
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 13 December 2023
Sec. Heart Valve Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1164104
This article is part of the Research Topic New Insights of Cardiac Rehabilitation: from Basic to Translational and Clinical Research Vol. II View all 5 articles
Transcatheter aortic valve replacement (TAVR) is a relatively new treatment method for aortic stenosis (AS) and has been demonstrated to be suitable for patients with varying risk levels. Indeed, among high-risk patients, TAVR outcomes are comparable to, or even better, than that of the traditional surgical aortic valve replacement (SAVR) method. TAVR outcomes, with respect to post-surgical functional capacity and quality of life, have also been found to be improved, especially when combined with cardiac rehabilitation (CR). CR is a multidisciplinary system, which integrates cardiology with other medical disciplines, such as sports, nutritional, mind-body, and behavioral medicine. It entails the development of appropriate medication, exercise, and diet prescriptions, along with providing psychological support, ensuring the cessation of smoking, and developing risk factor management strategies for cardiovascular disease patients. However, even with CR being able to improve TAVR outcomes and reduce post-surgical mortality rates, it still has largely been underutilized in clinical settings. This article reviews the usage of CR during both pre-and postoperative periods for valvular diseases, and the factors involved in influencing subsequent patient prognoses, thereby providing a direction for subsequent research and clinical applications.
Valvular heart disease (VHD), most commonly caused by rheumatic (RHD) and degenerative conditions, is prevalent in developed countries. In fact, over the past 60 years in those countries, VHD has shifted from being predominantly due to RHD to degenerative diseases, though RHD remains the main cause in developing countries. Concerning RHD, its most frequent pathological outcomes are mitral valve insufficiency and aortic stenosis (AS) (1). For instance, in 2017, ∼12.6 million cases, and 102,700 deaths, from calcific aortic valve disease, of which a severe form is AS, were documented worldwide (2). More generally, VHD incidence and mortality have been gradually increasing as the global population ages.
With regards to AS, it has been associated with high mortality rates, if left untreated once symptoms appear. Two main treatment options have been developed, the traditional surgical aortic valve replacement (SAVR), and the newer transcatheter aortic valve replacement (TAVR); other, more conservative approaches, only tackle the symptoms and are inadequate substitutes for SAVR/TAVR. Since TAVR was first successfully executed among human patients in 2002 (3), it has become an established, popular procedure for AS patients, though concerns regarding post-surgical patient health management are still present. Cardiac rehabilitation (CR) has thus been considered a possible solution for such health management.
CR has been defined as a multidisciplinary collaborative program, which includes baseline patient assessment, exercise training, modification of cardiac risk factors, such as lipid levels, hypertension, weight, diabetes, and smoking, as well as psychosocial assessment and evaluation of outcomes (4). Furthermore, cardiac rehabilitation of the patient is a holistic strategy for optimal medical, physiological, psychological, social, and vocational performance following an acute cardiac event, and the upscaling of optimal medical therapy is part of the CR program. Timely medication adjustments as needed during cardiac rehabilitation (5). CR has been proven to be beneficial for cardiovascular disease patients, particularly for those with heart failure (HF) or coronary artery disease (CAD) (4, 5). However, the utility of CR for VHD still does not have definitive guideline recommendations. Furthermore, CR's impact on TAVR patient prognosis, especially for the pre-and post-operation periods, needs to be further defined. This review summarizes the current knowledge on the application of CR among TAVR patients, both pre-and post-operation, and the factors that influence subsequent prognoses, to provide possible directions for future studies and clinical applications.
Since its first clinical application in France in 2002, TAVR has been established as the procedure of choice for elderly patients with severe AS and a high risk of perioperative death with SAVR (3), which yielded results comparable, or superior, to that of SAVR (6–11). TAVR also could serve as a viable treatment option for AS patients with clinical comorbidities (12), or multiple anatomical anomalies, particularly congenital ones, such as bicuspid aortic valves (13, 14). Furthermore, Makkar et al. showed that no significant difference was found in the incidence of death or disabling stroke 5 years after TAVR compared with surgical aortic valve replacement in patients with aortic stenosis who were at moderate surgical risk (15), indicating that it may become more accepted among younger patients with lower risk levels (7, 9, 16).
As more studies demonstrate the benefits of CR in alleviating valvular diseases (17, 18), more AS patients have been willing to undergo CR, particularly in conjunction with SAVR or TAVR. However, CR is still underutilized among both groups (19), with little change in overall participation over the years (20). And, currently, SAVR patients are more likely to undergo CR, not TAVR. Multiple reasons have contributed to this underutilization (20): (1) TAVR is a relatively new treatment modality; as a result, clear guidelines for recommending CR to TAVR patients have not been fully formulated, and few studies have been conducted to investigate the effectiveness of CR among these patients. Additionally, stereotypes regarding AS are still widely-held among physicians, such as excessive activity being recommended against, all of which contributes to the low referral rate for CR among AVR patients. (2) Compared to procedures requiring open surgery, TAVR patients have smaller incisions, shorter hospital stays, faster postoperative patient recovery, and a higher percentage of patients discharged to home, all of which contributes to a sentiment that CR is unnecessary for them. (3) Lack of clear influencing factors, such as age, preoperative health status, risk of depression, and comorbidities, where the lack of measurable effects of these variables can interfere with CR. (4) Hospital the TAVR patient is at may not have the relevant rehabilitation facilities. (5) Patient may not have health insurance coverage for CR. (6) Effect of the global coronavirus disease 2019 (COVID-19) pandemic (21). The COVID-19 pandemic prompted widespread dramatically reducing the delivery of non-essential outpatient services including CR (22, 23). All of these possibilities, stemming from uncertainties among physicians, patients, and their families, with respect to the effectiveness and feasibility of conducting CR in the context of TAVR have resulted in low referral rates for CR. In general, owing to increasing rates of VHD, and thus TAVR demand, it is predictable that CR utilization would likely increase, as part of optimizing post-surgical patient prognoses.
Hospitalized patients with AS generally are severely symptomatic; as a result, they may be unable to perform physical activities or even be allowed to get out of bed. Owing to the physical status of the patient being a strong influence on post-TAVR prognosis, it would therefore be a point of interest to investigate whether pre-operative CR could affect this prognosis, which could, in turn, increase patient referrals for such rehabilitative procedures. Currently, insufficient evidence is present for pre-operative CR effectiveness, though it has been noted that the association of poor prognoses and decreased quality of life among cardiovascular disease patients, particularly with VHD, is more owed to the physical and mental functional status associated with cardiovascular defects, rather than the defects themselves (24).
As the population ages, more individuals will experience natural physiological frailty, which has been documented to be an independent predictor of cardiovascular disease prognosis. Concerning TAVR, frailty status was also a significant predictor for 1-year mortality post-surgery, compared to the separate Society of Thoracic Surgeons (STS) score, as shown by Rogers et al. (25). In the combined model with the European System for Cardiac Operative Risk Evaluation (EuroSCORE) and STS scores, Schoenenberger et al. found that the frailty index accounted for 58.2% and 77.6% of the predictive information, respectively. This suggests that the combination of the frailty index with conventional STS, as well as the EuroSCORE score, could significantly improve the predictive capability for 1-year mortality post-TAVR (26), compared to those scores alone. additionally, preoperative frailty assessment is expected to be valuable in distinguishing the development of new postoperative complications from simple exacerbation of pre-existing disease (27). Several recent studies have shown that preoperative frailty is associated with death, or poor short-term functional recovery post-SAVR/TAVR (28–31); in the long term, it is also associated with higher likelihoods of the aortic valve and HF-related hospitalizations (32). Therefore, this frailty could affect CR conduct, such as the initiation, type, intensity, frequency, and patient compliance with exercise training (33). Physicians at rehabilitation centers should thus collaborate with geriatricians to develop precise CR interventions for complex frail patients to ensure the most optimal survival prognoses, as well as determine whether using frailty measurements could improve outcomes for elderly and frail patients subjected to CR, particularly post-AVR. Aside from frailty, other geriatric syndromes elderly patients could present with, pre-operation, include cognitive deficits, severe dependency, and depression, all of which are strongly associated with longer postoperative hospital stays, as well as poorer functional and clinical outcomes after discharge (27, 34). Indeed, a study by Khan et al. found that the presence of cognitive deficits predicted postoperative delirium and mortality after TAVR (35), emphasizing the value of screening for geriatric risk factors before TAVR to identify high-risk patients.
Preoperative CR could also potentially improve functional capacity and shorten hospital stay lengths for patients undergoing surgery (36). TAVR patients generally have moderate to high-risk status and are thus vulnerable to perioperative respiratory infections. Weber et al. showed that pre-interventional inspiratory muscle training (IMT), though, significantly improved inspiratory muscle function, along with a 75% reduction in pneumonia, and a 25% reduction in hospitalization length among patients who underwent this physiotherapy during the perioperative period (37). IMT is an important preoperative intervention that could reduce the incidence of postoperative pulmonary complications (PPCs) (38, 39). Additionally, among younger, low-to-middle-risk TAVR patients, IMT has also been shown to facilitate postoperative recovery, in the form of improving functional capacity submaximal and inspiratory muscle strength (40, 41).
Regularly-scheduled exercises, along with other interventions, such as medications, are an established component of CR, and could significantly complement the effects of TAVR. Several studies have shown intensive post-operative recovery regimens, such as short-term exercise-based CR, especially for Phase I during hospitalization, could increase functional capacity, quality of life (42–44), and exercise tolerance, particularly with respect to the distance in the 6 min walk test (6MWT) (18, 45) and maximum workload (46–48). These effects are coupled with lowered hospitalization lengths (49), frailty (50), anxiety, and disability. As TAVR patients are older and more associated with non-cardiovascular comorbidities (51), postoperative CR may thus be beneficial in reducing non-cardiovascular-related mortality risks, such as infection or unintentional injuries (52). Indeed, two recent randomized controlled trials of IMT performed postoperatively have shown that conventional CR improved 6MWT results, which was augmented when combined with IMT (53, 54). IMT itself, as shown by Xu Lin et al., was able to improve exercise tolerance, pulmonary ventilation function, and inspiratory muscle strength, along with shortening postoperative hospital stay and reducing postoperative complications among TAVR patients. All of these outcomes appear to have sustained effects on improving survival times (54), though, the study was limited by its lack of evaluation on hard endpoints, such as patient readmission rates and mortality, as well as a short post-discharge follow-up of 3 months, and a high rate of patients lost to follow-up (35.4% at 3 months). Therefore, the long-term efficacy of IMT still needs to be explored in future studies by examining longer follow-up periods and using hard endpoints, such as readmission rates and mortality.
Exercise-based CR is an essential component for long-term comprehensive patient management post-TAVR. 6MWT is a relatively good evaluation method for CR, as it is simple, safe, effective, and able to be used at home to guide CR for TAVR patients (55–57). The gold standard, though, is the cardiopulmonary exercise test (CPET), which is more suitable for use in developing CR protocol if the patient has satisfactory post-operative indicators. With respect to the efficacy and safety of exercise-based CR, a randomized pilot trial has shown that an 8-week moderate combined endurance and strength training was safe, with no adverse effects on valve prosthesis, kidney, or neurohumoral function (58), and was able to improve long-term oxygen consumption at anaerobic threshold (VO2AT). However, peak VO2, muscle strength, or quality of life was unchanged (59), which contradicts findings from several other studies demonstrating that post-operative exercise was able to improve peak VO2 (60–62), though a longer follow-up period may be required to fully confirm the effects of exercise training on muscle strength and other quality of life indicators. Nevertheless, despite the risk of post-exercise complications, such as arrhythmias, musculoskeletal injuries, or chest pain, patients after TAVR can undergo CR safely and successfully if monitored and guided by rehabilitation physicians (61). Indeed, exercise did not affect aortic regurgitation severity or other valve functional parameters, suggesting that short-term postoperative endurance or resistance training was safe for valve integrity; these training procedures could even be conducted in elderly cohorts including patients in their 90s. Furthermore, A randomized controlled trial by Tamulevičiūtė-Prascienė et al. indicated besides general aerobic exercise, specially-tailored resistance/balance training is also well-tolerated among TAVR patients (48).
As for CR protocols, early implementation was found by Sire et al. to enhance patient exercise tolerance and quality of life, in which physical training shortly after AVR yielded rapid, sustained improvements in work capacity, without corresponding increases in cardiac load. However, the return to work was less influenced by training and socio-occupational assistance (63). Other case studies also showed that developing individualized, medically-supervised training programs safely and effectively returned the strength and fitness levels of young patients to that of preoperative levels (64). Along with the early implementation of post-operative training, the regimen used should be staged, going from simple to complex, passive to active, and bed to the floor, as well as gradually increasing exercise types, amounts, and duration.
Overview of the main clinical studies on cardiac rehabilitation after transcatheter aortic valve replacement is shown in Table 1.
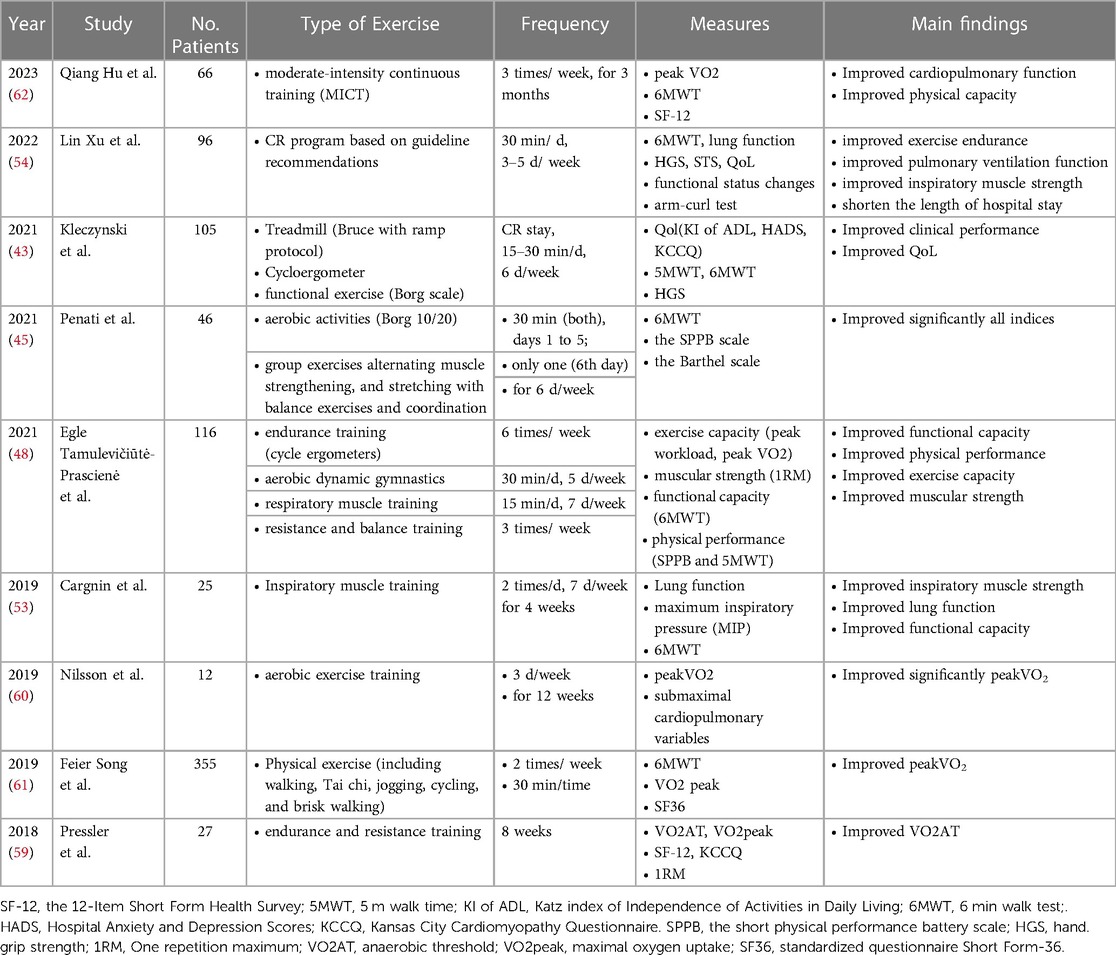
Table 1. Overview of the main clinical studies on cardiac rehabilitation after transcatheter aortic valve replacement.
Compared to SAVR, less evidence exists on CR utilization among TAVR patients, in which recommendations have been outlined to implement CR as soon as possible among SAVR patients. However, the lowest-functioning AVR patients have been noted to be more likely to be female, older, possess higher STS, be classified with New York Heart Association (NYHA) classification III/IV, use more home oxygen, exhibit more comorbidities as well as having higher fall frequencies, mortality, and stroke disability rates, compared to those with more active functional statues (29, 32, 65). This patient demographic profile would more likely benefit from TAVR, but their greater frailty has made it more difficult to obtain CR evidence. Nonetheless, a meta-analysis found that CR yielded similar improvements in the Barthel index and 6MWD post-SAVR and TAVR (66). Therefore, the application of CR as soon as possible after surgery is also highly recommended for TAVR patients, as it would aid in obtaining higher quality of life and functional exercise capacity improvements.
Aging, itself a risk factor, could affect post-surgical patient prognoses, in which TAVR patients tend to be older, as well as having more severe AS, comorbidities, and higher surgical risk; in particular, they are less able to tolerate open-heart surgery. A study from Attinger-Toller et al. found a linear trend between increasing age and all-cause mortality, stroke, and pacemaker implantation, during both the perioperative and long-term follow-up periods after TAVR (67). However, young patients are not risk-free from TAVR, as its risk is also related to the presence of comorbidities. Therefore, it is recommended, particularly for elderly patients, but also for young patients, to undergo CR post-TAVR, though the aging effect should also be taken into consideration for evaluating the effectiveness of CR.
The nutritional status of AVR patients is also an important factor behind post-surgical recovery and CR effectiveness. For instance, absolute iron deficiency is a common occurrence post-cardiac surgery, being diagnosed in up to 10% of the patient population. It is associated with prolonged postoperative stays in the intensive care unit, along with lowered exercise tolerance and increased safety risks (68, 69). Additionally, many AS patients have diabetes, which is also an independent risk factor for post-surgical iron deficiency, illustrating the importance of plasma glucose levels on post-AVR recovery and the development of possible complications. As a result, routine screening for iron deficiency is strongly recommended for patients referred to the CR unit. In light of this phenomenon, rehabilitation physicians should account for the lowered functional capacity of patients with absolute iron deficiency post-AVR when developing individualized CR protocols, with particular attention paid to specific nutritional and pharmacological requirements.
Another example of the importance of considering nutritional status is demonstrated in a study conducted by Hebeler et al., which compared various functional and nutritional markers, such as gait speed, hand grip strength, serum albumin, and Katz Activities of Daily Living, in a pre-TAVR risk assessment analysis for 1-year mortality assessment. There, it was found that albumin was the sole marker associated with higher mortality (70), and that pre-operative adjustment of serum albumin levels could lower TAVR risk scores. Furthermore, this adjustment could exert a protective effect among obese patients (71–73), yielding pronounced improvements in 6MWT results post-surgery (46). However, this result contradicts observations from other studies (74); thus, further research may be required to fully validate this claim.
All of these findings thus indicate that aside from exercises and medications, nutritional therapy should be one of the 5 core components prescribed for CR, and that beyond routine general nutritional support, rehabilitation practitioners should also focus on individualized nutritional therapy. Furthermore, future studies should be carried out to determine whether optimizing specific nutritional statuses could improve outcomes after TAVR.
CR has long been underutilized among women, with men being ∼1.5 times more likely to be referred to rehabilitation centers (75), owing to differences in regional distributions, religious cultures, and economics. Furthermore, CR referral rates for AVR women are even lower. With respect to gender differences for AVR, the gender-specific risk of SAVR is independently associated with adverse prognoses (76, 77). On the other hand, TAVR women, compared to men, have lower 1-year mortality rates, despite having higher incidences of vascular and bleeding complications (78–81). Therefore, it may be more advisable to recommend TAVR for female patients with AS (82, 83). Indeed, A meta-analysis by Straiton showed that older women are more common among TAVR patients (16), and being a woman is an independent predictor for post-TAVR admission to rehabilitation facilities. This is due to women, pre-TAVR, being frailer than men (84), in turn serving as a major obstacle for post-TAVR functional recovery and subsequently increasing rehabilitation demands. This greater frailty also contributes to TAVR women having a higher in-hospital mortality rate vs. men (85), as highly-frail women pre-TAVR become even frailer pos-TAVR, leading to longer hospital stays, as well as increased complications, mortality, and other adverse outcomes (32, 86, 87). However, pre-operative rehabilitation approaches could lower the likelihood of such adverse outcomes among TAVR women. Therefore, it is strongly recommended to increase CR utilization among such female patients.
The psychological state of the patient also has a significant impact on post-TAVR recovery. Psychological disorders, such as depression and anxiety, are prevalent among AS patients prior to surgery (88, 89). Combining adverse psychological factors such as depression and anxiety increases the risk of poorer prognosis and death after cardiac events, increases the risk of short-term postoperative functional decline (90–92), prolongs postoperative hospital stays or increases readmission rates within 30 days of discharge, and decreases adherence to medications and recommended treatments (93). Pre-operative depression is an independent risk factor for death after heart valve surgery. Michael Ho et al. studied an unadjusted 6-month mortality rate of 13.2% in patients with depression (94). In addition, patients with moderate to severe anxiety before cardiac surgery present with higher postoperative pain scores and a significantly increased need for intra- and postoperative analgesia, according to Muhammad Kashif et al. (95). SAVR or TAVR, though, could aid in reducing anxiety among elderly patients with AS (89, 96) and increasing confidence in their bodily states (16, 97), while CR could aid in improving depressive states (18, 98–100). Screening for psychological disorders should be incorporated into preoperative risk stratification (101), and future research must determine whether interventions to treat psychological disorders preoperatively or postoperatively can improve outcomes. Studies have shown that one in five adults has moderate to very severe mental health symptoms at the time of entry into a CR program, and these patients are significantly less likely to complete a CR program (102, 103). The challenge is to identify those patients who may have difficulty recovering on their own, and refer them to rehabilitation centers so that they could promptly receive appropriate CR, to maximize their abilities to perform daily living activities and enhance their sense of well-being.
Delays in detecting symptoms, referrals (77, 104, 105), as well as denial of symptoms, are common among AS patients (106, 107), especially in women (84). These late referrals and refusal of surgery, and subsequently the lack of appropriate care, could result in poorer outcomes among patients with VHD, which is characterized by high morbidity and mortality. Heart valve clinics, therefore, could help patients identify these risk factors early, as well as determine the best times to undergo surgery, via risk stratification (108). With respect to CR, the COVID-19 pandemic has significantly decreased its global utilization (22), though, with recent advances, remote technologies have become increasingly capable of assisting with CR administration. These technologies could also be useful for high-risk TAVR patients, as they typically are severely frail and impaired in mobility and cognition, which renders traditional outpatient CR infeasible and necessitates the usage of other modalities to ensure engagement and compliance (65). In light of current studies or guidelines, remote technologies may also be used to facilitate community- or home-based CR (HBCR) (109–113).
Recruiting and referring older post-TAVR participants for CR has been demonstrated in the literature to be feasible (114) and safe (69). With respect to specific referral approaches, both center-based and HBCR had similar positive effects at the end of the intervention period, but HBCR was better at promoting long-term behavioral changes, subsequently yielding more lasting improvements after the active intervention ended (115). HBCR patients, post-TAVR, also had higher compliance and physical activity levels, especially among those who did not participate in traditional center-based CR. All these findings thus suggest that HBCR could address poor compliance with center-based rehabilitation, among a subset of patients, particularly in older adults.
Patients undergoing TAVR have a high rehospitalization rate, nearly 50% after 1-year. The most common causes of rehospitalization are HF and bleeding (116). Despite TAVR being able to significantly improve symptoms and quality of life among AS patients, postoperative patient mortality remains high, being 12.2% in France (117), 5.4% in Italy (118), and 12.4% in Germany (119), 30 days post-operation. Furthermore, in Germany, non-home discharge post-TAVR is associated with a high 1-year mortality risk (120). Multiple risk factors are associated with non-home discharge, including older age, non-transfemoral access, being female, frailty status, chronic pulmonary history, pacemaker placement, and insulin-dependent diabetes mellitus. These risk factors should be of particular concern when these patients are referred to the relevant rehabilitation center for treatment.
Cessation of smoking has been noted to be the most cost-effective strategy to prevent cardiovascular disease. As a result, smoking is prohibited in patients after AVR and should be mandated as part of CR strategies (121).
No definitive guidelines currently exist for recommended pre- and post-operative rehabilitative strategies for TAVR. However, based on the current findings, both pre-and post-operative CR, including exercise training, nutritional modifications, cession of smoking, and medications, under the supervision of trained medical professionals could be highly beneficial for TAVR patients, by further improving functional capacity and quality of life post-surgery. These studies, though, lack data comparing TAVR patients who have undergone CR vs. those who did not, making it difficult to distinguish whether the beneficial effects are due to symptomatic relief, or from CR participation. Furthermore, there is a lack of comparisons regarding the impact of receiving CR pre- vs. post-operatively. Therefore, future multicenter studies should be conducted to further differentiate the effects of CR in general, as well as pre-and post-operatively, on TAVR outcomes, along with promoting its utilization to positively influence factors affecting patient prognoses.
JZ: Study design, data analysis, and writing the original draft. JY: Paper screening and data extraction. JL and QG: Quality assessment, writing-review, and editing. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation (Project #82200315), Guangdong Basic and Applied Basic Research Foundation (2021A1515111145), Sanming Project of Medicine in Shenzhen (No. SZSM201412012), Major scientific research project of Shenzhen People’s Hospital (SYWGSJCYJ202301). All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. (2021) 18:853–64. doi: 10.1038/s41569-021-00570-z
2. Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation. (2020) 141:1670–80. doi: 10.1161/CIRCULATIONAHA.119.043391
3. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106:3006–8. doi: 10.1161/01.cir.0000047200.36165.b8
4. Bozkurt B, Fonarow GC, Goldberg LR, Guglin M, Josephson RA, Forman DE, et al. Cardiac rehabilitation for patients with heart failure: JACC expert panel. J Am Coll Cardiol. (2021) 77:1454–69. doi: 10.1016/j.jacc.2021.01.030
5. Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American heart association scientific statement from the council on clinical cardiology (subcommittee on exercise, cardiac rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity), in collaboration with the American association of cardiovascular and pulmonary rehabilitation. Circulation. (2005) 111:369–76. doi: 10.1161/01.CIR.0000151788.08740.5C
6. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1093/eurheartj/ehx391
7. Jørgensen TH, Thyregod HGH, Ihlemann N, Nissen H, Petursson P, Kjeldsen BJ, et al. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur Heart J. (2021) 42:2912–9. doi: 10.1093/eurheartj/ehab375
8. Lin P-H, Wei H-J, Hsieh S-R, Tsai H-W, Yu C-L, Lee W-L, et al. One-year and five-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement in a Taiwanese elderly population. J Clin Med. (2023) 12:3429. doi: 10.3390/jcm12103429
9. UK TAVI Trial Investigators, Toff WD, Hildick-Smith D, Kovac J, Mullen MJ, Wendler O, Mansouri A, et al. Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all-cause mortality in patients with aortic stenosis: a randomized clinical trial. JAMA. (2022) 327:1875–87. doi: 10.1001/jama.2022.5776
10. Takeji Y, Taniguchi T, Morimoto T, Saito N, Ando K, Shirai S, et al. Transcatheter aortic valve implantation vs. Surgical aortic valve replacement for severe aortic stenosis in real-world clinical practice. Circ J. (2020) 84:806–14. doi: 10.1253/circj.CJ-19-0951
11. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, et al. 5-Year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. (2018) 72:2687–96. doi: 10.1016/j.jacc.2018.08.2146
12. Munir A, Wahab A, Khan M, Khan H, Htun WW, Schreiber TL. Transcatheter bicuspid aortic valve replacement in turner syndrome: a unique experience of interventional cardiologist. J Cardiol Cases. (2018) 17:29–32. doi: 10.1016/j.jccase.2017.08.016
13. Waksman R, Craig PE, Torguson R, Asch FM, Weissman G, Ruiz D, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe bicuspid aortic valve stenosis. JACC Cardiovasc Interv. (2020) 13(9):1019–27. doi: 10.1016/j.jcin.2020.02.008
14. Elbadawi A, Saad M, Elgendy IY, Barssoum K, Omer MA, Soliman A, et al. Temporal trends and outcomes of transcatheter versus surgical aortic valve replacement for bicuspid aortic valve stenosis. JACC Cardiovasc Interv. (2019) 12:1811–22. doi: 10.1016/j.jcin.2019.06.037
15. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. (2020) 382:799–809. doi: 10.1056/NEJMoa1910555
16. Straiton N, Jin K, Bhindi R, Gallagher R. Functional capacity and health-related quality of life outcomes post transcatheter aortic valve replacement: a systematic review and meta-analysis. Age Ageing. (2018) 47:478–82. doi: 10.1093/ageing/afx203
17. Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur J Prev Cardiol. (2020) 28(5):460–95. doi: 10.1177/2047487320913379
18. Jafri SH, Hushcha P, Dorbala P, Bousquet G, Lutfy C, Klein J, et al. Physical and psychological well-being effects of cardiac rehabilitation on patients following mitral valve and aortic valve procedures. J Cardiopulm Rehabil Prev. (2021) 42(2):90–6. doi: 10.1097/HCR.0000000000000609
19. Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, et al. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. (2020) 13:e005902. doi: 10.1161/CIRCOUTCOMES.119.005902
20. Guduguntla V, Yaser JM, Keteyian SJ, Pagani FD, Likosky DS, Sukul D, et al. Variation in cardiac rehabilitation participation during aortic valve replacement episodes of care. Circ: Cardiovasc Qual Outcomes. (2022) 15(7):e009175. doi: 10.1161/CIRCOUTCOMES.122.009175
21. Varghese MS, Beatty AL, Song Y, Xu J, Sperling LS, Fonarow GC, et al. Cardiac rehabilitation and the COVID-19 pandemic: persistent declines in cardiac rehabilitation participation and access among US medicare beneficiaries. Circ Cardiovasc Qual Outcomes. (2022) 15:e009618. doi: 10.1161/CIRCOUTCOMES.122.009618
22. de Melo Ghisi GL, Xu Z, Liu X, Mola A, Gallagher R, Babu AS, et al. Impacts of the COVID-19 pandemic on cardiac rehabilitation delivery around the world. Glob Heart. (2021) 16:43. doi: 10.5334/gh.939
23. Marzolini S, de Melo Ghisi GL, Hébert A-A, Ahden S, Oh P. Cardiac rehabilitation in Canada during COVID-19. CJC Open. (2021) 3:152–8. doi: 10.1016/j.cjco.2020.09.021
24. Moons P, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, et al. Physical functioning, mental health, and quality of life in different congenital heart defects: comparative analysis in 3538 patients from 15 countries. Can J Cardiol. (2021) 37:215–23. doi: 10.1016/j.cjca.2020.03.044
25. Rogers T, Alraies MC, Moussa Pacha H, Bond E, Buchanan KD, Steinvil A, et al. Clinical frailty as an outcome predictor after transcatheter aortic valve implantation. Am J Cardiol. (2018) 121:850–5. doi: 10.1016/j.amjcard.2017.12.035
26. Schoenenberger AW, Moser A, Bertschi D, Wenaweser P, Windecker S, Carrel T, et al. Improvement of risk prediction after transcatheter aortic valve replacement by combining frailty with conventional risk scores. JACC Cardiovasc Interv. (2018) 11:395–403. doi: 10.1016/j.jcin.2017.11.012
27. Vogt F, Wicklein S, Gosch M, Jessl J, Hitzl W, Fischlein T, et al. Functionality and outcome in older patients with severe aortic stenosis (FOOPAS): an interdisciplinary study concept for a prospective trial. Clin Interv Aging. (2018) 13:185–93. doi: 10.2147/CIA.S154234
28. Arnold SV, Zhao Y, Leon MB, Sathananthan J, Alu M, Thourani VH, et al. Impact of frailty and prefrailty on outcomes of transcatheter or surgical aortic valve replacement. Circ Cardiovasc Interv. (2022) 15:e011375. doi: 10.1161/CIRCINTERVENTIONS.121.011375
29. Kotajarvi BR, Schafer MJ, Atkinson EJ, Traynor MM, Bruce CJ, Greason KL, et al. The impact of frailty on patient-centered outcomes following aortic valve replacement. J Gerontol A Biol Sci Med Sci. (2017) 72:917–21. doi: 10.1093/gerona/glx038
30. Komaki K, Yoshida N, Satomi-Kobayashi S, Tsuboi Y, Ogawa M, Wakida K, et al. Preoperative frailty affects postoperative complications, exercise capacity, and home discharge rates after surgical and transcatheter aortic valve replacement. Heart Vessels. (2021) 36:1234–45. doi: 10.1007/s00380-021-01793-3
31. Bertschi D, Moser A, Stortecky S, Zwahlen M, Windecker S, Carrel T, et al. Evolution of basic activities of daily living function in older patients one year after transcatheter aortic valve implantation. J Am Geriatr Soc. (2021) 69:500–5. doi: 10.1111/jgs.16927
32. Tuttle MK, Kiaii B, Van Mieghem NM, Laham RJ, Deeb GM, Windecker S, et al. Functional Status after transcatheter and surgical aortic valve replacement. JACC: Cardiovasc Interv. (2022) 15:728–38. doi: 10.1016/j.jcin.2022.01.284
33. Vigorito C, Abreu A, Ambrosetti M, Belardinelli R, Corrà U, Cupples M, et al. Frailty and cardiac rehabilitation: a call to action from the EAPC cardiac rehabilitation section. Eur J Prev Cardiol. (2017) 24:577–90. doi: 10.1177/2047487316682579
34. Bobet AS, Brouessard C, Le Tourneau T, Manigold T, de Decker L, Boureau A-S. Length of stay in older patients undergoing transcatheter aortic valve replacement: value of a geriatric approach. Gerontology. (2022) 68:746–54. doi: 10.1159/000518821
35. Khan MM, Lanctôt KL, Fremes SE, Wijeysundera HC, Radhakrishnan S, Gallagher D, et al. The value of screening for cognition, depression, and frailty in patients referred for TAVI. Clin Interv Aging. (2019) 14:841–8. doi: 10.2147/CIA.S201615
36. Waite I, Deshpande R, Baghai M, Massey T, Wendler O, Greenwood S. Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J Cardiothorac Surg. (2017) 12(1):91. doi: 10.1186/s13019-017-0655-8
37. Weber M, Klein U, Weigert A, Schiller W, Bayley-Ezziddin V, Wirtz DC, et al. Use of pre- and intensified postprocedural physiotherapy in patients with symptomatic aortic stenosis undergoing transcatheter aortic valve replacement study (the 4P-TAVR study). J Interv Cardiol. (2021) 2021:8894223. doi: 10.1155/2021/8894223
38. Hulzebos EHJ, Helders PJM, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NLU. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. (2006) 296:1851–7. doi: 10.1001/jama.296.15.1851
39. Gempel S, Cohen M, Milian E, Vidret M, Smith A, Jones I, et al. Inspiratory muscle and functional performance of patients entering cardiac rehabilitation after cardiac valve replacement. J Cardiovasc Dev Dis. (2023) 10:142. doi: 10.3390/jcdd10040142
40. Cordeiro ALL, de Melo TA, Neves D, Luna J, Esquivel MS, Guimarães ARF, et al. Inspiratory muscle training and functional capacity in patients undergoing cardiac surgery. Braz J Cardiovasc Surg. (2016) 31:140–4. doi: 10.5935/1678-9741.20160035
41. Dsouza FV, Amaravadi SK, Samuel SR, Raghavan H, Ravishankar N. Effectiveness of inspiratory muscle training on respiratory muscle strength in patients undergoing cardiac surgeries: a systematic review with meta-analysis. Ann Rehabil Med. (2021) 45:264–73. doi: 10.5535/arm.21027
42. Sola M, Ramm CJ, Kolarczyk LM, Teeter EG, Yeung M, Caranasos TG, et al. Application of a multidisciplinary enhanced recovery after surgery pathway to improve patient outcomes after transcatheter aortic valve implantation. Am J Cardiol. (2016) 118(3):418–23. doi: 10.1016/j.amjcard.2016.05.015
43. Kleczynski P, Trebacz J, Stapor M, Sobczynski R, Konstanty-Kalandyk J, Kapelak B. Inpatient cardiac rehabilitation after transcatheter aortic valve replacement is associated with improved clinical performance and quality of life. J Clin Med. (2021) 10(10):2125. doi: 10.3390/jcm10102125
44. Russo N, Compostella L, Tarantini G, Setzu T, Napodano M, Bottio T, et al. Cardiac rehabilitation after transcatheter versus surgical prosthetic valve implantation for aortic stenosis in the elderly. Eur J Prev Cardiol. (2014) 21:1341–8. doi: 10.1177/2047487313494029
45. Penati C, Incorvaia C, Mollo V, Lietti F, Gatto G, Stefanelli M, et al. Cardiac rehabilitation outcome after transcatheter aortic valve implantation. Monaldi Arch Chest Dis. (2021) 91(2). doi: 10.4081/monaldi.2021.1621
46. Eichler S, Salzwedel A, Reibis R, Nothroff J, Harnath A, Schikora M, et al. Multicomponent cardiac rehabilitation in patients after transcatheter aortic valve implantation: predictors of functional and psychocognitive recovery. Eur J Prev Cardiol. (2017) 24:257–64. doi: 10.1177/2047487316679527
47. Tarro Genta F, Tidu M, Corbo P, Bertolin F, Salvetti I, Bouslenko Z, et al. Predictors of survival in patients undergoing cardiac rehabilitation after transcatheter aortic valve implantation. J Cardiovasc Med (Hagerstown). (2019) 20:606–15. doi: 10.2459/JCM.0000000000000829
48. Tamulevičiūtė-Prascienė E, Beigienė A, Thompson MJ, Balnė K, Kubilius R, Bjarnason-Wehrens B. Cardiac rehabilitation outcome after transcatheter aortic valve implantation. BMC Geriatr. (2021) 21:23. doi: 10.1186/s12877-020-01964-3
49. Furukawa H, Kangai K, Minami K, Ohura K, Ochi Y, Ikumoto H, et al. Initial clinical experience of early cardiac rehabilitation for very elderly patients over 85 years old following open heart surgery. Kyobu Geka Jpn J Thorac Surg. (2012) 65(6):440–5. Available at: https://pubmed.ncbi.nlm.nih.gov/22647324/
50. Lutz AH, Delligatti A, Allsup K, Afilalo J, Forman DE. Cardiac rehabilitation is associated with improved physical function in frail older adults with cardiovascular disease. J Cardiopulm Rehabil Prev. (2020) 40:310–8. doi: 10.1097/HCR.0000000000000537
51. Tarro Genta F, Tidu M, Bouslenko Z, Bertolin F, Salvetti I, Comazzi F, et al. Cardiac rehabilitation after transcatheter aortic valve implantation compared to patients after valve replacement. J Cardiovasc Med (Hagerstown). (2017) 18:114–20. doi: 10.2459/JCM.0000000000000494
52. Butter C, Groß J, Haase-Fielitz A, Sims H, Deutsch C, Bramlage P, et al. Impact of rehabilitation on outcomes after TAVI: a preliminary study. J Clin Med. (2018) 7:326. doi: 10.3390/jcm7100326
53. Cargnin C, Karsten M, da Costa Guaragna JCV, Dal Lago P. Inspiratory muscle training after heart valve replacement surgery improves inspiratory muscle strength, lung function, and functional capacity: a randomized controlled trial. J Cardiopulm Rehabil Prev. (2019) 39:E1–7. doi: 10.1097/HCR.0000000000000409
54. Xu L, Wei J, Liu J, Feng Y, Wang L, Wang S, et al. Inspiratory muscle training improves cardiopulmonary function in patients after transcatheter aortic valve replacement: a randomized clinical trial. Eur J Prev Cardiol. (2022) 30(2):191–202. doi: 10.1093/eurjpc/zwac269
55. Vitale G, Sarullo S, Vassallo L, Di Franco A, Mandalà G, Marazia S, et al. Prognostic value of the 6-min walk test after open-heart valve surgery: experience of a cardiovascular rehabilitation program. J Cardiopulm Rehabil Prev. (2018) 38:304–8. doi: 10.1097/HCR.0000000000000340
56. La Rovere MT, Pinna GD, Maestri R, Olmetti F, Paganini V, Riccardi G, et al. The 6-minute walking test and all-cause mortality in patients undergoing a post-cardiac surgery rehabilitation program. Eur J Prev Cardiol. (2015) 22:20–6. doi: 10.1177/2047487313502405
57. Imamura T, Narang N, Ushijima R, Sobajima M, Fukuda N, Ueno H, et al. Prognostic impact of baseline six-minute walk distance following trans-catheter aortic valve replacement. J Clin Med. (2023) 12:2504. doi: 10.3390/jcm12072504
58. Pressler A, Christle JW, Lechner B, Grabs V, Haller B, Hettich I, et al. Exercise training improves exercise capacity and quality of life after transcatheter aortic valve implantation: a randomized pilot trial. Am Heart J. (2016) 182:44–53. doi: 10.1016/j.ahj.2016.08.007
59. Pressler A, Förschner L, Hummel J, Haller B, Christle JW, Halle M. Long-term effect of exercise training in patients after transcatheter aortic valve implantation: follow-up of the SPORT:TAVI randomised pilot study. Eur J Prev Cardiol. (2018) 25:794–801. doi: 10.1177/2047487318765233
60. Nilsson H, Nylander E, Borg S, Tamás É, Hedman K. Cardiopulmonary exercise testing for evaluation of a randomized exercise training intervention following aortic valve replacement. Clin Physiol Funct Imaging. (2019) 39:103–10. doi: 10.1111/cpf.12545
61. Song F, Zhan H, Liang Y, He X, Guo L. Corrigendum to “cardiac rehabilitation improved oxygen uptake measured by cardiopulmonary exercise test in patients after aortic valve surgery”. Rev Cardiovasc Med. (2019) 20:109. doi: 10.31083/j.rcm.2019.01.3183corr
62. Hu Q, Li Y-S, Ren Q, Liang Y-C, Zhang J, Wang Y-X, et al. Efficacy and safety of moderate-intensity continuous training on the improvement of cardiopulmonary function in patients after transcatheter aortic valve replacement (ENERGY): a randomized controlled trial. J Am Med Dir Assoc. (2023) S1525-8610(23):00416–4. doi: 10.1016/j.jamda.2023.04.025
63. Sire S. Physical training and occupational rehabilitation after aortic valve replacement. Eur Heart J. (1987) 8:1215–20. doi: 10.1093/oxfordjournals.eurheartj.a062195
64. Jenkins N, Adams J, Bilbrey T, McCray S, Schussler JM. Specificity of training in cardiac rehabilitation to facilitate a patient’s return to strenuous work following aortic valve replacement. Proc (Bayl Univ Med Cent). (2018) 31:72–5. doi: 10.1080/08998280.2017.1401843
65. Kim DH, Afilalo J, Shi SM, Popma JJ, Khabbaz KR, Laham RJ, et al. Evaluation of changes in functional Status in the year after aortic valve replacement. JAMA Intern Med. (2019) 179:383–91. doi: 10.1001/jamainternmed.2018.6738
66. Ribeiro GS, Melo RD, Deresz LF, Dal Lago P, Pontes MR, Karsten M. Cardiac rehabilitation programme after transcatheter aortic valve implantation versus surgical aortic valve replacement: systematic review and meta-analysis. Eur J Prev Cardiol. (2017) 24:688–97. doi: 10.1177/2047487316686442
67. Attinger-Toller A, Ferrari E, Tueller D, Templin C, Muller O, Nietlispach F, et al. Age-Related outcomes after transcatheter aortic valve replacement: insights from the SwissTAVI registry. JACC Cardiovasc Interv. (2021) 14:952–60. doi: 10.1016/j.jcin.2021.01.042
68. Tramarin R, Pistuddi V, Maresca L, Pavesi M, Castelvecchio S, Menicanti L, et al. Surgical and clinical outcome research (SCORE) group. Patterns and determinants of functional and absolute iron deficiency in patients undergoing cardiac rehabilitation following heart surgery. Eur J Prev Cardiol. (2017) 24:799–807. doi: 10.1177/2047487317689975
69. Abdul-Jawad Altisent O, Puri R, Regueiro A, Chamandi C, Rodriguez-Gabella T, Del Trigo M, et al. Predictors and association with clinical outcomes of the changes in exercise capacity after transcatheter aortic valve replacement. Circulation. (2017) 136:632–43. doi: 10.1161/CIRCULATIONAHA.116.026349
70. Hebeler KR, Baumgarten H, Squiers JJ, Wooley J, Pollock BD, Mahoney C, et al. Albumin is predictive of 1-year mortality after transcatheter aortic valve replacement. Ann Thorac Surg. (2018) 106:1302–7. doi: 10.1016/j.athoracsur.2018.06.024
71. Smith RL, Herbert MA, Dewey TM, Brinkman WT, Prince SL, Ryan WH, et al. Does body mass index affect outcomes for aortic valve replacement surgery for aortic stenosis? Ann Thorac Surg. (2012) 93(3):742–6. doi: 10.1016/j.athoracsur.2011.11.027
72. Sharma A, Lavie CJ, Elmariah S, Borer JS, Sharma SK, Vemulapalli S, et al. Relationship of body mass Index with outcomes after transcatheter aortic valve replacement: results from the national cardiovascular data-STS/ACC TVT registry. Mayo Clin Proc. (2020) 95:57–68. doi: 10.1016/j.mayocp.2019.09.027
73. Potapov EV, Loebe M, Anker S, Stein J, Bondy S, Nasseri BA, et al. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur Heart J. (2003) 24:1933–41. doi: 10.1016/j.ehj.2003.09.005
74. Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. (1999) 282:1547–53. doi: 10.1001/jama.282.16.1547
75. Colella TJF, Gravely S, Marzolini S, Grace SL, Francis JA, Oh P, et al. Sex bias in referral of women to outpatient cardiac rehabilitation? A meta-analysis. Eur J Prev Cardiol. (2015) 22:423–41. doi: 10.1177/2047487314520783
76. Hernandez-Vaquero D, Rodriguez-Caulo E, Vigil-Escalera C, Blanco-Herrera O, Berastegui E, Arias-Dachary J, et al. Differences in life expectancy between men and women after aortic valve replacement. Eur J Cardiothorac Surg. (2021) 60:681–8. doi: 10.1093/ejcts/ezab140
77. Bienjonetti-Boudreau D, Fleury M-A, Voisine M, Paquin A, Chouinard I, Tailleur M, et al. Impact of sex on the management and outcome of aortic stenosis patients. Eur Heart J. (2021) 42:2683–91. doi: 10.1093/eurheartj/ehab242
78. Kodali S, Williams MR, Doshi D, Hahn RT, Humphries KH, Nkomo VT, et al. Sex-specific differences at presentation and outcomes among patients undergoing transcatheter aortic valve replacement: a cohort study. Ann Intern Med. (2016) 164(6):377–84. doi: 10.7326/M15-0121
79. Vlastra W, Chandrasekhar J, García Del Blanco B, Tchétché D, de Brito FS, Barbanti M, et al. Sex differences in transfemoral transcatheter aortic valve replacement. J Am Coll Cardiol. (2019) 74:2758–67. doi: 10.1016/j.jacc.2019.09.015
80. Denegri A, Romano M, Petronio AS, Angelillis M, Giannini C, Fiorina C, et al. Gender differences after transcatheter aortic valve replacement (TAVR): insights from the Italian clinical service project. J Cardiovasc Dev Dis. (2021) 8:114. doi: 10.3390/jcdd8090114
81. Leone PP, Gohar A, Pagnesi M, Mangieri A, Stefanini G, Cacia M, et al. Clinical outcomes in women and men with small aortic annuli undergoing transcatheter aortic valve implantation: a multicenter, retrospective, propensity score-matched comparison. Int J Cardiol. (2023) 379:16–23. doi: 10.1016/j.ijcard.2023.02.044
82. Tarantini G, Baumgartner H, Frank D, Husser O, Bleiziffer S, Rudolph T, et al. Four-year mortality in women and men after transfemoral transcatheter aortic valve implantation using the SAPIEN 3. Catheter Cardiovasc Interv. (2021) 97:876–84. doi: 10.1002/ccd.29257
83. Chaker Z, Badhwar V, Alqahtani F, Aljohani S, Zack CJ, Holmes DR, et al. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. (2017) 6:e006370. doi: 10.1161/JAHA.117.006370
84. Van Mieghem NM, Reardon MJ, Yakubov SJ, Heiser J, Merhi W, Windecker S, et al. Clinical outcomes of TAVI or SAVR in men and women with aortic stenosis at intermediate operative risk: a post hoc analysis of the randomised SURTAVI trial. EuroIntervention. (2020) 16:833–41. doi: 10.4244/EIJ-D-20-00303
85. Shah RM, Hirji SA, Jolissaint JS, Lander HL, Shah PB, Pelletier MP, et al. Comparison of sex-based differences in home or nonhome discharge utilization of rehabilitative services and outcomes following transcatheter aortic valve implantation in the United States. Am J Cardiol. (2019) 123:1983–91. doi: 10.1016/j.amjcard.2019.03.008
86. Pighi M, Piazza N, Martucci G, Lachapelle K, Perrault LP, Asgar AW, et al. Sex-specific determinants of outcomes after transcatheter aortic valve replacement. Circ Cardiovasc Qual Outcomes. (2019) 12:e005363. doi: 10.1161/CIRCOUTCOMES.118.005363
87. Elbaz-Greener G, Rahamim E, Abu Ghosh Z, Carasso S, Yarkoni M, Radhakrishnan S, et al. Sex difference and outcome trends following transcatheter aortic valve replacement. Front Cardiovasc Med. (2022) 9:1013739. doi: 10.3389/fcvm.2022.1013739
88. Modica M, Castiglioni P, Minotti A, Faini A, Racca V, Ferratini M. Psychological profile in coronary artery by-pass graft patients vs. valve replacement patients entering cardiac rehabilitation after surgery. Sci Rep. (2018) 8:14381. doi: 10.1038/s41598-018-32696-5
89. Wegermann ZK, Mack MJ, Arnold SV, Thompson CA, Ryan M, Gunnarsson C, et al. Anxiety and depression following aortic valve replacement. J Am Heart Assoc. (2022) 11:e024377. doi: 10.1161/JAHA.121.024377
90. Tang VL, Cenzer I, McCulloch CE, Finlayson E, Cooper Z, Silvestrini M, et al. Preoperative depressive symptoms associated with poor functional recovery after surgery. J Am Geriatr Soc. (2020) 68:2814–21. doi: 10.1111/jgs.16781
91. Jiang W. Anxiety in individuals with cardiovascular diseases: a narrative review and expert opinion. Heart Mind. (2022) 6:52. doi: 10.4103/hm.hm_5_22
92. Liu M. Spotlight on the relationship between heart disease and mental stress. Heart Mind. (2021) 5:1. doi: 10.4103/hm.hm_12_21
93. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the heart and soul study. Arch Intern Med. (2005) 165:2508–13. doi: 10.1001/archinte.165.21.2508
94. Ho PM, Masoudi FA, Spertus JA, Peterson PN, Shroyer AL, McCarthy M, et al. Depression predicts mortality following cardiac valve surgery. Ann Thorac Surg. (2005) 79:1255–9. doi: 10.1016/j.athoracsur.2004.09.047
95. Kashif M, Hamid M, Raza A. Influence of preoperative anxiety level on postoperative pain after cardiac surgery. Cureus. (2022) 14:e22170. doi: 10.7759/cureus.22170
96. Petersen J, Vettorazzi E, Winter L, Schmied W, Kindermann I, Schäfers H-J. Physical and mental recovery after conventional aortic valve surgery. J Thorac Cardiovasc Surg. (2016) 152:1549–1556.e2. doi: 10.1016/j.jtcvs.2016.07.072
97. Kirk BH, De Backer O, Missel M. Transforming the experience of aortic valve disease in older patients: a qualitative study. J Clin Nurs. (2019) 28:1233–41. doi: 10.1111/jocn.14732
98. Imran HM, Baig M, Mujib M, Beale C, Gaw A, Stabile L, et al. Comparison of phase 2 cardiac rehabilitation outcomes between patients after transcatheter versus surgical aortic valve replacement. Eur J Prev Cardiolog. (2018) 25:1577–84. doi: 10.1177/2047487318792099
99. Świątkiewicz I, Di Somma S, De Fazio L, Mazzilli V, Taub PR. Effectiveness of intensive cardiac rehabilitation in high-risk patients with cardiovascular disease in real-world practice. Nutrients. (2021) 13:3883. doi: 10.3390/nu13113883
100. Horn N, Gärtner L, Rastan AJ, Andrási TB, Lenz J, Böning A, et al. Preoperative optimization of cardiac valve patients’ expectations: study protocol of the randomized controlled ValvEx-trial. Front Cardiovasc Med. (2023) 10:1105507. doi: 10.3389/fcvm.2023.1105507
101. Guzelhan Y, Ugurlucan M, Oztas DM, Beyaz MO, Unal O, Bektas N, et al. Anxiety and health-related quality of life after cardiac surgery. Arch Med Sci Atheroscler Dis. (2020) 5:e27–35. doi: 10.5114/amsad.2020.94376
102. Rao A, Zecchin R, Newton PJ, Phillips JL, DiGiacomo M, Denniss AR, et al. The prevalence and impact of depression and anxiety in cardiac rehabilitation: a longitudinal cohort study. Eur J Prev Cardiol. (2020) 27:478–89. doi: 10.1177/2047487319871716
103. Pardaens S, De Smedt D, De Bacquer D, Willems A-M, Verstreken S, De Sutter J. Comorbidities and psychosocial characteristics as determinants of dropout in outpatient cardiac rehabilitation. J Cardiovasc Nurs. (2017) 32:14–21. doi: 10.1097/JCN.0000000000000296
104. Brennan JM, Lowenstern A, Sheridan P, Boero IJ, Thourani VH, Vemulapalli S, et al. Association between patient survival and clinician variability in treatment rates for aortic valve stenosis. J Am Heart Assoc. (2021) 10:e020490. doi: 10.1161/JAHA.120.020490
105. Flannery L, Etiwy M, Camacho A, Liu R, Patel N, Tavil-Shatelyan A, et al. Patient- and process-related contributors to the underuse of aortic valve replacement and subsequent mortality in ambulatory patients with severe aortic stenosis. J Am Heart Assoc. (2022) 11:e025065. doi: 10.1161/JAHA.121.025065
106. Lowenstern A, Sheridan P, Wang TY, Boero I, Vemulapalli S, Thourani VH, et al. Sex disparities in patients with symptomatic severe aortic stenosis. Am Heart J. (2021) 237:116–26. doi: 10.1016/j.ahj.2021.01.021
107. Tang L, Gössl M, Ahmed A, Garberich R, Bradley SM, Niikura H, et al. Contemporary reasons and clinical outcomes for patients with severe, symptomatic aortic stenosis not undergoing aortic valve replacement. Circ Cardiovasc Interv. (2018) 11:e007220. doi: 10.1161/CIRCINTERVENTIONS.118.007220
108. Zilberszac R, Lancellotti P, Gilon D, Gabriel H, Schemper M, Maurer G, et al. Role of a heart valve clinic programme in the management of patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. (2017) 18:138–44. doi: 10.1093/ehjci/jew133
109. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. Home-based cardiac rehabilitation: a scientific statement from the American association of cardiovascular and pulmonary rehabilitation, the American heart association, and the American college of cardiology. Circulation. (2019) 140:e69–89. doi: 10.1161/CIR.0000000000000663
110. Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol. (2015) 22:35–74. doi: 10.1177/2047487313501093
111. Bhattal GK, Park KE, Winchester DE. Home-based cardiac rehabilitation (HBCR) in post-TAVR patients: a prospective, single-center, cohort, pilot study. Cardiol Ther. (2020) 9:541–8. doi: 10.1007/s40119-020-00186-3
112. Ashikaga K, Doi S, Yoneyama K, Suzuki N, Kuwata S, Koga M, et al. Efficacy and safety of home-based cardiac telemonitoring rehabilitation in patients after transcatheter aortic valve implantation: single-center usability and feasibility study. JMIR Rehabil Assist Technol. (2023) 10:e45247. doi: 10.2196/45247
113. Nkonde-Price C, Reynolds K, Najem M, Yang S-J, Batiste C, Cotter T, et al. Comparison of home-based vs center-based cardiac rehabilitation in hospitalization, medication adherence, and risk factor control among patients with cardiovascular disease. JAMA Netw Open. (2022) 5:e2228720. doi: 10.1001/jamanetworkopen.2022.28720
114. Nusbickel AJ, Randall MH, Plasschaert JM, Brown MP, Anderson RD, Arnaoutakis GJ, et al. Cardiac rehabilitation referral after transcatheter aortic valve replacement. Crit Pathw Cardiol. (2022) 21:162–4. doi: 10.1097/HPC.0000000000000302
115. Lindman BR, Gillam LD, Coylewright M, Welt FGP, Elmariah S, Smith SA, et al. Effect of a pragmatic home-based mobile health exercise intervention after transcatheter aortic valve replacement: a randomized pilot trial. Eur Heart J Digit Health. (2021) 2:90–103. doi: 10.1093/ehjdh/ztab007
116. Czarnecki A, Austin PC, Fremes SE, Tu JV, Wijeysundera HC, Ko DT. Association between transitional care factors and hospital readmission after transcatheter aortic valve replacement: a retrospective observational cohort study. BMC Cardiovasc Disord. (2019) 19:23. doi: 10.1186/s12872-019-1003-9
117. Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, et al. Transcatheter aortic valve implantation: early results of the FRANCE (French aortic national CoreValve and edwards) registry. Eur Heart J. (2011) 32:191–7. doi: 10.1093/eurheartj/ehq261
118. Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. (2011) 123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533
119. Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. (2011) 32(2):198–204. doi: 10.1093/eurheartj/ehq339
120. Okoh AK, Haik N, Singh S, Kaur K, Fugar S, Cohen M, et al. Discharge disposition of older patients undergoing trans-catheter aortic valve replacement and its impact on survival. Catheter Cardiovasc Interv. (2019) 94:448–55. doi: 10.1002/ccd.28069
Keywords: cardiac rehabilitation, transcatheter aortic valve replacement, aortic stenosis, frailty, exercise training
Citation: Zou J, Yuan J, Liu J and Geng Q (2023) Impact of cardiac rehabilitation on pre- and post-operative transcatheter aortic valve replacement prognoses. Front. Cardiovasc. Med. 10:1164104. doi: 10.3389/fcvm.2023.1164104
Received: 12 February 2023; Accepted: 28 November 2023;
Published: 13 December 2023.
Edited by:
Evaldas Girdauskas, Augsburg University Hospital, GermanyReviewed by:
Laura Adelaide Dalla Vecchia, Scientific Clinical Institute Maugeri (ICS Maugeri), Italy© 2023 Zou, Yuan, Liu and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjin Liu MTkwODIxOTE4QHFxLmNvbQ== Qingshan Geng Z2VuZ3FzaEAxNjMubmV0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.