- 1Department for Angiology, Center for Internal Medicine I, Deutsches Angiologie Zentrum Brandenburg - Berlin, University Clinic Brandenburg, Brandenburg Medical School Theodor Fontane, Brandenburg an der Havel, Germany
- 2Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 3Institute for Pharmacology and Preventive Medicine, Cloppenburg, Germany
- 4Department for Cardiology, Center for Internal Medicine I, University Clinic Brandenburg, Brandenburg Medical School Theodor Fontane, Brandenburg an der Havel, Germany
- 5Faculty of Health Sciences Brandenburg, Joint Faculty of the Brandenburg University of Technology Cottbus – Senftenberg, The Brandenburg Medical School Theodor Fontane, University of Potsdam, Brandenburg an der Havel, Germany
- 6Institute of Physiology, Brandenburg Medical School Theodor Fontane, Neuruppin, Germany
- 7Institute of Biochemistry, Brandenburg Medical School Theodor Fontane, Brandenburg an der Havel, Germany
- 8Department of Cardiology, University Clinic Graz, Graz, Austria
Background: We investigated the association between leukocyte telomere length, mitochondrial DNA copy number, and endothelial function in patients with aging-related cardiovascular disease (CVD).
Methods: In total 430 patients with CVD and healthy persons were enrolled in the current study. Peripheral blood was drawn by routine venipuncture procedure. Plasma and peripheral blood mononuclear cells (PBMCs) were collected. Cell-free genomic DNA (cfDNA) and leukocytic genomic DNA (leuDNA) were extracted from plasma and PBMCs, respectively. Relative telomere length (TL) and mitochondrial DNA copy number (mtDNA-CN) were analyzed using quantitative polymerase chain reaction. Endothelial function was evaluated by measuring flow-mediated dilation (FMD). The correlation between TL of cfDNA (cf-TL), mtDNA-CN of cfDNA (cf-mtDNA), TL of leuDNA (leu-TL), mtDNA-CN of leuDNA (leu-mtDNA), age, and FMD were analyzed based on Spearman's rank correlation. The association between cf-TL, cf-mtDNA, leu-TL, leu-mtDNA, age, gender, and FMD were explored using multiple linear regression analysis.
Results: cf-TL positively correlated with cf-mtDNA (r = 0.1834, P = 0.0273), and leu-TL positively correlated with leu-mtDNA (r = 0.1244, P = 0.0109). In addition, both leu-TL (r = 0.1489, P = 0.0022) and leu-mtDNA (r = 0.1929, P < 0.0001) positively correlated with FMD. In a multiple linear regression analysis model, both leu-TL (β = 0.229, P = 0.002) and leu-mtDNA (β = 0.198, P = 0.008) were positively associated with FMD. In contrast, age was inversely associated with FMD (β = −0.426, P < 0.0001).
Conclusion: TL positively correlates mtDNA-CN in both cfDNA and leuDNA. leu-TL and leu-mtDNA can be regarded as novel biomarkers of endothelial dysfunction.
Introduction
Current trends in life expectancy and concomitant demographic change (1) are leading to an ever-increasing number of patients suffering from aging-related disease (ARD), in which, cardiovascular disease (CVD) remains the major cause of death in the elderly population worldwide (2). The dominant cause of aging-related CVD is atherosclerosis (AS), which refers to the pathological process in that intimal lipids and fibrous elements encroach on the lumen of large arteries (3). In this context, an accumulating body of research has highlighted that cellular senescence in AS are characterized by telomere attrition (4) and mitochondrial DNA depletion (5).
Indeed, telomeres and mitochondria play critical roles in premature biological aging (6) and aging-related CVD (7). More precisely, a telomere is a specific region of repetitive nucleotide sequences associated with specialized proteins at the termini of linear chromosomes. Telomeres protect the genome from nucleolytic degradation and interchromosomal fusion, thereby ensuring the integrity of linear chromosomes (8). Telomere attrition occurs during each DNA replication and ultimately triggers the senescence and apoptosis in cells. Therefore, telomere length (TL) has been considered a biological marker of aging (9–11). In terms of CVD, although atherosclerotic lesion develops focally, it usually results in chronic systemic inflammation, which increases the turnover and biological age of vascular cells (10) and circulating cells (12). Therefore, TL can be regarded as an individual prognostic marker for cardiovascular risk prediction (13).
A mitochondrion is a double-membrane-bound organelle, which can be found in most eukaryotic organisms. Mitochondria generate the majority of adenosine triphosphate (ATP) during aerobic respiration, thereby playing a critical role in cellular energy production (14). ARD is attributed to the deleterious effects of reactive oxygen species (ROS) on various cell components (15). Since the majority of ROS are generated by the mitochondrial electron transport chain (16), mitochondrial DNA is more prone to damage by ROS (17). Thus, the free radical theory was refined as the mitochondrial theory of ageing (15). Likewise, recent studies show that mitochondrial DNA damage widely occurs in both the vascular and circulating cells (5). Therefore, mitochondrial DNA copy number (mtDNA-CN) can be also regarded as an individual prognostic marker for cardiovascular risk prediction (18).
Endothelial function reflects the production of endothelium-derived factors that regulate cardiovascular homeostasis, such as vascular tone, blood flow and blood pressure (19). In contrast, endothelial dysfunction (ED) is a systemic pathological state characterized by imbalanced vasodilation and vasoconstriction of the endothelium (20). There is no doubt that AS is the leading cause of CVD (3), yet, numerous studies have shown that ED precedes the angiographic or ultrasonic evidence of AS in aging-related CVD (21, 22). It has been shown that ED accompanies multifactorial endothelial aging (23, 24). Beyond that, both telomeres and mitochondria may be essential for the key aspects of endothelial function (25, 26). Yet, the association between TL, mtDNA-CN and endothelial function is still unclear.
In this regard, flow-mediated vasodilatation (FMD) is the most widely used non-invasive approach for assessment of endothelial function by measuring the ability of the arteries respond to endothelium-derived nitric oxide (NO) during reactive hyperemia. NO-dependent vasodilation can be quantified as an index of vasomotor (endothelial) function. Furthermore, both circulating cell-free DNA and peripheral blood mononuclear cells (PBMCs) are widely used in aging research as they have reasonable prognostic or diagnostic potential (27, 28). Cell-free DNA is the fragmented double-strand DNA released from dying cells in circulating blood (29). In contrast, PBMCs consist of lymphocytes and monocytes, which are subsets of leukocytes (30). In this study, we analyzed TL and mtDNA-CN from both cell-free and leukocytic genomic DNA, and investigated the potential relationships between TL, mtDNA-CN and endothelial function in aging-related CVD.
Materials and methods
Study population
The WalkByLab registry (www.walkbylab.com) is an ongoing CVD screening trial. It aims to screen, diagnose and follow up patients with CVD in the non-metropolitan areas of the federal state of Brandenburg, Germany. A structured multimodal risk factor management standard has been set for measurement and assessment of vascular function in the WalkByLab (31). More than 1,000 participants have been examined in the WalkByLab subcenter of Brandenburg (University Clinic Brandenburg) from June 2018 to December 2022. Here, blood samples of 430 participants were randomly selected and used for the current study.
Isolation of plasma and peripheral blood mononuclear cells
Around 6 ml of peripheral blood were collected in the BD Vacutainer EDTA Blood Collection Tube (Becton Dickinson). Then, blood was transferred into a 50-ml conical centrifuge tube, an equal volume of 1 × PBS was added and mixed gently. Diluted blood was slowly layered onto the Ficoll-Paque density gradient media (GE Healthcare) at a ratio of 4:3, and centrifuged 25 min at room temperature (400 × g without brake). The upper layer of plasma was collected and immediately frozen at −80°C until genomic DNA extraction. The mononuclear cell layer was transferred into a new conical centrifuge tube and centrifuged 10 min at 450 × g. Supernatant was removed, the cell pellet was vortexed immediately after adding 1.5 ml erythrocyte lysis buffer (PAN Biotech GmbH), and then incubated in the dark at room temperature for 15 min. After washing with PBS, the cell pellet was immediately frozen at −80°C until genomic DNA extraction.
Cell-free and leukocytic genomic DNA extraction
For cell-free genomic DNA (cfDNA) extraction, 2 ml frozen plasma was thawed and centrifuged at room temperature for 10 min (20,000 × g) to remove cell debris. cfDNA was extracted by using QIAamp DNA Blood Mini Kit (Qiagen). For leukocytic genomic DNA extraction, frozen PBMCs pellet including 1 × 106 cells was thawed in 1 ml PBS at room temperature. Leukocytic genomic DNA (leuDNA) was extracted by using the DNeasy Blood & Tissue Kit (Qiagen). Quantitative analysis of genomic DNA (gDNA) was performed by using the Nanodrop™ Microvolume Spectrophotometer (Thermo Fisher Scientific). cfDNA and leuDNA were further diluted to final concentrations of 1 ng/µl and 10 ng/µl, respectively.
Analyses for telomere length and mitochondrial DNA copy number
For quantitative polymerase chain reaction (qPCR) amplification of cfDNA template, each reaction system contained 25 µl gDNA, 2 µl primer working solution, and 25 µl PowerTrack™ SYBR Green Master Mix (Thermo Fisher Scientific). For qPCR amplification of leuDNA template, each reaction system contained 5 µl gDNA, 1 µl primer working solution, 4 µl RNase/DNase-free water and 10 µl PowerTrack™ SYBR Green Master Mix (Thermo Fisher Scientific). 60 cycles of a two-step qPCR were performed. All of primers used in this study were synthesized by the Eurofins Genomics Germany GmbH. Primer sequences are shown in Table 1.
Telomere length (TL) was expressed as telomeric DNA (teloDNA) relative to acidic ribosomal phosphoprotein PO (36B4), 36B4 is a single copy gene and serves as internal reference. Here, TL was calculated according to the formula: TL = 2−ΔCT, ΔCT = CTteloDNA−CT36B4. Similarly, mitochondrial copy number (mtDNA-CN) was expressed as mitochondrial DNA relative to a single copy gene β2 microglobulin (B2M), B2M is a single copy gene and serves as internal reference. Here, mtDNA-CN was calculated according to the formula: mtDNA-CN = 2 × 2−ΔCT, and ΔCT = CTmtDNA−CTB2M. Data were expressed as a relative level by normalizing against mean value.
Evaluation of endothelial function by flow-mediated dilation
Endothelial function was evaluated by measuring FMD using AngioDefender™ system (Everist Health). The AngioDefender™ enables automatic and non-invasive measurement of brachial FMD. In brief, a proprietary software algorithm was used to analyze the high resolution continuous electrocardiogram-gated B-mode ultrasound imaging during reactive hyperemia in brachial artery. The equivalence of FMD determined by the AngioDefender™ and the classical ultrasound or Doppler flow based analysis has been verified (32).
Statistical analysis
All statistical analyses were performed by using IBM SPSS26 or R language. Clinical characteristics were given as mean ± standard deviation (SD), relative TL and mtDNA-CN were given as mean ± standard error of the mean (SEM). Correlation between variables was analyzed using Spearman's correlation coefficient. Three multiple linear regression analysis models (stepwise method) were used to investigate the relationship between major variables. P ≤ 0.05 was considered to indicate statistical significance.
Results
Clinical characteristics
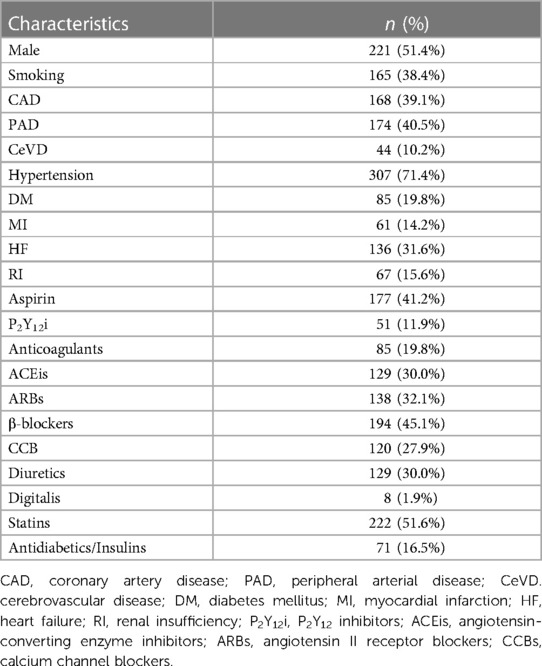
The mean age of 430 participants was 68.9 years. Specifically, 8 (1.9%) participants were between the age of 25 and 44 years (young age). 58 (13.5%) participants were between the age of 45 and 59 years (middle age). 226 (52.6%) participants were between the age of 60 and 74 years (elderly age). 138 (32.1%) participants were between the age of 75 and 89 years (senile age), respectively.
The mean FMD of 430 participants was 7.07 (±2.50) %. Specifically, the FMD of 111 participants were less than or equal to 5.50% (endothelial dysfunction), mean (±SD): 4.04 (±0.99) %. The FMD of 318 participants were more than 5.50% (normal endothelial function), mean (± SD): 8.13 (± 1.95) %. All other clinical characteristics are presented in Table 2.
Flow-mediated dilation inversely correlates with age
First, we analyzed the correlation between cf-TL, cf-mtDNA, leu-TL, leu-mtDNA, FMD, and age. Here, a significant inverse correlation existed between cf-mtDNA and age (r = −0.2207, P = 0.0031) (Figure 1B). Besides, cf-TL slightly positively correlated with age (Figure 1A), while both leu-TL and leu-mtDNA inversely correlated with age (Figures 1C,D). However, these results were without any statistical significance. Furthermore, a significant inverse correlation existed between FMD and age (r = −0.4085, P < 0.0001) (Figure 1E).
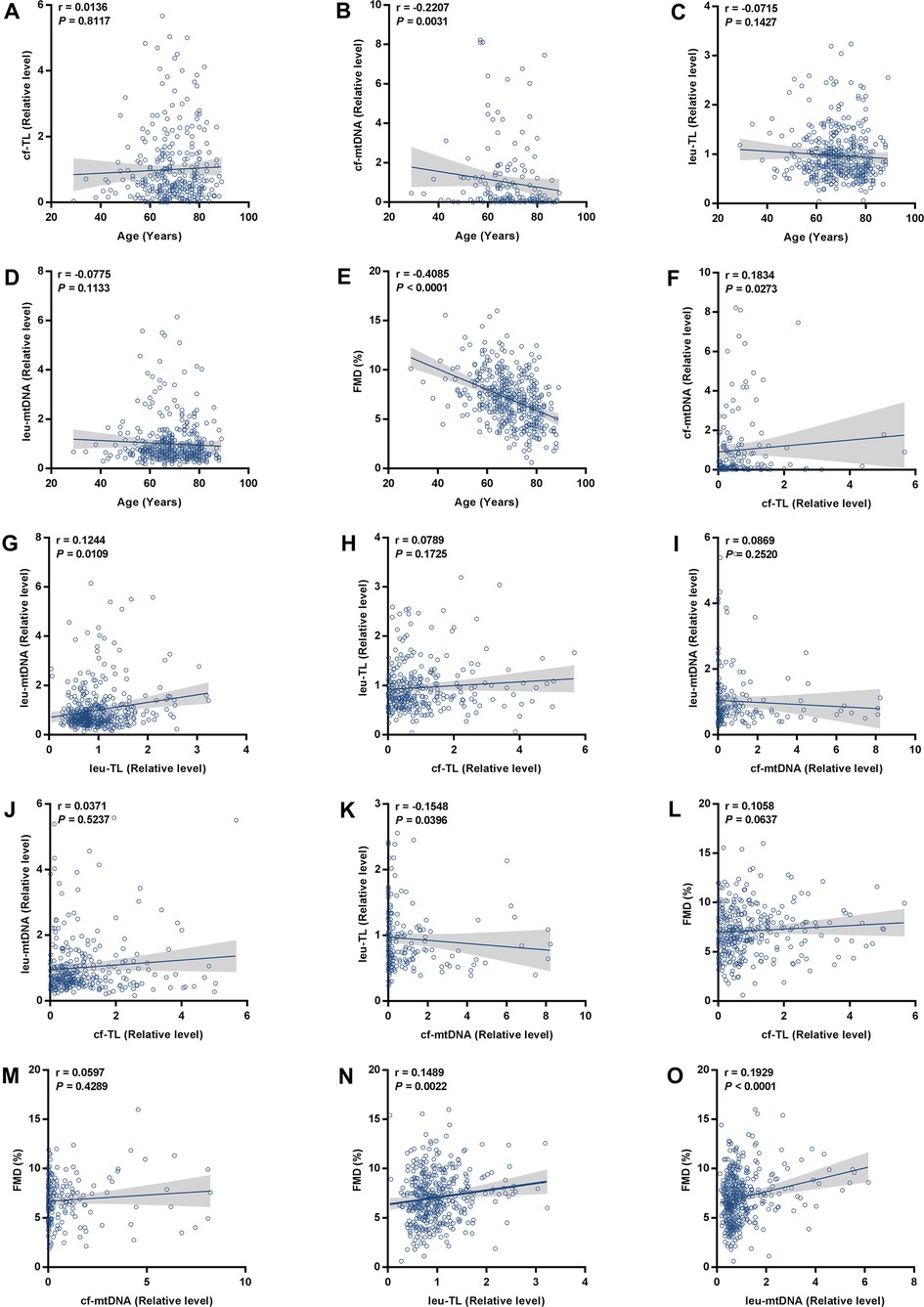
Figure 1. Correlation analyses. (A-E) Correlation analyses between cf-TL, cf-mtDNA, leu-TL, leu-mtDNA, FMD, and age. (F-K) Correlation analyses between cf-TL, cf-mtDNA, leu-TL, and leu-mtDNA. (L-O) Correlation analyses between cf-TL, cf-mtDNA, leu-TL, leu-mtDNA, and FMD.
Telomere length positively correlates with mitochondrial DNA copy number in both cell-free and leukocytic genomic DNA
Then, we analyzed the correlation between cf-TL, cf-mtDNA, leu-TL, and leu-mtDNA. Here, cf-TL positively correlated with cf-mtDNA (r = 0.1834, P = 0.0273) (Figure 1F). Similarly, leu-TL positively correlated with leu-mtDNA (r = 0.1244, P = 0.0109) (Figure 1G). Besides, positive correlations existed between cf-TL and leu-TL, and also, between cf-mtDNA and leu-mtDNA (Figures 1H,I). However, a significant difference was not reached. In addition, there was a significant inverse correlation between cf-mtDNA and leu-TL (r = −0.1548, P = 0.00396) (Figure 1K).
Leukocyte telomere length and mitochondrial DNA copy number positively correlate with flow-mediated dilation
Finally, we analyzed the correlation between cf-TL, cf-mtDNA, leu-TL, and leu-mtDNA, with FMD. Here, both leu-TL (r = 0.1489, P = 0.0022) and leu-mtDNA (r = 0.1929, P < 0.0001) positively correlated with FMD (Figures 1N,O). Although both cf-TL and cf-mtDNA positively correlated with FMD, no significant difference was reached (Figures 1L,M).
Multivariate correlates of flow-mediated dilation
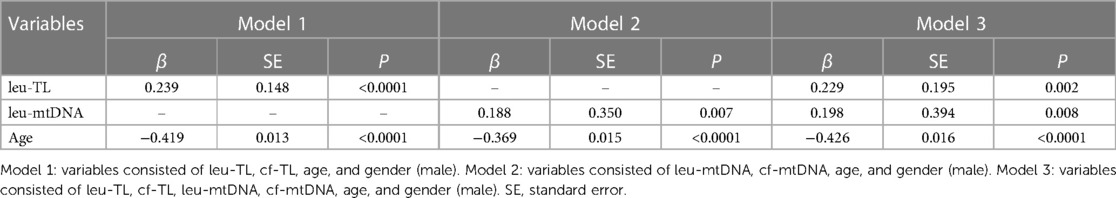
Adjusted covariates in model 1 consisted of cf-TL, leu-TL, age and gender (male). Here, leu-TL was positively associated with FMD (β = 0.239, SE = 0.148, P < 0.0001), while age was inversely associated with FMD (β = −0.419, SE = 0.013, P < 0.0001). Variates of cf-TL and gender (male) were excluded in this stepwise model (Table 3 and Figure 2A).
Figure 2. Multiple linear regression analyses. (A) Multiple linear regression analysis between leu-TL, age, and FMD. (B) Multiple linear regression analysis between leu-mtDNA, age, and FMD. (C) Multiple linear regression analysis between leu-TL, leu-mtDNA, age, and FMD.
Adjusted covariates in model 2 consisted of cf-mtDNA, leu-mtDNA, age and gender (male). Here, leu-mtDNA was positively associated with FMD (β = 0.188, SE = 0.350, P = 0.007), while age was inversely with FMD (β = −0.369, SE = 0.015, P < 0.0001). Variates of cf-mtDNA and gender (male) were excluded in this stepwise model (Table 3 and Figure 2B).
Adjusted covariates in model 3 consisted of cf-TL, leu-TL, cf-mtDNA, leu-mtDNA, age and gender (male). Here, both leu-TL (β = 0.229, SE = 0.195, P = 0.002) and leu-mtDNA (β = 0.198, SE = 0.394, P = 0.008) were positively associated with FMD. Again, age was inversely associated with FMD (β = −0.426, SE = 0.016, P < 0.0001). Variates of cf-TL, cf-mtDNA and gender (male) were excluded in this stepwise model (Table 3 and Figure 2C).
Discussion
First of all, our results showed that FMD inversely correlated with age in the patients with CVD. Consistent with our results, numerous studies have shown that FMD declines with age, which is an independent determinant of endothelial function (33–36). The endothelium is one of the largest human organs by area alone, and it interacts with nearly every system in the body (37). It is well accepted that age steadily impairs endothelial function through downregulating endothelial nitric oxide synthase (NOS) expression, inhibiting of NOS activity, and increasing NO degradation (38). Although the pathophysiology of age-dependent ED has not been fully revealed, a cause-and-effect relationship between diminished NO and ED has been confirmed (39, 40).
In addition, our results also indicate that both leu-TL and leu-mtDNA inversely correlate with age, yet a significant difference was not reached. One possible explanation could be that much more elderly aged participants than young and middle aged participants were recruited in this study. In fact, age-associated telomere attrition is a generally accepted finding based on numerous studies. In this regard, Nordfjäll et al. observed an age-related blood cell TL attrition with an interval of one-decade from 959 individuals (41). Spyridopoulos et al. demonstrated that leu-TL correlated with the progress of CVD, and it can be shown in all leukocyte populations, including peripheral blood stem cells and progenitor cells (42). Furthermore, Lee et al. reported that PBMC telomere fluorescence intensity was significantly decreased with age in healthy cynomolgus monkeys (43).
With regard to mtDNA-CN, Mengel-From et al. observed a tendency of fewer PBMC mtDNA-CN with aging by analyzing 1,067 subjects from a Danish cohort study (44). Furthermore, Foote et al. reported that arterial mtDNA-CN decreased with aging in mice (45). Indeed, because mtDNA is the major target of aging-associated mutation, age independently affects mtDNA-CN (46) and mitochondrial function (47).
Beyond that, an unexpected result was that cf-mtDNA inversely correlated with age. Here, it is well known that cf-mtDNA fragments are released extracellularly when dysfunctional mitochondria are accumulated in senescent cells (48). Therefore, cf-mtDNA fragments can be regarded as an aging biomarker (49). In this regard, Pinti et al. demonstrated that cf-mtDNA and proinflammatory cytokines increased gradually with age, which suggested that cf-mtDNA acts as the damage-associated molecular pattern in this context (50, 51). Furthermore, Ampo et al. demonstrated that cf-mtDNA was significantly increased in frail elderly subjects (52). However, these results are in contrast with our finding from the current study, the mechanism is still unclear.
This study showed a significant positive correlation between TL and mtDNA-CN in both cell-free and leukocyte genomic DNA. To the best of our knowledge, it is the first time to characterize the intimate relationship of TL and mtDNA-CN in patients with CVD. Specifically, higher levels of leu-TL and leu-mtDNA may indicate physiological condition or a compensation stage (11), while higher levels of cf-TL and cf-mtDNA may indicate pathological condition or a decompensation stage (50, 53). Here, a mounting number of studies have been performed to provide evidence that TL and mtDNA are coordinately regulated (54–57). Furthermore, co-regulation of telomeres and mitochondria play an important role in the pathophysiology process of chronic diseases and senescence (58, 59). Indeed, the interplay between telomeres and mitochondria was confirmed in recent studies (60). Therefore, the “telomere-mitochondrial axis” was proposed (61), which may serve as a target of molecular damage in aging (62) (Figure 3).
On the one hand, mitochondrial dysfunction leads to telomere attrition (63). During aging, damaged mitochondria produce indiscriminate amounts of ROS, which is known to cause irreversible damage to DNA by oxidizing cellular constituents (64). As a consequence, the normal redox signaling is disrupted and oxidative stress occurs (65). Accordingly, ROS also damages telomeric DNA. It has been shown that telomere attrition is largely caused by the repair inefficiency of a specific telomeric DNA single-strand (66). Therefore, mitochondrial dysfunction contributes to telomere attrition (67, 68). Here, Sanderson et al. demonstrated that telomere attrition in CD8+ T cells was suppressed by a ROS scavenger (67). Besides, Liu et al. demonstrated that telomere attrition in murine embryos was prevented by an antioxidant (68).
The other way around, telomere attrition leads to mitochondrial dysfunction (63). Mitochondrial content is regulated by mitochondrial biogenesis and mitophagy (75). Since mitochondria are sensitive to environmental cues, mitochondrial biogenesis could also be repressed due to telomere damage (59, 71). Telomere attrition induces DNA damage (6, 76, 77), thereby decreasing mtDNA-CN, while increasing ROS (6), which in turn damages both telomeres and mitochondria (59, 71). In addition, it has been known that p53 protein is a transcription factor responsible for preserving genomic integrity (69), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a transcription factor responsible for regulating mitochondrial biogenesis (70). In the case of telomere attrition, p53 inhibits PGC1α, thereby leading to mitochondrial dysfunction.
Furthermore, telomerase, an enzyme responsible for maintenance of telomere length by synthesizing terminal DNA, also appears to have the function of protecting against oxidative stress. In contrast, a mutant telomerase induces high levels of mitochondrial ROS, and thereby leading to mitochondrial dysfunction (78) (Figure 3).
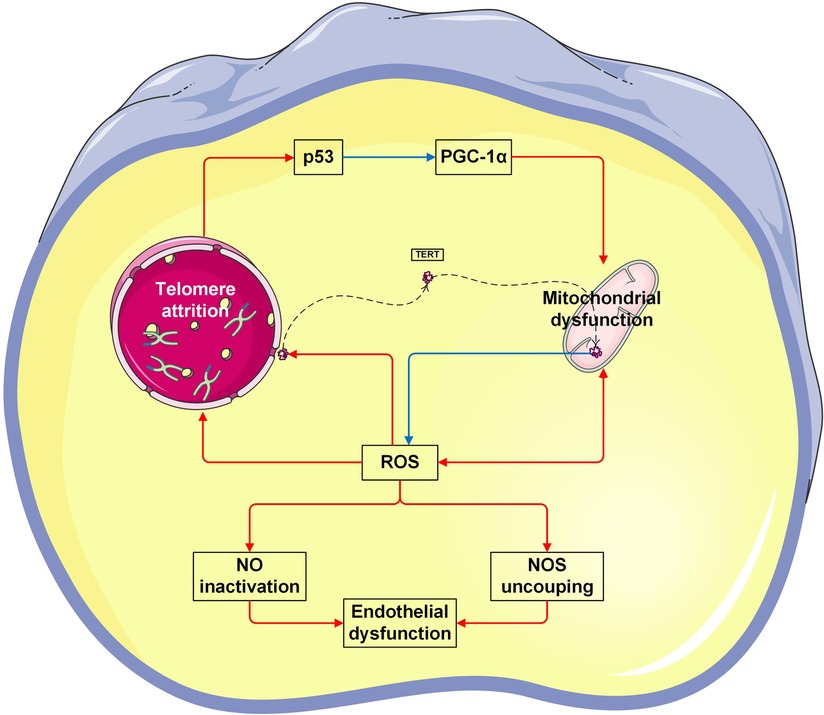
Figure 3. Vicious cycle between telomere attrition, mitochondrial dysfunction, and endothelial dysfunction. The p53 protein is a transcription factor responsible for preserving genomic integrity (69). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a transcription factor responsible for regulating mitochondrial biogenesis (70). In the case of telomere attrition, p53 inhibits PGC-1α, thereby leading to mitochondrial dysfunction. Vice versa, mitochondrial-derived ROS causes telomeric DNA damage, thereby leading to telomere attrition (61, 71). Telomerase reverse transcriptase (TERT) is the catalytic subunit of telomerase. In response to ROS, TERT is exported from the nucleus. In mitochondria, TERT binds to and protects mtDNA, thereby improving electron transport chain function and reducing ROS generation (72). On the other hand, excessive ROS suppresses the production of bioactive NO levels, but increases the production of by toxic peroxynitrite. Peroxynitrite uncouples endothelial NOS to form a dysfunctional superoxide-generating enzyme (73). Taken together, both NO inactivation and NOS uncoupling are enrolled in endothelial dysfunction (74). The Figures were partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Beyond that, this study showed that both leu-TL and leu-mtDNA, rather than cf-TL and cf-mtDNA, are positively associated with FMD in patients with aging-related CVD. Indeed, levels of leu-TL and leu-mtDNA may indicate the capacities of compensation (11). It has been demonstrated that telomeres and mitochondria (79) from immune cells play critical roles in peripheral arterial disease (80) and heart failure (81), respectively. In contrast, while cf-TL and cf-mtDNA may indicate stages of decompensation (50, 53). Therefore, both cf-TL and cf-mtDNA have been regarded as new biomarkers in cancer diagnosis and treatment (82–84). Yet, inverse linear correlations were not observed between cf-TL, cf-mtDNA and FMD in our current study.
Regarding the correlation between leu-TL and FMD, similar studies have been performed by Eguchi et al. (85) and Nakashima et al. (86), respectively. However, a significant correlation between leu-TL and FMD was not confirmed in their studies (85, 86). Nezu et al. reported that telomere G-tail length, but not total leu-TL, positively associated with FMD (87). Besides, Combrink et al. demonstrated that leu-TL positively correlated with plasma nitrite/nitrate levels in a bi-ethnic study (88), which are partially in support of our findings.
To date, former research mainly focused on telomerase activity and endothelial function. Here, it has been reported that activation of telomerase restored endothelial function in the human coronary and adipose arterioles (89). In addition, Bhayadia et al. demonstrated that endothelium-dependent vasodilation in telomerase deficient mice was impaired, which can be further restored by inhibiting oxidative stress (90).
It has been well accepted that endothelial function is NO-dependent, which fulfills a wide range of biological functions in cardiovascular homeostasis (39). NO inhibits telomere attrition by modulating telomerase activity (91). In fact, endothelial senescence and systemic vascular dysfunction are results of disruption of the delicate balance between NO and ROS (90, 92, 93). Therefore, a causal relationship between endothelial function and TL can be speculated based on our results (Figure 3).
Regarding the relationship between leu-mtDNA and FMD, Fetterman et al. reported that PBMC mitochondrial DNA damage inversely correlated to FMD in patients with diabetes mellitus and CVD, but a significant difference was not reached (94). In addition, Kakarla et al. demonstrated that mitochondrial membrane protein levels were positively associated with FMD in patients with type 2 diabetes mellitus (95).
Mitochondrial content is regulated by both mitochondrial biogenesis and mitophagy. Upregulation of mitochondrial biogenesis leads to increased mtDNA-CN correspondingly, thereby resulting in an enhanced metabolic capacity (96). In particular, endothelial mitochondrial energy production plays an important role in of vascular tone regulation (82). Research has shown that that mtDNA-CN negatively correlated with the disease severity and duration in healthy subjects (44, 97).
However, some research also indicated that mtDNA-CN positively correlated to cancer and CVD risk (98–100). Because the mtDNA is prone to DNA damage and susceptible to oxidative stress, increase of mtDNA-CN may serve as a possible compensatory mechanism to cope with mitochondrial dysfunction (101). In this regard, it has been reported that increased mtDNA-CN of gastrocnemius muscle was associated with lower ankle brachial index in patients with peripheral artery disease (100). In fact, within a certain level, ROS may increase mitochondria abundance and mtDNA content, thereby compensating for defective mitochondria to uphold the energy metabolism. Once beyond a threshold, ROS causes oxidative damage to mtDNA and elicits an irreversible apoptosis (102). Yet, the inner link between mitochondria in immune cells and endothelial function is still poorly understood. In conclusion, further studies are needed to investigate the putative causal relationship of mitochondria of immune cells and endothelial function in patients with CVD.
Conclusions
First, both leu-TL and mtDNA-CN positively correlate with FMD, while FMD negatively correlates with age. Second, TL positively correlates with mtDNA-CN in both leukocytic and cell-free genomic DNA. In conclusion, leu-TL and leu-mtDNA can be regarded as novel biomarkers of aging-related CVD.
Limitations
Because cfDNA is the fragmented double-strand DNA released from dying cells in circulating blood (29), the DNA concentrations were very low. Thus, the relevant Cq values in qPCR analysis were comparatively high, and finally affected the reliability of this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Association Ethics Committee of State Brandenburg (Ethikkommission der Landesärztekammer Brandenburg). The patients/participants provided their written informed consent to participate in this study.
Author contributions
PH, IB, CZ were responsible for designing the WalkByLab registry study, project supervision and monitoring. MS, PB, NP, OR, AP and EB were responsible for clinical examination and data collection. KL was responsible for performing all laboratory experiments and controling the quality of WalkByLab database. MD was responsible for performing statistical analysis with R. LL and ML were responsible for supporting laboratorial technics. KL, OG, AP and PH were responsible for drafting this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Faculty of Health Sciences Brandenburg (Fakultät für Gesundheitswissenschaften Brandenburg, FGW). The open-access publishing was funded by the Brandenburg Medical School Theodor Fontane (Medizinische Hochschule Brandenburg Theodor Fontane, MHB) publication funding supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG).
Acknowledgments
The authors thank Jeanett Förster and Vladlena Chirina for their serious and responsible work in the follow-up visits.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1157571/full#supplementary-material.
References
1. Crimmins EM. Lifespan and healthspan: past, present, and promise. Gerontologist. (2015) 55:901–11. doi: 10.1093/geront/gnv130
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. (2013) 11:117. doi: 10.1186/1741-7015-11-117
4. Huzen J, Peeters W, de Boer RA, Moll FL, Wong LS, Codd V, et al. Circulating leukocyte and carotid atherosclerotic plaque telomere length: interrelation, association with plaque characteristics, and restenosis after endarterectomy. Arterioscler Thromb Vasc Biol. (2011) 31:1219–25. doi: 10.1161/ATVBAHA.110.217158
5. Yu E, Calvert PA, Mercer JR, Harrison J, Baker L, Figg NL, et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. (2013) 128:702–12. doi: 10.1161/CIRCULATIONAHA.113.002271
6. Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. (2011) 470:359–65. doi: 10.1038/nature09787
7. Moslehi J, DePinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ Res. (2012) 110:1226–37. doi: 10.1161/CIRCRESAHA.111.246868
8. Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. (2011) 14:28–34. doi: 10.1097/MCO.0b013e32834121b1
9. von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. (2005) 5:197–203. doi: 10.2174/1566524053586545
10. Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. (2001) 358:472–3. doi: 10.1016/S0140-6736(01)05633-1
11. Zietzer A, Hillmeister P. Leucocyte telomere length as marker for cardiovascular ageing. Acta Physiol (Oxf). (2014) 211:251–6. doi: 10.1111/apha.12284
12. Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. (2007) 165:14–21. doi: 10.1093/aje/kwj346
13. Spyridopoulos I, Dimmeler S. Can telomere length predict cardiovascular risk? Lancet. (2007) 369:81–2. doi: 10.1016/S0140-6736(07)60042-7
14. Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. (2016) 61:654–66. doi: 10.1016/j.molcel.2016.01.028
15. Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med. (2008) 263:167–78. doi: 10.1111/j.1365-2796.2007.01905.x
16. Bartoli CG, Gómez F, Martínez DE, Guiamet JJ. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot. (2004) 55:1663–9. doi: 10.1093/jxb/erh199
17. Bonner MR, Shen M, Liu CS, Divita M, He X, Lan Q. Mitochondrial DNA content and lung cancer risk in Xuan Wei, China. Lung Cancer. (2009) 63:331–4. doi: 10.1016/j.lungcan.2008.06.012
18. Wang L, Zhang Q, Yuan K, Yuan J. mtDNA in the pathogenesis of cardiovascular diseases. Dis Markers. (2021) 2021:7157109. doi: 10.1155/2021/7157109
19. Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, et al. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. (2017) 174:1591–619. doi: 10.1111/bph.13517
20. Sun HJ, Wu ZY, Nie XW, Bian JS. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. (2019) 10:1568. doi: 10.3389/fphar.2019.01568
21. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. (2004) 109:Iii27–Iii32. doi: 10.1161/01.CIR.0000131515.03336.f8
22. El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol. (2012) 3:132. doi: 10.3389/fphys.2012.00132
23. Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev. (2010) 9:142–52. doi: 10.1016/j.arr.2009.07.002
24. Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. (2005) 66:286–94. doi: 10.1016/j.cardiores.2004.12.027
25. Minamino T, Komuro I. Role of telomere in endothelial dysfunction in atherosclerosis. Curr Opin Lipidol. (2002) 13:537–43. doi: 10.1097/00041433-200210000-00010
26. Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. (2013) 112:1171–88. doi: 10.1161/CIRCRESAHA.111.300233
27. Teo YV, Capri M, Morsiani C, Pizza G, Faria AMC, Franceschi C, et al. Cell-free DNA as a biomarker of aging. Aging Cell. (2019) 18:e12890. doi: 10.1111/acel.12890
28. Verschoor CP, Johnstone J, Millar J, Parsons R, Lelic A, Loeb M, et al. Alterations to the frequency and function of peripheral blood monocytes and associations with chronic disease in the advanced-age, frail elderly. PLoS One. (2014) 9:e104522. doi: 10.1371/journal.pone.0104522
29. Zemmour H, Planer D, Magenheim J, Moss J, Neiman D, Gilon D, et al. Non-invasive detection of human cardiomyocyte death using methylation patterns of circulating DNA. Nat Commun. (2018) 9:1443. doi: 10.1038/s41467-018-03961-y
30. He H, Tang L, Jiang N, Zheng R, Li W, Gu Y, et al. Characterization of peripheral blood mononuclear cells isolated using two kinds of leukocyte filters. Transfus Clin Biol. (2020) 27:10–7. doi: 10.1016/j.tracli.2019.11.001
31. Zemmrich C, Bramlage P, Hillmeister P, Sacirovic M, Buschmann I. Risk factor management in non-metropolitan patients with coronary and peripheral artery disease - A protocol of a prospective, multi-center, quality improvement strategy. Vasc Health Risk Manag. (2021) 17:267–72. doi: 10.2147/VHRM.S307014
32. Sharma MK, Chandan CB, Shukla A, Ameta D, Kurmi P. Assessment of flow mediated dilatation of brachial artery by angiodefender device as compared to brachial artery ultrasound imaging. IJMBS. (2019) 3:113-7. doi: 10.32553/ijmbs.v3i6.312
33. Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. (2006) 291:H3043–9. doi: 10.1152/ajpheart.00190.2006
34. Nakamura A, Kajitani S, Sato K, Kanazawa M, Kondo M, Endo H, et al. Decline of popliteal artery flow-mediated dilation with aging and possible involvement of asymmetric dimethylarginine in healthy men. J Med Ultrason (2001). (2019) 46:503–11. doi: 10.1007/s10396-019-00946-2
35. Langham MC, Zhou Y, Chirico EN, Magland JF, Sehgal CM, Englund EK, et al. Effects of age and smoking on endothelial function assessed by quantitative cardiovascular magnetic resonance in the peripheral and central vasculature. J Cardiovasc Magn Reson. (2015) 17:19. doi: 10.1186/s12968-015-0110-8
36. Bapir M, Untracht GR, Hunt JEA, McVey JH, Harris J, Skene SS, et al. Age-dependent decline in common femoral artery flow-mediated dilation and wall shear stress in healthy subjects. Life (Basel). (2022) 12. doi: 10.3390/life12122023
37. Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract. (2014) 3:291-308. doi: 10.5339/gcsp.2014.43
38. Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. (2012) 133:159–76. doi: 10.1016/j.pharmthera.2011.10.004
39. Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. (2012) 10:4–18. doi: 10.2174/157016112798829760
40. Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit Care Clin. (2020) 36:307–21. doi: 10.1016/j.ccc.2019.12.009
41. Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. (2009) 5:e1000375. doi: 10.1371/journal.pgen.1000375
42. Spyridopoulos I, Hoffmann J, Aicher A, Brümmendorf TH, Doerr HW, Zeiher AM, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. (2009) 120:1364–72. doi: 10.1161/CIRCULATIONAHA.109.854299
43. Lee WW, Nam KH, Terao K, Yoshikawa Y. Age-related telomere length dynamics in peripheral blood mononuclear cells of healthy cynomolgus monkeys measured by flow FISH. Immunology. (2002) 105:458–65. doi: 10.1046/j.1365-2567.2002.01386.x
44. Mengel-From J, Thinggaard M, Dalgård C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. (2014) 133:1149–59. doi: 10.1007/s00439-014-1458-9
45. Foote K, Reinhold J, Yu EPK, Figg NL, Finigan A, Murphy MP, et al. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell. (2018) 17:e12773. doi: 10.1111/acel.12773
46. Zhang R, Wang Y, Ye K, Picard M, Gu Z. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genomics. (2017) 18:890. doi: 10.1186/s12864-017-4287-0
47. Hudson EK, Hogue BA, Souza-Pinto NC, Croteau DL, Anson RM, Bohr VA, et al. Age-associated change in mitochondrial DNA damage. Free Radic Res. (1998) 29:573–9. doi: 10.1080/10715769800300611
48. Ma L, Westbrook R, Davalos M, Yang H, Walston J, Abadir P. Circulating cell-free apoptotic mitochondrial DNA fragments in frail old adults. Innov Aging. (2018) 2:61. doi: 10.1093/geroni/igy1023.1229
49. Nevalainen T. (2012) Evaluation of the cell-free mitochondrial DNA quantity in plasma as an aging biomarker candidate [dissertation/master's thesis]. [Tampere (Finland)]. University of Tampere
50. Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflamm-aging”. Eur J Immunol. (2014) 44:1552–62. doi: 10.1002/eji.201343921
51. Tumburu L, Ghosh-Choudhary S, Seifuddin FT, Barbu EA, Yang S, Ahmad MM, et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood. (2021) 137:3116–26. doi: 10.1182/blood.2020009063
52. Ampo E, Mendes-Silva AP, Goncalves V, Bartley JM, Kuchel GA, Diniz BS. Increased levels of circulating cell-free mtDNA in the plasma of subjects with late-life depression and frailty: a preliminary study. Am J Geriatr Psychiatry. (2022) 30:332–7. doi: 10.1016/j.jagp.2021.07.012
53. Zheng Z, Lian S, Lu C, Li F, Yu X, Ai L, et al. Early detection and disease monitoring of hepatocellular carcinoma using circulating telomere DNA. Research Square. (2022). doi: 10.21203/rs.3.rs-1836086/v1
54. Kim JH, Kim HK, Ko JH, Bang H, Lee DC. The relationship between leukocyte mitochondrial DNA copy number and telomere length in community-dwelling elderly women. PLoS One. (2013) 8:e67227. doi: 10.1371/journal.pone.0067227
55. Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, et al. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. (2015) 66:17–20. doi: 10.1016/j.exger.2015.04.002
56. Jung SJ, Cho JH, Park WJ, Heo YR, Lee JH. Telomere length is correlated with mitochondrial DNA copy number in intestinal, but not diffuse, gastric cancer. Oncol Lett. (2017) 14:925–9. doi: 10.3892/ol.2017.6197
57. Cruz-Ramos JA, López-Armas GDC, Díaz-Barba EI, Navarro-Meza M, Macías-Islas MA, Saldaña-Cruz AM, et al. Telomere shortening linked to disability and mitochondrial DNA copy number in patients with relapsing-remitting multiple sclerosis. Preprints.org. (2021). doi: 10.20944/preprints202112.0026.v1
58. Zhang R, Du J, Xiao Z, Jiang Y, Weng Q, Jin L. Combined effect of telomere length and mitochondrial DNA copy number as a potential biomarker indicating PE risk: a case-control study in a Chinese population. Research Square. (2022). doi:doi: 10.21203/rs.3.rs-1168394/v1
59. Birch J, Barnes PJ, Passos JF. Mitochondria, telomeres and cell senescence: implications for lung ageing and disease. Pharmacol Ther. (2018) 183:34–49. doi: 10.1016/j.pharmthera.2017.10.005
60. Derevyanko A, Skowronska A, Skowronski MT, Kordowitzki P. The interplay between telomeres, mitochondria, and chronic stress exposure in the aging egg. Cells. (2022) 11. doi: 10.3390/cells11162612
61. Van Der Stukken C, Nawrot TS, Alfano R, Wang C, Langie SAS, Plusquin M, et al. The telomere-mitochondrial axis of aging in newborns. Aging (Albany NY). (2022) 14:1627–50. doi: 10.18632/aging.203897
62. Wang L, Lu Z, Zhao J, Schank M, Cao D, Dang X, et al. Selective oxidative stress induces dual damage to telomeres and mitochondria in human T cells. Aging Cell. (2021) 20:e13513. doi: 10.1111/acel.13513
63. Zheng Q, Huang J, Wang G. Mitochondria, telomeres and telomerase subunits. Front Cell Dev Biol. (2019) 7:274. doi: 10.3389/fcell.2019.00274
64. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen Species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22094642
65. Sies H. Oxidative stress: concept and some practical aspects. Antioxidants (Basel). (2020) 9. doi: 10.3390/antiox9090852
66. Passos JF, Saretzki G, von Zglinicki T. DNA Damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. (2007) 35:7505–13. doi: 10.1093/nar/gkm893
67. Sanderson SL, Simon AK. In aged primary T cells, mitochondrial stress contributes to telomere attrition measured by a novel imaging flow cytometry assay. Aging Cell. (2017) 16:1234–43. doi: 10.1111/acel.12640
68. Liu L, Trimarchi JR, Smith PJ, Keefe DL. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell. (2002) 1:40–6. doi: 10.1046/j.1474-9728.2002.00004.x
69. Marei HE, Althani A, Afifi N, Hasan A, Caceci T, Pozzoli G, et al. P53 signaling in cancer progression and therapy. Cancer Cell Int. (2021) 21:703. doi: 10.1186/s12935-021-02396-8
70. Xiong S, Salazar G, Patrushev N, Ma M, Forouzandeh F, Hilenski L, et al. Peroxisome proliferator-activated receptor γ coactivator-1α is a central negative regulator of vascular senescence. Arterioscler Thromb Vasc Biol. (2013) 33:988–98. doi: 10.1161/ATVBAHA.112.301019
71. Gonzales-Ebsen AC, Gregersen N, Olsen RK. Linking telomere loss and mitochondrial dysfunction in chronic disease. Front Biosci (Landmark Ed). (2017) 22:117–27. doi: 10.2741/4475
72. Lundberg M, Millischer V, Backlund L, Martinsson L, Stenvinkel P, Sellgren CM, et al. Lithium and the interplay between telomeres and mitochondria in bipolar disorder. Front Psychiatry. (2020) 11:586083. doi: 10.3389/fpsyt.2020.586083
73. Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. (2010) 459:923–39. doi: 10.1007/s00424-010-0808-2
74. Vaziri ND. Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis. (2008) 2:1–10.19367002
75. Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol. (2014) 56:182–8. doi: 10.1016/j.exger.2014.01.021
76. Reaper PM, di Fagagna F, Jackson SP. Activation of the DNA damage response by telomere attrition: a passage to cellular senescence. Cell Cycle. (2004) 3:543–6. doi: 10.4161/cc.3.5.835
77. IJpma AS, Greider CW. Short telomeres induce a DNA damage response in Saccharomyces cerevisiae. Mol Biol Cell. (2003) 14:987–1001. doi: 10.1091/mbc.02-04-0057
78. Kovalenko OA, Caron MJ, Ulema P, Medrano C, Thomas AP, Kimura M, et al. A mutant telomerase defective in nuclear-cytoplasmic shuttling fails to immortalize cells and is associated with mitochondrial dysfunction. Aging Cell. (2010) 9:203–19. doi: 10.1111/j.1474-9726.2010.00551.x
79. Kirschner RH, Getz GS, Evans AE. Leukocyte mitochondria: function and biogenesis. Enzyme. (1972) 13:56–76. doi: 10.1159/000459649
80. Zietzer A, Buschmann EE, Janke D, Li L, Brix M, Meyborg H, et al. Acute physical exercise and long-term individual shear rate therapy increase telomerase activity in human peripheral blood mononuclear cells. Acta Physiol (Oxf). (2017) 220:251–62. doi: 10.1111/apha.12820
81. Kong CW, Hsu TG, Lu FJ, Chan WL, Tsai K. Leukocyte mitochondria depolarization and apoptosis in advanced heart failure: clinical correlations and effect of therapy. J Am Coll Cardiol. (2001) 38:1693–700. doi: 10.1016/S0735-1097(01)01601-1
82. Wilson C, Lee MD, Buckley C, Zhang X, McCarron JG. Mitochondrial ATP production is required for endothelial cell control of vascular tone. Function (Oxf). (2022)4:zqac063. doi: 10.1093/function/zqac063
83. North JP. (2018) Cell-free telomere DNA as a biomarker for treatment response and tumor burden in Glioblastoma. [dissertation/undergraduate thesis]. [Oxford (MS)]. University of Mississippi.
84. Yu M. Circulating cell-free mitochondrial DNA as a novel cancer biomarker: opportunities and challenges. Mitochondrial DNA. (2012) 23:329–32. doi: 10.3109/19401736.2012.696625
85. Eguchi K, Honig LS, Lee JH, Hoshide S, Kario K. Short telomere length is associated with renal impairment in Japanese subjects with cardiovascular risk. PLoS One. (2017) 12:e0176138. doi: 10.1371/journal.pone.0176138
86. Nakashima H, Ozono R, Suyama C, Sueda T, Kambe M, Oshima T. Telomere attrition in white blood cell correlating with cardiovascular damage. Hypertens Res. (2004) 27:319–25. doi: 10.1291/hypres.27.319
87. Nezu T, Hosomi N, Takahashi T, Anno K, Aoki S, Shimamoto A, et al. Telomere G-tail length is a promising biomarker related to white matter lesions and endothelial dysfunction in patients with cardiovascular risk: a cross-sectional study. EBioMedicine. (2015) 2:960–7. doi: 10.1016/j.ebiom.2015.05.025
88. Combrink JH. (2018) Leukocyte telomere length and its relation to nitric oxide metabolites in a bi-ethnic sample the SABPA study. [dissertation/master's thesis]. [Potchefstroom (South Africa)]. North-West University.
89. Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, et al. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res. (2016) 118:856–66. doi: 10.1161/CIRCRESAHA.115.307918
90. Bhayadia R, Schmidt BM, Melk A, Hömme M. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci. (2016) 71:161–9. doi: 10.1093/gerona/glv008
91. Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. (2000) 87:540–2. doi: 10.1161/01.RES.87.7.540
92. Matsui-Hirai H, Hayashi T, Yamamoto S, Ina K, Maeda M, Kotani H, et al. Dose-dependent modulatory effects of insulin on glucose-induced endothelial senescence in vitro and in vivo: a relationship between telomeres and nitric oxide. J Pharmacol Exp Ther. (2011) 337:591–9. doi: 10.1124/jpet.110.177584
93. McCann Haworth S. (2022) The role and regulation of nitric oxide and oxidative stress in cardiometabolic disease: focus on preeclampsia. [dissertation/ doctoral thesis]. [Stockholm (Sweden)]. Karolinska Institutet
94. Fetterman JL, Holbrook M, Westbrook DG, Brown JA, Feeley KP, Bretón-Romero R, et al. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc Diabetol. (2016) 15:53. doi: 10.1186/s12933-016-0372-y
95. Kakarla M, Puppala VK, Tyagi S, Anger A, Repp K, Wang J, et al. Circulating levels of mitochondrial uncoupling protein 2, but not prohibitin, are lower in humans with type 2 diabetes and correlate with brachial artery flow-mediated dilation. Cardiovasc Diabetol. (2019) 18:148. doi: 10.1186/s12933-019-0956-4
96. Ding Q, Qi Y, Tsang SY. Mitochondrial biogenesis, mitochondrial dynamics, and mitophagy in the maturation of cardiomyocytes. Cells. (2021) 10. doi: 10.3390/cells10092463
97. Lee JW, Park KD, Im JA, Kim MY, Lee DC. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta. (2010) 411:592–6. doi: 10.1016/j.cca.2010.01.024
98. Kumar B, Bhat ZI, Bansal S, Saini S, Naseem A, Wahabi K, et al. Association of mitochondrial copy number variation and T16189C polymorphism with colorectal cancer in North Indian population. Tumour Biol. (2017) 39:1010428317740296. doi: 10.1177/1010428317740296
99. Zhu X, Mao Y, Huang T, Yan C, Yu F, Du J, et al. High mitochondrial DNA copy number was associated with an increased gastric cancer risk in a Chinese population. Mol Carcinog. (2017) 56:2593–600. doi: 10.1002/mc.22703
100. McDermott MM, Peterson CA, Sufit R, Ferrucci L, Guralnik JM, Kibbe MR, et al. Peripheral artery disease, calf skeletal muscle mitochondrial DNA copy number, and functional performance. Vasc Med. (2018) 23:340–8. doi: 10.1177/1358863X18765667
101. Campa D, Barrdahl M, Santoro A, Severi G, Baglietto L, Omichessan H, et al. Mitochondrial DNA copy number variation, leukocyte telomere length, and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Breast Cancer Res. (2018) 20:29. doi: 10.1186/s13058-018-0955-5
Keywords: telomere length, mitochondrial DNA copy number, endothelial function, flow-mediated dilation, peripheral blood mononuclear cells, cell-free DNA, aging-related cardiovascular disease
Citation: Li K, Dai M, Sacirovic M, Zemmrich C, Pagonas N, Ritter O, Grisk O, Lubomirov LT, Lauxmann MA, Bramlage P, Persson AB, Buschmann E, Buschmann I and Hillmeister P (2023) Leukocyte telomere length and mitochondrial DNA copy number associate with endothelial function in aging-related cardiovascular disease. Front. Cardiovasc. Med. 10:1157571. doi: 10.3389/fcvm.2023.1157571
Received: 2 February 2023; Accepted: 22 March 2023;
Published: 5 June 2023.
Edited by:
Chen Xie, Sun Yan-sen University, ChinaReviewed by:
Zhang Canfeng, Sun Yat-Sen University, ChinaTingting Zhang, Sun Yat-sen University, China
Wenshu Zeng, Stanford University, United States
© 2023 Li, Dai, Sacirovic, Zemmrich, Pagonas, Ritter, Grisk, Lubomirov, Lauxmann, Bramlage, Persson, Buschmann, Buschmann and Hillmeister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Hillmeister cC5oaWxsbWVpc3RlckBrbGluaWt1bS1icmFuZGVuYnVyZy5kZQ== Ivo Buschmann aS5idXNjaG1hbm5Aa2xpbmlrdW0tYnJhbmRlbmJ1cmcuZGU=
Specialty Section: This article was submitted to Cardiovascular Biologics and Regenerative Medicine, a section of the journal Frontiers in Cardiovascular Medicine
 Kangbo Li
Kangbo Li Mengjun Dai
Mengjun Dai Mesud Sacirovic1
Mesud Sacirovic1 Claudia Zemmrich
Claudia Zemmrich Nikolaos Pagonas
Nikolaos Pagonas Olaf Grisk
Olaf Grisk Lubomir T. Lubomirov
Lubomir T. Lubomirov Martin A. Lauxmann
Martin A. Lauxmann Anja Bondke Persson
Anja Bondke Persson Eva Buschmann
Eva Buschmann