- 1The Second Department of Geriatrics, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2National Clinical Research Center for TCM Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Graduate School of Guangdong Pharmaceutical University, Guangzhou, China
Background: Growing evidence suggests that Coronary artery disease (CAD) is associated with cognitive impairment. However, these results from observational studies was not entirely consistent, with some detecting no such association. And it is necessary to explore the causal relationship between CAD and cognitive impairment.
Objective: We aimed to explore the potential causal relationship between CAD and cognitive impairment by using bidirectional two-sample mendelian randomization (MR) analyses.
Methods: Instrument variants were extracted according to strict selection criteria. And we used publicly available summary-level GWAS data. Five different methods of MR [random-effect inverse-variance weighted (IVW), MR Egger, weighted median, weighted mode and Wald ratio] were used to explore the causal relationship between CAD and cognitive impairment.
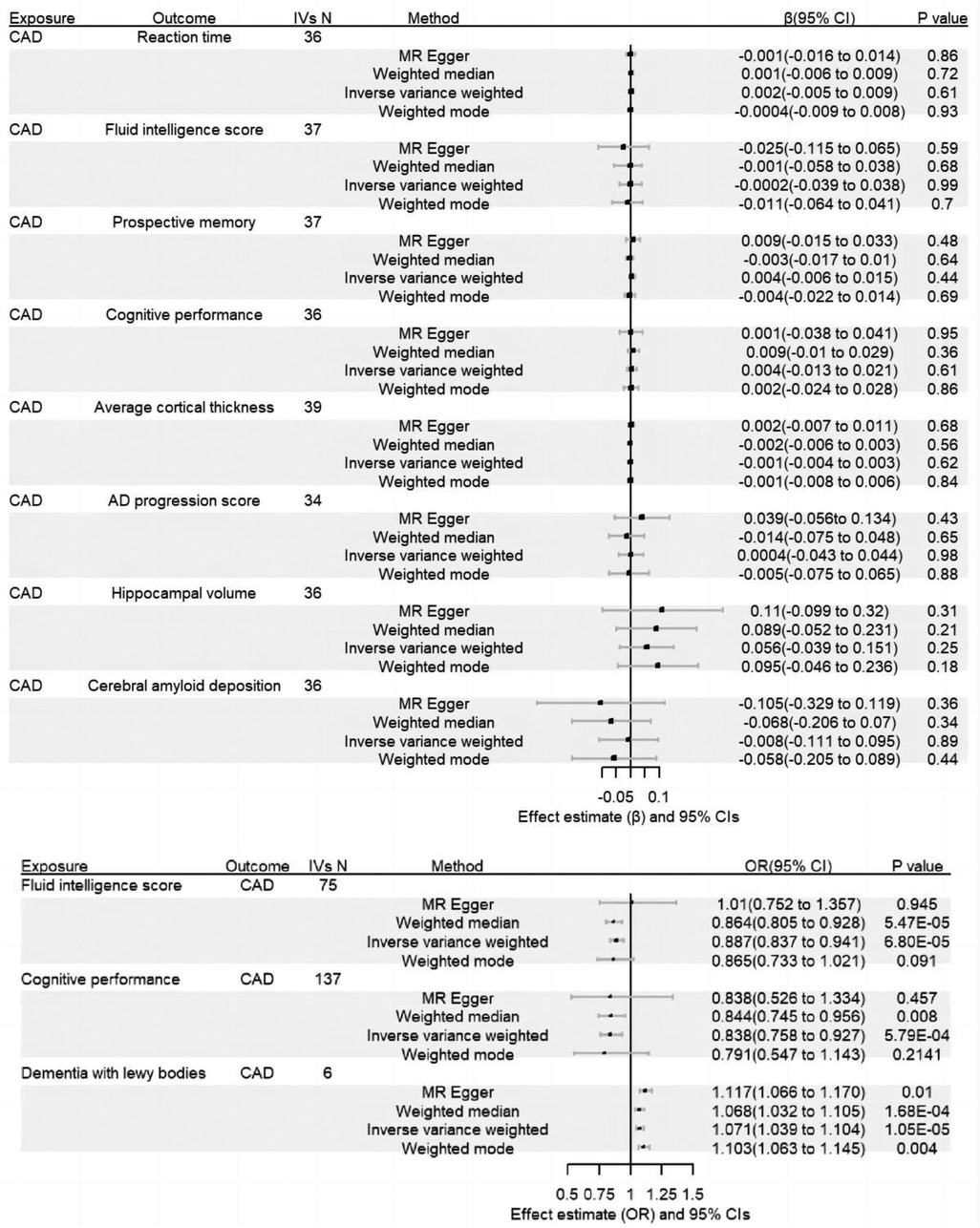
Results: There was little evidence to support a causal effect of CAD on cognitive impairment in the forward MR analysis. In the reverse MR analyses, We detect causal effects of fluid intelligence score (IVW: β = −0.12, 95% CI of −0.18 to −0.06, P = 6.8 × 10−5), cognitive performance (IVW: β = −0.18, 95% CI of −0.28 to −0.08, P = 5.8 × 10−4) and dementia with lewy bodies (IVW: OR = 1.07, 95% CI of 1.04–1.10, P = 1.1 × 10−5) on CAD.
Conclusion: This MR analysis provides evidence of a causal association between cognitive impairment and CAD. Our findings highlight the importance of screening for coronary heart disease in patients of cognitive impairment, which might provide new insight into the prevention of CAD. Moreover, our study provides clues for risk factor identification and early prediction of CAD.
1. Introduction
CAD remains one of the leading causes of mortality worldwide, and the risk of CAD increases with age (1, 2). Simultaneously, cognitive impairment has become one of the greatest global challenges for public health (3). Growing evidence suggests that CAD is associated with cognitive impairment. The study by Xie et al. (4) found that incident CAD was significantly associated with faster post-CAD-diagnosis cognitive decline by leveraging the longitudinal community-based data from the English Longitudinal Study of Aging. A 12-year follow-up in the Maastricht Aging Study (MAAS) reported that individuals with CAD show more cognitive decline in memory and processing speed than controls (5). However, these results from observational studies was not entirely consistent. Petrovitch H et al. (6) found that cognitive performance did not have significant association with ≥1 prior MI or history of coronary artery bypass graft surgery (CABG). And another study also found no significant correlation between CHD and more severe cognitive decline (7). CAD and cognitive impairment share many risk factors, such as elevated serum low-density lipoprotein cholesterol, high blood pressure, obesity, hyperglycemia and diabetes mellitus, and smoking (2, 3). The included individuals are often accompanied by these risk factors in observational studies, and the observational studies could not exclude the interference of these risk factors on the results. Therefore, it is unclear whether there is a causal relationship between CAD and cognitive impairment. This ambiguous relationship may confound some of the decisions made by clinicans, so it is necessary to explore the causal relationship between CAD and cognitive impairment.
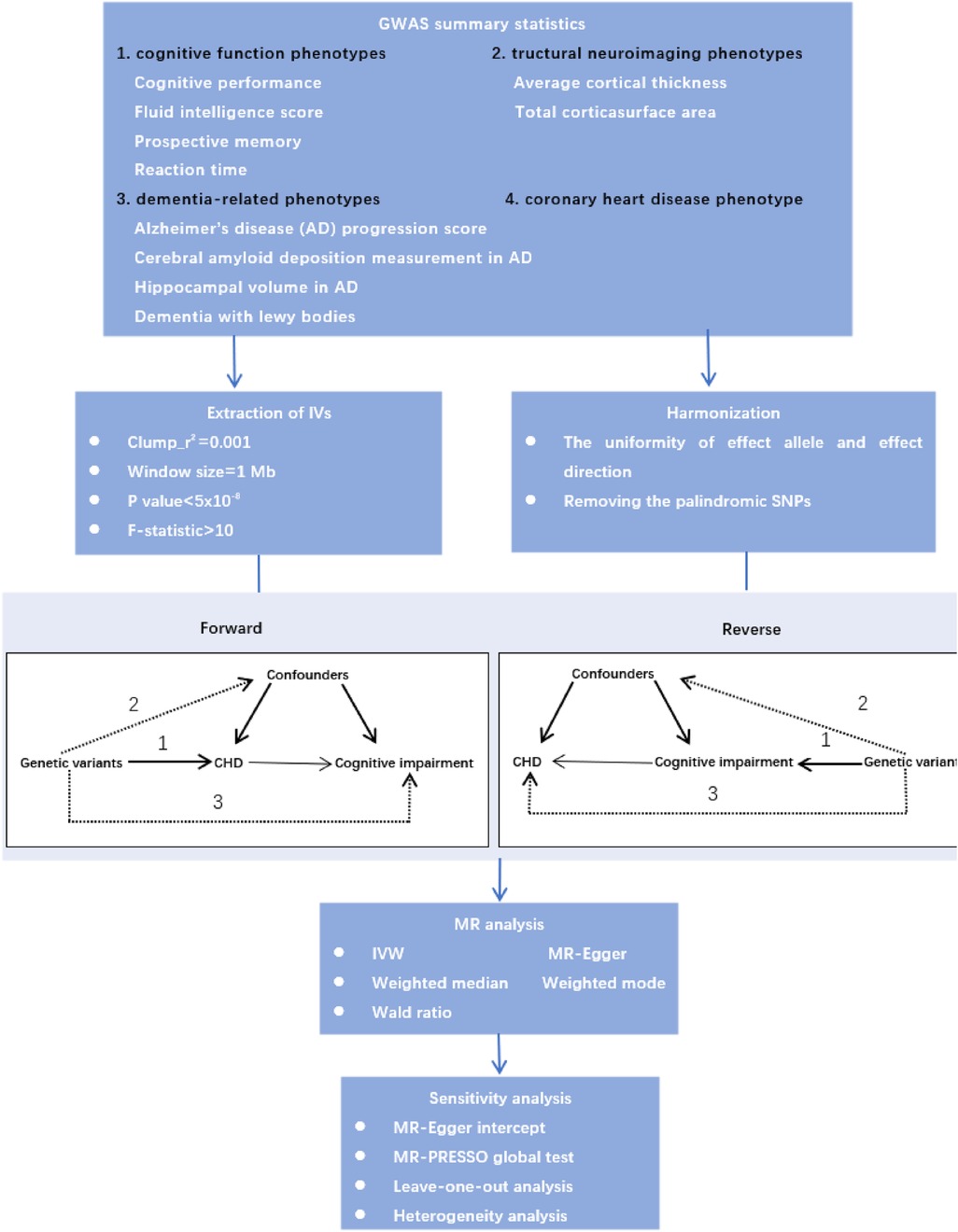
Mendelian randomization is a method that uses genetic variation associated with exposures to assess their possible causal relationship with outcomes, and it can reduce bias from confounding in epidemiological studies (8). Therefore, we performed bidirectional two-sample MR analyses to explore the causal relationship between CAD and cognitive impairment. Moreover, the underlying neurobiology of cognition is complex, we used detailed neurocognitive phenotypes, consisting of cognitive performance, fluid intelligence score, prospective memory, reaction time and structural neuroimaging phenotypes. In addition, cognitive impairment is a broad term that generally describes a decline in cognitive functions, and the severity of this impairment may range from mild symptoms to severe cognitive deficits that may be diagnosed as dementia. Therefore, we used the dementia-related phenotypes to explore the causal relationship between CAD and cognitive impairment from early to late stages. The study design is shown in Figure 1.
2. Materials and methods
2.1. GWAS summary data
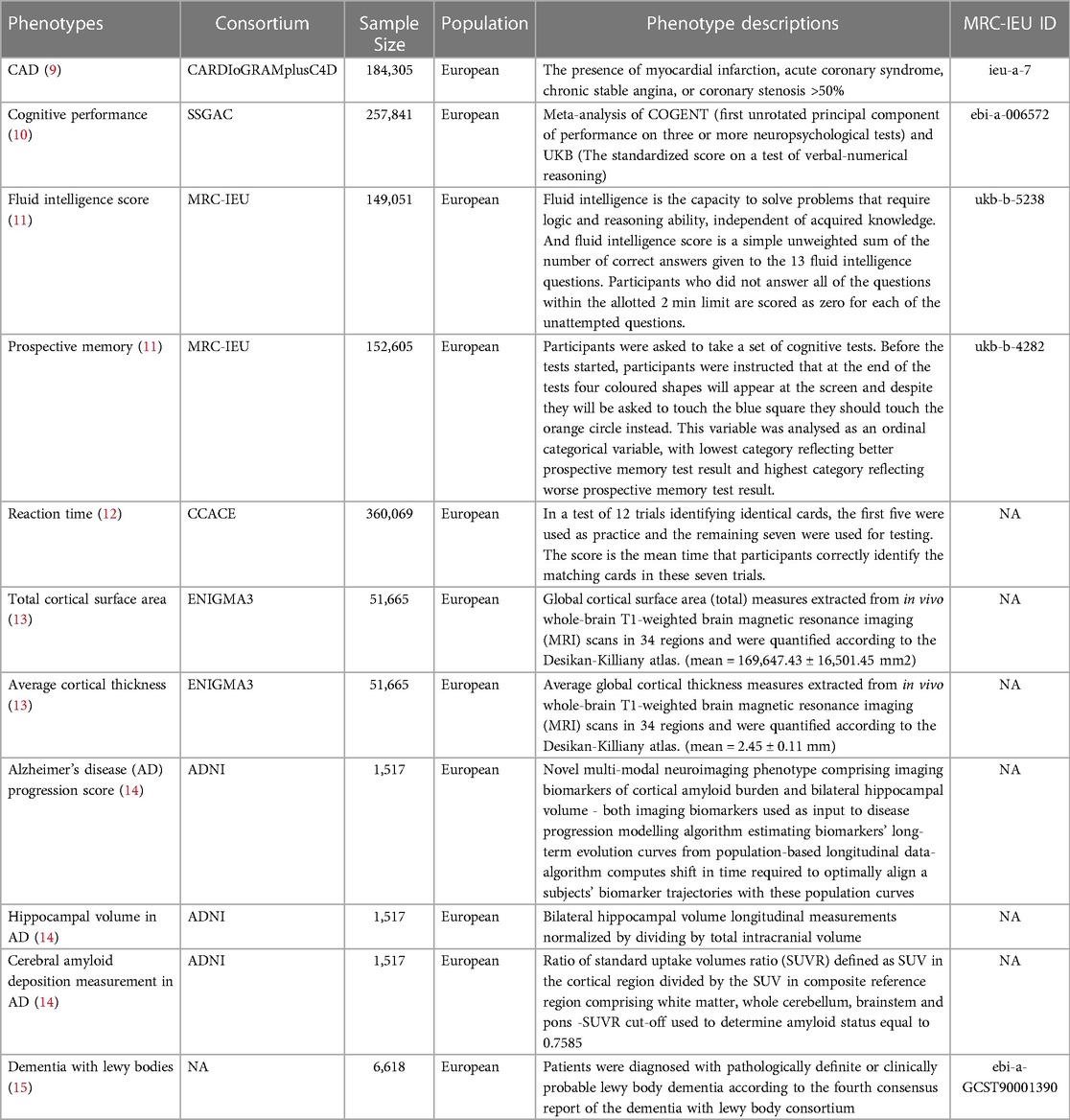
We used publicly available summary-level GWAS data, and the detailed information is presented in Table 1. Summary-level data for CAD were obtained from the Coronary Artery Disease Genome-Wide Replication and Meta-analysis plus the Coronary Artery Disease (CARDIoGRAMplusC4D) Consortium cohort which assembled 60,801 cases and 123,504 controls from 48 studies (9). The data of cognitive performance from the Social Science Genetic Association Consortium (SSGAC) GWAS meta-analyzing published results combined a general cognitive ability study from the COGENT Consortium with a new genome-wide association analysis of cognitive performance in the UK Biobank (10). The data of fluid intelligence score and prospective memory were obtained from the Medical Research Council Integrated Epidemiology Unit (MRC-IEU) GWAS Pipeline (11). For reaction time, the summary-level GWAS data were extracted from Center for Cognitive Ageing and Cognitive Epidemiology (CCACE) analyzing the scores obtained on the reaction time test and genetic data of 330,069 UK Biobank participants (12). Average cortical thickness and total cortical surface area were included as structural neuroimaging phenotypes in the study, and the full GWAS summary statistic were extracted from a recent neuroimaging GWAS study including 51,665 participants (13). This study performed a genome-wide association meta-analysis of brain magnetic resonance imaging data to identify genetic variants affecting cortical structure. We also used dementia-related phenotypes, including Alzheimer's disease (AD) progression score, cerebral amyloid deposition measurement in AD, hippocampal volume in AD and lewy body dementia, the first three phenotypes from the genetic study of multimodal imaging Alzheimer's disease progression score (14), and the data of lewy body dementia from a recent study of GWAS associated with Lewy body dementia (15).
2.2. Instrument variant (IV) selection
We extracted qualified IVs according to strict selection criteria. First of all, the SNPs with P < 5.00E-08 were extracted as candidate IVs. Secondly, the SNPs which were in linkage disequilibrium (r2 < 0.001, within a 10,000 kb window) would be excluded, and the SNPs were eliminated if they were palindromic with intermediate allele frequencies. Thirdly, the SNPs were excluded if they were not available in the outcome GWAS, and we did not use proxy SNPs in the study. In addition, we only used summary-level GWAS data from European ancestry to select the qualified IVs. Finally, we calculated the F statistics to further extract IVs strongly associated with phenotypes (F statistic >10), and these SNPs were considered valid and reliable IVs.
2.3. Mendelian randomization analysis
MR analysis must satisfy the following three assumptions: (1) the selected IVs must be strongly associated with the exposure; (2) the selected IVs should not be associated with potential confounders; (3) the selected IVs could only influence the outcomes through the exposure, but not other pathways. We chose five different methods of MR [random-effect inverse-variance weighted (IVW), MR Egger, weighted median, weighted mode and Wald ratio] to explore the causal relationship, and IVW was used as the major outcome, which is equivalent to fitting a weighted linear regression of the geneoutcome associations on the gene-exposure associations, with the intercept term constrained to zero (16). The estimated causal effects obtained from IVW method were compared with those obtained from MR-Egger regression, weighted median and weighted mode. MR-Egger regression allows for an unconstrained intercept term and provides a robust causal effect estimate, after adjusting for horizontal pleiotropy (17). The weighted median estimator can be considered an causal effect estimate without bias when up to 50% of the instruments are invalid, by estimating the causal effect as the median of the weighted ratio estimates (18). The weighted mode detects weaker causal effects than IVW, but presents less bias and lower type-I error rates (19). When the number of IVs extracted is less than 3, we used the Wald ratio to estimate the causal relationship.
2.4. Sensitivity analyses
We performed sensitivity analyses to verify the causalities obtained with bidirectional MR. First, the Cochran's Q tests were performed to detect the heterogeneity of the IVW method, and P < 0.05 in the Cochran's Q test indicated heterogeneity. Second, we performed the MR-egger intercept and the global MR pleiotropy residual sum and outlier (MR-PRESSO) to test and correct the potential horizontal pleiotropy of the selected IVs. The MREgger method was used to assess potential directional pleiotropy. A statistically significant intercept suggests directional pleiotropy, violating the instrumental variable assumptions (17). MR-PRESSO has three components which are detection of horizontal pleiotropy, correction for horizontal pleiotropy via outlier removal and testing of significant differences in the causal estimates before and after correction for outliers (20). Third, we conducted leave-one out analysis to explore the influence of each genetic variant on the outcome (21). All in all, the reliability of our causal effects were confirmed by the sensitivity analyses in both the forward and reverse MR results.
3. Results
A total of 611 SNPs were used as IVs for MR tests according to the selection criteria of IVs. And when average cortical thickness total corticasurface area, hippocampal volume in AD, AD progression score and cerebral amyloid deposition measurement in AD were used as exposures, the F values of the extracted SNPs were less than 10. Therefore, we did not performed reverse MR analysis when the above phenotypes were used as exposures. The full lists of IVs used for forward and reverse MR tests are provided in in Supplementary Table S1.
According to the results of the forward MR anaylsis, there was little evidence to support a causal effect of CAD on cognitive impairment. Further, we failed to find evidence that CAD impacted dementia-related outcome. In the reverse MR analyses, we detect causal effects of fluid intelligence score (IVW: β = −0.12, 95% CI of −0.18 to −0.06, P = 6.8 × 10−5), cognitive performance (IVW β=−0.18, 95% CI of −0.28 to −0.08, P = 5.8 × 10−4) and dementia with lewy bodies (IVW: OR = 1.07, 95% CI of 1.04–1.10, P = 1.1 × 10−5) on CAD. Complete results are presented in Supplementary Table S2. And the results were presented in Figure 2.
The sensitivity analyses confirmed the reliability of our result in both the forward and reverse MR. First of all, the MR-PRESSO test and the MR-egger intercept did not detect any evidence of horizontal pleiotropy. Second, Cochran's IVW Q test showed the P-values of these IVs from fluid intelligence score and cognitive performance were less than 0.05, but the results have less impact on causality because we chose a random-effect IVW. Third, leave-one-out analyses showed that no single SNP drove the causal estimates. Complete results of sensitivity analyses are presented in Supplementary Tables S3,S4.
4. Discussion
Observational studies had reported that CAD are associated with cognitive impairment. However it is undeniable that the results obtained from observational studies are largely influenced by confounding factors, such as hypertension (22), so it is difficult to distinguish whether the effect on cognition is caused by CAD or other factors. In the present study, we performed bidirectional two-sample MR analyses to systematically explore the causal relationship between CAD and cognitive impairment by controlling for confounding factors. And we did not identified CAD has a causal influence on cognitive impairment and dementia in the forward MR analysis. The clinical implication of these data is that the treatment of CAD may not directly protect against cognitive impairment. However, it is worth noting that the cardiovascular risk factors associate with cognitive impairment (23–25), the treatment strategies and lifestyle interventions currently recommended for CAD may indirectly have beneficial effects on the risk of cognitive impairment by improving these risk factors.
Moreover, we detect causal effects of fluid intelligence score, cognitive performance, and dementia with lewy bodies on CAD. Similar to our results, several observational studies also showed that cognitive impairment contributes to increased risk of coronary heart disease and in-hospital mortality (26, 27). However, these observational studies consistent with our results cannot completely exclude the influence of other confounding factors, so this result needs to be viewed with caution. The mechanism for the effect of cognitive impairment on CAD is still uncertain. A previous study showed that measuring blood levels of amyloid-beta 1–40 (Aβ40) were significantly and independently associated with arterial stiffness progression, incident subclinical atherosclerosis, and incident CAD (28). Stamatelopoulos et al. found that circulating Aβ40 is a predictor of mortality and improves risk stratification of patients with Non-ST-Segment Elevation Acute Coronary Syndrome (29). Amyloid-beta (Aβ) highly associated with cognitive impairment and dementia (30–33). The Aβ produced by the brain is able to cross the blood-brain barrier and thus enter the bloodstream (34–36), and then cause non-neurological amyloid lesions by depositing in distal organs and blood vessels (37, 38). Increasing evidence from animal studies have shown that Aβ plays a central role in vascular inflammation pathophysiology (39–43). It can promotes the secretion of cytokines, leading to oxidative stress, and activate a cascade of other proinflammatory responses, which ultimately lead to vascular disease (41, 44, 45). In summary, the causal relationship between cognitive impairment and CAD may be due to a series of pathological processes centered on Aβ. And further studies are needed to verify this association and to understand the mechanisms behind it.
The limitations of our study should be addressed. Frist, the small amount of data from ANDI may be underpowered enough to prevent potential relationships from being detected. When biometrics of AD from ANDI were used as exposures in the forward MR analysis, IVs could not be extracted, and this may be precisely due to insufficient data volume. Therefore, these analyses using biometrics of AD should be repeated when larger datasets become available. Second, we only analyzed the causal relationship between average cortical thickness and total corticasurface area and CAD. However different cortical regions have functional specializations (13), therefore, a more fine-grained analysis of cortical regions is needed to discover potential causal links. Third, the data we used were all from European ancestry, so our results may not be generalizable to populations of other ancestry. Finally, although IVs were obtained after strict screening criteria, caution is still needed. We performed the MR-egger intercept and MR-PRESSO to test and correct the potential horizontal pleiotropy of the selected IVs. However, there are limitations to theses approaches. Only if the gene pleiotropy is directional, and MR-egger intercept can detect the potential horizontal pleiotropy. In some cases, correction strategies of MR-PRESSO cannot completely eliminate the horizontal pleiotropy. Therefore, we look forward to better approaches to test and correct the potential horizontal pleiotropy of the IVs in the future.
In conclusion, we performed bidirectional two-sample MR analyses to systematically estimate the underlying causal relationships between CAD and cognitive impairment. We found some evidence to support a causal role of cognitive impairment in the pathogenesis of CAD. Future studies are needed to profoundly investigate the relationships between CAD and cognitive impairment, which might provide new insight into the prevention of CAD. Our findings highlight the importance of screening for coronary heart disease in patients of cognitive impairment. Moreover, our study provides clues for risk factor identification and early prediction of CAD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YL and FX: conceptualization, methodology and writing - review and editing. SX and YL: investigation, resources, data curation and writing - original draft. QW and FL: data curation and visualization. All authors contributed to the article and approved the submitted version.
Funding
This study is supported through the key project of scientific and technological innovation project sponsored by Chinese Academy of Chinese Medical Sciences (grant no. CI2021A01406) and the Special Project for Outstanding Young Talents of China Academy of Chinese Medical Sciences (grant no. ZZ15-YQ-017 and ZZ13-YQ-001-A1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1150432/full#supplementary-material.
References
1. GBD 2019 Diseases and Injuries. Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. (2022) 145(8):e153–e639. doi: 10.1161/CIR.0000000000001052
3. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. (2017) 390:2673–734. doi: 10.1016/S0140-6736(17)31363-6
4. Xie W, Zheng F, Yan L, Zhong B. Cognitive decline before and after incident coronary events. J Am Coll Cardiol. (2019) 73:3041–50. doi: 10.1016/j.jacc.2019.04.019
5. Schievink SHJ, van Boxtel MPJ, Deckers K, van Oostenbrugge RJ, Verhey FRJ, Köhler S. Cognitive changes in prevalent and incident cardiovascular disease: a 12-year follow-up in the Maastricht aging study (MAAS). Eur Heart J. (2017) 43:e2–e9. doi: 10.1093/eurheartj/ehx365. [E-pub ahead of print].
6. Petrovitch H, White L, Masaki KH, Ross GW, Abbott RD, Rodriguez BL, et al. Influence of myocardial infarction, coronary artery bypass surgery, and stroke on cognitive impairment in late life. Am J Cardiol. (1998) 81:1017–21. doi: 10.1016/S0002-9149(98)00082-4
7. Zhu L, Viitanen M, Guo Z, Winblad B, Fratiglioni L. Blood pressure reduction, cardiovascular diseases, and cognitive decline in themini-mental state examination in a community population of normal very old people: a three-year follow-up. J Clin Epidemiol. (1998) 51:385–91. doi: 10.1016/S0895-4356(98)00003-1
8. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326(16):1614–21. doi: 10.1001/jama.2021.18236
9. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47:1121–30. doi: 10.1038/ng.3396
10. Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. (2018) 50(8):1112–21. doi: 10.1038/s41588-018-0147-3
11. Haworth S, Mitchell R, Corbin L, Wade KH, Dudding T, Budu-Aggrey A, et al. Apparent latent structure within the UK biobank sample has implications for epidemiological analysis. Nat Commun. (2019) 10(1):333. doi: 10.1038/s41467-018-08219-1
12. Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun. (2018) 9(1):2098. doi: 10.1038/s41467-018-04362-x
13. Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, et al. The genetic architecture of the human cerebral cortex. Science. (2020) 367:eaay6690. doi: 10.1126/science.aay6690
14. Scelsi MA, Khan RR, Lorenzi M, Christopher L, Greicius MD, Schott JM, et al. Genetic study of multimodal imaging Alzheimer’s disease progression score implicates novel loci. Brain. (2018) 141(7):2167–80. doi: 10.1093/brain/awy141
15. Chia R, Sabir MS, Bandres-Ciga S, Saez-Atienzar S, Reynolds RH, Gustavsson E, et al. Genome sequencing analysis identifies new loci associated with lewy body dementia and provides insights into its genetic architecture. Nat Genet. (2021) 53(3):294–303. doi: 10.1038/s41588-021-00785-3
16. Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG; EPIC- InterAct Consortium. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30(7):543–52. doi: 10.1007/s10654-015-0011-z
17. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
18. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40(4):304–14. doi: 10.1002/gepi.21965
19. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46(6):1985–98. doi: 10.1093/ije/dyx102
20. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mndelian randomization between complex traits and diseases. Nat Genet. (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
21. Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-egger method. Eur J Epidemiol. (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
22. Peters R, Xu Y, Fitzgerald O, Aung HL, Beckett N, Bulpitt C, et al. Dementia rIsk REduCTion (DIRECT) collaborationet al.Blood pressure lowering and prevention of dementia: an individual patient data meta-analysis. Eur Heart J. (2022) 43(48):4980–90. doi: 10.1093/eurheartj/ehac584
23. Rovio SP, Pahkala K, Nevalainen J, Juonala M, Salo P, Kähönen M, et al. Cardiovascular risk factors from childhood and midlife cognitive performance: the young Finns study. J Am Coll Cardiol. (2017) 69(18):2279–89. doi: 10.1016/j.jacc.2017.02.060
24. Yaffe K, Vittinghoff E, Hoang T, Matthews K, Golden SH, Zeki Al Hazzouri A. Cardiovascular risk factors across the life course and cognitive decline: a pooled cohort study. Neurology. (2021) 96(17):e2212–9. doi: 10.1212/WNL.0000000000011747
25. Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR Jr, Lewis CE, et al. Cardiovascular risk factors and accelerated cognitive decline in midlife: the CARDIA study. Neurology. (2020) 95(7):e839–46. doi: 10.1212/WNL.0000000000010078
26. Bagai A, Chen AY, Udell JA, Dodson JA, McManus DD, Maurer MS, et al. Association of cognitive impairment with treatment and outcomes in older myocardial infarction patients: a report from the NCDR chest pain-MI registry. J Am Heart Assoc. (2019) 8(17):e012929. doi: 10.1161/JAHA.119.012929
27. Leng X, Espeland MA, Manson JE, Stefanick ML, Gower EW, Hayden KM, et al. Cognitive function and changes in cognitive function as predictors of incident cardiovascular disease: the women's health initiative memory study. J Gerontol A Biol Sci Med Sci. (2018) 73(6):779–85. doi: 10.1093/gerona/glx138
28. Stamatelopoulos K, Sibbing D, Rallidis LS, Georgiopoulos G, Stakos D, Braun S, et al. Amyloid-beta (1–40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J Am Coll Cardiol. (2015) 65(9):904–16. doi: 10.1016/j.jacc.2014.12.035
29. Stamatelopoulos K, Mueller-Hennessen M, Georgiopoulos G, Sachse M, Boeddinghaus J, Sopova K, et al. Amyloid-β (1–40) and mortality in patients with non-ST-segment elevation acute coronary syndrome: a cohort study. Ann Intern Med. (2018) 168(12):855–65. doi: 10.7326/M17-1540
30. Elman JA, Panizzon MS, Gustavson DE, Franz CE, Sanderson-Cimino ME, Lyons MJ, et al.Alzheimer’s disease neuroimaging initiative. Amyloid-β positivity predicts cognitive decline but cognition predicts progression to amyloid-β positivity. Biol Psychiatry. (2020) 87(9):819–28. doi: 10.1016/j.biopsych.2019.12.021
31. Yoo HS, Jeon S, Cavedo E, Ko M, Yun M, Lee PH, et al. Association of β-amyloid and basal forebrain with cortical thickness and cognition in Alzheimer and Lewy body disease Spectra. Neurology. (2022) 98(9):e947–57. doi: 10.1212/WNL.0000000000013277
32. Wagner J, Degenhardt K, Veit M, Louros N, Konstantoulea K, Skodras A, et al. Medin co-aggregates with vascular amyloid-β in Alzheimer’s disease. Nature. (2022) 612(7938):123–31. doi: 10.1038/s41586-022-05440-3
33. Ossenkoppele R, Pichet Binette A, Groot C, Smith R, Strandberg O, Palmqvist S, et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med. (2022) 28(11):2381–7. doi: 10.1038/s41591-022-02049-x
34. Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. (1996) 66:11–23. doi: 10.1006/nlme.1996.0039
35. Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. (2003) 10:463–70. doi: 10.1038/sj.mn.7800212
36. Pitschke M, Prior R, Haupt M, Riesner D. Detection of single amyloid beta-protein aggregates in the cerebrospinal fluid of Alzheimer’s patients by fluorescence correlation spectroscopy. Nat Med. (1998) 4:832–4. doi: 10.1038/nm0798-832
37. Kuo YM, Kokjohn TA, Watson MD, Woods AS, Cotter RJ, Sue LI, et al. Elevated Aβ42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AβPP metabolism. Am J Pathol. (2000) 156:797–805. doi: 10.1016/S0002-9440(10)64947-4
38. Troncone L, Luciani M, Coggins M, Wilker EH, Ho CY, Codispoti KE, et al. Aβ amyloid pathology affects the hearts of patients with Alzheimer’s disease: mind the heart. J Am Coll Cardiol. (2016) 68(22):2395–407. doi: 10.1016/j.jacc.2016.08.073
39. Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. (2006) 12(9):1005–15. doi: 10.1038/nm1484
40. Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. (2003) 9(7):907–13. doi: 10.1038/nm890
41. Puglielli L, Friedlich AL, Setchell KD, Nagano S, Opazo C, Cherny RA, et al. Alzheimer Disease beta-amyloid activity mimics cholesterol oxidase. J Clin Invest. (2005) 115(9):2556–63. doi: 10.1172/JCI23610
42. Tibolla G, Norata GD, Meda C, Arnaboldi L, Uboldi P, Piazza F, et al. Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis. (2010) 210(1):78–87. doi: 10.1016/j.atherosclerosis.2009.10.040
43. Van De Parre TJ, Guns PJ, Fransen P, Martinet W, Bult H, Herman AG, et al. Attenuated atherogenesis in apolipoprotein E-deficient mice lacking amyloid precursor protein. Atherosclerosis. (2011) 216(1):54–8. doi: 10.1016/j.atherosclerosis.2011.01.032
44. Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, et al. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. (2009) 34(1):95–106. doi: 10.1016/j.nbd.2008.12.007
Keywords: causal effect, genome-wide association studies, mendelian randomization, cognitive impairment, coronary artery disease
Citation: Xu S, Liu Y, Wang Q, Liu F, Xu F and Liu Y (2023) Mendelian randomization study reveals a causal relationship between coronary artery disease and cognitive impairment. Front. Cardiovasc. Med. 10:1150432. doi: 10.3389/fcvm.2023.1150432
Received: 27 January 2023; Accepted: 9 May 2023;
Published: 23 May 2023.
Edited by:
Jingkai Wei, University of South Carolina, United StatesReviewed by:
Xuan Zhang, National Institute on Aging (NIH), United StatesChao Yang, University of Texas MD Anderson Cancer Center, United States
© 2023 Xu, Liu, Wang, Liu, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengqin Xu ZHIueHVmZW5ncWluQGhvdG1haWwuY29t Yue Liu bGl1eXVlaGVhcnRAaG90bWFpbC5jb20=
†These authors share first authorship
 Shihan Xu
Shihan Xu Yanfei Liu
Yanfei Liu Qing Wang
Qing Wang Fenglan Liu
Fenglan Liu Fengqin Xu1,2*
Fengqin Xu1,2* Yue Liu
Yue Liu