy, Tasly Holding Group Co., Ltd., Tianjin, China
- 3School of Public Health, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background: Long-term use of nitrates for treating stable angina pectoris (SAP) may lead to patients' tolerance to nitrates. As a traditional Chinese medicine, Compound danshen dropping pills (CDDP) is beneficial for patients with SAP. This study aimed to critically assess the efficacy and safety of CDDP vs. nitrates for SAP.
Methods: PubMed, Embase, Web of Science, Cochrane library, CNKI, Wanfang Digital Periodicals, and Chinese Science and Technology Periodicals database were searched from inception to April 2023. Randomized controlled trials (RCTs) comparing CDDP with nitrates for SAP were included. The meta-analysis was conducted to estimate the pooled effect.
Results: Twenty-nine studies were included for the statistical analysis. The meta-analyses with the random-effect model indicated that CDDP could significantly increase the effective rate in symptom improvement compared with nitrates (Pooled 9 RCTs, OR = 1.95, 95% CI: 1.25–3.05, P = 0.003, duration of 4 weeks; Pooled 4 RCTs, OR = 3.45, 95% CI: 1.84–6.48, P = 0.0001, duration of 6 weeks; Pooled 13 RCTs, OR = 4.02, 95% CI: 2.14–7.57, P < 0.0001, duration of 8 weeks). The meta-analyses with the random-effect model indicated that CDDP could significantly increase the effective rate in electrocardiogram improvement compared with nitrates (Pooled 5 RCTs, OR = 1.60, 95% CI: 1.02–2.52, P = 0.04, duration of 4 weeks; Pooled 3 RCTs, OR = 2.47, 95% CI: 1.60–3.82, P < 0.0001, duration of 6 weeks; Pooled 11 RCTs, OR = 3.43, 95% CI: 2.68–4.38, P < 0.00001, duration of 8 weeks). The incidence of adverse drug reactions in the CDDP group was lower than that in the nitrates group (Pooled 23 RCTs, OR = 0.15, 95% CI: 0.1–0.21, P < 0.00001). The results of the meta-analyses with fixed-effect model were similar with above results. The levels of the evidence ranged from very low to low.
Conclusion: The present study suggests that CDDP with the duration of at least 4 weeks can be considered as an alternative to nitrates for treating SAP. However, more high-quality RCTs are still needed to confirm these findings.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022352888, identifier [CRD42022352888].
1. Introduction
Stable angina pectoris (SAP) is one of the most common manifestations of coronary artery disease (CAD) (1). A survey reported that the prevalence of chronic SAP was 7.7% in Iran (2). A prospective study showed that 20% of patients with CAD had symptoms related with angina pectoris, which may be associated with an increased risk of adverse cardiovascular outcomes (3). A cohort study found that the high frequency of angina pectoris was associated with the high incidence of secondary cardiovascular events and death in patients with stable coronary heart disease (4).
At present, the goals of treatments for SAP mainly focus on the reduction in cardiovascular adverse events and the improvement in the quality of life (5). There are many antianginal therapies, such as nitrates, beta-blockers, calcium channel blockers and ranolazine (5). Nitrates as standard antianginal treatments have been used in the management of angina pectoris for many years (6). A systematic review found that nitrates could significantly reduce the angina attacks and improve the exercise duration (6). However, some adverse events associated with nitrates are reported, such as headache and dizziness (6). Moreover, long-term use may lead to patients' tolerance to nitrates and the reduction of protection duration (7).
Traditional Chinese medicine (TCM) is beneficial for the management of angina pectoris. A systematic review showed that TCM could reduce the frequency of angina pectoris, shorten the duration of angina attack and improve the quality of life (8). Compound danshen dropping pills (CDDP) is a TCM formula, and is composed of Salvia miltiorrhiza Bunge, Panax notoginseng (Burkill) F.H. Chen and Dryobalanops aromatica C.F. Gaertn. TCM network pharmacology is a new approach to understanding herb formula (9, 10). A study based on the network pharmacology and molecular docking found that the mechanisms of CDDP for treating SAP might be associated with the regulation of inflammatory response, apoptosis signal pathway, etc. (11). Some clinical trials comparing CDDP with nitrates for SAP have been published in recent years. However, the relative advantages and disadvantages of CDDP compared with nitrates for SAP were not systematically evaluated. Therefore, we conducted this systematic review to critically assess the efficacy and safety of CDDP vs. nitrates for SAP.
2. Methods
This study was registered on PROSPERO (No. CRD42022352888) available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022352888. It followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (12).
2.1. Inclusion and exclusion criteria
2.1.1. Type of included studies
Randomized controlled trials (RCTs) were considered for inclusion, regardless of publication date and language. Quasi-RCTs, cross-over trials, on-going trials, abstracts and letters were excluded.
2.1.2. Patients
Patients diagnosed with SAP were included. Age, gender, race and nationality were unrestricted. SAP should be assessed according to the internationally recognized criteria or guidelines, such as National Institute for Health and Care Excellence or European Society of Cardiology guidelines (13).
2.1.3. Interventions
CDDP was used to treat SAP in the experimental group. Nitrates were used for the management of SAP in the control group. According to the instruction of CDDP, patients should take CDDP ten pills a time, and three times a day for at least 4 weeks. The dosage, frequency and course of nitrates were unlimited. The types of nitrates such as isosorbide dinitrate and isosorbide nitrate were also unrestricted. Other specific interventions for treating SAP were inhibited in the two groups. Patients with SAP may be accompanied with other diseases, such as coronary heart disease. Conventional therapies for the management of these accompanied diseases were unlimited.
2.1.4. Outcomes
The primary outcomes were the effective rate and markedly effective rate in symptom improvement (14). The secondary outcomes included the effective rate and markedly effective rate in electrocardiogram (ECG) improvement, and adverse drug reactions. The patients were labeled as “effective” in symptom improvement when the frequency of angina attacks and nitroglycerin consumption were reduced by more than 50%, and “markedly effective” in symptom improvement when symptoms associated with SAP were disappeared, the frequency of angina attacks or nitroglycerin consumption was reduced by more than 80% or 90% after treatment. The patients were labeled as “effective” in ECG improvement when S-T segment was elevated by 0.5 mm, however, not recovered completely after treatment, or defined by guidelines. The patients were labeled as “markedly effective” in ECG improvement when resting electrocardiogram returned to normal level after treatment, or defined by guidelines. The effective rate is equal to the number of patients labeled as “effective” or “markedly effective” divided by the total number of patients in one group. The markedly effective rate is equal to the number of patients labeled as “markedly effective” divided by the total number of patients in one group.
2.2. Search strategy
Two reviewers (JZ and MZ) independently searched PubMed, Embase, Web of Science, Cochrane library, China National Knowledge Infrastructure, Wanfang Digital Periodicals, and Chinese Science and Technology Periodicals database from inception to April 2023. The search terms included (“compound danshen dripping pills” OR “fufang danshen diwan” OR “Cardiotonic Pills” OR “dantonic” OR T89) AND (nitrate OR “isosorbide dinitrate” OR “isosorbide nitrate”) AND (“stable angina” OR “angina” OR “angina pectoris” OR “stenocardia” OR “angor pectoris”). Moreover, ClinicalTrials.gov, World Health Organization International Clinical trials Registry platform, and Chinese Clinical Trial Registry were also be searched to identify potentially grey literatures. The detailed search strategies are attainable in Supplementary Material.
2.3. Study selection
The studies collected from the comprehensive literature search were imported into EndNote X9 software to remove duplicates. Then, two reviewers (JZ and MZ) independently checked titles and abstracts to delete obviously ineligible studies according to the inclusion and exclusion criteria. They checked the full texts of the remaining studies to further identify eligible studies. Disagreements were handled in consultation with a third reviewer (YH). A PRISMA flow diagram was used to describe the screening process.
2.4. Data extraction
The important data were extracted and imported into Microsoft Excel 2013 by two authors (JZ and MZ) independently. The information included characteristic of included studies (first author, publication year, country, sample size, design), patients (age, gender, race, nationality), interventions (type, dosage, frequency, duration), and outcomes (primary and secondary outcomes). The information on risk of bias assessment (randomization, allocation, blinding, loss to follow-up) were also extracted synchronously.
2.5. Assessment of risk of bias
The risk of bias for included RCTs was assessed by the Cochrane “risk of bias” tool. The selection bias, performance bias, detection bias, attrition bias, and reporting bias for each included RCT was classified to low, high or unclear level. Two reviewers (JZ and MZ) were independently assessed the risk of bias. Any disagreement was resolved by a third author (YH).
2.6. Statistical analysis
Odds ratio (OR) with 95% confidence intervals (CIs) was used to estimate the effect size for the dichotomous variables. The meta-analysis with the random-effect model as the primary analysis was conducted by Review Manager 5.4.1 software. Then, the meta-analysis with the fixed-effect model was conducted as the sensitivity analysis. P < 0.05 indicated a statistically significant difference between the two groups. Subgroup analyses were conducted based on the duration. The publication bias was assessed by the funnel plot. The level of evidence for each outcome was assessed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
3. Results
3.1. Literature search
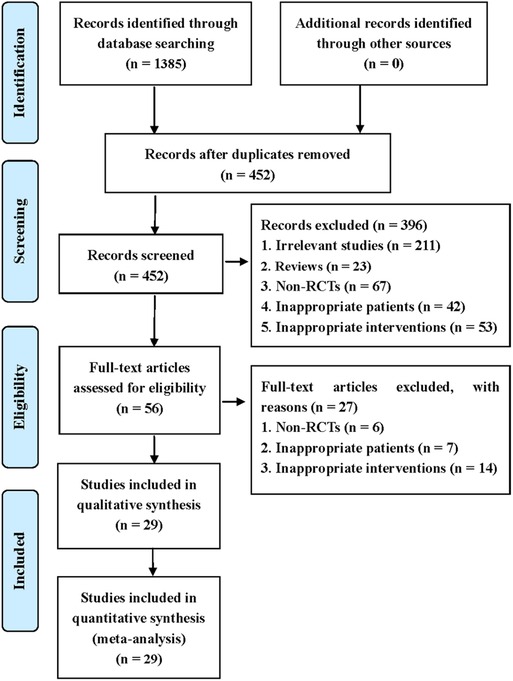
This systematic review identified 1,385 potentially eligible studies via the extensive search. Nine hundred and thirty-three duplicate studies were removed using EndNote software. Then, 396 irrelevant studies were deleted by checking the titles and abstracts. After checking full-texts, 29 studies were finally included for the statistical analysis. The process of screening eligible studies is described in Figure 1 (15–43).
3.2. Characteristics of included studies
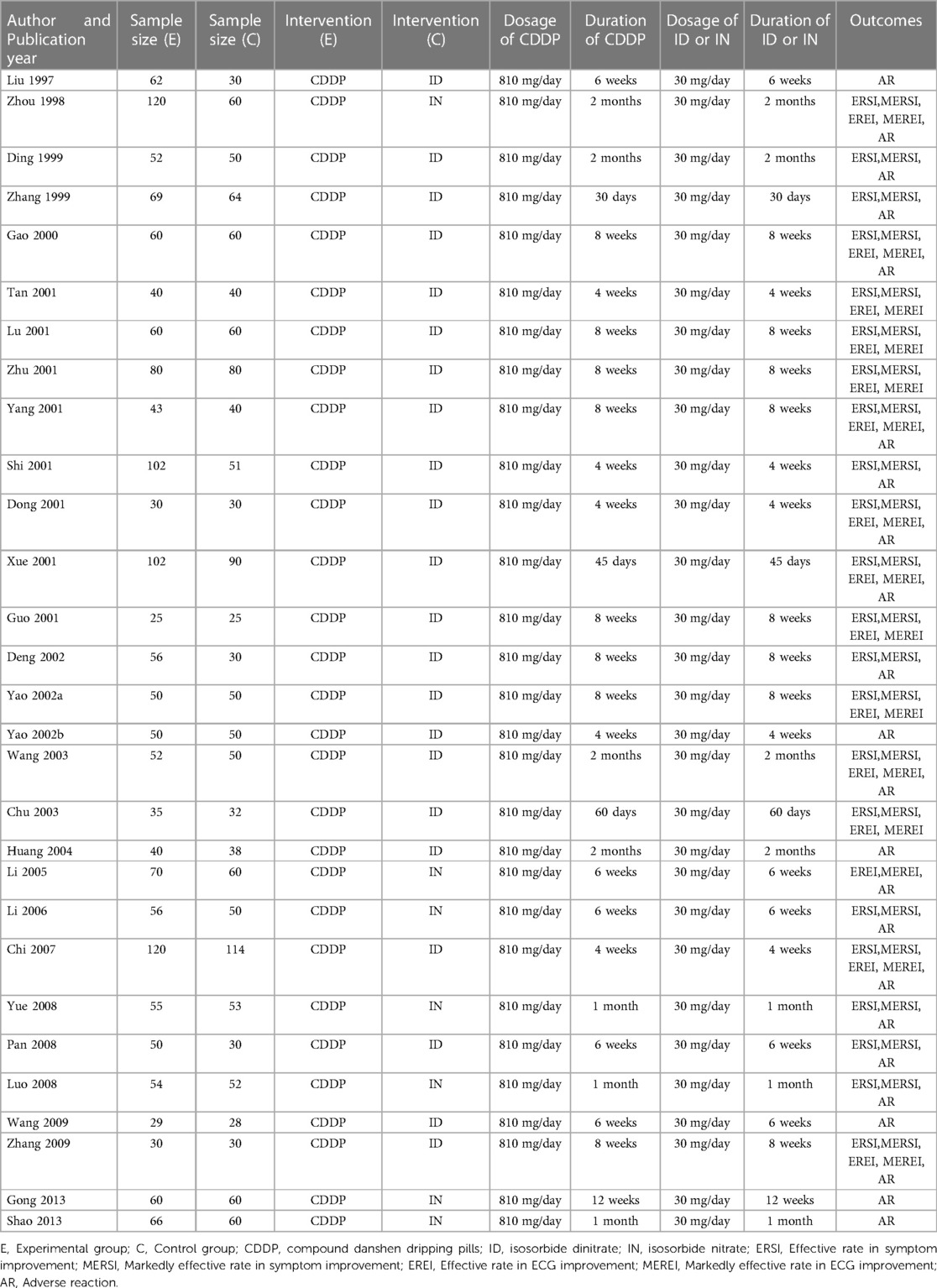
The characteristics of the included studies are summarized in Table 1. They involved 3,185 patients and were published between 1997 and 2013. Sample size ranged from 25 to 120 in the experimental group and 25–114 in the control group. Isosorbide nitrate was used in 7 trials (15, 16, 19, 21, 23, 24, 42), and isosorbide dinitrate was used in other studies. There was the same dosage of CDDP or nitrates across the included studies. The duration of CDDP or nitrates ranged from 4 weeks to 12 weeks. However, patients took CDDP or nitrates with the same duration in the same study. Twenty-two RCTs reported the effective rate and the markedly effective rate in symptom improvement (17, 19–23, 26, 27, 29–42). Fifteen RCTs reported the effective rate and the markedly effective rate in ECG improvement (17, 22, 24, 26, 27, 29, 31–34, 36–39, 42). Twenty-three RCTs reported the adverse drug reactions (15–26, 28, 30, 32, 33, 35, 38–43).
3.3. Assessment of risk of bias
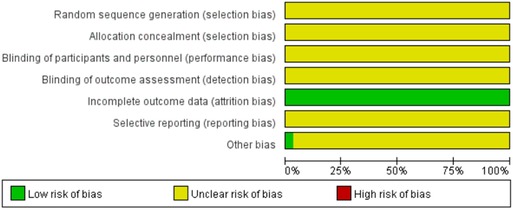
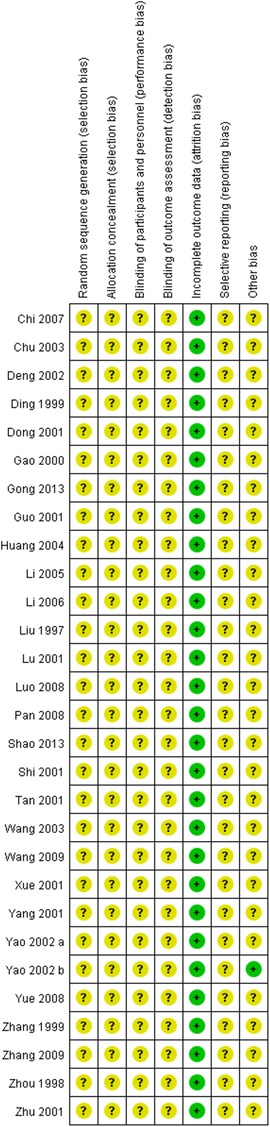
Figures 2, 3 show the results of risk of bias assessment. The risk of selection bias was graded as unclear in all of the included studies because no specific random sequence generation and allocation concealment methods were reported. The risk of performance bias was also classified as unclear in all of the included studies because no blinding of participants, personnel or outcome assessment was clearly reported. The risk of attrition bias was low in all of the included studies due to the complete outcome data. The reporting bias and other bias were categorized as unclear risk because of the insufficient information.
3.4. Symptom improvement
3.4.1. Effective rate in symptom improvement
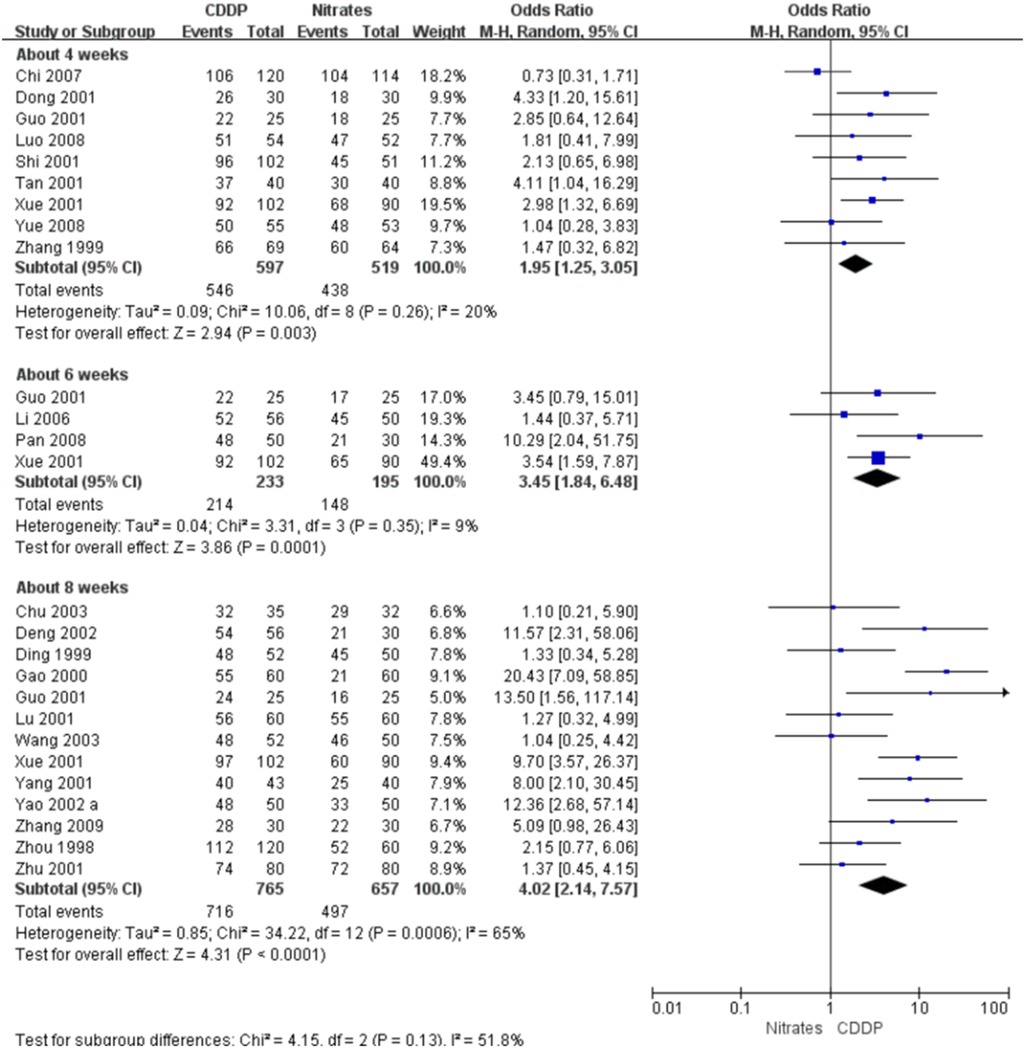
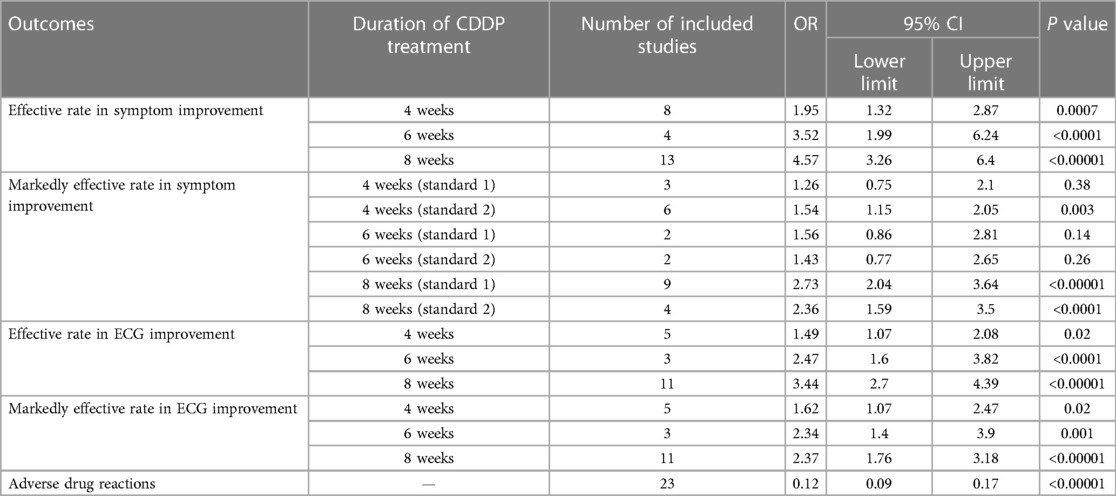
Twenty-two RCTs reported the effective rate in symptom improvement. The meta-analyses with the random-effect model indicated that CDDP could significantly increase the effective rate in symptom improvement compared with nitrates (Pooled 9 RCTs, OR = 1.95, 95% CI: 1.25–3.05, P = 0.003, duration of about 4 weeks; Pooled 4 RCTs, OR = 3.45, 95% CI: 1.84–6.48, P = 0.0001, duration of about 6 weeks; Pooled 13 RCTs, OR = 4.02, 95% CI: 2.14–7.57, P < 0.0001, duration of about 8 weeks; Figure 4). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2. In addition, one RCT (29) reported that the effective rate in symptom improvement in the CDDP group was higher than that in the nitrates group (OR = 1.5, 95% CI: 0.68–3.31, P = 0.32) after 5 weeks of CDDP treatment.
3.4.2. Markedly effective rate in symptom improvement
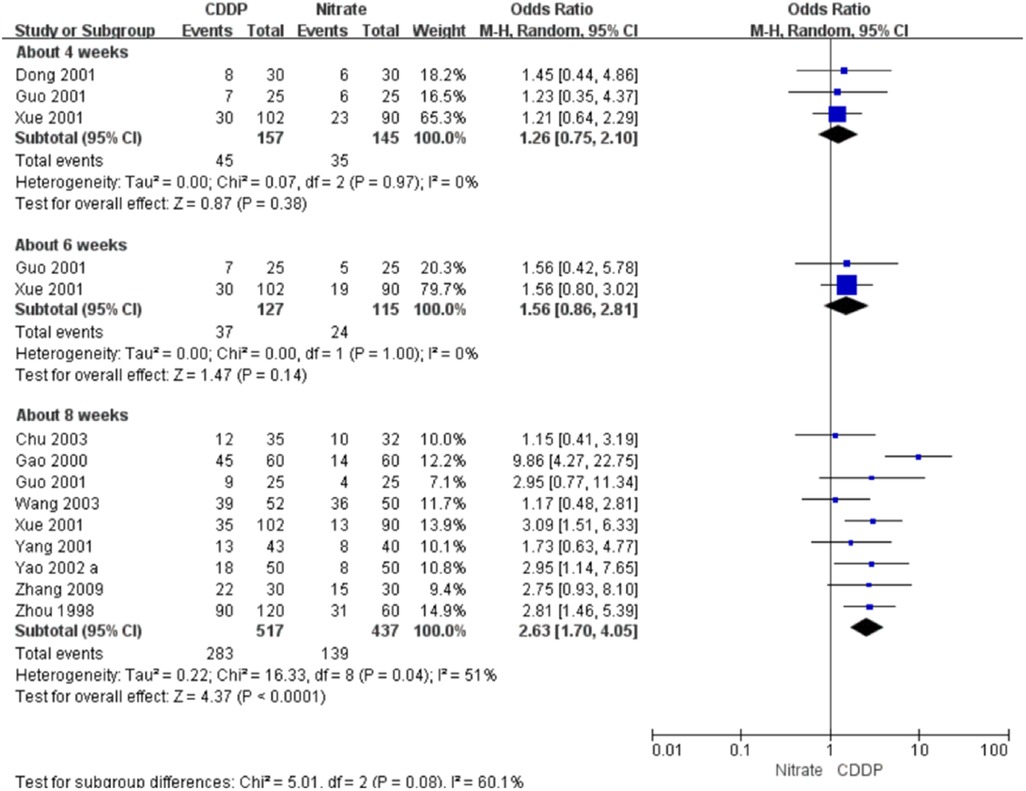
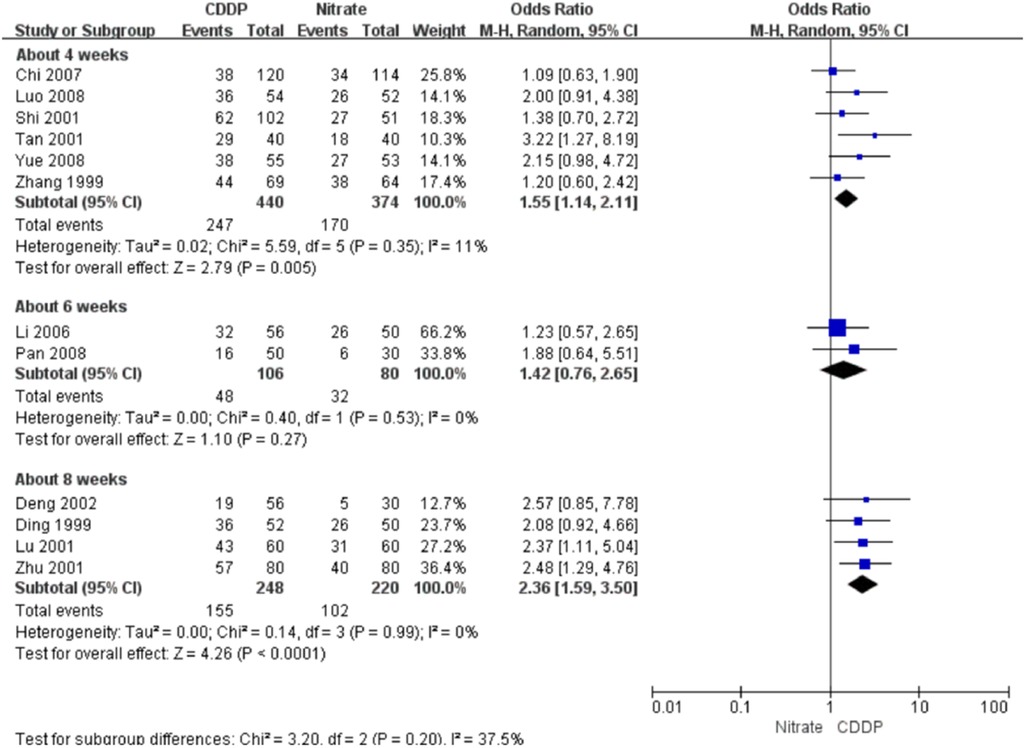
Twenty-two RCTs reported the markedly effective rate in symptom improvement. When the markedly effective in symptom improvement was defined as “symptoms associated with SAP were disappeared, the frequency of angina attacks or nitroglycerin consumption was reduced by more than 80% after treatment” (standard 1), the meta-analyses with the random-effect model indicated that CDDP compared with nitrates could increase the markedly effective rate in symptom improvement with no statistical significance at 4 and 6 weeks after treatment (Pooled 3 RCTs, OR = 1.26, 95% CI: 0.75–2.10, P = 0.38, duration of about 4 weeks; Pooled 2 RCTs, OR = 1.56, 95% CI: 0.86–2.81, P = 0.14, duration of about 6 weeks; Figure 5) and with the statistical significance at 8 weeks after treatment (Pooled 9 RCTs, OR = 2.63, 95% CI: 1.70–4.05, P < 0.0001, duration of about 8 weeks; Figure 5). When the markedly effective in symptom improvement was defined as “symptoms associated with SAP were disappeared, the frequency of angina attacks or nitroglycerin consumption was reduced by more than 90% after treatment” (standard 2), the meta-analyses with the random-effect model indicated that CDDP compared with nitrates could increase the markedly effective rate in symptom improvement with no statistical significance at 6 weeks after treatment (Pooled 2 RCTs, OR = 1.42, 95% CI: 0.76–2.65, P = 0.27, duration of about 6 weeks; Figure 6) and with the statistical significance at 4 and 8 weeks after treatment (Pooled 6 RCTs, OR = 1.55, 95% CI: 1.14–2.11, P = 0.005, duration of about 4 weeks; Pooled 4 RCTs, OR = 2.36, 95% CI: 1.59–3.50, P < 0.0001, duration of about 8 weeks; Figure 6). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2.
3.5. ECG improvement
3.5.1. Effective rate in ECG improvement
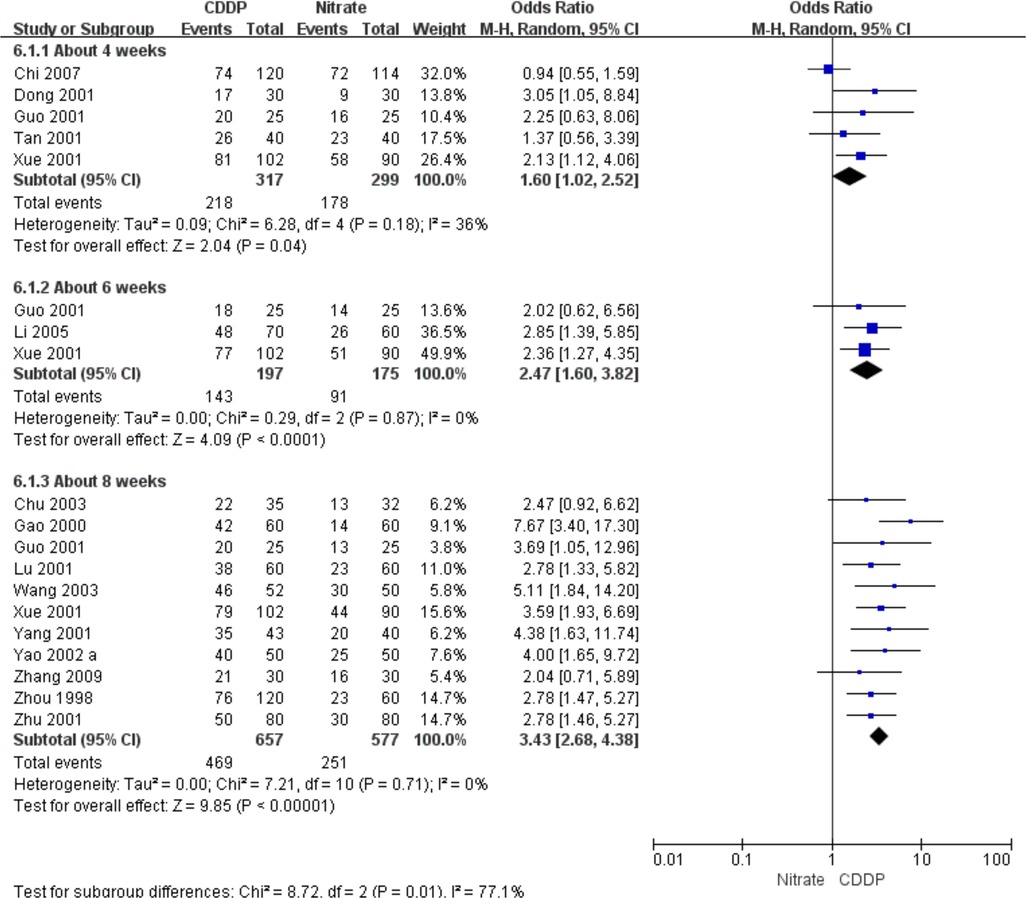
Fifteen RCTs reported the effective rate in ECG improvement. The meta-analyses with the random-effect model indicated that CDDP could significantly increase the effective rate in ECG improvement compared with nitrates (Pooled 5 RCTs, OR = 1.60, 95% CI: 1.02–2.52, P = 0.04, duration of about 4 weeks; Pooled 3 RCTs, OR = 2.47, 95% CI: 1.60–3.82, P < 0.0001, duration of about 6 weeks; Pooled 11 RCTs, OR = 3.43, 95% CI: 2.68–4.38, P < 0.00001, duration of about 8 weeks; Figure 7). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2.
3.5.2. Markedly effective rate in ECG improvement
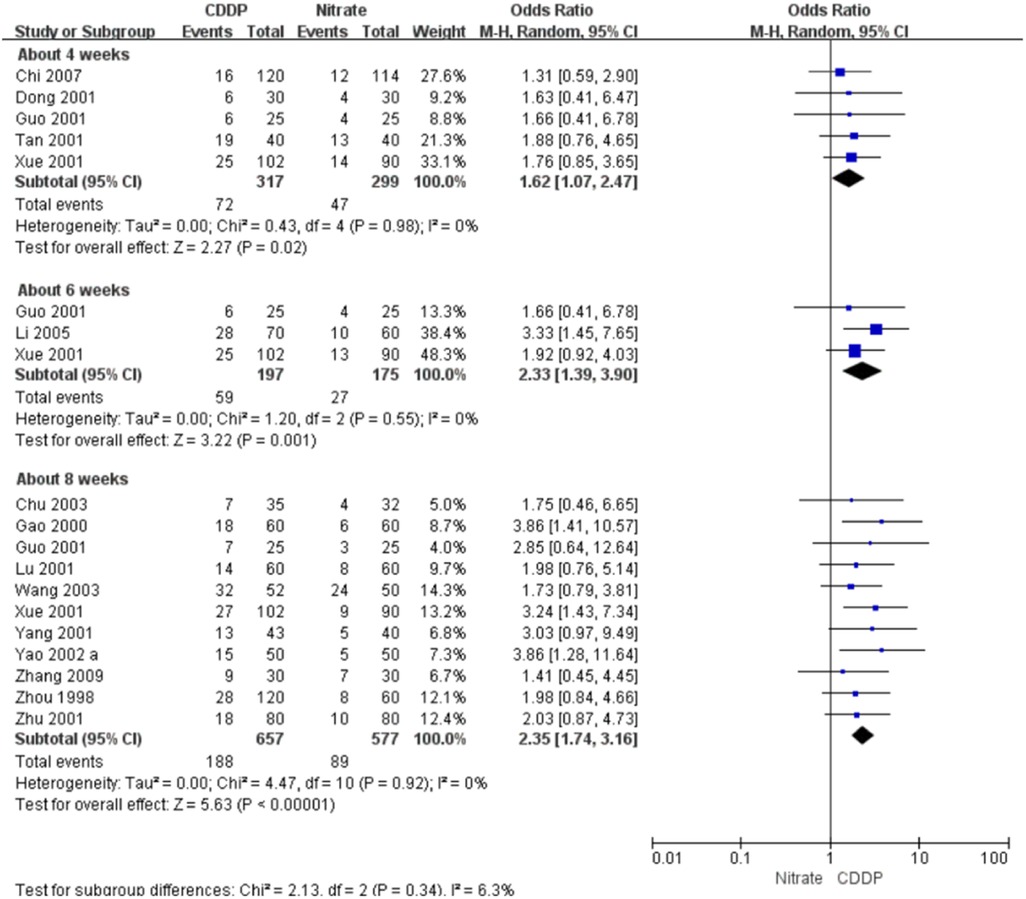
Fifteen RCTs reported the markedly effective rate in ECG improvement. The meta-analyses with the random-effect model indicated that CDDP could significantly increase the markedly effective rate in ECG improvement compared with nitrates (Pooled 5 RCTs, OR = 1.62, 95% CI: 1.07–2.47, P = 0.02, duration of about 4 weeks; Pooled 3 RCTs, OR = 2.33, 95% CI: 1.39–3.90, P = 0.001, duration of about 6 weeks; Pooled 11 RCTs, OR = 2.35, 95% CI: 1.74–3.16, P < 0.00001, duration of about 8 weeks; Figure 8). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2.
3.6. Adverse drug reactions
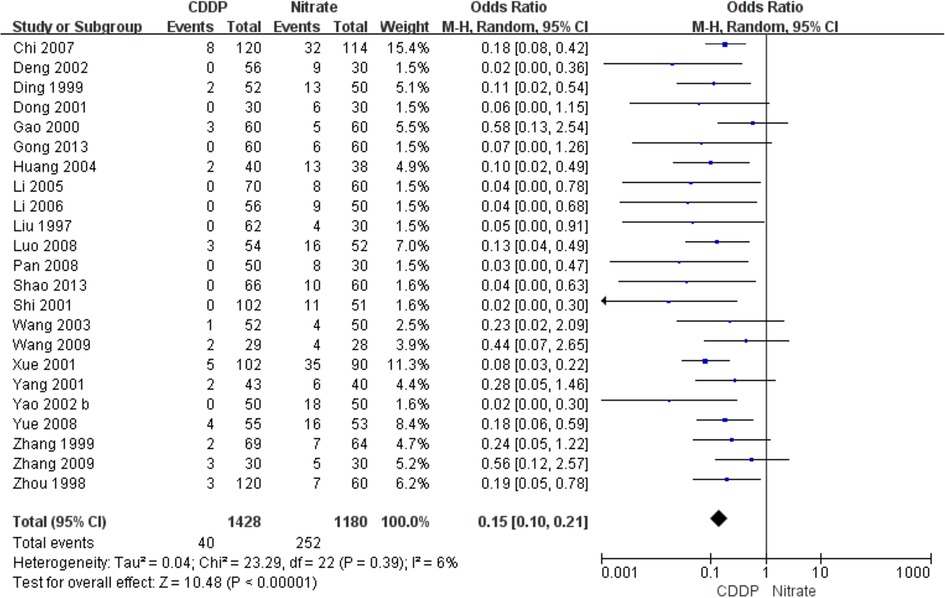
Twenty-three RCTs reported the adverse drug reactions. The pooled result showed that the incidence of adverse drug reactions in the CDDP group was lower than that in the nitrates group (Pooled 23 RCTs, OR = 0.15, 95% CI: 0.1–0.21, P < 0.00001, Figure 9). Specifically, adverse drug reactions included stomach discomfort in the CDDP group. In the nitrate group, adverse drug reactions included headache, dizziness, facial burning, nausea, etc.
3.7. Publication bias
Funnel plots of the meta-analyses are presented in Figure 10. Publication bias may be existed because of the asymmetry in some funnel plots. It may be associated with small sample size, poor methodological quality, etc.
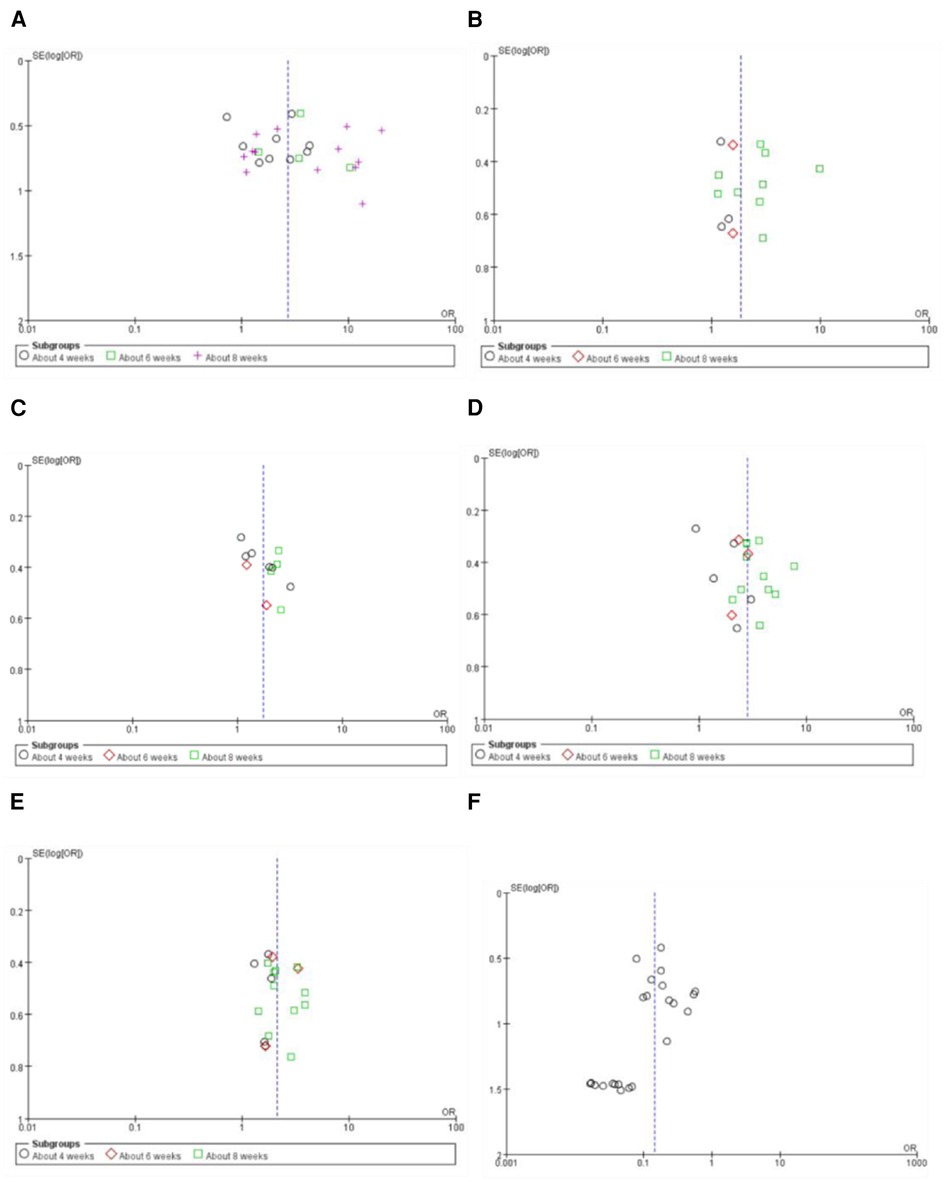
Figure 10. Funnel plot of the meta-analyses on effective rate in symptom improvement (A), markedly effective rate in symptom improvement (standard 1) (B), markedly effective rate in symptom improvement (standard 2) (C), effective rate in ECG improvement (D), markedly effective rate in ECG improvement (E), and the adverse drug reaction (F).
3.8. Grade of evidence
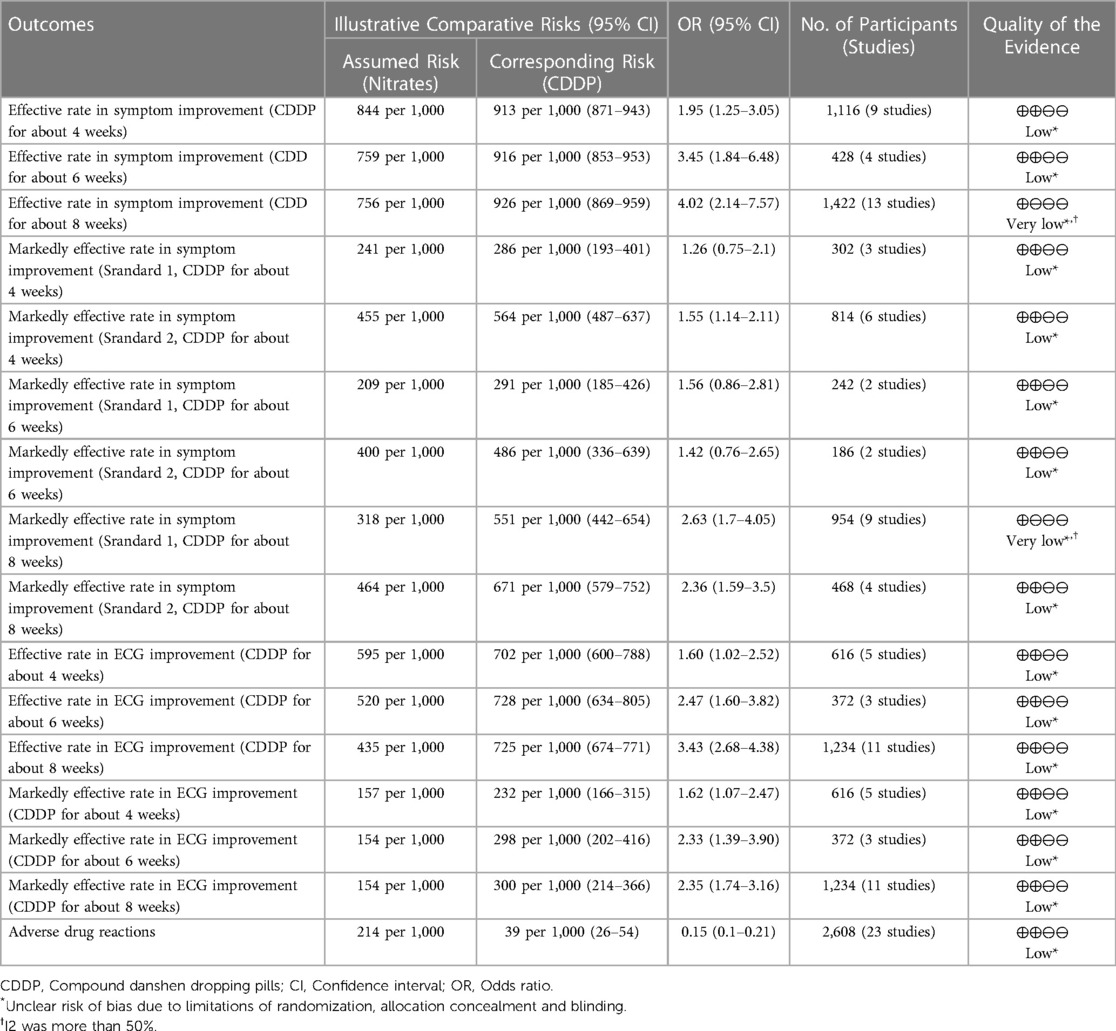
The grade of evidence for each outcome was presented in Table 3. The levels of the evidence for the effective rate and the markedly effective rate in symptom improvement ranged from very low to low. The levels of the evidence for the effective rate and markedly effective rate in ECG improvement were classified as low.
4. Discussion
Nitrates have been widely used in the management of angina in the clinical practice (44). However, the long-term use of nitrates may be limited because of the tolerance to nitrates (44). It may be caused by multiple factors, such as a burst of oxygen free radicals and vascular peroxynitrite formation (45–47). Many efforts are made to reduce the tolerance to nitrates, such as the intermittent nitrate therapy and long-acting nitrates (48). However, intermittent nitrate therapy may bring about the rebound ischemia (49), and long-acting nitrates may be associated with the endothelial dysfunction (48). At present, novel nitrate drugs are still under development (50, 51). Some side effects associated with nitrates for treating angina have been reported, such as headache, dizziness, and hypotension (52). Therefore, alternative therapies for angina are still needed.
CDDP has been approved for treating angina associated with coronary heart disease by the National Medical Products Administration in China for over twenty years. Many RCTs comparing CDDP with nitrates for treating angina has been published in recent years. A systematic review in 2015 suggested that CDDP were more effective than isosorbide dinitrate in improving the symptoms and ECG in patients with angina (53). However, some limitations on that study can't be ignored. For example, only isosorbide dinitrate as one type of nitrates was considered as the control intervention. The safety was not assessed and the subgroup analysis based on stable and unstable angina was not conducted. Another systematic review in 2021 did not conduct subgroup analyses based on the duration of the drugs, and did not report adverse drug reactions (54). In this study, an updated systematic review with the strict eligibility criteria was conducted to critically evaluate the efficacy and safety of CDDP vs. nitrates for treating SAP. The meta-analyses were conducted based on the duration of CDDP. The pooled results showed that the effective rate in symptom improvement and ECG improvement were significantly increased at about 4, 6 or 8 weeks after CDDP treatment compared to nitrates. The incidence of adverse reactions in the CDDP group was lower than that in the nitrates group. It means that CDDP with the duration of at least 4 weeks has the relative advantages in relieving symptoms associated with SAP, reducing the frequency of angina attacks or nitroglycerin consumption, and has relatively fewer adverse effects compared with nitrates. It provides a new insight into the management of SAP. CDDP with the duration of at least 4 weeks may be considered as an alternative to nitrates for treating SAP.
Several limitations should be taken into consideration when interpreting above results. Firstly, the effect size may be overestimated or underestimated due to the small sample size (less than 100) in most of included studies. Secondly, the risks of selection bias, performance bias, reporting bias and other bias for most of included studies were graded as unclear because of insufficient information. Thirdly, the mechanisms of CDDP for treating SAP aren't fully understood due to the lack of pharmacological evidence.
5. Conclusion
The present study suggests that CDDP with the duration of at least 4 weeks can be considered as an alternative to nitrates for treating SAP. However, more high-quality RCTs are still needed to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
MZ: methodology, investigation, data curation, formal analysis, writing—original draft, writing—review and editing. WW: writing—original draft, writing—review and editing. HS: writing—original draft, writing—review and editing. JZ: conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing. YH: conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
MZ, WW, HS and YH were employed by Tasly Pharmaceutical Group Co., Ltd. HS was also employed by Tasly Holding Group Co., Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1168730/full#supplementary-material.
References
1. Ohman EM. Clinical practice. Chronic stable angina. N Engl J Med. (2016) 374(12):1167–76. doi: 10.1056/NEJMcp1502240
2. Abbasi M, Neishaboury M, Koohpayehzadeh J, Etemad K, Meysamie A, Asgari F, et al. National prevalence of self-reported coronary heart disease and chronic stable angina pectoris: factor analysis of the underlying cardiometabolic risk factors in the SuRFNCD-2011. Glob Heart. (2018) 13(2):73–82 e1. doi: 10.1016/j.gheart.2018.01.001
3. Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, et al. Prospective observational longitudinal registry of patients with stable coronary artery disease I. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the international observational CLARIFY registry. JAMA Intern Med. (2014) 174(10):1651–9. doi: 10.1001/jamainternmed.2014.3773
4. Beatty AL, Spertus JA, Whooley MA. Frequency of angina pectoris and secondary events in patients with stable coronary heart disease (from the heart and soul study). Am J Cardiol. (2014) 114(7):997–1002. doi: 10.1016/j.amjcard.2014.07.009
5. Joshi PH, de Lemos JA. Diagnosis and management of stable angina: a review. JAMA. (2021) 325(17):1765–78. doi: 10.1001/jama.2021.1527
6. Wei J, Wu T, Yang Q, Chen M, Ni J, Huang D. Nitrates for stable angina: a systematic review and meta-analysis of randomized clinical trials. Int J Cardiol. (2011) 146(1):4–12. doi: 10.1016/j.ijcard.2010.05.019
7. Thadani U. Challenges with nitrate therapy and nitrate tolerance: prevalence, prevention, and clinical relevance. Am J Cardiovasc Drugs. (2014) 14(4):287–301. doi: 10.1007/s40256-014-0072-5
8. Chen W, Wang B, Ge Y, Xu H, Jiang C, Yu P, et al. A systematic review and meta-analysis of clinical research on treating angina pectoris of coronary heart disease with traditional Chinese medicine to promote blood circulation and remove blood stasis. Ann Palliat Med. (2021) 10(10):10506–14. doi: 10.21037/apm-21-2233
9. Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. (2013) 11(2):110–20. doi: 10.1016/S1875-5364(13)60037-0
10. Li S. Mapping ancient remedies: applying a network approach to traditional Chinese medicine. Science. (2015) 350(6262):S72–4.
11. Chen K, Chen M, Wang Q, Dong J, Ma S, Zhao X, et al. Potential molecular mechanism of compound danshen dripping pills in treatment of angina pectoris based on network pharmacology and molecular docking. Yaowu Pingjia Yanjiu. (2022) 45(7):1282–93.
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Archbold RA. Comparison between national institute for health and care excellence (NICE) and European society of cardiology (ESC) guidelines for the diagnosis and management of stable angina: implications for clinical practice. Open Heart. (2016) 3(1):e000406. doi: 10.1136/openhrt-2016-000406
14. Li S, Wang R, Zhang Y, Zhang X, Layon AJ, Li Y, et al. Symptom combinations associated with outcome and therapeutic effects in a cohort of cases with SARS. Am J Chin Med (Gard City N Y). (2006) 34(6):937–47. doi: 10.1142/S0192415X06004417
15. Shao Z, Ji L, Zhang H. Effect of compound danshen dripping pills on stable angina pectoris. People's Military Surgeon. (2013) 56(4):423–4.
16. Gong L. Curative effect of compound danshen dripping pills on 60 cases of stable angina pectoris. Chin J Geriatr Care. (2013) 11(2):54–5. doi: 10.3969/j.issn.1672-4860.2013.02.028
17. Zhang S. Therapeutic effect of compound danshen dripping pills on stable angina pectoris in the elderly. J Changchun Univ Chin. (2009) 25(1):89. doi: 10.1007/s11802-009-0089-6
18. Wang J. Curative effect of compound dasnhen dripping pills on 29 cases of stable angina pectoris. J Shanxi Univ Chin Med. (2009) 10(2):32–3. doi: 10.3969/j.issn.1671-0258.2009.02.013
19. Yue X. Effect of compound danshen dripping pills on stable angina pectoris of coronary heart disease. J Mod Med Health. (2008) 24(19):2888–9.
20. Pan C. Effect of compound Salvia miltiorrhiza dripping pills on stable angina pectoris. Tianjin Pharm. (2008) 20(4):56–7. doi: 10.3969/j.issn.1006-5687.2008.04.029
21. Luo J. Clinical observation of compound danshen dripping pills in the treatment of stable angina pectoris. China Med Her. (2008) 5(22):82. doi: 10.3969/j.issn.1673-7210.2008.22
22. Chi B, He T, Yang Y. Treatment of 120 cases of stable angina pectoris with compound danshen dripping pills. Shaanxi J Tradit Chin Med. (2007) 28(10):1283–5. doi: 10.3969/j.issn.1000-7369.2007.10.007
23. Li Y, Xi Y, Zhou C, Fang L, Liu A. Effect of compound danshen dripping pills on stable angina pectoris. Clin J Med Off. (2006) 34(6):673–4. doi: 10.3969/j.issn.1671-3826.2006.06.004
24. Li L, Yang H. Therapeutic effect of compound danshen dripping pills on 70 cases of stable angina pectoris. J Handan Med Coll. (2005) 18(1):34–5.
25. Huang S, Zou X, Li P. Clinical observation of compound danshen dripping pills and nitrate in the treatment of stable angina pectoris. Mod Diagn Treat. (2004) 15(6):349–50. doi: 10.3969/j.issn.1001-8174.2004.06.013
26. Wang X, Shen L, Yan L. Clinical analysis of compound danshen dripping pills in treatment of stable angina pectoris. J Inner Mongolia Med Univ. (2003) 25(3):204–5. doi: 10.3969/j.issn.1004-2113.2003.03.023
27. Chu X, Geng J. Clinical analysis of compound danshen dripping pills in treatment of stable angina pectoris. Heilongjiang Med J. (2003) (4):16–7.
28. Yao X. Clinical observation of compound danshen dripping pills in treating 50 cases of angina pectoris of coronary heart disease. J Emerg Tradit Chin. (2002) 11(1):26–7. doi: 10.3969/j.issn.1004-745X.2002
29. Yao F, Zhang Y, Huang M, Liu H, Sun Y. Treatment of 50 cases of stable angina pectoris with compound danshen dripping pills. Shandong J Tradit Chin. (2002) 21(3):147–9. doi: 10.3969/j.issn.0257-358X.2002.03.011
30. Deng G, He J, Li B. Treatment of stable angina pectoris with compound danshen dripping pills. Tianjin Pharm. (2002) 14(4):48. doi: 10.3969/j.issn.1006-5687.2002.04.029
31. Zhu Y. Comparison of curative effect of compound danshen dripping pill combined with nitrate in treating stable angina pectoris. West J Tradit Chin Med. (2001) 2:52–3. doi: 10.3969/j.issn.1004-6852.2001.02.046
32. Yang S, Huang T, Wang H. Effect of compound danshen dripping pills and nitrate on angina pectoris. J Wannan Med Coll. (2001) 20(3):198. doi: 10.3969/j.issn.1002-0217.2001.03.023
33. Xue F, Liu S. Clinical observation of compound danshen dripping pills in the treatment of stable angina pectoris. J Hunan Univ Chin Med. (2001) 21(2):36–8. doi: 10.3969/j.issn.1674-070X.2001.02.016
34. Tan S, Liu C. Clinical observation of compound danshen dripping pills in the treatment of stable angina pectoris. Heilongjiang Med J. (2001) 14(2):125. doi: 10.3969/j.issn.1006-2882.2001.02
35. Shi Y, Zhang Z, Li R, Guan Y. Analysis of curative effect of compound danshen dripping pills on stable angina pectoris. Inner Mongolia J Tradit Chin. (2001) 05:6. doi: 10.16040/j.cnki.cn15-1101.2001.05.008
36. Lu J, Sheng H. Treatment of stable angina pectoris with compound danshen dripping pills. West J Tradit Chin Med. (2001) 14(6):22–3. doi: 10.3969/j.issn.1004-6852.2001.06.016
37. Guo L, Li S, Zhang W. Clinical observation of curative effect of compound danshen dripping pills on angina pectoris. J Shandong Med Coll. (2001) 23(2):113–5. doi: 10.3969/j.issn.1674-0947.2001.02.011
38. Dong X, Zhang S. Effect of compound danshen dripping pills on stable angina pectoris. J Clin Med Pract. (2001) 2(3):143–5.
39. Gao B, Wu Y. Curative effect of compound danshen dripping pills on 60 cases of stable angina pectoris. J Binzhou Med Univ. (2000) 23(1):68.
40. Zhang G, Cao Y LUX, Liu Z, Liu W, Li W. Treatment of 69 cases of stable angina pectoris of coronary heart disease with compound danshen dripping pills. Jilin J Chin Med. (1999) 03:12.
41. Ding X, Jia L, Wang C. Comparison of the efficacy of compound danshen dripping pills in the treatment of stable angina pectoris. Suzhou Univ J Med Sci. (1999) 19(5):512–3.
42. Zhou Y. Treatment of 120 cases of stable angina pectoris with compound danshen dripping pills. Chin J New Drugs Clin Rem. (1998) 17(6):56–7.
43. Liu Z, Gao S, Deng J, Li H. Effect of compound danshen dropping pills on angina pectoris of coronary heart disease. Chin Tradit Pat Med. (1997) 19(07):20–1+53.
44. Klemenska E, Beręsewicz A. Bioactivation of organic nitrates and the mechanism of nitrate tolerance. Cardiol J. (2009) 16(1):11–9.19130411
45. Hink U, Oelze M, Kolb P, Bachschmid M, Zou M-H, Daiber A, et al. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J Am Coll Cardiol. (2003) 42(10):1826–34. doi: 10.1016/j.jacc.2003.07.009
46. Kosmicki MA. Long-term use of short- and long-acting nitrates in stable angina pectoris. Curr Clin Pharmacol. (2009) 4(2):132–41. doi: 10.2174/157488409788185016
47. Facchini E, Degiovanni A, Cavallino C, Lupi A, Rognoni A, Bongo AS. Beta-Blockers and nitrates pharmacotherapy and indications. Cardiovasc Hematol Agents Med Chem. (2015) 13(1):25–30. doi: 10.2174/1871525713666141219114708
48. Giuseppe C, Paul J, Hans-Ulrich I. Use of nitrates in ischemic heart. Expert Opin Pharmacother. (2015) 16(11):1567–72. doi: 10.1517/14656566.2015.1052742
49. Parker J. Potential problems with intermittent nitrate therapy. Can J Cardiol. (1996) 12(Suppl C):22C–4C. PMID: 8634920.8634920
50. Munzel T, Daiber A. Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vasc Pharmacol. (2018) 102:1–10. doi: 10.1016/j.vph.2017.11.003
51. Liang J, Zhang P, Yang H, Zhang Y, Yao T, Liu K, et al. Design, synthesis and biological evaluation of novel nitric oxide donors with antioxidative activity. Eur J Med Chem. (2022) 236:114331. doi: 10.1016/j.ejmech.2022.114331
52. Thadani U, Rodgers T. Side effects of using nitrates to treat angina. Expert Opin Drug Saf. (2006) 5(5):667–74. doi: 10.1517/14740338.5.5.667
53. Yao Y, Feng Y, Lin W. Systematic review and meta-analysis of randomized controlled trials comparing compound danshen dripping pills and isosorbide dinitrate in treating angina pectoris. Int J Cardiol. (2015) 182:46–7. doi: 10.1016/j.ijcard.2014.12.112
Keywords: compound danshen dripping pills (CDDP), nitrates, stable angina pectoris (SAP), systematic review, meta-analysis
Citation: Zhang M, Wang W, Sun H, Zhai J and Hu Y (2023) Compound danshen dripping pills vs. nitrates for stable angina pectoris: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1168730. doi: 10.3389/fcvm.2023.1168730
Received: 21 February 2023; Accepted: 24 April 2023;
Published: 22 May 2023.
Edited by:
Yaozu Xiang, Tongji University, ChinaReviewed by:
Zehuai Wen, Guangzhou University of Chinese Medicine, ChinaJing Hu, Beijing TCM hospital, China
© 2023 Zhang, Wang, Sun, Zhai and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingbo Zhai emhhaWppbmdib0Bmb3htYWlsLmNvbQ== Yunhui Hu dHNsLWh1eXVuaHVpQHRhc2x5LmNvbQ==
 Mengying Zhang1
Mengying Zhang1 He Sun
He Sun Jingbo Zhai
Jingbo Zhai Yunhui Hu
Yunhui Hu