
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 15 March 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1144333
This article is part of the Research Topic Comprehensive Risk Prediction in Cardiomyopathies: New genetic and imaging markers of risk, Volume II View all 6 articles
 Jun-Yan Zhu1,2,3
Jun-Yan Zhu1,2,3 Xin-Chao Wang4,5
Xin-Chao Wang4,5 Nan Huang3
Nan Huang3 Xiao-Qian Li3
Xiao-Qian Li3 Yan Cheng3,5
Yan Cheng3,5 Zhi-Fang Wu3,5
Zhi-Fang Wu3,5 Yuan-Yuan Li1,2,6
Yuan-Yuan Li1,2,6 Ping Wu3,5
Ping Wu3,5 Li Li3,5
Li Li3,5 Hua Wei3
Hua Wei3 Si-Jin Li1,3*
Si-Jin Li1,3* Ji-Min Cao1,2*
Ji-Min Cao1,2*
Background: The prognosis of patients with dilated cardiomyopathy (DCM) is poor and new indicators are urgently needed to predict lethal cardiac events. This study aimed to investigate the value of summed motion score (SMS) in predicting cardiac death of DCM patients using gated single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI).
Methods and results: Eighty-one patients with DCM who underwent 99mTc-MIBI gated SPECT MPI were retrospectively enrolled and were divided into cardiac death and survivor groups. The functional parameters of left ventricle including SMS were measured using quantitative gated SPECT software. During the follow-up period of 44 (25, 54) months, 14 (17.28%) cardiac deaths were observed. Compared with the survivor group, SMS was significantly higher in the cardiac death group. Multivariate cox regression analysis showed that SMS was an independent predictor for cardiac death (HR 1.34, 95% CI 1.02–1.77, P = 0.034). SMS also provided incremental prognostic value over other variables in the multivariate model as determined by likelihood ratio global chi-squared test. In the Kaplan-Meier survival analysis, the event-free survival rate was significantly lower in the high-SMS (HSMS) group than the low-SMS (LSMS) (log-rank P < 0.001). Furthermore, the area under curve (AUC) of SMS was larger than that of LVEF at the 12th month of follow-up (0.85 vs. 0.80, P = 0.045).
Conclusion: SMS is an independent predictor of cardiac death in DCM patients and provides incremental prognostic value. SMS might have higher predictive value than LVEF for early cardiac death.
Dilated cardiomyopathy (DCM) is one of the most common causes of heart failure, second only to ischemic cardiomyopathy, and is the most common indication for heart transplantation worldwide (1, 2). DCM has a poor prognosis, with a 1-year mortality rate of 25%–30% and a 5-year survival rate of 50%. Despite advances in DCM treatment, the 10-year survival rate is less than 60% (2, 3). Current studies have shown that many variables are associated with adverse DCM outcomes, and the functional parameters of left ventricle obtained by whatever imaging techniques remain the major prognosticators, but their values in estimating the prognosis of DCM are limited (2, 4). Therefore, there is still an urgent need for new markers to predict the outcome of DCM patients so as to prolong their survival.
Studies have shown that regional wall motion obtained from gated single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) provides important prognostic information on cardiovascular outcome events (5–7). For example, in patients with known or suspected coronary artery disease (CAD), abnormalities of post-stress reversible regional wall motion are the most powerful predictive parameters of cardiac events including cardiac death, nonfatal myocardial infarction, unstable angina, and early or late coronary revascularization (8). Furthermore, SPECT has the advantage of high reproducibility and repeatability and can be used for semi-quantitative analysis of ventricular wall motion to obtain summed motion score (SMS) (9–11). SMS can be considered as an independent prognostic factor in the risk stratification of patients with suspected CAD (12). SMS can also be used as a predictor of cardiac events and have a value for clinical risk stratification of diabetic patients with normal perfusion (13). However, to date, there is no report documenting the value of SMS in predicting the prognosis of DCM. Accordingly, this study aimed to investigate the value and incremental value of SMS in the prognosis of patients with DCM using gated SPECT MPI.
Eighty-one patients with DCM were retrospectively enrolled in this study at the First Hospital of Shanxi Medical University from January 2015 to July 2020. These patients were eligible to be enrolled after their diagnosis were confirmed, based on medical history, clinical, electrocardiographic, and echocardiographic findings according to the recommendation criteria (14–16). Patients with the following conditions were excluded (17, 18): (1) CAD with lumen stenosis greater than 50% or a history of myocardial infarction or stent placement; (2) valvular heart disease, alcoholism, inflammatory cardiomyopathy, or specific cardiomyopathy secondary to any known systemic disease; (3) patients with a history of cardiac resynchronization therapy or implantable cardioverter defibrillator; (4) patients with malignant tumors. All the enrolled patients underwent coronary angiography or coronary computed tomography angiography, with coronary artery stenosis ≤50%, and had complete data of demography, electrocardiography, echocardiography, and gated SPECT MPI. The study was approved by the Ethics Review Committee of the First Hospital of Shanxi Medical University, and informed consent was obtained from all patients.
The 99mTc was provided by Beijing Atomic High-tech Co. LTD, with radiochemical purity >95%; MIBI was purchased from Jiangsu Institute of Atomic Medicine. To perform gated SPECT MPI, each patient underwent intravenous injection of 20–30 mCi of 99mTc-MIBI in an overnight fast state, followed by a fatty meal 20 min after tracer injection, and resting gated SPECT MPI scan was performed 60 min after injection. The MPI images were acquired on a dual-collimator instrument (Siemens Symbia T16, Siemens) using a standard protocol. Image acquisition parameters: SMART-ZOOM collimator, energy peak 140 keV, matrix 128 × 128, magnification 1.0, acquisition of myocardial images in ECG gated tomography mode, and ECG window width 20%. The two probes were rotated at an angle of 76° and images were acquired total for 208° from a right anterior oblique 38° to a left posterior oblique 66°. The acquisition speed was 25 sec per frame, and 34 frames were acquired in approximately 8 min. The ordered-subsets expectation maximization (OSEM) method was used to reconstruct the image (iteration number 12, subset 5) and to obtain the myocardial tomographic images at the left ventricular (LV) short axis, horizontal long axis and vertical long axis.
Gated SPECT tomograms were reconstructed and reoriented using automated software (Autoquant software, Cedars Sinai Medical Center, Los Angeles, California, United States). A 17-segment model (American Heart Association, AHA) was used to analyze the gated SPECT data (19, 20). Quantitative gated SPECT (QGS) software was used to analyze: (1) LV global functional parameters, including LV ejection fraction (LVEF) (LVEF was also measured by conventional echocardiography in this study), LV end diastolic volume (EDV), and LV end systolic volume (ESV); (2) LV regional function parameters, including regional myocardial wall motion (RWM) and regional myocardial wall thickening (RWT); (3) LV mechanical systolic synchrony parameters: phase standard deviation (PSD), phase histogram bandwidth (PBW), mean and phase entropy (PE). Segmental wall motion was graded according to the 6-point scoring system (0-normal, 1-mild hypokinesia, 2-moderate hypokinesia, 3-severe hypokinesia, 4-akinesia, 5-dyskinesia). Wall thickening was graded using the 4-point scoring system (0-normal, 1-mild, 2-moderate to severe, 3-absent) (12, 21). The scores of the 17 segments were added up to obtain the summed motion score (SMS) and summed thickening score (STS), respectively (13).
Follow-up data were obtained through phone contact with patients or their relatives, and other information of patients were obtained from the records of the hospitalized case system. The endpoint was cardiac death, including cardiac arrest or death from circulatory failure occurring within the first hour or refractory chronic heart failure (17). The mean follow-up period was 44 (25, 54) months.
R4.1.2 software and IBM SPSS Statistics 28.0 were used for statistical analysis. Package “survminer” was used to find the cut-off value for SMS, and the population was divided into high-SMS (HSMS) and low-SMS (LSMS) groups based on the cut-off value of SMS. The quantitative data adopted the Mann–Whitney U test and were expressed as M (P25, P75). The categorical variable adopted the chi-square test or nonparametric Fisher’s exact test and was presented as number and percentage.
The Kaplan-Meier method was used to obtain the event-free survival curve, and the log-rank test was used for comparison; univariable cox survival analysis and least absolute shrinkage and selection operator (LASSO) regression were performed to assess the association of each variable with survival outcome. The method of LASSO was used to select LV function parameters. Univariable cox regression analysis was used to calculate the hazard ratio (HR) and the 95% confidence interval (95% CI) of each variable. Multivariable cox regression analysis was used to determine independent predictors of adverse events. The deviation residuals were drawn for continuous variables to check the hypothesis of proportional hazards. Variables with P < 0.05 in univariable cox regression analysis were included in multivariable Cox regression analysis. For any variable included in the cox model, the proportional hazard assumption was not rejected. In addition, Spearman’s correlation was used to illustrate the strength of an association between imaging indicators.
We also used global likelihood-ratio Chi-square statistics to evaluate the incremental value of different models including clinical variables (clin) only, clin + SMS, clin + conventional LV function parameters (con LV), and clin + SMS + con LV. In addition, time-dependent receiver operating characteristic (ROC) curves were used to evaluate the performance of the indicators; Package “time ROC” was used to draw ROC curves between different imaging indicators in different times and to compare the area under the curve (AUC) of them. P < 0.05 was considered to be statistically significant.
Table 1 presents the demographic and clinical characteristics of the 81 participating patients [54.0 (42.0; 62.0) years old, 72.8% male]. Patients were divided into two groups: the cardiac death group and the survivor group. Patients in the cardiac death group were older and had lower BMI than those in the survivor group (P < 0.05). Of all the patients, 4 (5.0%) were classified as having NYHA functional class I, 10 (12.3%) as NYHA class II, 39 (48.1%) as NYHA class III, and 28 (34.6%) as NYHA class IV. All patients received guideline-directed medication for heart failure. The use of β-blocker in the survivor group was significantly higher than that in the cardiac death group (47, 70.1% vs. 5, 35.7%, P = 0.033). There was no significant difference in the use of ACE inhibitors/ARBs, diuretics and digoxin between the two groups. There was also no significant difference in hypertension, diabetes, hyperlipidemia, LBBB, mitral regurgitation and LVEF measured by echocardiography (LVEFecho) between the two groups. The QRS duration was longer in the cardiac death group than in the survivor group (130.0 [106.0; 165.0] ms vs. 100.0 [90.5; 113.0] ms, P = 0.007).
Table 2 describes the LV function parameters from gated SPECT MPI. Compared with the survivor group, the cardiac death group had significantly lower LVEF (13.0 [9.00; 15.5]% vs. 18.0 [15.0; 25.0]%, P = 0.001) and greater EDV and ESV (EDV: 298.0 [254.0; 385.0] ml vs. 197.0 [166.0; 260.0] ml, P = 0.001; ESV: 258.0 [224.0; 348.0] ml vs. 159.0 [125.0; 220.0] ml, P = 0.001). Compared with the survivor group, the cardiac death group had higher SMS (56.5 [53.0; 58.8] vs. 47.0 [37.5; 53.5], P < 0.001), higher STS (34.0 [32.2; 37.0] vs. 31.0 [27.5; 33.5], P = 0.003), and larger PE (66.0 [63.5; 72.5] vs. 62.0 [55.0; 67.0], P = 0.015). There was no significant difference in other mechanical contraction synchrony parameters PBW, PSD and mean between the two groups. Figure 1 shows the wall motion and wall thickening of a representative case in both groups.
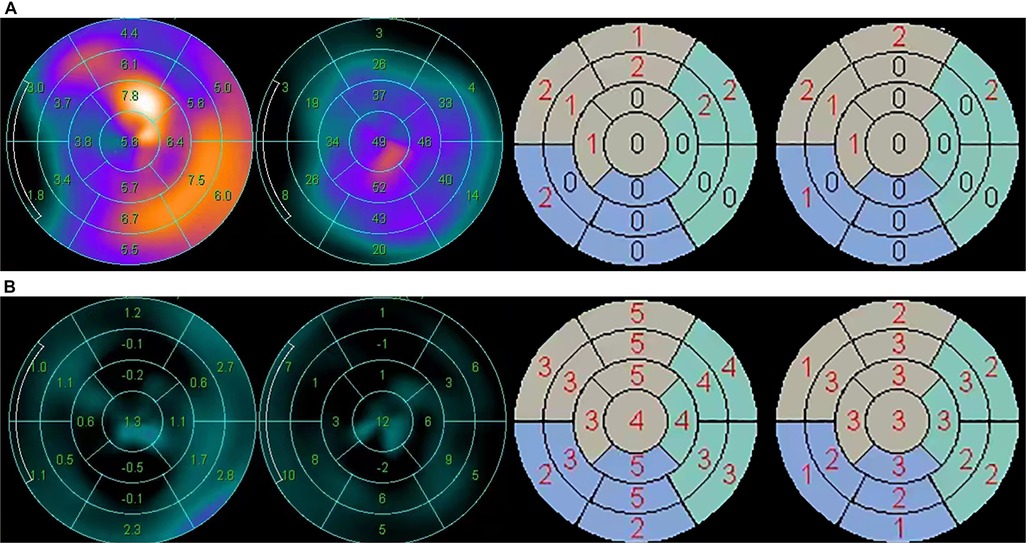
Figure 1. Wall motion and wall thickening analysis in two typical cases of the survivor group and the cardiac death group, respectively. (A) The wall motion (mm), wall thickening (%), wall motion score and wall thickening score of a 72-year-old male patient in the survivor group. SMS = 13, STS = 9. (B) The wall motion (mm), wall thickening (%), wall motion score and wall thickening score of a 65-year-old male patient in the cardiac death group. SMS = 63, STS = 39.
Table 3 shows the baseline characteristics and LV function parameters of patients grouped according to SMS. Patients were dichotomized into two groups according to the cut-off value of SMS: 44 patients were assigned to the LSMS group (≤50), and 37 patients were assigned to the HSMS group (>50). Compared with the LSMS group, patients in the HSMS group had lower BMI (P = 0.035), longer QRS duration (P = 0.019), more NYHA class III/IV cases (P = 0.045) and lower LVEFecho (P = 0.001). There was no statistical difference in age, sex, hypertension, diabetes, hyperlipidemia, LBBB, mitral regurgitation and medication use (ACE inhibitors/ARBs, β-blockers, diuretics and digoxin) between LSMS and HSMS groups. Compared with the LSMS group, the HSMS group had significantly lower LVEF (P < 0.05), significantly higher SMS (P < 0.001), and significantly larger values of EDV, ESV, STS, mean and PE (P < 0.05). The differences in PBW and PSD between the LSMS and HSMS groups were not statistically significant.
Univariable cox regression analysis showed that age, BMI, QRS duration (ms), β-blockers, EDV, ESV, LVEF, SMS, STS and PE were associated with cardiac death (total P < 0.05) (Table 4). These parameters were included in the multivariate analysis. In addition, LASSO regression could select variables by performing a penalized regression on all variable coefficients so that coefficients of relatively insignificant independent variables became zero. To eliminate possible collinearity among LV function parameters, we used LASSO regression to screen variables (Figure 2), and finally selected SMS, ESV and mean from 10 variables and then put these three variables into the multivariate cox regression model. The multivariate cox regression analysis revealed that SMS was an independent predictor of cardiac death (HR 1.34, 95% CI 1.02–1.77, P = 0.034). Age and β-blockers were also influential factors for cardiac death (Age: HR 1.08, 95% CI 1.01–1.15, P = 0.017; β-blockers: HR 0.13, 95% CI 0.02–0.76, P = 0.024) (Table 4).
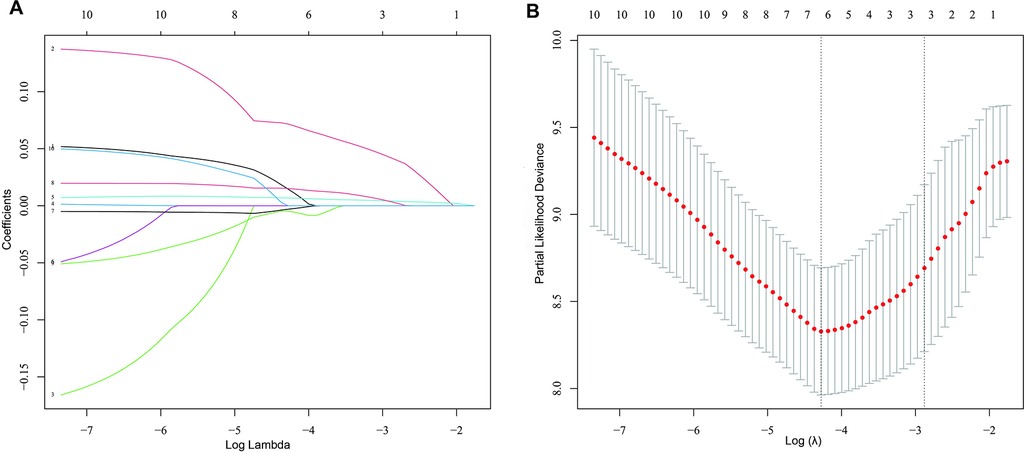
Figure 2. Screening for variables using the LASSO regression model. (A) Results of the Lasso regression. The 10 colored lines marked by Arabic numerals represent 10 different variables, lines 1–10 represented LVEFecho, SMS, STS, EDV (ml), ESV (ml), LVEF (%), PBW (°), Mean, PSD (°), and PE, respectively. By the LASSO regression, three variables (SMS, ESV and mean) were finally selected from the 10 variables. (B) LASSO coefficient profiles of the 10 prognostic factors for cardiac death. The optimal value of λ was determined by 10-fold cross-validation to find the minimum mean squared error (minMSE) + 1 standard error of minMSE backwards along the λ path.
Table 5 shows the hazard ratio (HR) of SMS on cardiac death after correction for potential confounders including age, gender, BMI, hypertension, diabetes, hyperlipidemia, QRS duration, NYHA class, ACE inhibitors/ARBs, β-blockers, diuretics, digoxin, LVEFecho, EDV, ESV, LVEF, STS, PBW, mean, PSD and PE. In addition, our results showed that SMS was significantly associated with LVEF (r = −0.917, P < 0.001) (Figure 3). To clarify whether the effect of SMS on the prognosis was influenced by LVEF, we further explored the interaction between SMS and LVEF, but did not find interaction between them in model 3 (P = 0.972) (Table 5), indicating that SMS was always an independent predictor of cardiac death in DCM patients (total P < 0.05).
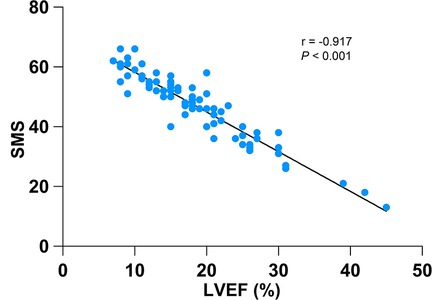
Figure 3. Relationship between SMS and LVEF. A significant inverse correlation was observed between SMS and LVEF (r = −0.917, P < 0.001).
Adding SMS increased the global Chi-square from 12.845 to 28.120 (P < 0.001) and adding the conventional LV function parameters (con LV) increased the global Chi-square from 12.845 to 42.624 (P < 0.001) compared to clinical variables only. Adding SMS to the model that included clinical variables and con LV still improved the global Chi-square (42.624 vs. 44.240, P = 0.047) (Figure 4).
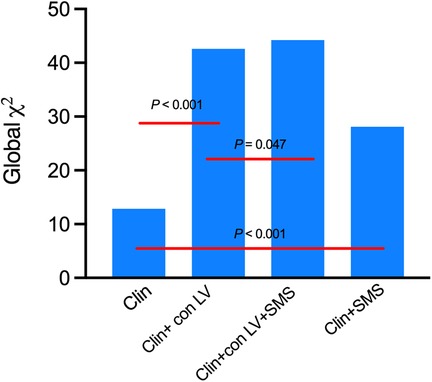
Figure 4. Incremental association between clinical variables (Clin), conventional LV function parameters (con LV), SMS and cardiac death as indicated by the statistics in serial multivariable adjusted models. Clinical variables include age, gender, BMI, Hypertension, Diabetes, Hyperlipidemia, QRS duration and NYHA class. LV index included EDV, ESV, LVEF, STS, Mean and PE.
During the follow-up period of 44 (25, 54) months, cardiac death occurred in 14 (17.28%) of the total 81 patients. The Kaplan-Meier event-free survival curve showed that the HSMS group had a lower event-free survival rate than the LSMS group (log-rank P < 0.001). The higher the SMS, the worse the prognosis (Figure 5). The time-dependent ROC curve of SMS showed that the area under the curve (AUC) at 12 months, 36 months and 60 months was 0.853, 0.785 and 0.871, respectively (Figure 6A). The time-dependent ROC curve of LVEF revealed that the AUC at 12 months, 36 months and 60 months was 0.795, 0.828 and 0.812, respectively (Figure 6B). The time-dependent ROC curves showed that the AUC of SMS was larger than that of LVEF at the 12th month of follow-up (P = 0.045), and there was no significant difference in the AUC between SMS and LVEF at other follow-up times (Figure 6C).
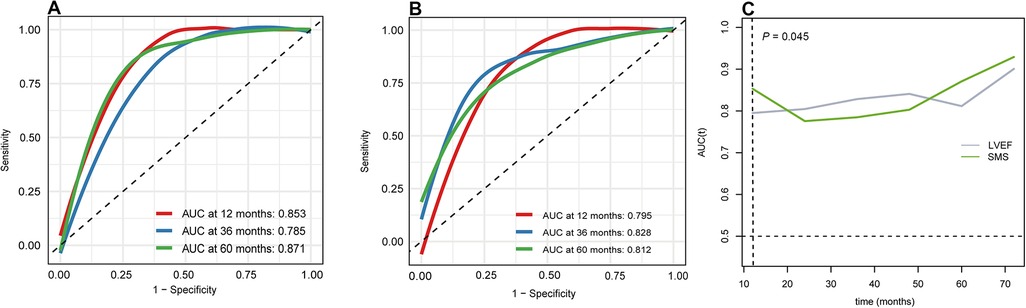
Figure 6. Time-independent receiver operating characteristic (ROC) curve. (A) ROC curve of SMS in different time. (B) ROC curve of LVEF in different time. (C) the AUC of SMS and LVEF in different time.
In this study, we investigated the prognostic value of SMS obtained by gated SPECT MPI in patients with DCM. To our knowledge, we demonstrated for the first time that SMS is an independent predictor of cardiac death in patients with DCM. On the basis of clinical variables and conventional LV functional parameters, SMS may also provide incremental value in predicting cardiac death in patients with DCM. In addition, we demonstrated that SMS may be more predictive of early cardiac death compared with LVEF.
Many previous studies have shown that abnormal ventricular wall motion is associated with poor prognosis (cardiac death, non-fatal myocardial infarction, heart failure) in cardiovascular disease (22–25). There are also studies showing that wall motion is associated with poor prognosis in patients with DCM, but most of them merely analyzed the wall motion of each segment obtained by echocardiography, radionuclide imaging and left ventriculography (26–28). However, at present, no study has used SMS to evaluate the prognosis of DCM. Gated SPECT imaging has unique capabilities to provide accurate, reproducible and operator independent quantitative data (29). This study evaluated the prognostic value of the SMS obtained by gated SPECT MPI in patients with DCM. After taking SMS-LVEF interaction into account and correcting for potential confounders (age, gender, BMI, hypertension, diabetes, hyperlipidemia, QRS duration, NYHA class, ACE inhibitors/ARBs, β-blockers, diuretics, digoxin, LVEF measured by echocardiography, EDV, ESV, LVEF, STS, PBW, Mean, PSD and PE), we still confirmed that SMS was an independent predictor of cardiac death in patients with DCM. Previous studies (18, 30, 31) found that LV dyssynchrony parameters are predictors of cardiac death in DCM patients. However, the present study suggested that these LV dyssynchrony parameters (such as PE shown in Table 4) are likely not independent predictors of cardiac death in DCM patients. Other factors, such as patient selection and study methods, may also lead to disagreement in different studies. These issues warrant further studies. In addition, we found that age and β-blockers were also influential factors for cardiac death in DCM, which is consistent with previous findings (32, 33).
The pathophysiological mechanisms of segmental ventricular wall motion abnormalities in DCM are unclear. Based on previous studies, we considered that several factors may be involved in the mechanisms. First, LV structural and functional parameters, including partial volume effects caused by myocardial wall thinning and increased local wall stress, reduction of blood perfusion due to cardiomyocyte loss, increase of intra-diastolic myocardial pressure, and myocardial fibrotic changes (26, 34, 35), may be mechanisms of abnormal segmental ventricular wall motion. Second, regional myocardial sympathetic denervation and/or hyperinnervation may partially contribute to the abnormalities of segmental ventricular wall motion, because spatiotemporal changes of cardiac sympathetic innervation often occur in cardiac diseases including DCM and these neuronal changes would inevitably affect myocardial contractility, wall stress and motion, and tension of coronary arteries. The severity of these changes is partially associated with local ventricular wall motion abnormalities and myocardial perfusion abnormalities (36). Third, microcirculatory dysfunction may also be contributable to segmental ventricular wall motion. Coronary microvascular damage, loss of endothelium-dependent relaxation of coronary microvessels and inflammation due to viral cardiomyopathy have been associated with segmental ventricular wall motion abnormalities (37–39).
Our study also found the incremental value of SMS in predicting cardiac death in DCM. Compared to a model with only clinical variables (age, gender, BMI, hypertension, diabetes, hyperlipidemia, QRS duration and NYHA class), adding SMS increased the global Chi-square from 12.845 to 28.120 (P < 0.001) (shown in Figure 4); adding conventional LV function parameters (EDV, ESV, LVEF, STS, mean and PE) into the model increased the global Chi-square from 12.845 to 42.624 (P < 0.001) (Figure 4). The addition of SMS continued to improve the global Chi-square compared to a model that included both clinical variables and conventional LV function parameters (42.624 vs. 44.240, P = 0.047) (Figure 4). We also found that the higher the SMS, the worse the prognosis of the DCM patients. These findings suggest that for patients with DCM, in addition to the conventional clinical and imaging indicators, additional analysis of the LV function parameters, especially the SMS, may help clinicians to better assess the conditions of patients and may provide better guidance for patient treatment, and thus may prolong the survival of patients.
In the follow-up of the 81 DCM patients for 44 (25, 54) months, we found that the AUC of the ROC curve of SMS was relatively stable around 0.80 at all timepoints, suggesting that SMS is relatively stable in predicting cardiac death. It is well known that the LV global functional parameter LVEF is an important predictor of cardiac death in patients with DCM (2, 17). The global and regional functions are closely related and the correlation between global systolic wall motion score measured by echocardiography (echocardiographic wall motion score index, WSMI) and LVEF obtained by radionuclide ventriculography is high (r = 0.72) (40, 41). Our present study showed a higher correlation between LVEF and SMS (r = 0.917), possibly because both of the two parameters were obtained through gated SPECT MPI and were consistent. Our study also showed a reduction in LVEF in the HSMS group compared to the LSMS group (21.5% vs. 12.0%, P < 0.001). However, LVEF reflects the global function, and the hypercontractile cardiomyopathy segments may offset the hypokinetic cardiomyopathy segments, thereby preserving total LV systolic function (42). Studies have reported that WSMI is a stronger powerful predictor of adverse cardiac outcomes in patients with acute myocardial infarction (43–45). In addition, WSMI is the only significant independent predictor of cardiac events in patients with chronic congestive heart failure (41). The present study also showed similar results. For patients with DCM, SMS and LVEF were both predictors in the univariate model, while only SMS was an independent predictor of cardiac death in the multivariate model. Here we also found that there was no significant difference in LVEFecho between the survivor group and the cardiac death group, while there was a significant difference in LVEF between the two groups as measured by gated SPECT MPI. The reason for this phenomenon may be that echocardiography is more affected by the operator, and gated SPECT MPI is less affected by the operator and show higher reliability.
Interestingly, we found that the AUC of the ROC curve for SMS was larger than that of LVEF at the 12th month after follow-up, suggesting that SMS is more predictive of early cardiac death in DCM. One possible explanation of this phenomenon is that compensatory hyperkinesia of non-involved myocardium may not affect the ventricular wall motion score while limit the overall LVEF reduction, therefore the sensitivity of the ventricular wall motion score is higher than LVEF in detecting myocardial injury, especially in the early stage of cardiac diseases (46, 47).
Our study had some limitations. First, the study was retrospective with recall bias. Second, the study was a single-center study, thus the findings might not be generalizable to a broad population. Third, the relatively smaller sample size may have limited the statistical power. Forth, this study used a relatively high tracer dose and an all-purpose gamma camera. In the future, we may improve these limitations by conducting similar studies in multicentered and larger population using new equipment.
We report for the first time that SMS can independently predict cardiac death in patients with DCM and provide incremental prognostic value. These findings may be clinically important to anticipate patients’ conditions in advance, to increase clinical attention, and to prolong patient survival. In addition, we found that SMS might have higher predictive value than LVEF for early cardiac death.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of First Hospital of Shanxi Medical University. The patients/participants provided their written informed consent to participate in this study.
JYZ conceived the study design. JYZ, NH, XQL, YC and YYL performed the data collection and completed the patient communication. JYZ and XCW summarized and analyzed the data. JYZ, ZFW, PW, LL and HW contributed to the data interpretation and the discussion. JYZ drafted the manuscript. SJL and JMC guided the study and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was financed by Shanxi “1331” Project Quality and Efficiency Improvement Plan (1331KFC) and partially by grants from the National Natural Science foundation of China (82170523, 81901785, 82001873, U22A6008).
We thank the First Hospital of Shanxi Medical University for the collaboration with data acquisition.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DCM, dilated cardiomyopathy; EDV, end diastolic volume; ESV, end systolic volume; HR, hazard ratio; LVEF, left ventricular ejection fraction; MPI, myocardial perfusion imaging; PE, phase entropy; ROC, receiver operating characteristic; SMS, summed motion score; SPECT, single photon emission computed tomography; STS, summed thickening score.
1. Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet. (2017) 390:400–14. doi: 10.1016/S0140-6736(16)31713-5
2. Donal E, Delgado V, Bucciarelli-Ducci C, Galli E, Haugaa KH, Charron P, et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: an expert consensus document from the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2019) 20:1075–93. doi: 10.1093/ehjci/jez178
3. Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. (1994) 331:1564–75. doi: 10.1056/NEJM199412083312307
4. Seferovic PM, Polovina M, Bauersachs J, Arad M, Gal TB, Lund LH, et al. Heart failure in cardiomyopathies: a position paper from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2019) 21:553–76. doi: 10.1002/ejhf.1461
5. Karacavus S, Celik A, Tutus A, Kula M, Oguzhan A, Kalay N. Can left ventricular parameters examined by gated myocardial perfusion scintigraphy and strain echocardiography be prognostic factors for major adverse cardiac events? Hell J Nucl Med. (2014) 17:10–1. doi: 10.1967/s002449910112
6. Noordzij W, Slart RH. Clinical value of quantitative measurements derived from GATED SPECT: motion and thickening, volumes and related LVEF. Q J Nucl Med Mol Imaging. (2018) 62:321–4. doi: 10.23736/S1824-4785.16.02868-X
7. Shen TY, Chang MC, Hung GU, Kao CH, Hsu BL. Prognostic value of functional variables as assessed by gated thallium-201 myocardial perfusion single photon emission computed tomography for Major adverse cardiac events in patients with coronary artery disease. Acta Cardiol Sin. (2013) 29:243–50. PMID: 2712271327122713
8. Petix NR, Sestini S, Marcucci G, Coppola A, Arena A, Nassi F, et al. Can the reversible regional wall motion abnormalities on stress gated tc-99m sestamibi SPECT predict a future cardiac event? J Nucl Cardiol. (2005) 12:20–31. doi: 10.1016/j.nuclcard.2004.09.017
9. Shiroodi MK, Shafiei B, Baharfard N, Gheidari ME, Nazari B, Pirayesh E, et al. 99 mTc-MIBI washout as a complementary factor in the evaluation of idiopathic dilated cardiomyopathy (IDCM) using myocardial perfusion imaging. Int J Cardiovasc Imaging. (2012) 28:211–7. doi: 10.1007/s10554-010-9770-5
10. Germano G, Kiat H, Kavanagh P, Moriel M, Mazzanti M, Su H, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. (1995) 36:2138–47. PMID: 74726117472611
11. Berman D, Germano G, Lewin H, Kang X, Kavanagh P, Tapnio P, et al. Comparison of post-stress ejection fraction and relative left ventricular volumes by automatic analysis of gated myocardial perfusion single-photon emission computed tomography acquired in the supine and prone positions. J Nucl Cardiol. (1998) 5:40–7. doi: 10.1016/S1071-3581(98)80009-3
12. Chavoshi M, Fard-Esfahani A, Fallahi B, Emami-Ardekani A, Beiki D, Hassanzadeh-Rad A, et al. Assessment of prognostic value of semiquantitative parameters on gated single photon emission computed tomography myocardial perfusion scintigraphy in a large middle eastern population. Indian J Nucl Med. (2015) 30:233–8. doi: 10.4103/0972-3919.151651
13. Jeong HJ, Lee DS, Lee HY, Choi S, Han YH, Chung JK. Prognostic value of normal perfusion but impaired left ventricular function in the diabetic heart on quantitative gated myocardial perfusion SPECT. Nucl Med Mol Imaging. (2013) 47:151–7. doi: 10.1007/s13139-013-0213-9
14. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. (2008) 29:270–6. doi: 10.1093/eurheartj/ehm342
15. Mathew T, Williams L, Navaratnam G, Rana B, Wheeler R, Collins K, et al. Diagnosis and assessment of dilated cardiomyopathy: a guideline protocol from the British society of echocardiography. Echo Res Pract. (2017) 4:G1–G13. doi: 10.1530/ERP-16-0037
16. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Bohm M, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. (2016) 37:1850–8. doi: 10.1093/eurheartj/ehv727
17. Wang C, Tang H, Zhu F, Jiang Z, Shi J, Zhou Y, et al. Prognostic value of left-ventricular systolic and diastolic dyssynchrony measured from gated SPECT MPI in patients with dilated cardiomyopathy. J Nucl Cardiol. (2020) 27:1582–91. doi: 10.1007/s12350-018-01468-z
18. Kano N, Okumura T, Isobe S, Sawamura A, Watanabe N, Fukaya K, et al. Left ventricular phase entropy: novel prognostic predictor in patients with dilated cardiomyopathy and narrow QRS. J Nucl Cardiol. (2018) 25:1677–87. doi: 10.1007/s12350-017-0807-1
19. Kuslu D, Ozturk E. A comparison of iterative reconstruction and prone imaging in reducing the Inferior wall attenuation in tc-99 m sestamibi myocardial perfusion SPECT. Mol Imaging Radionucl Ther. (2017) 26:110–5. doi: 10.4274/mirt.83007
20. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. American Heart association writing group on myocardial S and registration for cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation. (2002) 105:539–42. doi: 10.1161/hc0402.102975
21. Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. (1995) 26:639–47. doi: 10.1016/0735-1097(95)00218-S
22. Espersen C, Modin D, Platz E, Jensen GB, Schnohr P, Prescott E, et al. Global and regional wall motion abnormalities and incident heart failure in the general population. Int J Cardiol. (2022) 357:146–51. doi: 10.1016/j.ijcard.2022.03.027
23. Cortigiani L, Huqi A, Ciampi Q, Bombardini T, Bovenzi F, Picano E. Integration of wall motion, coronary flow velocity, and left ventricular Contractile reserve in a single test: prognostic value of vasodilator stress echocardiography in patients with diabetes. J Am Soc Echocardiogr. (2018) 31:692–701. doi: 10.1016/j.echo.2017.11.019
24. Xiang L, Wang M, You T, Jiao Y, Chen J, Xu W. Prognostic value of ventricular wall motion score and global registry of acute coronary events score in patients with acute myocardial infarction. Am J Med Sci. (2017) 354:27–32. doi: 10.1016/j.amjms.2017.03.029
25. Rigo F, Sicari R, Gherardi S, Djordjevic-Dikic A, Cortigiani L, Picano E. The additive prognostic value of wall motion abnormalities and coronary flow reserve during dipyridamole stress echo. Eur Heart J. (2008) 29:79–88. doi: 10.1093/eurheartj/ehm527
26. Iida Y, Inomata T, Kaida T, Fujita T, Ikeda Y, Nabeta T, et al. Prognostic impact of segmental wall motion abnormality in patients with idiopathic dilated cardiomyopathy. Int Heart J. (2017) 58:544–50. doi: 10.1536/ihj.16-582
27. Wallis D, O'Connell J, Henkin R, Costanzo-Nordin M, Scanlon P. Segmental wall motion abnormalities in dilated cardiomyopathy: a common finding and good prognostic sign. J Am Coll Cardiol. (1984) 4:674–9. doi: 10.1016/S0735-1097(84)80392-7
28. Pratali L, Otasevic P, Neskovic A, Molinaro S, Picano E. Prognostic value of pharmacologic stress echocardiography in patients with idiopathic dilated cardiomyopathy: a prospective, head-to-head comparison between dipyridamole and dobutamine test. J Card Fail. (2007) 13:836–42. doi: 10.1016/j.cardfail.2007.07.011
29. Abidov A, Germano G, Hachamovitch R, Slomka P, Berman DS. Gated SPECT in assessment of regional and global left ventricular function: an update. J Nucl Cardiol. (2013) 20:1118–43; quiz 1144–6. doi: 10.1007/s12350-013-9792-1
30. Wang L, Yang MF, Cai M, Zhao SH, He ZX, Wang YT. Prognostic significance of left ventricular dyssynchrony by phase analysis of gated SPECT in medically treated patients with dilated cardiomyopathy. Clin Nucl Med. (2013) 38:510–5. doi: 10.1097/RLU.0b013e318292eedf
31. Park SM, Kim HD, Cho DH, Kim MN, Shim WJ. Impact of left bundle branch block on left atrial dyssynchrony and its relationship to left ventricular diastolic function in patients with heart failure and dilated cardiomyopathy. Int J Heart Fail. (2019) 1:42–52. doi: 10.36628/ijhf.2019.0001
32. Dziewiecka E, Gliniak M, Winiarczyk M, Karapetyan A, Wisniowska-Smialek S, Karabinowska A, et al. Mortality risk in dilated cardiomyopathy: the accuracy of heart failure prognostic models and dilated cardiomyopathy-tailored prognostic model. ESC Heart Fail. (2020) 7:2455–67. doi: 10.1002/ehf2.12809
33. Miura K, Matsumori A, Nasermoaddeli A, Soyama Y, Morikawa Y, Sakurai M, et al. Prognosis and prognostic factors in patients with idiopathic dilated cardiomyopathy in Japan. Circ J. (2008) 72:343–8. doi: 10.1253/circj.72.343
34. Juilliere Y, Marie PY, Danchin N, Gillet C, Paille F, Karcher G, et al. Radionuclide assessment of regional differences in left ventricular wall motion and myocardial perfusion in idiopathic dilated cardiomyopathy. Eur Heart J. (1993) 14:1163–9. doi: 10.1093/eurheartj/14.9.1163
35. Gaitonde RS, Subbarao R, Michael MA, Dandamudi G, Bhakta D, Mahenthiran J, et al. Segmental wall-motion abnormalities of the left ventricle predict arrhythmic events in patients with nonischemic cardiomyopathy. Heart Rhythm. (2010) 7:1390–5. doi: 10.1016/j.hrthm.2010.05.039
36. Parthenakis FI, Prassopoulos VK, Koukouraki SI, Zacharis EA, Diakakis GF, Karkavitsas NK, et al. Segmental pattern of myocardial sympathetic denervation in idiopathic dilated cardiomyopathy: relationship to regional wall motion and myocardial perfusion abnormalities. J Nucl Cardiol. (2002) 9:15–22. doi: 10.1067/mnc.2002.118239
37. Factor SM, Minase T, Cho S, Dominitz R, Sonnenblick EH. Microvascular spasm in the cardiomyopathic Syrian hamster: a preventable cause of focal myocardial necrosis. Circulation. (1982) 66:342–54. doi: 10.1161/01.CIR.66.2.342
38. Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, et al. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. (1990) 81:772–9. doi: 10.1161/01.CIR.81.3.772
39. Bowles N, Richardson P, Olsen E, Archard L. Detection of coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. (1986) 1:1120–3. doi: 10.1016/S0140-6736(86)91837-4
40. McClements BM, Weyman AE, Newell JB, Picard MH. Echocardiographic determinants of left ventricular ejection fraction after acute myocardial infarction. Am Heart J. (2000) 140:284–9. doi: 10.1067/mhj.2000.107543
41. Madsen BK, Videbaek R, Stokholm H, Mortensen LS, Hansen JF. Prognostic value of echocardiography in 190 patients with chronic congestive heart failure. A comparison with New York heart association functional classes and radionuclide ventriculography. Cardiology. (1996) 87:250–6. doi: 10.1159/000177096
42. Kilcullen NM, Uthamalingam S, Gurm GS, Gregory SA, Picard MH. The prognostic significance of resting regional left ventricular function in patients with varying degrees of myocardial ischemia. Cardiol Res. (2013) 4:178–85. doi: 10.4021/cr240w
43. Jurado-Roman A, Agudo-Quilez P, Rubio-Alonso B, Molina J, Diaz B, Garcia-Tejada J, et al. Superiority of wall motion score index over left ventricle ejection fraction in predicting cardiovascular events after an acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. (2019) 8:78–85. doi: 10.1177/2048872616674464
44. Galasko GI, Basu S, Lahiri A, Senior R. A prospective comparison of echocardiographic wall motion score index and radionuclide ejection fraction in predicting outcome following acute myocardial infarction. Heart. (2001) 86:271–6. doi: 10.1136/heart.86.3.271
45. Carluccio E, Tommasi S, Bentivoglio M, Buccolieri M, Prosciutti L, Corea L. Usefulness of the severity and extent of wall motion abnormalities as prognostic markers of an adverse outcome after a first myocardial infarction treated with thrombolytic therapy. Am J Cardiol. (2000) 85:411–5. doi: 10.1016/S0002-9149(99)00764-X
46. Moller JE, Hillis GS, Oh JK, Reeder GS, Gersh BJ, Pellikka PA. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J. (2006) 151:419–25. doi: 10.1016/j.ahj.2005.03.042
Keywords: dilated cardiomyopathy, prognostics, outcome, wall motion, gated SPECT
Citation: Zhu J, Wang X, Huang N, Li X, Cheng Y, Wu Z, Li Y, Wu P, Li L, Wei H, Li S and Cao J (2023) Prognostic value of summed motion score assessed by gated SPECT myocardial perfusion imaging in patients with dilated cardiomyopathy. Front. Cardiovasc. Med. 10:1144333. doi: 10.3389/fcvm.2023.1144333
Received: 14 January 2023; Accepted: 28 February 2023;
Published: 15 March 2023.
Edited by:
Giovanni Quarta, Papa Giovanni XXIII Hospital, ItalyReviewed by:
Yuetao Wang, First People's Hospital of Changzhou, China© 2023 Zhu, Wang, Huang, Li, Cheng, Wu, Li, Wu, Li, Wei, Li and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Si-Jin Li bGlzam5tMTIzQDE2My5jb20=; Ji-Min Cao Y2FvamltaW5Ac3htdS5lZHUuY24=
Specialty Section: This article was submitted to Cardiovascular Imaging, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.