- 1Department of Cardiology, Thoraxcentrum Twente, Medisch Spectrum Twente, Enschede, Netherlands
- 2Department of Health Technology and Services Research, Faculty BMS, Technical Medical Centre, University of Twente, Enschede, Netherlands
- 3Department of Cardiology, Haga Hospital, The Hague, Netherlands
- 4Department of Cardiology, Treant Zorggroep, Scheper Hospital, Emmen, Netherlands
- 5Department of Cardiology, Hillel Yaffe Medical Center, Hadera and B. Rappaport-Faculty of Medicine, Israel, Institute of Technology, Haifa, Israel
- 6Department of Cardiology, Rijnstate Hospital, Arnhem, Netherlands
- 7Department of Cardiology, Jessa Hospital, Hasselt, Belgium
- 8Department of Cardiology, Centre Hospitalier Universitaire de Charleroi, Charleroi, Belgium
- 9Department of Cardiology, Ziekenhuisgroep Twente, Almelo, Hengelo, Netherlands
Objectives: We assessed differences in risk profile and 3-year outcome between patients undergoing percutaneous coronary intervention (PCI) for premature and non-premature coronary artery disease (CAD).
Background: The prevalence of CAD increases with age, yet some individuals develop obstructive CAD at younger age.
Methods: Among participants in four randomized all-comers PCI trials, without previous coronary revascularization or myocardial infarction (MI), we compared patients with premature (men <50 years; women <55 years) and non-premature CAD. Various clinical endpoints were assessed, including multivariate analyses.
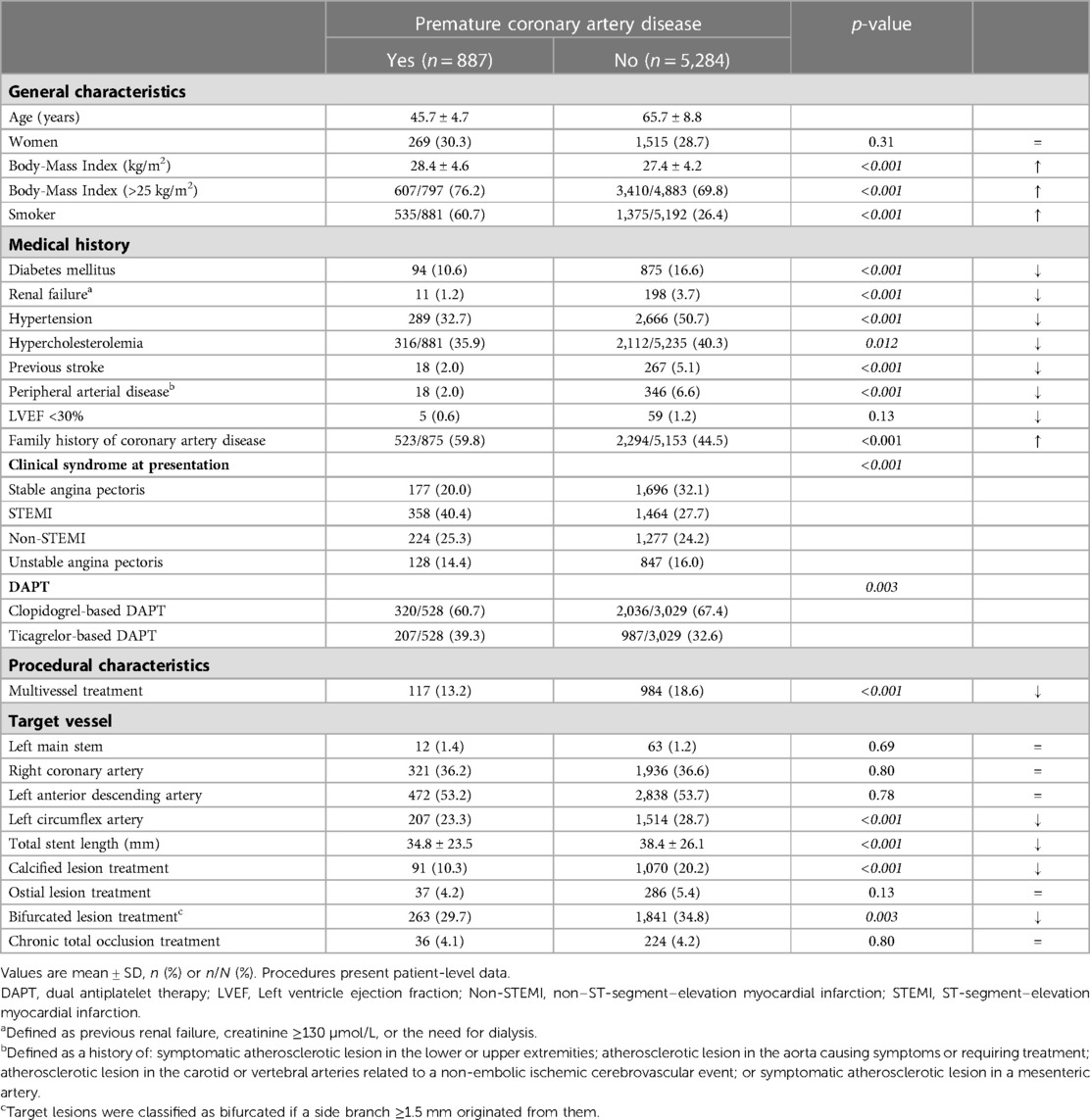
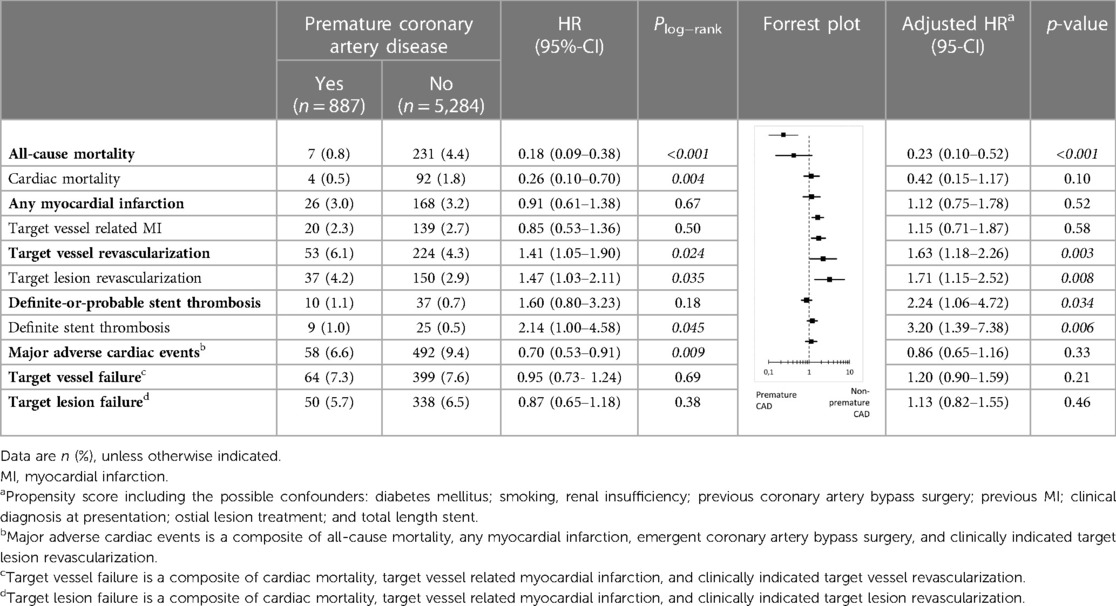
Results: Of 6,171 patients, 887(14.4%) suffered from premature CAD. These patients had fewer risk factors than patients with non-premature CAD, but were more often smokers (60.7% vs. 26.4%) and overweight (76.2% vs. 69.8%). In addition, premature CAD patients presented more often with ST-segment elevation MI and underwent less often treatment of multiple vessels, and calcified or bifurcated lesions. Furthermore, premature CAD patients had a lower all-cause mortality risk (adj.HR:0.23, 95%-CI: 0.10–0.52; p < 0.001), but target vessel revascularization (adj.HR:1.63, 95%-CI: 1.18–2.26; p = 0.003) and definite stent thrombosis risks (adj.HR:2.24, 95%-CI: 1.06–4.72; p = 0.034) were higher. MACE rates showed no statistically significant difference (6.6% vs. 9.4%; adj.HR:0.86, 95%-CI: 0.65–1.16; p = 0.33).
Conclusions: About one out of seven PCI patients was treated for premature CAD. These patients had less complex risk profiles than patients with non-premature CAD; yet, their risk of repeated revascularization and stent thrombosis was higher. As lifetime event risk of patients with premature CAD is known to be particularly high, further efforts should be made to improve modifiable risk factors such as smoking and overweight.
Clinical Trial Registration: [clinicaltrials.gov], TWENTE [NCT01066650]; DUTCH PEERS [NCT01331707]; BIO-RESORT [NCT01674803]; BIONYX [NCT02508714].
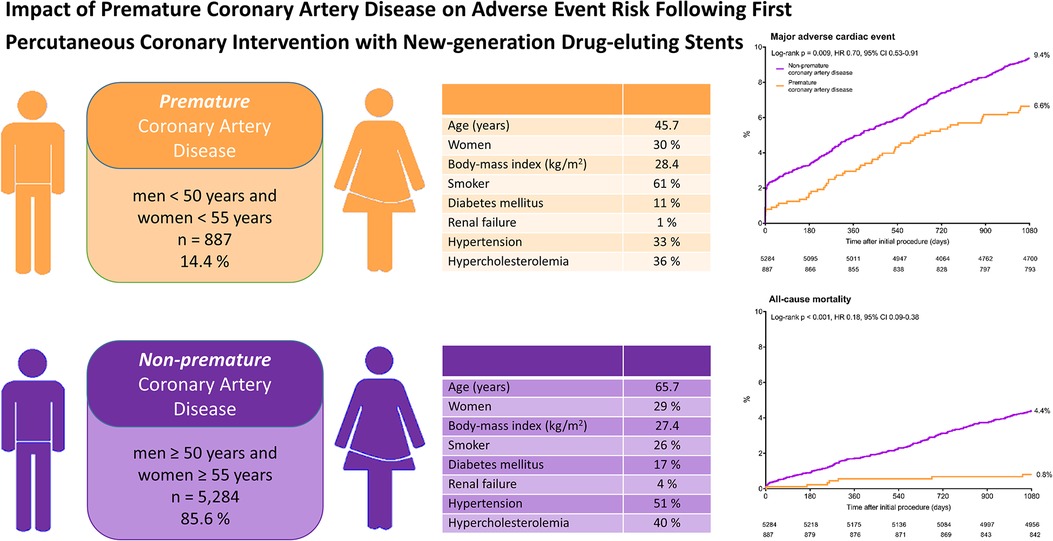
Graphical Abstract
Impact of premature coronary artery disease on adverse event risk following percutaneous coronary intervention with New-generation drug-eluting stents.
Kaplan-Meier cumulative event curves for major adverse cardiac events and all-cause mortality of patients with premature (orange) and non-premature (purple) coronary artery disease. CI, confidence interval; HR, hazard ratio.
1. Introduction
While coronary artery disease (CAD) has a high prevalence in elderly individuals, for some patients the disease already starts at a young age. Only a small percentage of young individuals develops a stage of CAD that requires coronary revascularization. In individuals aged 40–59 years, the American Heart Association has reported the prevalence of coronary heart disease to be somewhat less than 7% (1). The definition of premature CAD has been changing over time. Earlier studies in adult patients classified CAD as premature, if patients were younger than 45 (or even less than 35) years (2–9), while more recent studies used higher age-thresholds of 50 or 55 years in men (10, 11). As the female hormonal state before menopause delays the progression of atherosclerosis, it makes sense to use a somewhat higher age-threshold in women (12).
Premature atherosclerosis has previously been related to risk factors such a smoking, diabetes, dyslipidemia, and abuse of drugs, such as cocaine or cannabis (2, 7, 9). During the last decades, the cardiovascular risk profile in young individuals has shown subtle alterations. Overall, the prevalence of smoking decreased, and in women the prevalence of hypertension and diabetes increased (13). At the same time, young patients with chronic or acute coronary syndromes do not always have traditional cardiovascular risk factors (14). Awareness of the modifiable risk factors in patients with premature CAD can help set the target for prevention campaigns, in order to reduce the risk of CAD in young individuals. Yet, recent data on the risk profile of all-comers with premature CAD, who underwent percutaneous coronary intervention (PCI) in the current era of new-generation drug-eluting stents (DES) and refined concomitant pharmacological therapy, is scarce.
Therefore, in this pooled patient-level analysis of data from four large-scale randomized PCI trials with contemporary DES, we aimed to assess potential differences in risk profile between patients with premature (men <50 and women <55 years) and non-premature (men ≥50 and women ≥55 years) CAD at the time of their first coronary revascularization procedure. In addition, we assessed and compared the 3-year clinical outcome of both patient groups.
2. Methods
2.1. Study design
For this study, original patient-level data of all-comer patients enrolled in the TWENTE (clinicaltrials.gov: NCT01066650), DUTCH PEERS (TWENTE II, NCT01331707), BIO-RESORT (TWENTE III, NCT01674803), and BIONYX (TWENTE IV, NCT02508714) trials were analyzed. Details of the original trials have been reported previously (15–18). Briefly, these investigator-initiated, patient-blinded, randomized stent trials included 9,204 all-comer patients who underwent a PCI. Trial participants were enrolled in 6 Dutch centers, 2 Belgian centers, and 1 Israeli center. Patients were eligible for trial enrolment if they were older than 18 years, able to give informed consent, and required a PCI with DES implantation. Randomization between stent types was performed in a 1:1 fashion in the TWENTE, DUTCH PEERS, and BIONYX trials, whereas the 3-arm BIO-RESORT trial had a 1:1:1 randomization between 3 stent types. Further details of the four trials are described in the Supplemental material. A custom-designed computer program with random block sizes of 4 and 8 was used for web-based randomizations. BIO-RESORT and BIONYX stratified for diabetes, while TWENTE and BIONYX stratified for sex. All original trials were approved by the Medical Ethics Committee Twente and the Institutional Review Boards of all participating centers and complied with the Declaration of Helsinki. Written informed consent was provided by all trial participants.
In the present analysis, we compared the cardiovascular risk factors and clinical outcomes between patients with premature CAD and patients with non-premature CAD in those without previous revascularization procedure (by means of PCI or coronary artery bypass surgery) and without previous myocardial infarction (MI). Men were classified as patients with premature CAD if they underwent the index PCI at an age <50 years and likewise women at an age <55 years. Information on cardiovascular risk factors and comorbidities was collected at the time of the index PCI from the medical record or via questionnaires. For all patients, we pooled demographical, clinical, and angiographic characteristics, and outcome data from the four original randomized clinical trials.
2.2. Procedures, follow-up, and monitoring
PCI procedures were performed according to international medical guidelines and the operator's judgment. Technical details of the implanted contemporary DES have been published (15–18). In general, dual antiplatelet therapy was prescribed for 12 months in case of an acute coronary syndrome and for 6 months following PCI for a chronic coronary syndrome. Clinical follow-up was obtained via questionnaires, patient visits to outpatient clinics, or by telephone follow-up. The trials were monitored, and adverse events were externally adjudicated by independent blinded clinical event committees.
2.3. Definitions
Clinical endpoints were prespecified according to recommendations of the Academic Research Consortium (19, 20). Various clinical endpoints were assessed, including the composite endpoints major adverse cardiac events (MACE; all-cause mortality, any MI, emergent coronary bypass surgery, or clinically indicated target lesion revascularization), target vessel failure (cardiac mortality, target vessel MI, or clinically indicated target vessel revascularization), and target lesion failure (cardiac mortality, any MI, or clinically indicated target lesion revascularization). In addition to each individual endpoint of the composite clinical endpoints, definite and definite-or-probable stent thrombosis were assessed.
2.4. Statistical analysis
Chi-square test was used to assess differences in categorical variables, and differences in continuous variables were assessed with the Wilcoxon Rank Sum test or Student t-test, as appropriate. Time to endpoints was assessed with the Kaplan-Meier statistics, and the log-rank test was applied for between-group comparisons. Hazard ratios (HR) were computed by Cox proportional hazards analysis, and 2-sided confidence intervals were calculated. Potential confounders were identified if in univariate analysis a p-value of <0.15 was found. All potential confounders were included in the first pass of a multivariate Cox regression model. Potential confounders were: diabetes mellitus; body mass index; smoking; renal insufficiency; hypertension; hypercholesterolemia; previous stroke; peripheral arterial disease; family history of coronary artery disease; clinical syndrome at presentation; multivessel treatment; calcified lesion treatment; bifurcated lesion treatment; total stent length and included trial. Stepwise backward selection was used to exclude variables with a non-significant association with the main endpoint. The model consisted of: diabetes mellitus; body mass index; smoking; renal insufficiency; hypertension; family history of coronary artery disease; bifurcated lesion treatment; and total length stent. Finally, a multivariate Cox regression model was used to adjust for the propensity score. Separate, analyses of the events between 1- and 2-year and between 2- and 3-year follow-up were performed. In addition, men and women were divided in 5 percentiles and afterwards one-way analyses of variance were performed to assess between-group difference. P-values and confidence intervals were two-sided and p-values <0.05 were considered significant. SPSS version 24.00 (IBM, Armonk, NY) was used for the statistical analyses.
3. Results
3.1. Study population
Of all participants in the four randomized clinical trials (n = 9,204), 3,033 had a previous coronary revascularization (bypass surgery in 764 patients, and PCI in 1,803 patients) or MI (n = 1,896) and were therefore excluded from the present analysis. The current study population consists of 6,171 patients who had no previous coronary revascularization or MI. A total of 887 (14.4%) of these patients had premature CAD (Figure 1), including 269 (30.3%) women <55 years and 618 (69.7%) men <50 years.
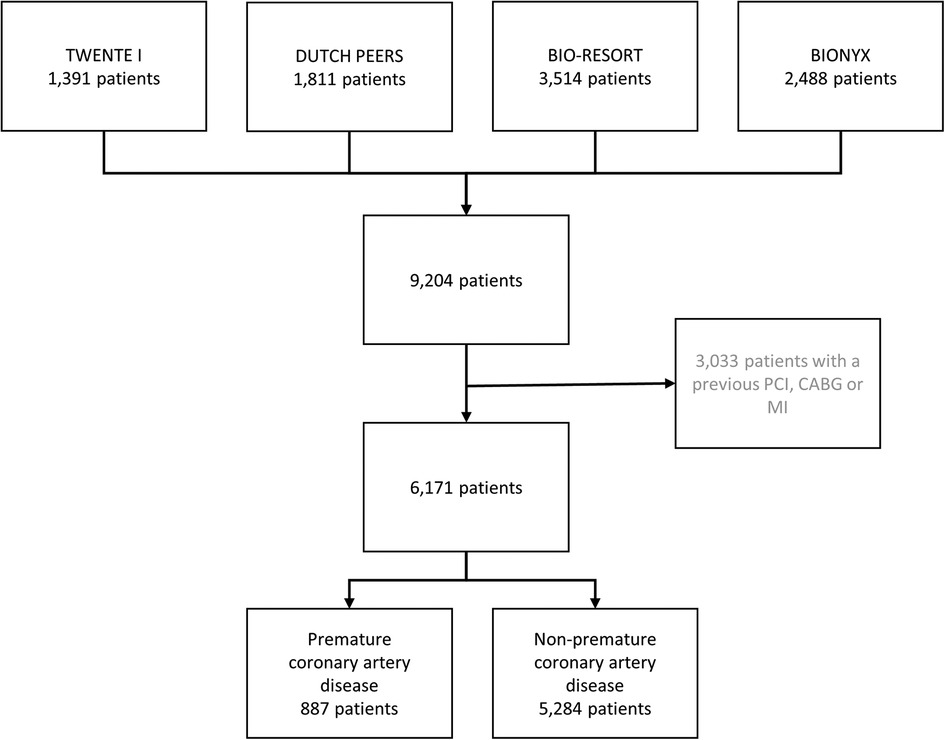
Figure 1. Flowchart. The number of patients with premature coronary artery disease. CABG, coronary artery bypass surgery; MI, myocardial infarction; PCI, percutaneous coronary intervention.
3.2. Characteristics of patients and procedures
The mean age of patients with premature CAD was 45.7 ± 4.7 years, whereas patients with non-premature CAD were on average 65.7 ± 8.8 years (Table 1). Patients with premature CAD had a lower prevalence of diabetes mellitus, renal failure, hypertension, hypercholesterolemia, peripheral arterial disease, and previous stroke than patients with non-premature CAD. In addition, patients with premature CAD had more often a family history of CAD (59.8% vs. 44.5%), were more often smokers (60.7% vs. 26.4%) and overweight (76.2% vs. 69.8%; body-mass index >25 kg/m2). The clinical syndrome at presentation differed between groups: patients with premature CAD presented more often with an ST-segment elevation myocardial infarction (STEMI), while patients with non-premature CAD more frequently presented with stable angina pectoris. This may explain the higher rate of ticagrelor-based dual antiplatelet therapy in patients with premature CAD as compared to patients with non-premature CAD (39.3% vs. 32.6%). In addition, patients with premature CAD were less often treated for multivessel, bifurcated, or calcified coronary lesions, requiring a total stent length that was shorter than in patients with non-premature CAD (Table 1).
3.3. Clinical outcome at 3-year follow-up
During 3-year follow-up, the composite clinical endpoint MACE occurred in 58/887 (6.6%) patients with premature CAD and in 492/5,284 (9.4%) patients with non-premature CAD (p = 0.009; Figure 2). In addition, patients with premature CAD had lower rates of all-cause mortality (HR:0.18, 95%-CI: 0.09–0.38; p < 0.001) and cardiac mortality (HR:0.26, 95%-CI: 0.10–0.70; p = 0.004) than patients with non-premature CAD. In contrast, patients with premature CAD had higher rates of definite stent thrombosis (HR:2.14, 95%-CI: 1.00–4.58; p = 0.045), target vessel revascularization (HR:1.41, 95%-CI: 1.05–1.90; p = 0.024), and target lesion revascularization (HR:1.47, 95%-CI: 1.03–2.11; p = 0.035; Table 2). Very late (>1 year) definite stent thrombosis showed no statistically significant difference between both groups (5/887 vs. 12/5,284 patients; p = 0.08). Inspection of the Kaplan-Meier time-to-event curves suggested potential between-group differences for MI and target vessel revascularization during follow-up, which triggered the calculation of annual adverse event rates that are presented Supplemental material.
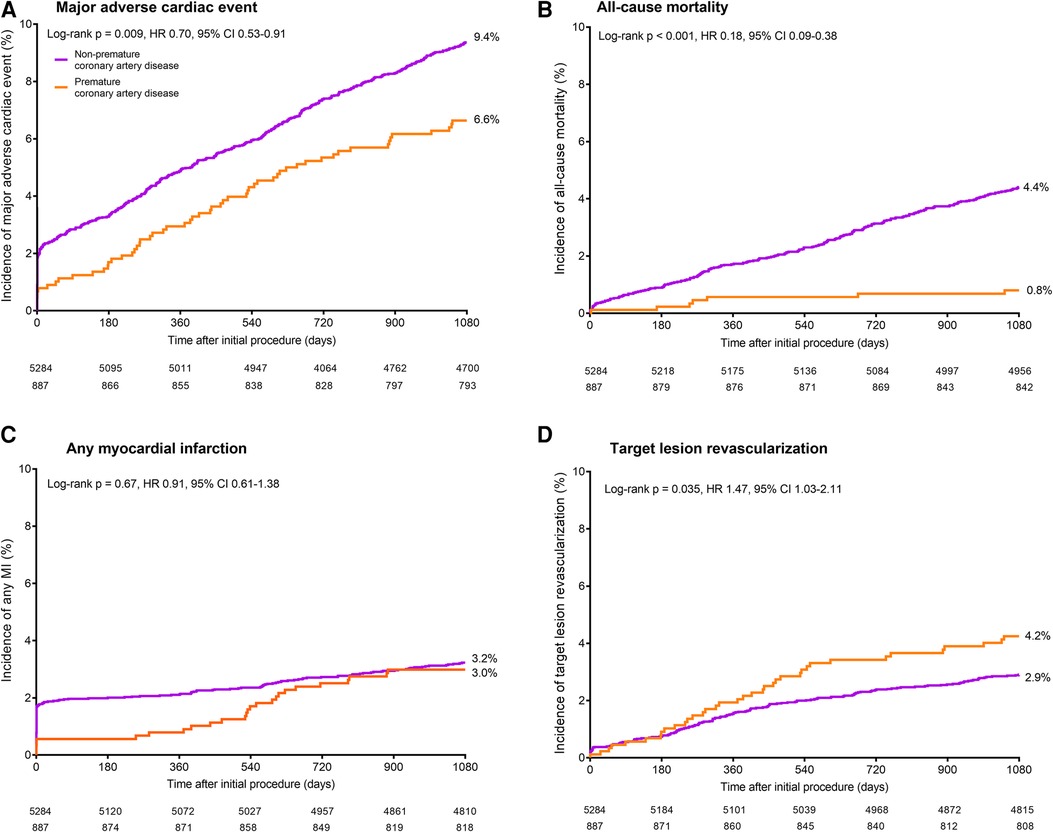
Figure 2. Kaplan-Meier cumulative event curves for the endpoint major adverse cardiac events and its individual components at 3-year follow-up. Kaplan-Meier cumulative incidence curves for: (A) the primary endpoint major adverse cardiac events, a composite of all-cause mortality (B), any myocardial infarction (C), emergent coronary bypass surgery, or clinically indicated target lesion revascularization (D). Patients with premature (orange) and non-premature (purple) coronary artery disease. HR, hazard ratio; MI, myocardial infarction.
After adjustment for confounders, the risk for all-cause mortality was significantly lower in patients with premature CAD (adjHR:0.23, 95%-CI: 0.10–0.52; p < 0.001), while in these patients the risks of target vessel revascularization (adjHR:1.63, 95%-CI: 1.18–2.26; p = 0.003), target lesion revascularization (adjHR:1.71, 95%-CI: 1.15–2.52; p = 0.008), definite-or-probable stent thrombosis (adjHR:2.24, 95%-CI: 1.06–4.72; p = 0.034), and definite stent thrombosis (adjHR:3.20, 95%-CI: 1.39–7.38; p = 0.006) were significantly higher than in patients with non-premature CAD.
When dividing men and women into 5 age percentiles, the rates of MACE, and all-cause and cardiac mortality were found to be lower in younger as compared to older men and women (Table 3). Similar results were found when men and women were divided in 7-age groups (Supplementary Table S2).
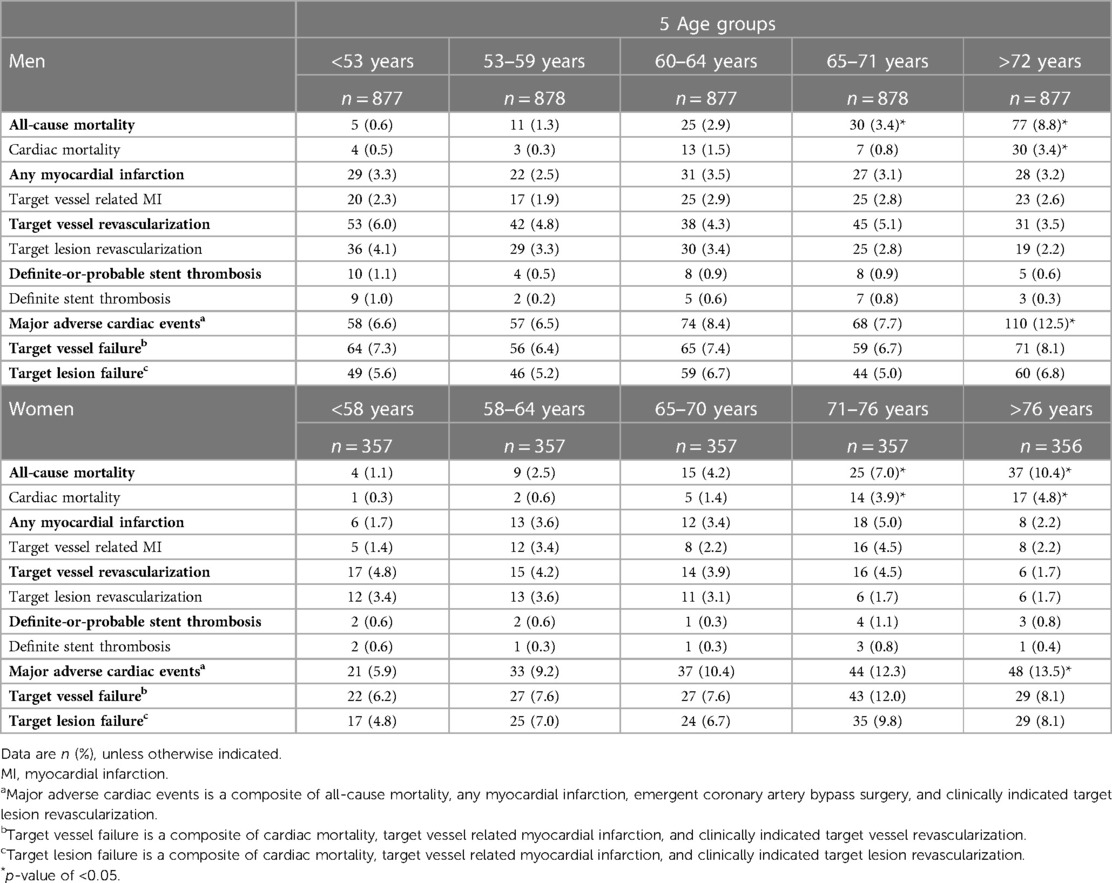
Table 3. Clinical outcomes in men and women at 3-year follow-up divided in 5 percentiles based on age.
4. Discussion
4.1. Main findings
Patients with premature CAD had less often diabetes mellitus, renal failure, hypertension, hypercholesterolemia, and a history of stroke; yet, they had on average a higher body-mass index and were more often smokers. Furthermore, patients with premature CAD underwent less often treatment of multiple vessels and of calcified or bifurcated coronary lesions. At 3-year follow-up, the rate of MACE differed between patients with premature and non-premature CAD (6.6% vs. 9.4%), but the presence of premature coronary atherosclerosis was not an independent predictor of MACE. In addition, 3-year all-cause mortality was lower in patients with premature CAD, while the rates of repeated revascularization and stent thrombosis were higher. When men and women were divided into age percentiles, the rates of MACE, and all-cause and cardiac mortality were found to be lower in younger patients.
4.2. Definition of premature coronary artery disease
The definition of “premature CAD” (or “premature atherosclerosis” or “young adults with an MI”) varies between studies and several different age-thresholds have been used. In most recent studies, age-thresholds of 45, 50, or 55 years were used for premature atherosclerosis (10, 11, 21). Yet, some studies applied a lower age-threshold of 35 or 40 years (6, 22), while other researchers divided their patients in 3 groups: very young, young, and older (23, 24). A study based on the autopsy reports of 1,139 men with sudden coronary atherosclerotic death, recommended an age-threshold of 49 years for the definition of premature CAD (25). Yet, as the female hormonal state delays the progression of atherosclerosis, women generally develop atherosclerosis at a more advanced age. Therefore, it makes sense to apply somewhat different age-thresholds in men and women. A previous study also used age-thresholds of 50 years in men and 55 years in women to define premature atherosclerosis (26). The definition of premature CAD in our present manuscript is in line with that definition.
4.3. Prevalence of premature coronary artery disease
From 2015 to 2018, the American Heart Association reported the prevalence of coronary heart disease in men and women aged 40 to 59 years to be 6.9% and 6.6% (1). The prevalence of premature CAD differs between studies; it is about 20 to 30% in patients presenting with a MI (10, 13). It may differ between the various regions of the world and among ethnicities. For instance, an Asian study of patients with premature CAD performed in India found a prevalence of 26.5% (27). In mostly European patients without previous revascularization or MI, assessed in the present study, this rate was 14.4%. Over the years, a stabilization or even an increase in the number of young adults with obstructive CAD have been observed (1, 10, 13), which may be explained by an increase in cardiac risk profile and relevant comorbidities (28), which emphasizes the importance of assessing modifiable cardiovascular risk factors and optimizing their treatment in young adults.
4.4. Risk factors
In the present analysis, patients with premature CAD had significantly less often hypertension, hypercholesterolemia, and diabetes, which is in line with previous studies (6, 8, 22, 29–31). In addition, our patients with premature CAD had on average a higher body-mass index, presented more often with STEMI, and underwent less often multivessel treatment than patients with non-premature CAD. Furthermore, patients with premature CAD were significantly more often current smokers.
Previous studies found that patients with premature CAD, who required PCI, were more likely to be men, as compared to patients with non-premature CAD. Yet, patients with premature CAD suffered less often from heart failure, hypertension, diabetes, or a history of coronary revascularization (6, 8, 22, 27, 29–31). In addition, patients with premature CAD had on average a higher body-mass index (6, 7). Furthermore, they presented more often with STEMI, had more often single-vessel disease, and a lower total plaque burden (22, 24, 29, 30). The data on smoking status were contradictory: one study found no difference in the current smoking status (29), while other studies found a higher proportion of current smokers among the premature CAD patients (6, 22, 30, 31).
In addition, three large-scale studies that assessed only young patients with coronary artery disease found a high prevalence of smoking, hypercholesterolemia, obesity, and family history of CAD (32–34). This is consistent with the findings of the present analysis.
4.5. Chronological vs. biological aging
It is still unclear why some patients develop obstructive CAD at a much younger age than others. Yet, it is likely that a combination of genetic predisposition, for instance the risk for familial hypercholesterolemia, and cardiovascular risk factors accounts for the premature occurrence of CAD (35). Observations have indicated that biological or physiological aging, which relates to a decline in function, differs from chronological aging, which only involves time. Physiological aging involves, for instance: telomere shorting and attrition (36); DNA methylation which is a epigenetic mechanism that regulates gene expression (37); and accumulation of mutations in DNA of somatic cells (38). In addition, a low-grade inflammation, measured by elevated proinflammatory molecules, is also a characteristic of aging (39). Furthermore, changes in the levels of proteins, metabolites, and other biomolecules have been observed (40). With aging, structural changes in arteries such as elastin fragmentation, collagen accumulation, medial vascular smooth muscle cell loss, and atherosclerosis have been reported (41).
In addition, several differences in plaques characteristics have been observed between patients with premature and non-premature CAD, which may be related to differences in the pathophysiological mechanisms involved and may explain the differences in clinical outcome. In the present study, plaque characteristics were not assessed in a detailed way. Yet, other studies observed that young adults have fewer atherosclerotic lesions and relative lack of acellular scar tissue, but their plaques have more inflammatory features and contain more lipid-containing foam cells (2, 7). Furthermore, young adults with STEMI showed a higher prevalence of plaque erosion and a lower degree of luminal obstruction, while other plaque features (e.g., calcification, cholesterol crystals, and thin-cap fibroatheroma) were less often seen (31).
4.6. Clinical outcome and implications
The present study found in patients with premature CAD a lower 3-year all-cause mortality risk than in patients with non-premature CAD. Yet, premature CAD patients had higher risks of target vessel revascularization, target lesion revascularization, and definite stent thrombosis. The number of patients with a repeated revascularization may be higher due to the fact that generally, young patients are more physically active and therefore more likely to develop symptoms of angina. In addition, in young adults with symptoms, physicians may be more inclined to choose an invasive treatment, such as a PCI, than choosing pharmacological therapy only.
A previous study which assessed patients aged ≤40 years who underwent coronary revascularization found 6-months and 1-year mortality rates that were lower than in non-premature CAD patients (8). This is in line with the results of the current analysis at 3-year follow-up. Similarly, the Norwegian Myocardial Infarction Register found that young MI patients (<45 years) had a 40% lower risk of all-cause mortality after a median follow-up of 2.4 years than MI patients aged between 45 and 59 years (30).
It is important to identify individuals who are at a particularly high risk of secondary adverse events in order to take more aggressive preventive measures. In the present analysis of patients undergoing their first coronary revascularization, a significant proportion had modifiable risk factors, such as hypertension, hypercholesterolemia, overweight, or smoking. As the prevalence of premature CAD increases with the number of cardiovascular risk factors (1, 28), increasing patient awareness about modifiable risk factors will support secondary cardiovascular prevention and is likely to improve the clinical outcome in PCI patients with premature CAD. In addition, rigorous screening for modifiable risk factors and the presence of atherosclerosis (primary prevention) in asymptomatic descendants of patients with premature CAD could be beneficial.
4.7. Limitations
Based on the post-hoc nature of the present analysis, our findings should be considered hypothesis generating. The analysis assessed patients without previous revascularization or previous MI in order to prevent that patients of the non-premature CAD group could have met the criteria of premature CAD previously. While using this criterion was indispensable, it might have resulted in the exclusion of some patients with extensive atherosclerotic burden. In addition, comparison between studies can be difficult due to dissimilarities between the various used definitions of premature CAD and the study populations. As other studies, we cannot exclude the presence of undetected or unmeasured confounders. Data about medication compliance were not available and could therefore not be assessed. The present analysis, similar to previous research, used chronological age instead of biological age, as biological age estimators are more complex and quite impractical in the context of most studies.
5. Conclusions
About one out of seven PCI patients was treated for premature CAD. These patients had less complex risk profiles than patients with non-premature CAD; yet, their risk of repeated revascularization and stent thrombosis was higher. As lifetime event risk of patients with premature CAD is known to be particularly high, further efforts should be made to improve modifiable risk factors such as smoking and overweight.
Data availability statement
The datasets presented in this article are not readily available because of the privacy of the participants. Researchers with a specific research question can send it to the corresponding author. The request will be assessed individually by a group of searchers consisting of members of the steering committees of the trials. Requests to access the datasets should be directed to C. von Birgelen,Yy52b25iaXJnZWxlbkBtc3Qubmw=.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee Twente. The patients/participants provided their written informed consent to participate in this study.
Author contributions
THP: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing—Original Draft, Visualization, Project administration. EHP: Validation, Data Curation, Investigation, Writing—Original Draft, Project administration. CJMD: Conceptualization, Formal analysis Methodology, Writing—Original Draft. PZ: Investigation, Resources, Writing—Review and Editing. MH: Conceptualization, Investigation, Resources, Writing—Review and Editing. CES: Investigation, Resources, Writing—Review and Editing. RLA: Investigation, Resources, Writing—Review and Editing. AR: Investigation, Resources, Writing—Review and Editing. PWD: Investigation, Resources, Writing—Review and Editing. EB: Investigation, Resources, Writing—Review and Editing. AdA: Investigation, Resources, Writing—Review and Editing. GCML: Investigation, Resources, Writing—Review and Editing. CvB: Conceptualization, Methodology, Investigation, Resources, Writing—Original Draft, Visualization, Supervision, Project administration, Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The TWENTE I trial was equally funded by Abbott Vascular and Medtronic. The DUTCH PEERS trial was equally funded by Boston Scientific and Medtronic. The BIO-RESORT trial was equally funded by Biotronik, Boston Scientific, and Medtronic. The BIONYX trial was equally funded by Biotronik and Medtronic.
Conflict of interest
CvB reports that the research department of Thoraxcentrum Twente has received research grants provided by Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. RLA reports a teaching grant from Biotronik, a license from Sanofi, a speaking fee from Abiomed and support from Amgen for attending a meeting, all outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1160201/full#supplementary-material.
References
1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. (2022) 145(8):e153–639. doi: 10.1161/CIR.0000000000001052
2. Chen L, Chester M, Kaski JC. Clinical factors and angiographic features associated with premature coronary artery disease. Chest. (1995) 108(2):364–9. doi: 10.1378/chest.108.2.364
3. Cole JH, Miller JI, Sperling LS, Weintraub WS. Long-term follow-up of coronary artery disease presenting in young adults. J Am Coll Cardiol. (2003) 41(4):521–8. doi: 10.1016/s0735-1097(02)02862-0
4. Kofflard MJ, de Jaegere PP, van Domburg R, Ruygrok P, van den Brand M, Serruys PW, et al. Immediate and long-term clinical outcome of coronary angioplasty in patients aged 35 years or less. Br Heart J. (1995) 73(1):82–6. doi: 10.1136/hrt.73.1.82
5. Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the pathobiological determinants of atherosclerosis in youth (PDAY) research group. JAMA. (1990) 264(23):3018–24. doi: 10.1001/jama.1990.03450230054029
6. Hassan A, Jaffe R, Rubinshtein R, Karkabi B, Halon DA, Flugelman MY, et al. Characterization of coronary artery disease in young adults and assessment of long-term outcomes. Isr Med Assoc J. (2018) 20(10):613–8.30324777
7. Sagris M, Antonopoulos AS, Theofilis P, Oikonomou E, Siasos G, Tsalamandris S, et al. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc Res. (2022) 118(10):2281–92. doi: 10.1093/cvr/cvab264
8. Mukherjee D, Hsu A, Moliterno DJ, Lincoff AM, Goormastic M, Topol EJ. Risk factors for premature coronary artery disease and determinants of adverse outcomes after revascularization in patients < or =40 years old. Am J Cardiol. (2003) 92(12):1465–7. doi: 10.1016/j.amjcard.2003.08.062
9. Zimmerman F, Cameron A, Fisher LD, Ng G. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (coronary artery surgery study registry). J Am Coll Cardiol. (1995) 26(3):654–61. doi: 10.1016/0735-1097(95)00254-2
10. Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. Am Coll Cardiol. (2014) 64(4):337–45. doi: 10.1016/j.jacc.2014.04.054
11. Leifheit-Limson EC, D’Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, et al. Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: the VIRGO study. J Am Coll Cardiol. (2015) 66(18):1949–57. doi: 10.1016/j.jacc.2015.08.859
12. Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. (2007) 14(3 Pt 1):373–84. doi: 10.1097/GME.0b013e31803c764d
13. Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. (2019) 139(8):1047–56. doi: 10.1161/CIRCULATIONAHA.118.037137
14. Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, et al. Cardiovascular risk and statin eligibility of young adults after an MI: partners YOUNG-MI registry. J Am Coll Cardiol. (2018) 71(3):292–302. doi: 10.1016/j.jacc.2017.11.007
15. von Birgelen C, Basalus MW, Tandjung K, van Houwelingen KG, Stoel MG, Louwerenburg JH, et al. A randomized controlled trial in second-generation zotarolimus-eluting resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. (2012) 59(15):1350–61. doi: 10.1016/j.jacc.2012.01.008
16. von Birgelen C, Zocca P, Buiten RA, Jessurun GAJ, Schotborgh CE, Roguin A, et al. Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt-chromium strut, bioresorbable polymer-coated (Orsiro) drug-eluting stents in allcomers with coronary artery disease (BIONYX): an international, single-blind, randomised non-inferiority trial. Lancet. (2018) 392(10154):1235–45. doi: 10.1016/S0140-6736(18)32001-4
17. von Birgelen C, Sen H, Lam MK, Danse PW, Jessurun GA, Hautvast RW, et al. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet. (2014) 383(9915):413–23. doi: 10.1016/S0140-6736(13)62037-1
18. von Birgelen C, Kok MM, van der Heijden LC, Danse PW, Schotborgh CE, Scholte M, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. (2016) 388(10060):2607–17. doi: 10.1016/S0140-6736(16)31920-1
19. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
20. Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, et al. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. (2010) 5(7):871–4. doi: 10.4244/eijv5i7a146
21. Lu Y, Li SX, Liu Y, Rodriguez F, Watson KE, Dreyer RP, et al. Sex-specific risk factors associated with first acute myocardial infarction in young adults. JAMA Netw Open. (2022) 5(5):e229953. doi: 10.1001/jamanetworkopen.2022.9953
22. Wittlinger T, Seifert C, Simonis G, Gerlach M, Strasser RH. Prognosis in myocardial infarction of young patients: results of a prospective registry. Int J Cardiol. (2020) 300:1–6. doi: 10.1016/j.ijcard.2019.10.037
23. Qureshi WT, Kakouros N, Fahed J, Rade JJ. Comparison of prevalence, presentation, and prognosis of acute coronary syndromes in ≤35 years, 36–54 years, and ≥55 years patients. Am J Cardiol. (2021) 140:1–6. doi: 10.1016/j.amjcard.2020.10.054
24. Yang J, Biery DW, Singh A, Divakaran S, DeFilippis EM, Wu WY, et al. Risk factors and outcomes of very young adults who experience myocardial infarction: the partners YOUNG-MI registry. Am J Med. (2020) 133(5):605–612.e1. doi: 10.1016/j.amjmed.2019.10.020
25. Abderrahman HA, Al-Abdallat IM, Idhair AK. Age threshold for proper definition of premature coronary artery disease in males. J Forensic Leg Med. (2018) 58:45–9. doi: 10.1016/j.jflm.2018.04.011
26. Vikulova DN, Grubisic M, Zhao Y, Lynch K, Humphries KH, Pimstone SN, et al. Premature atherosclerotic cardiovascular disease: trends in incidence, risk factors, and sex-related differences, 2000 to 2016. J Am Heart Assoc. (2019) 8(14):e012178. doi: 10.1161/JAHA.119.012178
27. Sharma SK, Makkar JS, Bana A, Sharma K, Kasliwal A, Sidana SK, et al. Premature coronary artery disease, risk factors, clinical presentation, angiography and interventions: hospital based registry. Indian Heart J. (2022) 74(5):391–7. doi: 10.1016/j.ihj.2022.08.003
28. Izadnegahdar M, Singer J, Lee MK, Gao M, Thompson CR, Kopec J, et al. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Womens Health (Larchmt). (2014) 23(1):10–7. doi: 10.1089/jwh.2013.4507
29. Mahjoob MP, Sadeghi S, Khanaman HF, Naderian M, Khaheshi I. Comparison of coronary risk factors and angiographic findings in younger and older patients with significant coronary artery disease. Rom J Intern Med. (2018) 56(2):90–5. doi: 10.1515/rjim-2017-0048
30. Jortveit JA-O, Pripp AH, Langørgen J, Halvorsen S. Incidence, risk factors and outcome of young patients with myocardial infarction. Heart. (2020) 106(18):1420–6. doi: 10.1136/heartjnl-2019-316067
31. Fang C, Dai J, Zhang S, Wang Y, Wang J, Li L, et al. Culprit lesion morphology in young patients with ST-segment elevated myocardial infarction: a clinical, angiographic and optical coherence tomography study. Atherosclerosis. (2019) 289:94–100. doi: 10.1016/j.atherosclerosis.2019.08.011
32. Collet JP, Zeitouni M, Procopi N, Hulot JS, Silvain J, Kerneis M, et al. Long-term evolution of premature coronary artery disease. J Am Coll Cardiol. (2019) 74(15):1868–78. doi: 10.1016/j.jacc.2019.08.1002
33. Zeitouni M, Clare RM, Chiswell K, Abdulrahim J, Shah N, Pagidipati NP, et al. Risk factor burden and long-term prognosis of patients with premature coronary artery disease. J Am Heart Assoc. (2020) 9(24):e017712. doi: 10.1161/JAHA.120.017712
34. Iyengar SS, Gupta R, Ravi S, Thangam S, Alexander T, Manjunath CN, et al. Premature coronary artery disease in India: coronary artery disease in the young (CADY) registry. Indian Heart J. (2017) 69(2):211–6. doi: 10.1016/j.ihj.2016.09.009
35. Roberts R, Chang CC. A journey through genetic architecture and predisposition of coronary artery disease. Curr Genomics. (2020) 21(5):382–98. doi: 10.2174/1389202921999200630145241
36. De Meyer T, Nawrot T, Bekaert S, De Buyzere ML, Rietzschel ER, Andrés V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J Am Coll Cardiol. (2018) 72(7):805–13. doi: 10.1016/j.jacc.2018.06.014
37. Bell JT, Tsai PC, Yang TP, Pidsley R, Nisbet J, Glass D, et al. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. (2012) 8(4):e1002629. doi: 10.1371/journal.pgen.1002629
38. Fuster JJ, Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res. (2018) 122(3):523–32. doi: 10.1161/CIRCRESAHA.117.312115
39. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15(9):505–22. doi: 10.1038/s41569-018-0064-2
40. Strazhesko ID, Tkacheva ON, Akasheva DU, Dudinskaya EN, Plokhova EV, Pykhtina VS, et al. Growth hormone, insulin-like growth factor-1, insulin resistance, and leukocyte telomere length as determinants of arterial aging in subjects free of cardiovascular diseases. Front Genet. (2017) 8:198. doi: 10.3389/fgene.2017.00198
Keywords: coronary artery disease, drug-eluting stent (DES), percutaneous coronary intervention (or PCI), premature coronary artery disease, obstructive coronary artery disease
Citation: Pinxterhuis TH, Ploumen EH, Zocca P, Doggen CJM, Schotborgh CE, Anthonio RL, Roguin A, Danse PW, Benit E, Aminian A, Hartmann M, Linssen GCM and von Birgelen C (2023) Impact of premature coronary artery disease on adverse event risk following first percutaneous coronary intervention. Front. Cardiovasc. Med. 10:1160201. doi: 10.3389/fcvm.2023.1160201
Received: 6 February 2023; Accepted: 5 June 2023;
Published: 19 June 2023.
Edited by:
Tommaso Gori, University Medical Centre, Johannes Gutenberg University Mainz, GermanyReviewed by:
Anthony Matta, Centre Hospitalier Universitaire de Toulouse, FranceNiya Mileva, Aleksandrovska University Hospital, Bulgaria
© 2023 Pinxterhuis, Ploumen, Zocca, Doggen, Schotborgh, Anthonio, Roguin, Danse, Benit, Aminian, Hartmann, Linssen and von Birgelen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clemens von Birgelen Yy52b25iaXJnZWxlbkBtc3Qubmw=
Abbreviations: CAD, coronary arterial disease; DES, drug-eluting stents; MACE, major adverse cardiac events; MI, myocardial infarction; Non-STEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
 Tineke H. Pinxterhuis1,2
Tineke H. Pinxterhuis1,2 Rutger L. Anthonio
Rutger L. Anthonio Adel Aminian
Adel Aminian Clemens von Birgelen
Clemens von Birgelen