
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 12 May 2023
Sec. Heart Failure and Transplantation
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1143658
Background: It remains controversial whether sodium-glucose cotransporter-2 inhibitors (SGLT-2is) are effective in treating heart failure with preserved ejection fraction (HFpEF).
Purpose: The objective of this umbrella review is to provide a summary of the available evidence regarding the efficacy and safety of SGLT-2is for the treatment of HFpEF.
Methods: We extracted pertinent systematic reviews and meta-analyses (SRs/MAs) from PubMed, EMBASE, and the Cochrane Library that were published between the inception of the database and December 31, 2022. Two independent investigators assessed the methodological quality, risk of bias, report quality, and evidence quality of the included SRs/MAs in randomized controlled trials (RCTs). We further evaluated the overlap of the included RCTs by calculating the corrected covered area (CCA) and assessed the reliability of the effect size by performing excess significance tests. Additionally, the effect sizes of the outcomes were repooled to obtain objective and updated conclusions. Egger's test and sensitivity analysis were used to clarify the stability and reliability of the updated conclusion.
Results: This umbrella review included 15 SRs/MAs, and their methodological quality, risk of bias, report quality, and evidence quality were unsatisfactory. The total CCA for 15 SRs/MAs was 23.53%, indicating a very high level of overlap. The excess significance tests did not reveal any significant results. Our updated MA demonstrated that the incidence of the composite of hospitalization for heart failure (HHF) or cardiovascular death (CVD), first HHF, total HHF, and adverse events as well as the Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS) and 6 min-walk distance (6MWD) were all substantially improved in the SGLT-2i intervention group compared to the control group. However, there was limited evidence that SGLT-2is could improve CVD, all-cause death, plasma B-type natriuretic peptide (BNP) level, or plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) level. Egger's test and sensitivity analysis proved that the conclusion was stable and reliable.
Conclusions: SGLT-2 is a potential treatment for HFpEF with favourable safety. Given the dubious methodological quality, reporting quality, evidence quality, and high risk of bias for certain included SRs/MAs, this conclusion must be drawn with caution.
Systematic Review Registration: https://inplasy.com/, doi: 10.37766/inplasy2022.12.0083, identifier INPLASY2022120083.
Heart failure with preserved ejection fraction (HFpEF), as measured by left ventricular ejection fraction (LVEF), is observed in approximately 50% of all patients with heart failure and is regarded as a significant subtype (1, 2). HFpEF is more common in females and the elderly. The incidence of HFpEF increases with age, and the proportion of females is higher than that of males in all age groups (3–5). In addition to the high prevalence, HFpEF is related to a significant decline in quality of life (6), and both the mortality risk and hospital readmission rates of HFpEF are higher than those of heart failure with reduced ejection fraction (HFrEF) (7–9). The combination of these two elements makes HFpEF a serious public health concern and places a significant burden on society and families (10, 11). However, as a heterogeneous disease, the complex pathogenic factors and various pathophysiological characteristics of HFpEF present challenges to the formulation of treatment (12–14). Although advancements have been reported for certain phenotypes of patients with HFpEF, unequivocal class Ia and Ib obligatory prescription recommendations to decrease mortality and morbidity in patients with HFpEF have not been reported according to the 2021 ESC guidelines and 2022 AHA/ACC/HFSA guidelines (1, 15).
Sodium-glucose cotransporter-2 inhibitors (SGLT-2is) are a new family of oral hypoglycaemic drugs that decrease serum glucose by inhibiting glucose reabsorption by proximal renal tubules and enhancing urine glucose excretion (16). Multiple randomized controlled trials (RCTs) have indicated that SGLT-2is have cardioprotective and renoprotective effects regardless of hyperglycaemia and decrease the incidence of hospitalization for heart failure (HHF) and cardiovascular death (CVD) in patients with HFrEF (17–19). As a result, SGLT-2is are recommended as the foundation for HFrEF therapy (1, 15). Nevertheless, the benefits of SGLT-2is in treating HFpEF remain controversial, and the results of several large-scale RCTs have been inconsistent. For example, the EMPEROR-Preserved trial (20–22) found that SGLT-2is reduced the risk of first HHF and the composite of HHF or CVD in patients with HFpEF, whereas the VERTIS-CV trial (23) showed opposite outcomes. Similar trends were observed for plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) level and 6 min-walk distance (6MWD). SGLT-2is did not improve the value of 6WMD in the EMPERIAL-Preserved trial (24), which was opposite in the PRESERVED-HF trial (25). The EMPEROR-Preserved trial (20–22) found that SGLT-2is helped to reduce NT-ProBNP levels in patients with HFpEF, whereas the PRESERVED-HF trial (25) showed the opposite outcomes. However, this situation has drastically changed with the recent publications of several cases of large-scale RCTs. For instance, the DELIVER trial, an international, multicenter, double-blind, randomized, placebo-controlled trial done in 350 healthcare centers and hospitals across 20 countries, has shown that SGLT-2is could reduce the risk of first HHF and the composite of HHF or CVD in patients with HFpEF but has no significant improvement in reducing the incidence of CVD and all-cause death (26–28). Notably, based on the result of the “Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction”, the 2022 AHA/ACC/HFSA guidelines have assigned a recommended grade of II to SGLT-2is, which has attracted a considerable amount of attention from researchers, clinicians and patients (20).
As a result of the growing attention to SGLT-2is for the treatment of HFpEF, researchers have performed systematic reviews and meta-analyses (SRs/MAs) to assess the therapeutic benefits. By appropriately adhering to the relevant research guidelines, the SRs/MAs provide reliable medical evidence (29). Unfortunately, the strength of the conclusions is diminished to some degree by the existing lack of methodological quality assessment of SRs/MAs related to SGLT-2is for the treatment of HFpEF. Umbrella reviews offer a novel approach to combining SRs/MAs by assessing their methodological quality and reestimating outcomes, which may offer high-quality evidence for clinical decision-making. Consequently, the purpose of this research is to combine existing evidence, assess the quality of prior SRs/MAs pertaining to the efficacy of SGLT-2is in treating patients with HFpEF, and recalculate the effect size by an umbrella review.
This umbrella review of SRs/MAs follows the guidelines outlined by the Cochrane Handbook (30) and other high-quality umbrella reviews (31, 32). We registered a protocol for this study on the INPLASY platform (DOI: 10.37766/inplasy2022.12.0083; Registration Number: INPLASY2022120083).
PubMed, EMBASE and the Cochrane Library were searched for relevant studies. Searches were conducted by two study investigators (RM-L and H-G) independently from inception to December 31, 2022. We combined keyword search with free word search as strategy, and the keywords included “Meta-Analysis as Topic”, “Systematic review”, “Sodium-Glucose Transporter 2 Inhibitors”, and “Heart Failure, Preserved Ejection Fraction”. Based on this, both the websites for study registration (ClinicalTrials.gov) and the reference lists of included SRs/MAs were manually examined to identify additional relevant studies for this umbrella review. There were no restrictions placed on language use or region of publication. All the detailed strategies were shown in Supplementary Table S1.
The following were the criteria for inclusion: (a) study design: this umbrella review included publicly published SRs/MAs based on RCTs concerning the efficacy of SGLT-2is in treating patients with HFpEF; (b) population: patients with HFpEF were defined based on the ESC guidelines (1), AHA/ACC/HFSA guidelines (15) or Chinese guidelines for the diagnosis and treatment of heart failure 2018 (33) with an LVEF of ≥40% or 50%; (c) intervention and comparison: the intervention group was given SGLT-2is, while the control group was given placebo or conventional treatment (CT); (d) outcomes: main outcomes included first or total HHF, CVD, all-cause death, and the composite of HHF and CVD. Based on this, additional outcomes included plasma B-type natriuretic peptide (BNP) level, NT-proBNP level, change of NT-proBNP, 6MWD, the Kansas City Cardiomyopathy Questionnaire Scores, including the Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS), the Kansas City Cardiomyopathy Questionnaire Physical Limitation (KCCQ-PL), the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), and the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OSS), the ratio of early mitral inflow velocity to mitral annular early diastolic velocity (E/e′) and adverse events (hypoglycaemia, diabetic ketoacidosis, renal events, urinary infection, and any unfavourable or unintended signs, symptoms, or disease, including abnormal laboratory values).
The following were the criteria for exclusion: (a) cell or animal-based studies; (b) study protocols, conference abstracts, editorials, case reports, letters, narrative reviews, and umbrella reviews; and (c) unavailability of data required for this umbrella review.
Identified articles were imported into EndNote X9, and duplicates were eliminated. Two study investigators (WL-G and H-G) independently reviewed the titles and abstracts to screen the potentially eligible articles. After examining the full text, the included studies were finally confirmed. The following information was extracted by two independent investigators (XM-W and H-G): the authors, country, year of publication, number of included RCTs and participants contained, intervention, comparison, risk of bias assessment tool, outcomes, overall conclusions, and relevant data of the included RCTs. Any discrepancies in these two workflows were settled by consultation and arbitration with the third investigator (LL-R).
The methodological quality of the included SRs/MAs was evaluated by independent investigators (RM-L and XM-W) utilizing A Measure Tool to Assess Systematic Reviews 2 (AMSTAR-2) (34). The critical areas were assessed by seven items (2, 4, 7, 9, 11, 13, 15). Each item was classified as “no,” “partial yes,” or “yes” based on its conformance to the criteria. The overall level of methodological quality was categorized as “high,” “moderate,” “low,” or “critically low.” Disparities that arose throughout the evaluation were resolved through discussion by a third investigator (LL-R).
In this umbrella review, the risk of bias in the review process, the results, and the conclusions of included SRs/MAs was determined by investigators (RM-L and HW-Q) with the assistance of The Risk of Bias in Systematic Review (ROBIS) scale (35, 36). The scale was completed in three phases: (a) assessing relevance, (b) identifying concerns with the review process, and (c) judging the risk of bias. Throughout the evaluation, discrepancies were resolved through discussion by a third investigator (LL-R).
The reporting quality was evaluated by independent investigators (HW-Q and XM-W) using the 27-item Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (37). There were two possible responses for each item: “yes” or “no”. Points were given based on each response. Any disagreements that arose over the process of the assessment were discussed and settled by a third investigator (WL-G).
The evidence quality for outcomes were assessed by The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (38). Bias risk, inconsistency, indirectness, imprecision, and publication bias were five factors that could lower the quality of the evidence. There were four levels of evidence quality in this system: high, moderate, low, and very low. XM-W and HW-Q were responsible for the specific evaluation, whereas WL-G was responsible for discussing and settling any disagreements in this process.
A considerable number of SRs/MAs published in a short time frame that concentrate on the same field may contain numerous duplicate RCTs, which may introduce bias into the overall results. To evaluate the possible effect caused by including the same RCTs, we calculated the amount of overlap using the corrected covered area (CCA). The primary RCTs served as rows and the included SRs/MAs served as columns in the matrix, as described by Pieper et al. (39). The total number of RCTs included in SRs/MAs, RCTs, and included SRs/Mas were denoted by “N” (repetition permitted), “r,” and “c,” then CCA = (N − r)/[(r × c) − r]. Minor overlap was indicated by a CCA value between 0% and 5%, moderate overlap by 6% to 10%, high overlap by 11% to 15%, and very high overlap by >15% (40).
To determine if the significance of combined effect size was due to chance or bias, the excess significance tests on categorical variable outcomes in the included SRs/MAs were performed. Excess significance bias was determined by comparing the observed number (O) with the expected number (E); the greater the discrepancy between the two values, the more severe the bias. A P-value of less than 0.10 suggested an excess significance for a single SR/MA (32, 41).
Utilizing data from individual RCTs, we repooled various outcome indicators with incongruent SR/MA effect sizes [e.g., risk ratio (RR), hazard ratio (HR), odds ratio (OR), or standard mean difference (SMD); when applicable, the confidence interval (CI) was also calculated]. At the same time, for participants with type 2 diabetes (T2D), chronic kidney disease (CKD), acute heart failure (AHF), non-AHF, and intervention with SGLT-1/2is or SGLT-2is, we conducted subgroup meta-analyses to determine the potential sources of heterogeneity. The significance threshold was established at P < 0.05. In cases where no heterogeneity was identified, a fixed effects model was utilized, whereas the random effects model was used otherwise. If the P-value for the Q test was less than 0.10 and the I2 value was more than 25%, then we concluded that there existed heterogeneity (42). Egger's test was utilized to assess the evidence for small-study effects (43). Additionally, we performed a sensitivity analysis to assess the robustness and reliability of the combined results. R 4.1.1 (http://www.R-project.org) and Stata 16.0 (StataCorp LLC) were utilized for all statistical analyses.
Following the research strategy, 127 potentially relevant records were obtained, and 23 of them were excluded after eliminating duplicates. After title and abstract-based screening, we eliminated 81 records. The remaining 23 records were then retrieved for full-text evaluation. Eight records were eliminated at this stage. Finally, 15 records (44–58) were included in this umbrella review. Figure 1 showed the literature screening procedure. Supplementary Table S2 provided detailed information on the excluded literature.
The characteristics of the 15 SRs/MAs were summarized in Table 1. A total of 17 primary RCTs (20–28, 59–70) were included across 15 SRs/MAs, and their corresponding relationships were shown in Supplementary Table S3. The overall CCA value was 23.53%, indicating a very high level of overlap. This suggested that a considerable amount of attention was devoted to research on SGLT-2i treatment of HFpEF, and there was a lack of relevant RCTs. The specific calculation process was also shown in Supplementary Table S3. The included SRs/MAs had a range of 2 to 12 RCTs, with sample sizes from 1,810 to 15,989 participants per trial, and all were published between 2020 and 2022. All included SRs/MAs were published in English, and the researchers were mainly from Asia and North America. Seven SRs/MAs were performed in China (45, 46, 42–54, 57, 58), 4 in The United States (44, 48, 50, 51), and one each in India (47), Canada (49), Japan (55), and United Kingdom (57). Regarding intervention modality, the control group was given CT or placebo, whereas the intervention group was given various types of SGLT-2is, including “Canagliflozin,” “Dapagliflozin,” “Empagliflozin,” “Ertugliflozin,” “Ipragliflozin,” “Luseogliflozin,” and “Sotagliflozin”. For the risk and bias assessment of the included RCTs, all the SRs/MAs selected the Cochrane criteria except Jhund et al. (57).
Seven SRs/MAs were assessed as critically low quality (44, 49, 50, 54, 55, 57, 58), 7 were assessed as low quality (45–48, 51–53), and 1 was assessed as high quality (56) using the AMSTAR-2. Item 2 [lack of protocol before the study (10/15, 66.67%)], Item 7 [lack of excluded trials list (14/15, 93.33%)], and Item 15 [lack of an adequate investigation and discussion of publication bias (7/15, 46.67%)] were the most common absence of the 7 critical items. Table 2 provided the evaluation results of the AMSTAR-2 assessment for each study.
Regarding the ROBIS evaluation outcomes, phase 1 examined the relevance of study topics, while phase 2 domain 1 evaluated study eligibility criteria. For both items, all SRs/MAs were assessed as low risk of bias. For the included SRs/MAs, in domain 2, 12 were assessed as low risk of bias (12/15, 80.00%) (44–46, 48–50, 52–56, 58), in domain 3, 11 were assessed as low risk of bias (11/15, 73.33%) (44, 46–51, 53, 55, 56, 58), and in domain 4, only 1 was assessed as low risk of bias (1/15, 6.67%) (56). In phase 3, 10 SRs/Mas had a low risk of bias (10/15, 66.67%) (45–48, 51–53, 56–58). The details of the ROBIS assessments were shown in Supplementary Table S4.
Supplementary Table S5 provided details on the report quality. Despite the fact that the titles, introductions, and discussions of the SRs/MAs included in this umbrella review were reported completely, reporting problems were discovered in other aspects. In the abstract section, Item 12 (registration) had a 33.33% response rate. In the methods section, the response rates for Item 7 (search strategy), Item 13(e) and (f) (synthesis methods), Item 14 (reporting bias assessment), and Item 15 (certainty assessment) were 80.00%, 66.67%, 80.00%, 53.33%, and 20.00%, respectively. In the results section, Item 16(b) (study selection), Item 20(c) and (d) (results of syntheses), Item 21 (reporting biases), and Item 22 (certainty of the evidence) exhibited less than 80% response rates. The quality assessment of Items 24(a) and (b) (registration and protocol) was inadequate since only 5 (50, 51, 55, 56, 58) (5/15, 33.33%) SRs/MAs provided information on research protocol registration.
Table 3 summarized the results of the evidence quality assessment for 70 outcomes among the 15 included SRs/MAs. The evidence quality was assessed as very low in 11 cases (11/70, 15.71%), low in 36 cases (36/70, 51.43%), moderate in 22 cases (22/70, 31.43%), and high in one case (1/70, 1.43%). Publication bias (n = 69) was the most prevalent factor for downgrading, followed by imprecision (n = 47) and inconsistency (n = 11). Table 3 provided details on downgrades for each GRADE domain by the outcome.
In this umbrella review, we conducted a test for excess significance effect, a narrative description, and a reestimation of the quantitatively assessed outcome indicators by the SRs/MAs. Detailed information was provided in Table 4 and Figures 2, 3.
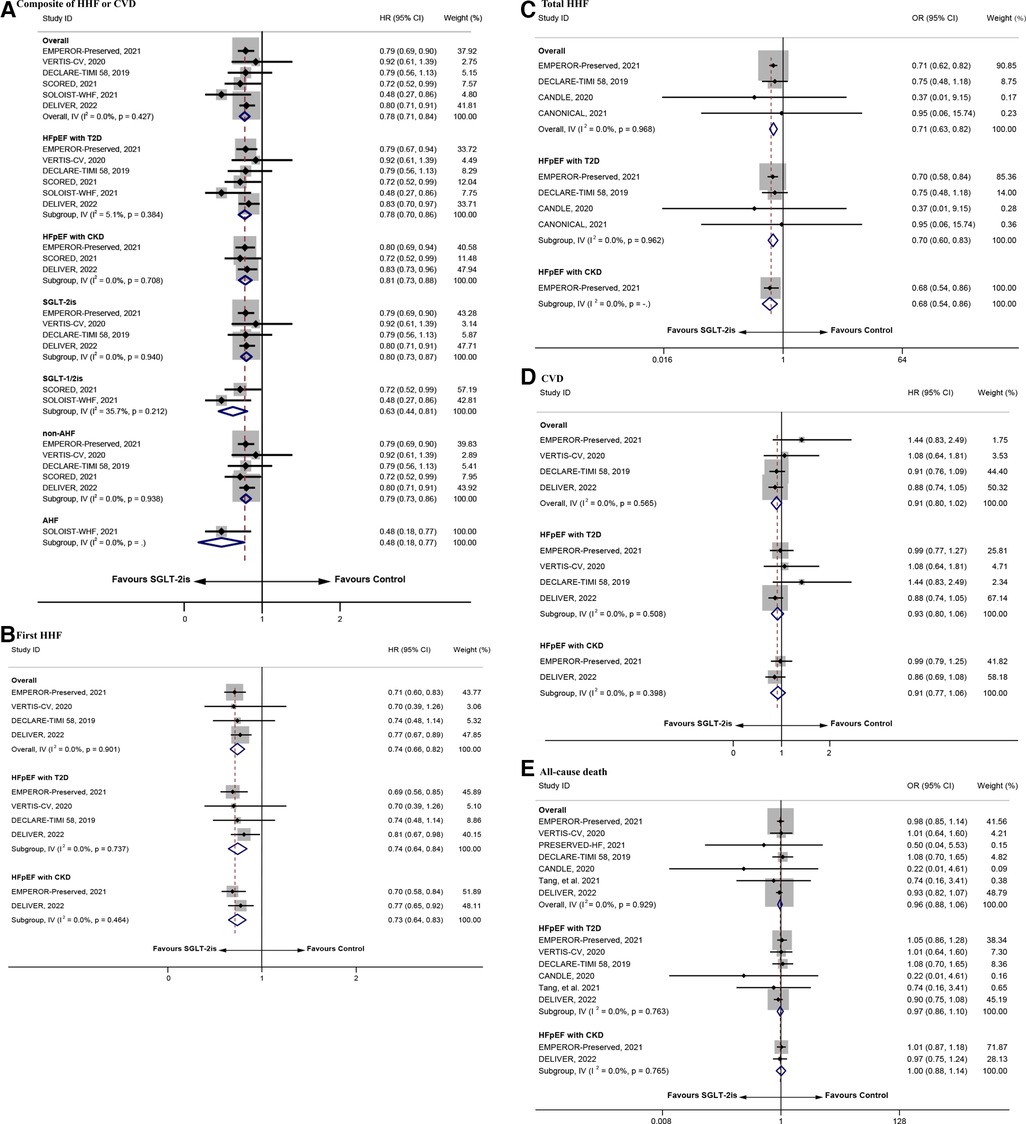
Figure 2. The repooled effect size of the composite of HHF or CVD, first HHF, total HHF, CVD, and all-cause death. HHF, hospitalization for heart failure; CVD, cardiovascular death.

Figure 3. The repooled effect size of NT-proBNP level, BNP level, KCCQ-TSS, 6MWD, and adverse events. BNP, B-type natriuretic peptide; NT-proBNP, N-terminal pro-B-type natriuretic peptide; 6MWD: 6 min-walk distance; KCCQ-TSS: the Kansas City Cardiomyopathy Questionnaire Total Symptom Score.
The main outcomes included first or total HHF, CVD, all-cause death, and the composite of HHF and CVD. No excess significant effects were found in the main outcome indicators (Table 4). There were 14 (44, 45, 47–58) SRs/MAs involving the composite of HHF or CVD. Eleven (47–56, 58) of these reviews indicated that SGLT-2is reduced the occurrence of this indicator relative to placebo or CT. Ten SRs/MAs (44, 46, 50, 52–58) reported the effect of SGLT-2is on CVD, but none of them found a significantly reduced CVD rate in patients with HFpEF. In addition to the MA from Jhund et al. (57), the remaining 5 SRs/MAs (44, 50, 52, 53, 56) demonstrated that SGLT-2is significantly reduce the incidence of first HHF. Four (54–57, 58) of the 6 (46, 54–58) SRs/MAs involving total HHF revealed that SGLT-2is were associated with a decrease in its incidence. In addition, none of the 9 SRs/MAs (44, 46, 50, 52, 54–58) supported the use of SGLT-2is to reduce all-cause death.
As shown in Figure 2, the repooled HRs (95% CI) were 0.78 (0.71–0.84) and 0.74 (0.66–0.82) for the composite of HHF or CVD and first HHF, respectively, suggesting that the risk in the intervention group was 22% and 26% lower than that in the control group. Subgroup analysis revealed that participants with diagnosis of T2D or CKD (HR = 0.78, 95% CI = 0.70–0.86 and HR = 0.81, 95% CI = 0.73–0.88), with diagnosis of non-AHF or AHF (HR = 0.79, 95% CI = 0.73–0.86 and HR = 0.48, 95% CI = 0.18–0.77), and treated with SGLT-2is or SGLT-1/2is (HR = 0.80, 95% CI = 0.73–0.87 and HR = 0.63, 95% CI = 0.44–0.81), had lower risk of the composite of HHF or CVD in the intervention group compared with the control group. Similar to the above results, participants with diagnosis of T2D or CKD (HR = 0.74, 95% CI = 0.64–0.84 and HR = 0.73, 95% CI = 0.64–0.83) had lower risk of first HHF in the intervention group. The repooled OR (95% CI) for total HHF was 0.71 (0.63–0.82), reflecting a 29% reduction in the total HHF rate with SGLT-2is. However, for CVD and all-cause death, there was no significant improvement in the intervention group (HR = 0.91, 95% CI = 0.80–1.02 and OR = 0.96, 95% CI = 0.88–1.06). Subgroup analysis revealed that participants with diagnosis of T2D or CKD in the intervention group had lower risk in the total HHF [OR = 0.70, 95% CI = 0.60–0.83 and OR = 0.68, 95% CI = 0.54–0.86)], but for participants with diagnosis of T2D or CKD, there were no significant improvement in CVD (HR = 0.93, 95% CI = 0.80–1.06 and HR = 0.91, 95% CI = 0.77–1.06) and all-cause death (OR = 0.97, 95% CI = 0.86–1.10 and OR = 1.00, 95% CI = 0.88–1.14) compared with the control group.
Egger's test showed no significant small-study effect on the composite of HHF or CVD, first HHF, total HHF, CVD, and all-cause death (Supplementary Table S6). The sensitivity analysis showed high reliability of the conclusions (Supplementary Figures S1A–E).
The markers of heart failure symptoms included NT-proBNP level, change in NT-proBNP, and BNP level. In the meanwhile, indicators of cardiac function outcomes included 6MWD and E/e′ level (Table 4). Two (54, 56) of the 3 SRs/MAs (54–56) that involved NT-proBNP levels found that SGLT-2is did not decrease the level of NT-proBNP. Furthermore, Zhou et al. (56) revealed that SGLT-2is were not associated with change in NT-proBNP. Similarly, 2 SRs/MAs (55, 56) reported that SGLT-2is did not significantly reduce BNP levels. The conclusions of the 3 SRs/MAs (54–56) on 6MWD were different. Fukuta et al. (55) suggested that SGLT-2is increased the 6MWD value, whereas Yang et al. (54) and Zhou et al. (56) reported the opposite results. In an extended study, Zhou et al. (56) found that SGLT-2is contribute to the reduction of E/e′ level.
Indicators of health status and life quality outcomes included the KCCQ-TSS, the KCCQ-PL, the KCCQ-CSS, and the KCCQ-OSS. All 3 SRs/MAs (50, 54, 55) showed that SGLT-2is increase the level of KCCQ-TSS, showing high consistency. Vaduganathan et al. (50) believed that SGLT-2is help improve the level of KCCQ-CSS and KCCQ-OSS, which was contrary to the research conclusion of Yang et al. (54). In addition, 1 review (54) showed no clear association between SGLT-2is and increased KCCQ-PL levels.
The research on adverse events by Vaduganathan et al. (50) showed that the intervention group had fewer cases of amputation, diabetic ketoacidosis, hypoglycemia, renal events, and any serious adverse events compared with the control group. MAs were not performed due to differences in the definition of adverse events among RCTs. Zhou et al. and Wang et al. (56, 58) demonstrated that the incidence of adverse events in the intervention group was significantly lower than that in the control group.
As shown in Figure 3, the repooled SMDs (95% CI) of NT-proBNP level, BNP level, 6MWD, and KCCQ-TSS were −0.17 (−0.35–0.01), −0.01 (−0.21–0.18), 0.19 (0.07–0.30), and 0.19 (0.06–0.32), respectively. Except for 6MWD and KCCQ-TSS, the above data did not support the significant positive effect of SGLT-2is on the outcome indicators (P > 0.05). Subgroup analysis revealed that participants with diagnosis of T2D or CKD in the intervention group had significant positive effect on NT-ProBNP level (SMD = −0.23, 95% CI = −0.44 to −0.01 and SMD = −0.45, 95% CI = −0.53 to −0.40). Similar to that, participants with diagnosis of T2D had significant positive effect on 6MWD (SMD = 1.54, 95% CI = 0.96–2.12). For adverse events, the incidence of adverse events was significantly lower in the intervention group than in the control group (OR = 0.84, 95% CI = 0.71–0.99). Similar conclusion was observed in the subgroup of patients with CKD (OR = 0.78, 95% CI = 0.64–0.95). However, this conclusion could not be applied to patients with T2D (OR = 0.76, 95% CI = 0.51–1.12). Moreover, Egger's test revealed no significant small-study effects for NT-proBNP level, 6MWD, and adverse events (Supplementary Table S6). The sensitivity analysis indicated that the conclusions were highly reliable (Supplementary Figure S1 F-J).
It is now increasingly recognized that the cardiovascular protective effects of SGLT-2is may be facilitated by blood pressure reductions, diuretic and natriuretic effects, attenuation of cardiac inflammation and fibrosis, reduction in left ventricular preload and afterload, improvement of endothelial function, reductions in oxidative stress and arterial stiffness (71, 72). In HFrEF, the effect of SGLT-2is on the composite outcome of HHF and CVD has been demonstrated. However, the efficacy of SGLT-2is in HFpEF remains controversial. For example, in terms of reducing the risk of first HHF and the composite of HHF or CVD, the outcomes of the EMPEROR-Preserved trial (20–22) are the opposite of the VERTIS-CV trial (23). In the meantime, SGLT-2is did not improve the value of 6WMD in the EMPERIAL-Preserved trial (24), which was opposite in the PRESERVED-HF trial (25). The EMPEROR-Preserved trial (20–22) found that SGLT-2is could reduce NT-ProBNP levels in patients with HFpEF, whereas the PRESERVED-HF trial (25) showed the opposite outcome. Numerous relevant SRs/MAs have been published to provide further evidence-based medical evidence, but their quality has not been evaluated. The current comprehensive umbrella review aimed to systematically assess the quality of SRs/MAs that examined the clinical efficacy of SGLT-2is in the treatment of HFpEF. We used the AMSTAR-2, ROBIS, PRISMA guidelines, GRADE framework, CCA, and excess significance tests to summarize and evaluate the dependability of study outcomes. Furthermore, to clarify the size and direction of the impact of SGLT-2is on patients with HFpEF, we repooled the primary RCTs of SRs/MAs to obtain updated conclusions. Our study provides methodological application warnings for relevant SR/MA studies and evidence-based medical evidence of the efficacy of SGLT-2is in treating HFpEF.
To the best of our knowledge, this study is the first umbrella review of SRs/MAs on the effects of SGLT-2is on HFpEF. The 15 included SRs/MAs were all published in the last three years, whereas the RCTs involved in each SR/MA were published in 2019 and later. This figure indicates that the therapeutic effect of SGLT-2is on HFpEF is becoming a research hotspot. However, as revealed by the assessment of methodological quality, risk of bias, reporting quality, and evidence quality, most of the included SRs/MAs were unsatisfactory.
The methodological quality assessment showed that 14 SRs/MAs performed poorly on the 7 critical items of the AMSTAR-2. These studies were classified into critically low quality or low quality, accounting for 93.33% of all the included SRs/MAs. The major shortcomings are highlighted as follows: (1) 10 SRs/MAs lacked research protocol registration, which may lead to significant modifications in the research process; weaken the standardization, rigor, and transparency of the SRs/MAs; and increase the possibility of selective report bias (73); (2) none of the 14 SRs/MAs offered an excluded literature list, reducing the transparency of the SRs/MAs and undermining the trustworthiness of the results; (3) although most studies evaluated publication bias, 7 SRs/MAs did not use funnel plots, Egger's test, Begg's test, and other tools to evaluate publication bias. This conclusion might be attributed to the lack of adequate RCTs for the outcome indicators, the irregular use of methodology still reduces the confidence in the findings; (4) additionally, regarding Item 14, 6 SRs/MAs did not analyze and discuss the heterogeneity of the RCT results and lacked necessary measures, such as subgroup analysis or meta-regression, which resulted in decreased reliability of the combined calculation. All of the mentioned methodological limitations reduce the reliability of SRs/MAs.
When reviewing the risk bias assessment results obtained by the ROBIS tool, it was found that the high-risk bias was mainly derived from Phase 2 domains 2–4 and Phase 3. For Phase 2, the high-risk bias mainly stems from the lack of an effective retrieval scheme for grey literature (domain 2); lack of partial research features (domain 3); lack of registration of the research protocol; lack of sensitivity analysis and heterogeneity intervention (domain 4); and inadequate assessment of publication bias (Phase 3). The above factors affect the authenticity and credibility of SR/MA results. Based on the PRISMA, the absence of protocol registration, publication bias, incomplete retrieval strategy, insufficient evaluation of heterogeneity, the lack of sensitivity analysis, and lack of certainty assessment of evidence for outcomes were important reasons to reduce the reporting quality of SRs/MAs.
The evidence quality assessment of 70 outcomes based on the GRADE tool showed that inconsistency, imprecision, and publication bias were the main factors of evidence grade reduction. Of these outcomes, the quality of evidence was very low for 11 (11/70, 15.71%), low for 36 (36/70, 51.43%), moderate for 22 (22/70, 31.43%), and high for 1 (1/70, 1.43%). Specifically, clinical and methodological discrepancies across the included RCTs may be responsible for the high inconsistency. Differences in the age, sex, and ejection fraction of the included patients; the variety, dosage, and intervention duration of SGLT-2is; and the definition and measurement of outcome variables may all contribute to the significant heterogeneity and diminished credibility of the results. Furthermore, the insufficient sample size included in a single effect size was also a significant factor in the severe imprecision and deterioration of the evidence quality. By analyzing the reasons for this phenomenon, we believe that part of the clinical data included in SRs/MAs was derived from the subgroup analysis of large RCTs, so the lack of a study population for some specific outcomes also became an important factor affecting the quality of evidence. Regarding the decline in evidence quality due to the improper management of publication bias, according to our hypothesis, this low quality of evidence may be due to the insufficient RCTs included in the pertinent outcome measures.
Given the flawed methodology and evidence quality of the included SRs/MAs, conclusions may be biased in comparison to reality. Caution should be exercised when recommending SGLT-2is for HFpEF. Therefore, it is necessary to reintegrate and evaluate the existing evidence.
The high level of overlap between the included SRs/MAs means that they could not be considered independent and ideal sets of evidence, although excess significance tests indicated that there was no bias. Therefore, extracting relevant data from the primary RCTs and reestimating controversial outcome indicators is helpful to avoid the errors caused by overlap and obtain higher-quality evidence-based conclusions (29). Our updated MA showed that SGLT-2is had significant effects on first HHF, total HHF, composite of HHF or CVD, 6MWD, KCCQ-TSS, and adverse events in patients with HFpEF but did not significantly affect CVD, all-cause death, NT-proBNP level, or BNP level. The results were consistent across subgroups of composite of HHF or CVD, first HHF, total HHF, CVD, all-cause death, and 6MWD. However, the impact of SGLT-2is on NT-proBNP levels showed improvement in participants with a diagnosis of T2D or CKD. The impacts of SGLT-2is on adverse events appeared to be negative in participants with a diagnosis of T2D compared with the control group. Egger's test and sensitivity analysis indicated the stability of the results.
Our umbrella review may serve as a valuable reference for future research in the following three aspects.
First, regarding the SR/MA methodology, researchers should register research protocols promptly to ensure rigorous research procedures. Regarding the literature search strategy, we should pay attention to the grey literature retrieval method and the excluded literature list to ensure the comprehensiveness of the search and the reproducibility of the research. Before quantitative analysis, the heterogeneity of the included studies should be evaluated, and its influence on the outcome should be mitigated using subgroup analysis, meta-regression, and other techniques. To ensure the stability of the results, quantitative calculations of effect size should focus on excluding the results of a single study and analyzing the sensitivity of the included study. In addition, funnel plots, Egger's test, Begg's test, and other methods were used to evaluate publication bias, which also contributed to improving the accuracy of the MA results.
Second, a large number of RCTs specifically focused on treating HFpEF with SGLT-2is need to be implemented to avoid the problem of insufficient study samples and low evidence quality due to the inclusion of subgroup analysis. In addition, various comorbidities of patients with HFpEF also need to be fully considered when designing RCTs to clarify the efficacy of SGLT-2is in different populations. Given the available evidence, SGLT-2is can significantly reduce the incidence of HHF and adverse events as well as improve activity tolerance and quality of life in patients with HFpEF, but they do not significantly reduce the incidence of CVD and all-cause death. Future RCTs should focus on CVD and all-cause death and supplement the indicators of the impacts of SGLT-2is on the cardiac function, structure, and serum biochemistry of patients with HFpEF, thereby laying the foundation for pharmacological research. In addition, given the curative efficacy of SGLT-2is for patients with HFpEF, the design of placebo controls or blank controls should be limited in future RCTs, and for ongoing RCTS, such as “EMPAGUM research” (74), “SGLT2 Inhibitors, Ketones, and Cardiovascular Benefit Research Plan” (75), and “Sotagliflozin in Heart Failure With Preserved Ejection Fraction (HFpEF) Patients” (76), modification of the study protocol should be considered to prevent any potential ethical problems.
Finally, significant deficiencies were found in all aspects of the SR/MA report, which may be due to researchers' unfamiliarity with the relevant tools, such as AMSTAR-2, ROBIS, PRISMA, and GRADE. Therefore, evidence-based medicine education should be popularized in universities. Especially, the Cochrane Handbook and several generally recognized scales, such as AMSTAR-2, ROBIS, PRISMA, and GRADE, should be employed in these studies.
Our umbrella review is the first to use the AMSTAR-2, ROBIS, PRISMA guidelines, GRADE framework, CCA, and excess significance tests to summarize and assess SRs/MAs with respect to the efficacy of SGLT-2is on HFpEF. Based on our updated MA, SGLT-2is may represent an effective therapy for HFpEF by reducing HHF and adverse events and improving 6MWD and KCCQ-TSS levels. In addition, the assessment procedure showed evident limits of the present relevant SRs/MAs and RCTs, which may improve the quality of future clinical studies. However, this study is subjective concerning methodological evaluation. Although our assessment was analysed and reviewed by separate researchers, different researchers may have their unique perspectives on each item, resulting in variable outcomes.
According to the available evidence, SGLT-2is appear to have a beneficial impact on HFpEF while maintaining a high level of security. Concerning the low methodological quality, reporting quality, evidence quality, and high risk of bias of the SRs/MAs supporting these findings, we should carefully draw this conclusion. Therefore, more rigorous, standardized, and comprehensive SRs/MAs are needed in related fields. More importantly, we must pay attention to the outcomes of recently updated, prospective randomized controlled, double-blind clinical trials with rigorous design and proper conduct, since they contain the least amount of bias and provide the highest level of evidence.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
RL and GD participated in the research design. RL, GH, WG, XW, and LR conducted a literature search and screened data extraction. RL, LR, XW, HQ, and WG evaluate the quality of the literature and analyzed the data. RL wrote the manuscript. RL and GD were involved in the manuscript's revision. Each author has read the manuscript. All authors contributed to the article and approved the submitted version.
This project was funded by the National Key Research and Development Program of China (Nos. 2019YFC1710400; 2019YFC1710401); The National Natural Science Foundation of China (Nos. 81774047; 82174172).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1143658/full#supplementary-material.
6MWD, 6 min-walk distance; AHF, acute heart failure; AMSTAR-2, A Measure Tool to Assess Systematic Reviews 2; BNP, B-type natriuretic peptide; CCA, corrected covered area; CI, confidence interval; CKD, chronic kidney disease; CT, conventional treatment; CVD, cardiovascular death; E, expected number;
1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
2. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. (2012) 126:65–75. doi: 10.1161/circulationaha.111.080770
3. Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. (2011) 26:562–8. doi: 10.1097/HCO.0b013e32834b7faf
4. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14:591–602. doi: 10.1038/nrcardio.2017.65
5. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. (2019) 124:1598–617. doi: 10.1161/circresaha.119.313572
6. Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. (2020) 22:1009–18. doi: 10.1002/ejhf.1788
7. McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, et al. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:602–11. doi: 10.1016/j.jacc.2018.11.033
8. Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. (2018) 71:339–51. doi: 10.1016/j.jacc.2017.11.019
9. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. (2011) 8:30–41. doi: 10.1038/nrcardio.2010.165
10. Clark H, Rana R, Gow J, Pearson M, van der Touw T, Smart N. Hospitalisation costs associated with heart failure with preserved ejection fraction (HFpEF): a systematic review. Heart Fail Rev. (2022) 27:559–72. doi: 10.1007/s10741-021-10097-7
11. Toth PP, Gauthier D. Heart failure with preserved ejection fraction: disease burden for patients, caregivers, and the health-care system. Postgrad Med. (2021) 133:140–5. doi: 10.1080/00325481.2020.1842621
12. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. (2012) 59:998–1005. doi: 10.1016/j.jacc.2011.11.040
13. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. (2014) 64:2281–93. doi: 10.1016/j.jacc.2014.08.036
14. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. (2013) 62:263–71. doi: 10.1016/j.jacc.2013.02.092
15. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e263–e421. doi: 10.1016/j.jacc.2021.12.012
16. Siamashvili M, Davis SN. Sodium-glucose cotransporter 2 inhibitors for the management of type 2 diabetes. Expert Opin Pharmacother. (2021) 22:2181–98. doi: 10.1080/14656566.2021.1967320
17. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
18. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
19. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24. doi: 10.1056/NEJMoa2022190
20. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
21. Filippatos G, Butler J, Farmakis D, Zannad F, Ofstad AP, Ferreira JP, et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation. (2022) 146:676–86. doi: 10.1161/CIRCULATIONAHA.122.059785
22. Sharma A, Ferreira JP, Zannad F, Pocock SJ, Filippatos G, Pfarr E, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from the EMPEROR-preserved trial. Eur J Heart Fail. (2023). doi: 10.1002/ejhf.2857
23. Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. (2020) 142:2205–15. doi: 10.1161/circulationaha.120.050255
24. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. (2021) 42:700–10. doi: 10.1093/eurheartj/ehaa943
25. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. (2021) 27:1954–60. doi: 10.1038/s41591-021-01536-x
26. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387:1089–98. doi: 10.1056/NEJMoa2206286
27. Inzucchi SE, Claggett BL, Vaduganathan M, Desai AS, Jhund PS, de Boer RA, et al. Efficacy and safety of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction by baseline glycaemic status (DELIVER): a subgroup analysis from an international, multicentre, double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. (2022) 10:869–81. doi: 10.1016/S2213-8587(22)00308-4
28. Mc Causland FR, Claggett BL, Vaduganathan M, Desai AS, Jhund P, de Boer RA, et al. Dapagliflozin and kidney outcomes in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified analysis of the DELIVER randomized clinical trial. JAMA Cardiol. (2023) 8:56–65. doi: 10.1001/jamacardio.2022.4210
29. Bougioukas KI, Vounzoulaki E, Mantsiou CD, Savvides ED, Karakosta C, Diakonidis T, et al. Methods for depicting overlap in overviews of systematic reviews: an introduction to static tabular and graphical displays. J Clin Epidemiol. (2021) 132:34–45. doi: 10.1016/j.jclinepi.2020.12.004
30. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
31. Dong C, Shi H, Liu P, Si G, Yan Z. A critical overview of systematic reviews and meta-analyses of light therapy for non-seasonal depression. Psychiatry Res. (2022) 314:114686. doi: 10.1016/j.psychres.2022.114686
32. Poprom N, Wilasrusmee C, Attia J, McEvoy M, Thakkinstian A, Rattanasiri S. Comparison of postoperative complications between open and laparoscopic appendectomy: an umbrella review of systematic reviews and meta-analyses. J Trauma Acute Care Surg. (2020) 89:813–20. doi: 10.1097/ta.0000000000002878
33. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Cardiology. Chinese Guidelines for the diagnosis and treatment of heart failure 2018. Chin J Cardiol. (2018) 46:760–89. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004
34. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Br Med J. (2017) 358:j4008. doi: 10.1136/bmj.j4008
35. Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. (2016) 69:225–34. doi: 10.1016/j.jclinepi.2015.06.005
36. Bühn S, Mathes T, Prengel P, Wegewitz U, Ostermann T, Robens S, et al. The risk of bias in systematic reviews tool showed fair reliability and good construct validity. J Clin Epidemiol. (2017) 91:121–8. doi: 10.1016/j.jclinepi.2017.06.019
37. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
38. Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. An algorithm was developed to assign GRADE levels of evidence to comparisons within systematic reviews. J Clin Epidemiol. (2016) 70:106–10. doi: 10.1016/j.jclinepi.2015.08.013
39. Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. (2014) 67:368–75. doi: 10.1016/j.jclinepi.2013.11.007
40. Hennessy EA, Johnson BT. Examining overlap of included studies in meta-reviews: guidance for using the corrected covered area index. Res Synth Methods. (2020) 11:134–45. doi: 10.1002/jrsm.1390
41. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. (2007) 4:245–53. doi: 10.1177/1740774507079441
42. Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. (2008) 37:1158–60. doi: 10.1093/ije/dyn204
43. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
44. Butler J, Usman MS, Khan MS, Greene SJ, Friede T, Vaduganathan M, et al. Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Heart Fail. (2020) 7:3298–309. doi: 10.1002/ehf2.13169
45. Lu Y, Li F, Fan Y, Yang Y, Chen M, Xi J. Effect of SGLT-2 inhibitors on cardiovascular outcomes in heart failure patients: a meta-analysis of randomized controlled trials. Eur J Intern Med. (2021) 87:20–8. doi: 10.1016/j.ejim.2021.03.020
46. Zheng C, Lin M, Chen Y, Xu H, Yan L, Dai H. Effects of sodium-glucose cotransporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. Cardiovasc Diabetol. (2021) 20:83. doi: 10.1186/s12933-021-01272-z
47. Singh AK, Singh R, Misra A. Do SGLT-2 inhibitors exhibit similar cardiovascular benefit in patients with heart failure with reduced or preserved ejection fraction? J Diabetes. (2021) 13:596–600. doi: 10.1111/1753-0407.13182
48. Cardoso R, Graffunder FP, Ternes CMP, Fernandes A, Rocha AV, Fernandes G, et al. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: a systematic review and meta-analysis. EClinicalMedicine. (2021) 36:100933. doi: 10.1016/j.eclinm.2021.100933
49. Pandey AK, Dhingra NK, Hibino M, Gupta V, Verma S. Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a meta-analysis. ESC Heart Fail. (2022) 9:942–6. doi: 10.1002/ehf2.13805
50. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. (2022) 400:757–67. doi: 10.1016/s0140-6736(22)01429-5
51. Razuk V, Chiarito M, Cao D, Nicolas J, Pivato CA, Camaj A, et al. SGLT-2 inhibitors and cardiovascular outcomes in patients with and without a history of heart failure: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2022) 8:557–67. doi: 10.1093/ehjcvp/pvac001
52. Cao Y, Li P, Li Y, Han Y. Sodium-glucose cotransporter-2 inhibitors in heart failure: an updated meta-analysis. ESC Heart Fail. (2022) 9:1942–53. doi: 10.1002/ehf2.13905
53. Zhao L, Guo W, Huang W, Wang L, Huang S. Benefit of sodium-glucose cotransporter-2 inhibitors on survival outcome is related to the type of heart failure: a meta-analysis. Diabetes Res Clin Pract. (2022) 187:109871. doi: 10.1016/j.diabres.2022.109871
54. Yang D, Zhang Y, Yan J, Liu M, An F. SGLT-2 inhibitors on prognosis and health-related quality of life in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:942125. doi: 10.3389/fcvm.2022.942125
55. Fukuta H, Hagiwara H, Kamiya T. Sodium-glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Int J Cardiol Heart Vasc. (2022) 42:101103. doi: 10.1016/j.ijcha.2022.101103
56. Zhou H, Peng W, Li F, Wang Y, Wang B, Ding Y, et al. Effect of sodium-glucose cotransporter 2 inhibitors for heart failure with preserved ejection fraction: a systematic review and meta-analysis of randomized clinical trials. Front Cardiovasc Med. (2022) 9:875327. doi: 10.3389/fcvm.2022.875327
57. Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. (2022) 28:1956–64. doi: 10.1038/s41591-022-01971-4
58. Wang Y, Gao T, Meng C, Li S, Bi L, Geng Y, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: an updated systematic review and meta-analysis. Eur J Med Res. (2022) 27:314. doi: 10.1186/s40001-022-00945-z
59. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. (2019) 139:2528–36. doi: 10.1161/circulationaha.119.040130
60. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. (2021) 384:129–39. doi: 10.1056/NEJMoa2030186
61. Tanaka A, Hisauchi I, Taguchi I, Sezai A, Toyoda S, Tomiyama H, et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE). ESC Heart Fail. (2020) 7:1585–94. doi: 10.1002/ehf2.12707
62. Ejiri K, Miyoshi T, Kihara H, Hata Y, Nagano T, Takaishi A, et al. Effect of luseogliflozin on heart failure with preserved ejection fraction in patients with diabetes mellitus. J Am Heart Assoc. (2020) 9:e015103. doi: 10.1161/jaha.119.015103
63. Ovchinnikov A, Borisov A, Zherebchikova KY, Ryabtseva OY, Gvozdeva A, Masenko V, et al. Effects of empagliflozin on exercise tolerance and left ventricular diastolic function in patients with heart failure with preserved ejection fraction and type 2 diabetes: a prospective single-center study. Russ J Cardiol. (2021) 26:4304. doi: 10.15829/1560-4071-2021-4304
64. Sun H. Effects of dapagliflozin on preserved ejection fraction heart failure with type 2diabetes. [Master’s thesis]. Nanchang City, China: Nanchang University (2021).
65. Tang X, Fan Y. A study of sodium-glucose co-transporter inhibitor in improving the prognosis of type 2 diabetes with heart failure with preserved ejection fraction patients. J Clin Cardiol. (2021) 384:117–28. doi: 10.13201/j.issn.1001-1439.2021.01.014
66. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. (2021) 384:117–28. doi: 10.1056/NEJMoa2030183
67. Ueda T, Kasama S, Yamamoto M, Nakano T, Ueshima K, Morikawa Y, et al. Effect of the sodium-glucose cotransporter 2 inhibitor canagliflozin for heart failure with preserved ejection fraction in patients with type 2 diabetes. Circ Rep. (2021) 3:440–8. doi: 10.1253/circrep.CR-21-0030
68. Akasaka H, Sugimoto K, Shintani A, Taniuchi S, Yamamoto K, Iwakura K, et al. Effects of ipragliflozin on left ventricular diastolic function in patients with type 2 diabetes and heart failure with preserved ejection fraction: the EXCEED randomized controlled multicenter study. Geriatr Gerontol Int. (2022) 22:298–304. doi: 10.1111/ggi.14363
69. Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. (2022) 28:809–13. doi: 10.1038/s41591-022-01703-8
70. AstraZeneca AB. Determine-Preserved-Dapagliflozin Effect on Exercise Capacity Using a 6-Minute Walk Test in Patients with Heart Failure with Preserved Ejection Fraction. ClinicalTrials.gov (2022). Available at: https://wwwclinicaltrialsgov/ct2/show/NCT03877224 (Accessed December 15, 2022).
71. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. (2020) 17:761–72. doi: 10.1038/s41569-020-0406-8
72. Abdelmasih R, Abdelmaseih R, Thakker R, Faluk M, Ali A, Alsamman MM, et al. Update on the cardiovascular benefits of sodium-glucose co-transporter-2 inhibitors: mechanism of action, available agents and comprehensive review of literature. Cardiol Res. (2021) 12:210–8. doi: 10.14740/cr1268
73. Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. (2012) 1:7. doi: 10.1186/2046-4053-1-7
74. Sun Z. EMPAGUM: Effects of Empagliflozin on Gut Microbiota in Heart Failure With a Preserved Ejection Fraction. ClinicalTrials.gov (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05584319 (Accessed March 1, 2023).
75. Qin Y. SGLT2 Inhibitors, Ketones, and Cardiovascular Benefit Research Plan. ClinicalTrials.gov (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05057806 (Accessed March 1, 2023).
76. Badimon J. Sotagliflozin in Heart Failure With Preserved Ejection Fraction (HFpEF) Patients. ClinicalTrials.gov (2023). Available at: https://ClinicalTrials.gov/show/NCT05562063 (Accessed March 1, 2023).
Keywords: sodium-glucose cotransporter-2 inhibitor, heart failure with preserved ejection fraction, umbrella review, overview, systematic review, meta-analysis, evidence quality assessment
Citation: Li R, Dai G, Guan H, Gao W, Ren L, Wang X and Qu H (2023) Scientific evidence of sodium-glucose cotransporter-2 inhibitors for heart failure with preserved ejection fraction: an umbrella review of systematic reviews and meta-analyses. Front. Cardiovasc. Med. 10:1143658. doi: 10.3389/fcvm.2023.1143658
Received: 13 January 2023; Accepted: 2 May 2023;
Published: 12 May 2023.
Edited by:
Inna P. Gladysheva, University of Arizona College of Medicine—Phoenix, United StatesReviewed by:
Matthew Lee, University of Glasgow, United Kingdom© 2023 Li, Dai, Guan, Gao, Ren, Wang and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohua Dai ZGFpZ2gyb280QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.