- 1IBD Center, Department of Gastroenterology, IRCCS Humanitas Research Hospital, Milan, Italy
- 2Department of Biomedical Sciences, Humanitas University, Milan, Italy
- 3Division of Colon and Rectal Surgery, IRCCS Humanitas Research Hospital, Milan, Italy
- 4Department of Cardiovascular Medicine, IRCCS Humanitas Research Hospital, Milan, Italy
Atherosclerotic cardiovascular disease and stroke are the leading causes of morbidity and mortality worldwide. Along to the traditional risk factors for these diseases, chronic inflammation is known to be an important player in accelerating the process of atherosclerosis, which can result in an increased incidence of arterial thromboembolic events. As in other chronic inflammatory diseases, in the past few years, several studies suggested that subjects affected by inflammatory bowel diseases (IBD) may also be at an incremented risk of atherosclerotic disease, especially during the periods of disease's flare. Therefore, IBD treatment may assume an important role for achieving both disease remission and the control of the atherosclerotic risk. In this article we aimed to perform a comprehensive review on evidence on the increased risk of arterial thromboembolic events in patients affected by IBD and discuss the potential role of IBD therapy in reducing this risk.
1. Introduction
Inflammatory bowel diseases (IBD), specifically Crohn's disease (CD) and ulcerative colitis (UC), are a group of life-long disorders characterized by chronic relapsing inflammation of the gastrointestinal tract (1, 2). The pathogenesis of IBD is not yet fully understood. CD and UC are immune-mediated diseases that originate from an abnormal immune response to the gut microbiota in genetically susceptible hosts (3). Therefore, the inflammation of the intestinal mucosa leads to the development of symptoms such as diarrhea, abdominal pain, bloody stools, and weight loss (4). In addition to gastrointestinal symptoms, CD and UC can also be associated with various immune-mediated extraintestinal manifestations which can affect the joints, skin, or eyes and whose mechanisms can be ascribed to an extension of the intestinal immune response or an independent inflammatory event perpetuated by IBD (5, 6). Furthermore, similar to other immune-mediated diseases, IBD can also be associated with other extraintestinal comorbidities which are a direct or indirect result of bowel inflammation and that are linked to a reduced quality of life and outcomes due to hospitalization, surgery complications, and mortality (5, 7). The perception of comorbidities in IBD is recently emerging and, in the late years, there has been plentiful interest on this topic (7). Among extraintestinal comorbidities, atherosclerotic cardiovascular disease (ASCVD) is one of the most relevant (7). Indeed, ASCVD remains the leading cause of morbidity and mortality worldwide (8). IBD has so far been overlooked as a risk factor for ASCVD, since, similarly to other chronic inflammatory diseases (i.e., rheumatoid arthritis), chronic inflammation is a main driver for the development of accelerated atherosclerosis and some studies suggest that IBD may display an incremented risk for arterial thrombotic events (9–11).
The aim of this review is to highlight the current evidence on the increased risk of ASCVD in patients affected by IBD, and discuss the shared physiopathology, present and future therapeutical implications.
2. Methods
We carried out a literature search in the PubMed database until November 2022 to identify relevant studies investigating the shared mechanisms and association between ASCVD and IBD. The key words used were: “inflammatory bowel disease” OR “Crohn's disease” OR “ulcerative colitis” AND “cardiovascular disease” OR “atherosclerosis” OR “ischemic heart disease” OR “ischemic stroke”. In addition, references of original articles and relevant reviews were screened to find additional publications. No publication date restrictions were applied. We excluded case reports, case series and any irrelevant abstract.
3. Pathological mechanisms shared by IBD and atherosclerotic cardiovascular diseases
Chronic inflammation and inflammatory cytokines displayed by chronic immune-mediated diseases have been pointed as important players in the pathogenesis of atherosclerosis and rapid coronary arterial disease evolution (12). For its being chronic inflammatory diseases, also IBD are associated with an upregulation of several cytokines (13). It has been shown that IBD patients with active disease, compared to subjects with quiescent disease and healthy controls, exhibit higher levels of vascular endothelial growth factor (VEGF) which is a potent angiogenic and vascular permeability-enhancing cytokine (14–16). Also, other inflammatory molecules that have an important role in the pathogenesis of IBD, such as tumor necrosis alpha (TNF-α), can generate endothelial alterations and can damage vascular functionality by reducing the nitric oxide (NO) availability (16, 17). Furthermore, TNF-α promotes the interconnection between the endothelium and circulating leukocytes by the upregulation of adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) (10). High levels of VCAM-1 and ICAM-1 have been described as an independent risk of major adverse cardiac events (18). The expression of ICAM-1 and VCAM-1 is associated with intestinal disease activity, demonstrated by the reduction of their serum concentration after treatment (10, 19). In addition, interleukin-6 (IL-6), whose levels are increased in IBD, is linked to endothelial dysfunction, early atherosclerosis, and coronary heart disease (20–23). Moreover, both innate and adaptive cells are involved in the linkage between IBD and atherogenesis (16). It has been acknowledged that in IBD, the disruption of the intestinal mucosal barrier promotes the translocation of microbial lipopolysaccharides (LPS) that can stimulate the production of proinflammatory molecules and the oxidation of low-density lipoproteins through Toll-like receptors (TLRs) signaling, leading to endothelial injury and atherosclerosis (24). TLRs are fundamental in both IBD and atherosclerosis pathogenesis and have been observed in atherosclerotic plaques (16, 24). LPS as well are present in atherosclerotic arteries but not in normal arteries. In atherosclerotic plaques, the pro-inflammatory status induced by LPS can also lead to plaque instability (25). Beyond the innate immune system, also the adaptative immune system plays an important role in the network between IBD and atherogenesis. IBD patients are characterized by activated T cells (26) which are crucial also in the pathogenesis of atherosclerosis (16, 27). Indeed, activated T cells has been observed in atherosclerotic lesions (16), similarly as in Crohn's disease. The T cells infiltrating atherosclerotic plaques exhibit a Th1 profile which activates macrophages and expands pro-inflammatory cytokines such as IFN-γ. IFN-γ reduces the production of collagen, making the atherosclerotic fibrous cap more susceptible to rupture (27). Also genetics factors are implicated in the pathogenesis of IBD and atherosclerosis and some gene variants may be shared by both diseases (16). Indeed, it has been observed that some nucleotide-binding oligomerization domain-containing protein 2 (NOD2)/CARD15 polymorphisms (2 missense mutations: ARG702TRP, GLY908ARG; 1 frameshift mutation: 1,007 fs) implicated in the regulation of CD were associated also with the development of coronary atherosclerosis (28–30). Conversely, a polymorphism of IL-6 receptor (rs2228145) seems to possess a protective role in both coronary heart disease and IBD (31). In the future, more studies are needed to assess the role of these or other genetic variants in the predisposition to atherosclerosis in individuals with IBD. Finally, the alterations in the gut microbiota of IBD patients and the microbiota-derived processes may be associated to atherosclerosis (32). Interestingly, an increased microbiota composition of Enterobacteriaceae species (mainly Escherichia coli) have been described in both IBD and cardiovascular disease even if the connection between these findings have not been cleared (32–34). Furthermore, butyrate-producing bacteria (i.e., Faecalibacterium prausnitzii) can be decreased in the microbiota of either IBD and CVD individuals and it is known that butyrate possess an atheroprotective role and can hamper the intestinal epithelial cell apoptosis, therefore reducing LPS translocation (32, 35–37). Figure 1 summarizes the shared pathological mechanisms between IBD and ASCVD.
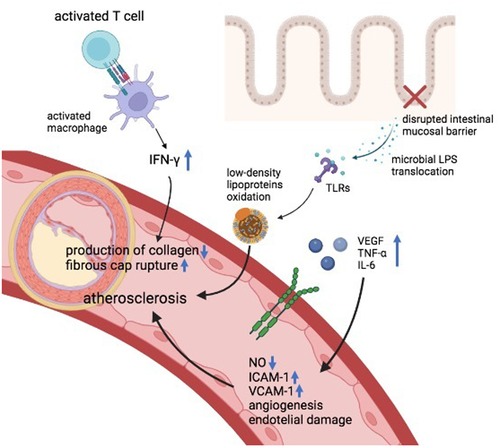
Figure 1. Pathological mechanisms shared by IBD and atherosclerotic cardiovascular diseases. IFN-γ, interferon gamma, TLRs, toll-like receptors, VEGF, vascular-endothelial growth factor, TNF-α, tumor necrosis factor, IL, interleukin, NO, nitric oxide, ICAM, intercellular adhesion molecule, VCAM, vascular cell adhesion protein, LPS, lipopolysaccharides.
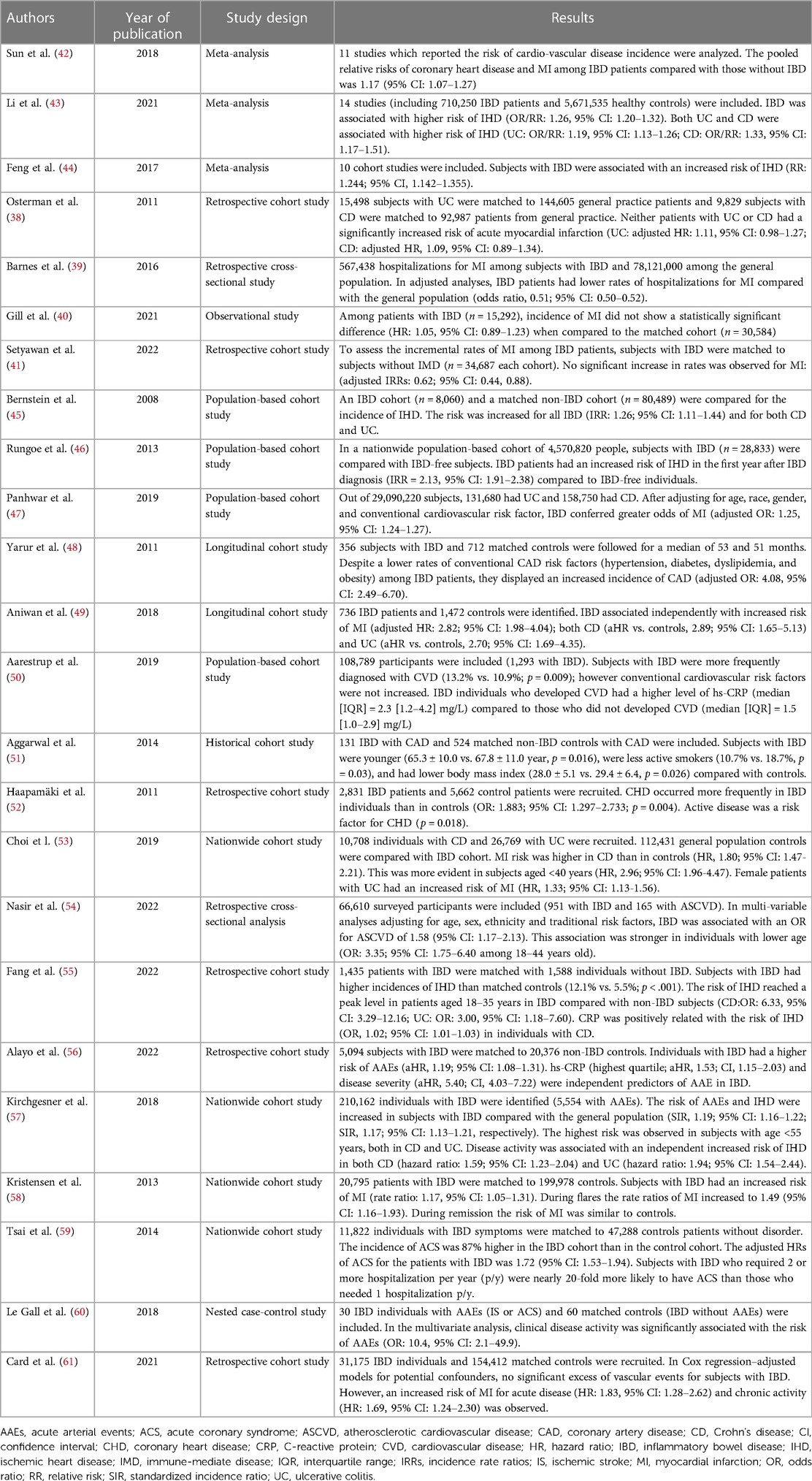
4. Risk of ischemic heart disease in IBD
Several studies assessed the interconnection between IBD and the risk of ischemic heart disease (IHD) with heterogeneous results. Although some retrospective studies did not find a significant association between IBD and IHD (38–41), many other studies and meta-analyses demonstrated a positive correlation between the two diseases (11, 42–44). A landmark population-based matched-cohort study conducted by Bernstein et al. (8,072 IBD patients and 80,489 non-IBD controls) found an increased incidence rate ratio of IHD in the IBD cohort (IRR: 1.26; 95% CI: 1.11–1.44) (45).
Subsequently, also a large nationwide Danish population-based cohort study (4,570,820 people, 28,833 with IBD) showed that IBD patients have an increased risk of IHD in the first year after IBD diagnosis (IRR = 2.13, 95% CI: 1.91–2.38) compared to IBD-free individuals. Furthermore, this study showed that in the long term, during 1–13 years of follow-up, the risk was 1.22 (95% CI: 1.14–1.30) (46). Similarly, a more recent large population-based study from the United States (29,090,220 people, 290,430 with IBD) found that IBD confer a higher risk of myocardial infarction (adjusted odds ratio: 1.25; 95% CI: 1.24–1.27) (47). Comparable results were noted also in a longitudinal cohort study with matched controls conducted by Yarur et al. (48). In this study, 356 IBD patients and 712 controls were followed for a median of 53 and 51 months, respectively. An increased incidence of coronary artery disease was observed in subjects with IBD (adjusted hazard ratio: 4.08; 95% CI: 2.49–6.70) (48). Interestingly, it was also found that IBD patients had lower rates of traditional coronary artery disease risk factors (hypertension, diabetes, dyslipidemia, and obesity) (48). These findings were corroborated by other studies. The study of Aniwan et al., showed that IBD was independently associated with an increased risk of acute myocardial infarction (AMI) (adjusted hazard ratio: 2.82; 95% CI: 1.98–4.04), despite a lower prevalence of conventional risk factors for AMI (49). Similar results were observed also by Aarestrup et al. (50). In their population-based study of >100.000 individuals (1,203 with IBD), the Authors observed that, even though subjects with IBD were diagnosed more frequently with cardiovascular diseases (13.2% vs. 10.9%; p = 0.009), conventional cardiovascular risk factors were not increased. Furthermore, among subjects with IBD, those who developed cardiovascular diseases displayed higher levels of C-reactive protein (CRP) (50). In addition, another study found that IBD patients diagnosed with coronary artery disease (CAD) were younger, less active smokers and had lower body mass index compared to non-IBD patients diagnosed with CAD, despite similar prevalence of hypertension, hyperlipidemia and diabetes was exhibited in both groups (51). This trend was observed as well by Haapamäki et al., which showed an increased frequency of coronary heart disease (CHD) in subjects with IBD compared to controls (2.2% vs. 1.4%; p = 0.004) despite a younger age of the IBD group (52). Again, in another nationwide study, Choi et al. observed that the risk of myocardial infarction was higher in subjects with CD than in the general population (hazard ratio: 1.80; 95% CI: 1.47–2.21) and that this tendency was stronger in female patients and those ones aged under 40 years (53). Similarly, Nasir et al. demonstrated an increased odds of ASCVD in IBD compared to non-IBD (odds ratio: 1.58; 95% CI: 1.17–2.13) with a significant interaction by age whereby the younger people displayed a stronger association with ASCVD (adjusted OR among 18–44 year-olds 3.35; 95% CI: 1.75–6.40) (54). These evidences reinforce the hypothesis that conventional cardiovascular risk factors might not be enough to estimate the risk of CAD in subjects with IBD and that intestinal chronic inflammation likely play a crucial role in the pathogenesis of atherosclerosis (50, 51). Indeed, several studies demonstrated a positive relation between CRP, an important quantifiable marker of systemic inflammation, and the risk of IHD or acute arterial events (50, 55, 56).
Likewise, a large nationwide French cohort study (n = 210,162 IBD patients) conducted by Kirchgesner et al. observed that disease activity was associated with an independent increased risk of IHD in both CD (hazard ratio: 1.59; 95% CI: 1.23–2.04) and UC (hazard ratio: 1.94; 95% CI: 1.54–2.44) (57). Other several studies showed that acute coronary disease was correlated to IBD disease activity (58–61). Therefore, a recent international consensus stated that IBD patients, especially young subjects with active disease, harbor an increased risk of arterial thrombotic events, recommended to strive for intestinal disease remission in order to reduce its atherosclerotic risk and suggested to perform an active screening and control of traditional cardiovascular disease risks in the setting of IBD (11). Interestingly, a meta-analysis conducted by Sun et al. (42) observed a higher relative risk of CHD in women with IBD compared to the increment of the relative risk in male with IBD (RR: 1.27; 95% CI: 1.12–1.45 vs. RR: 1.13; 95% CI: 1.09–1.17, respectively) and this may be partially explained by the variance in the gender distribution of traditional IHD risk factors and by the fact that inflammation may play a higher role in women (62, 63). Furthermore, the same meta-analysis also observed that subjects with IBD were not associated with an increased cardiovascular mortality. Possible explanations for this result is that cardiovascular mortality may not be a good surrogate for IHD incidence and that the advancement of medical therapy and health care may reduce the mortality from cardiovascular diseases (42). Further prospective studies are needed to evaluate if the increased incidence of IHD in IBD will also result in an increased cardiovascular mortality. Table 1 summarizes the studies which evaluated the risk of IHD in IBD.
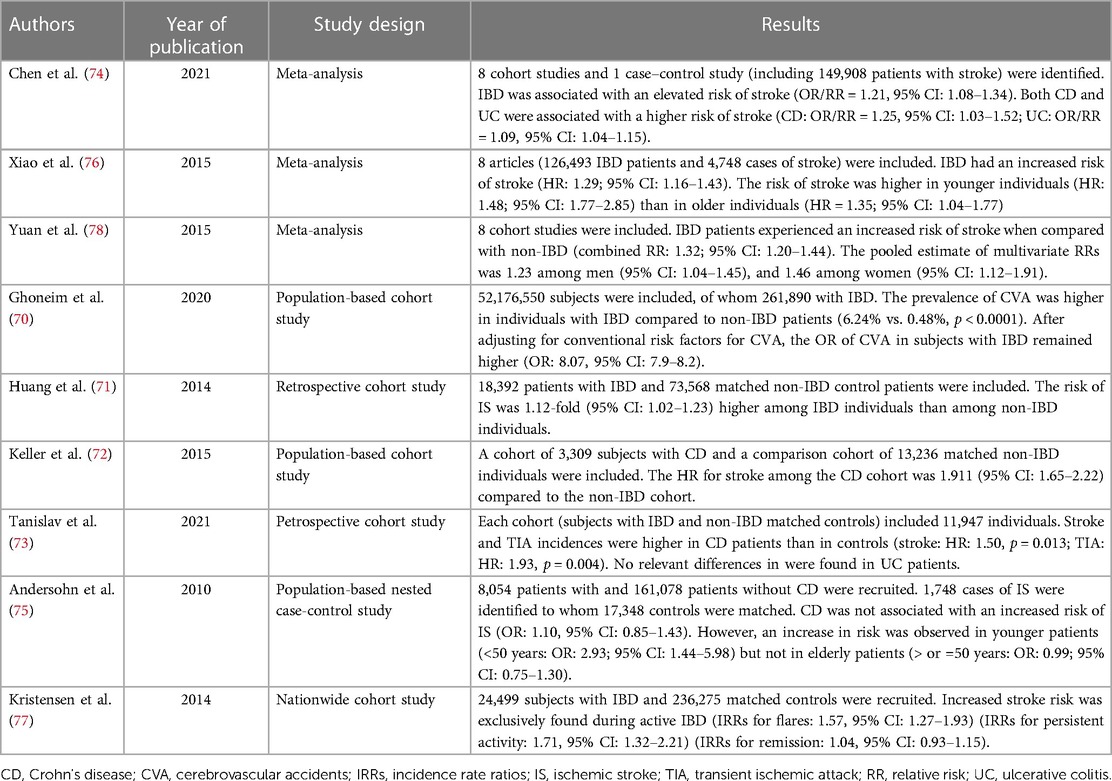
5. Risk of cerebrovascular ischemic disease in IBD
Stroke is the second leading cause of both disability and death worldwide (64) and arterial occlusion represents its etiology in 87% of cases (65). Similarly to IHD, also a higher risk of stroke has been associated with chronic inflammatory diseases (66, 67), and to increased concentrations of systemic inflammatory factors such as CRP (68). These findings suggest that chronic inflammation may as well play a relevant role in the development of cerebrovascular ischemic accidents. Indeed, IBD patients have evidence of premature cerebrovascular disease (10). An interesting study conducted by Biondi et al. observed that IBD patients had a 6.45-fold higher risk of carotid atherosclerotic plaque compared to healthy controls (95% CI: 1.035–40.216; p < 0.046) (69). These findings may have an implication in the daily clinical practice as a large population-based study (52,176,550 patients, 261,890 with IBD) showed that IBD is an independent risk factor for cerebrovascular accidents (OR: 8.07, 95% CI: 7.9–8.2) (70). Another population-based cohort study recruiting 18,392 IBD patients and 73,568 matched controls showed that the risk of ischemic stroke was 1.12-fold higher among the IBD cohort (95% CI: 1.02–1.23) (71). Similarly, Keller et al. observed that CD patients had a higher hazard ratio (HR) for stroke compared to non-IBD patients (HR: 1.91; 95% CI: 1.65–2.22) (72) and this was supported also by the results achieved in the study of Tanislav et al. (HR: 1.50; p = 0.013) (73). In accordance with the above-mentioned studies, a recent meta-analysis of cohort and case-control studies confirmed that IBD was associated with an elevated risk of stroke (OR/RR: 1.21; 95% CI: 1.08–1.34). Furthermore, both CD and UC were significantly associated with stroke (CD: OR/RR: 1.25. UC: OR/RR: 1.09) (74). Likewise as IHD, studies showed that especially younger patients display an increased risk of ischemic stroke (75, 76) and that the risk of stroke is significantly increased during IBD flares but not during remission (77). Furthermore, it seems that the risk of incidence of stroke is higher in female IBD patients compared to male ones, as it was observed in the metanalysis by Yuan et al. (RRs of stroke incidence: men 1.23, 95% CI: 1.04–1.45; women 1.46, 95% CI: 1.12–1.91) (78). Gender differences in the risk of stroke may be explained by a major role of systemic inflammation and hormonal discrepancy in women and by the fact that men have a higher background risk which could exceed the independent risk of IBD for stroke (11). Finally, it is important to note that also atrial fibrillation (AF), which incidence is increased in IBD, may partially contribute to the increased risk of stroke in IBD patients (77). However, in the nationwide study of Kristensen et al., the relative impact on stroke risk attributable to AF was significantly lower among individuals with IBD compared with the matched controls (77). Table 2 summarizes the studies which evaluated the risk of cerebrovascular ischemic disease in IBD.
6. IBD therapies and cardiovascular risk
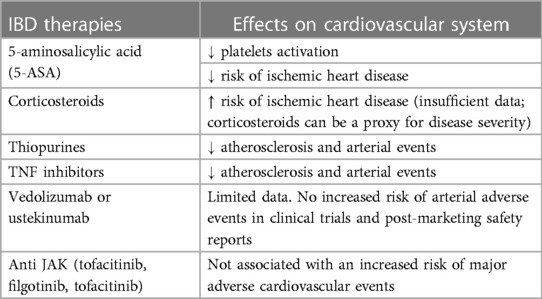
The increased risk of arterial thrombosis in IBD must be taken into account also during the prescription of treatment, because the risk of thrombosis may be affected by these therapies (11, 16) as some IBD drugs display an intrinsic pro or anti-thrombotic effect. One of the most common therapy used in IBD is 5-aminosalicylic acid (5-ASA). This drug shares many pharmacological features of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin (79). It can inhibit platelet activation (80) and may decrease the risk of IHD in subjects affected by IBD (11). Indeed, in a large Danish nationwide population-based study of Rungoe et al., the risk of IHD was lower among IBD patients using 5-ASA (IRR = 1.16; 95% CI: 1.06–1.26) than among non-users (IRR = 1.36; 95% CI: 1.22–1.51) (p = 0.02). Furthermore, long-term users of 5-ASA showed an even lower risk of IHD (IRR = 1.08; 95% CI: 0.98–1.19) (46). On the other hand, in the same study, the authors found that the subjects requiring oral corticosteroids had a higher risk of IHD (IRR = 1.37; 95% CI: 1.25–1.50) compared to the subjects who never used corticosteroids (IRR = 1.23; 95% CI: 1.12–1.36) (p < 0.01) (46). In fact, corticosteroids may exacerbate the risk of cardiovascular disease in subjects with IBD since they can aggravate some traditional risk factors such as hypertension, obesity, hyperlipidemia and insulin resistance (32). In another retrospective study, subjects affected by CD (but not UC) exposed to a prolonged assumption of corticosteroids were associated with increased rates of myocardial infarction compared to anti tumor necrosis factor (TNF)-alfa use (81). However, corticosteroids can be a proxy for disease severity, which itself is associated with incremented risk of IHD (46) and a clear causal association cannot entirely be determined (11).
Regarding TNF inhibitors, studies suggest a potential protective role of anti TNF-alfa drugs toward atherosclerosis and IHD (82). In an in vitro experiment, infliximab showed an atheroprotective effect by restoring the reverse cholesterol transport proteins which counteract foam cell formation by ridding cells of excess cholesterol (83). These findings appear to be confirmed also in clinical studies.
In a large population-based nationwide cohort of IBD patients, the exposure to anti-TNFs was associated with a decreased risk of acute arterial events (AAEs) compared to non-exposure (HR: 0.79, 95% CI: 0.66–0.95) (84). Furthermore, exposure to anti-TNFs was associated also with a decreased risk of recurrent acute arterial events (HR: 0.75, 95% CI: 0.63–0.90) in IBD patients with a previous history of AAEs (85). Thiopurines as well seem to possess an atherosclerotic protective feature since they also were associated with a decreased risk of recurrent AAEs (85). In addition, dos Santos et al. demonstrated that the use of azathioprine in association with 5-ASA, more than 5-ASA alone, can control the production of anti-inflammatory cytokines such as TGF-beta and IL-10, which participate to the modulation of the endothelial cells activation (86).
In regard to the more recently approved biologic agents for IBD (i.e., vedolizumab, ustekinumab), data concerning their role on AAEs are scarce (11). Data from clinical trials and post-marketing safety reports did not observe any safety signal of an augmented risk of AAEs in subjects with IBD treated with vedolizumab or ustekinumab (87–91) and no changes were observed in the serum lipid levels during the treatment with this classes of drugs (92).
Finally, Janus kinase inhibitors (JAK) (i.e., tofacitinib, filgotinib, upadacitinib) are part of one of the most recent class of drugs approved for the treatment of moderate-severe UC (93–95). In clinical studies, treatment with tofacitinib has been associated with generally reversible increases in serum lipids during the first few months of therapy, particularly in total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol (96–102). Hypercholesterolemia is a risk factor for cardiovascular events and the potential occurrence of major adverse cardiovascular events (MACE) with has long been a concern (11). In the Oral Rheumatoid Arthritis Trial (ORAL) Surveillance study, a randomized safety endpoint trial which involved active rheumatoid arthritis (RA) patients who were 50 years of age or older with the presence of at least one cardiovascular risk factor and that compared combined tofacitinib doses (5 mg BID and 10 mg BID) and TNF inhibitor, the incidences of MACE (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) were higher in the tofacitinib groups than TNF inhibitor (HR: 1.33; 95% CI: 0.91–1.94) (103). As a result, the European Medicines Agency (EMA)'s human medicines committee (CHMP) has endorsed the measures recommended by the Pharmacovigilance Risk Assessment Committee (PRAC) which stated that tofacitinib (and all approved JAK inhibitors) should be used only if no suitable treatment alternatives are available in patients at increased risk of major cardiovascular problems (such as heart attack or stroke) (104). However, in the setting of UC, a long-term extension study of the OCTAVE open observed an incidence rate (IR) for MACE of 0.16 (95% CI: 0.04–0.42) among all patients (2,440.8 patient-years of exposure) (105). This result is in line with the IR of MACE observed with the use of TNF inhibitors in subjects with UC (incidence rate: 0.51, 95% CI: 0.31–0.79) (11). In addition, also real-life studies observed that tofacitinib did not confer a significantly elevated risk for MACE compared to TNF inhibitors (106, 107). Nevertheless, consistent with the ORAL surveillance trial, in the previously reported long-term extension study of the OCTAVE open the four adjudicated MACE occurred in patients aged ≥55 years, and three of the four subjects had a prior medical history that included risk factors for cardiovascular disease (105).
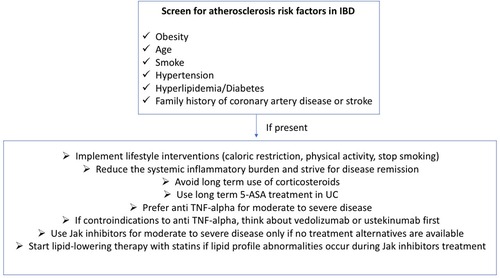
Less data are available on the cardiovascular safety profile of filgotinib or upadacitinib. In the registrative trials, events of MACEs were reported infrequently and no difference was observed with placebo (94, 95). Furthermore, in a meta-analysis assessing the safety of JAK inhibitors in subjects with either IBD or other immune-mediated inflammatory diseases (IMIDs), 30 studies evaluated MACEs on 32,765 patients exposed to JAK inhibitors (17 tofacitinib; 6 upadacitinib; 4 baricitinib; 3 filgotinib). The incidence rate was 0.67 per 100 patient-years. In addition, in the pooled analysis of 22 controlled studies, the RR of MACEs was 1.07 (0.56–2.03) (108). To our best knowledge, real-life safety data of filgotinib or upadacitinib in UC are missing. Table 3 summarizes the relationship between IBD therapies and atherosclerotic cardiovascular disease while Figure 2 provides recommendations for the management of the atherosclerotic risk in subjects with IBD.
Finally, in regards of medical prevention of atherosclerotic cardiovascular disease, even if the atherosclerotic risk is increased in IBD and although low-dose aspirin is not contraindicated in subjects with IBD and does not seem to increase disease flares (32, 109, 110), to date the indication for low-dose aspirin in either primary or secondary prevention in IBD does not change from those in general population (32). Concerning the drug category of statins, which are used for primary and secondary prevention of cardiovascular diseases, it has been suggested that this class of drugs may also exert complex immunomodulatory properties and might be beneficial agents for inflammatory conditions (111). However, even though statins can decrease inflammation in animal models of colitis (112), clinical studies evaluating their disease-modifying and preventive efficacy in IBD have shown conflicting results, being insufficient to support their use for preventing or treating IBD (111, 113, 114).
7. Conclusions
Increasing evidence suggests that IBD patients harbor an increased risk of arterial thrombotic events, especially the young subjects with active disease. This presumably may not be explained by traditional cardiovascular risk factors, but rather by chronic systemic inflammation. Therefore, the control of intestinal inflammation is an important outcome to reduce the risk of atherosclerosis and cardiovascular events. Generally, exposure to IBD's therapies does not seem to be associated with an increased risk of mayor adverse cardiovascular events. On the other hand, some classes of drugs, especially TNF inhibitors and azathioprine, may reduce the risk of arterial thrombotic events. JAK inhibitors are an important extension of the armamentarium against IBD and, unlike in other IMIDs, they do not seem to confer an additional risk of mayor adverse cardiovascular events. Further studies are needed to assess their cardiovascular safety profile in IBD.
Author contributions
RG performed the research, RG wrote the manuscript. AA, ADB, EM, VS, AR and AS critically reviewed the content of the paper. AA conceived the subject of the paper, contributed to the critical interpretation and supervised the project. All authors contributed to the article and approved the submitted version.
Acknowledgments
The Figure 1 was created with BioRender.com. This work was partially supported by “Ricerca Corrente” funding from Italian Ministry of Health to IRCCS Humanitas Research Hospital.
Conflict of interest
AA has received consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, and Takeda; lecture and/or speaker bureau fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mitsubishi Tanabe, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, and Tigenix; and research grants from MSD, Pfizer, Takeda and Biogen. AS has served as a speaker, consultant or advisory board member for Ethicon, Takeda, Pfizer, Sofar, and Oasis. AR received a consultancy fee from Medtronic. RG has received speaker fees from Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. (2017) 389:1741–55. doi: 10.1016/S0140-6736(16)31711-1
2. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. (2017) 389:1756–70. doi: 10.1016/S0140-6736(16)32126-2
3. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. (2015) 12:205–17. doi: 10.1038/nrgastro.2015.34
4. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. (2019) 1:7247238. doi: 10.1155/2019/7247238
5. Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology. (2021) 161:1118–32. doi: 10.1053/j.gastro.2021.07.042
6. Hedin CRH, Vavricka SR, Stagg AJ, Schoepfer A, Raine T, Puig L, et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohns Colitis. (2019) 13:541–54. doi: 10.1093/ecco-jcc/jjy191
7. Argollo M, Gilardi D, Peyrin-Biroulet C, Chabot JF, Peyrin-Biroulet L, Danese S. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol. (2019) 4:643–54. doi: 10.1016/S2468-1253(19)30173-6
8. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 140:e563–95. doi: 10.1161/CIR.0000000000000677
9. Cainzos-Achirica M, Glassner K, Zawahir HS, Dey AK, Agrawal T, Quigley EMM, et al. Inflammatory bowel disease and atherosclerotic cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. (2020) 76:2895–905. doi: 10.1016/j.jacc.2020.10.027
10. Sleutjes JAM, van Lennep JER, van der Woude CJ, de Vries AC. Thromboembolic and atherosclerotic cardiovascular events in inflammatory bowel disease: epidemiology, pathogenesis and clinical management. Ther Adv Gastroenterol. (2021) 14:17562848211032126. doi: 10.1177/17562848211032126
11. Olivera PA, Zuily S, Kotze PG, Regnault V, Al Awadhi S, Bossuyt P, et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2021) 18:857–73. doi: 10.1038/s41575-021-00492-8
12. Panico C, Condorelli G. Unmet needs in the pathogenesis and treatment of cardiovascular comorbidities in chronic inflammatory diseases. Clin Rev Allergy Immunol. (2018) 55:254–27. doi: 10.1007/s12016-017-8624-5
13. Leppkes M, Neurath MF. Cytokines in inflammatory bowel diseases—update 2020. Pharmacol Res. (2020) 158:104835. doi: 10.1016/j.phrs.2020.104835
14. Griga T, Tromm A, Spranger J, May B. Increased serum levels of vascular endothelial growth factor in patients with inflammatory bowel disease. Scand J Gastroenterol. (1998) 33:504–8. doi: 10.1080/00365529850172070
15. Griga T, Gutzeit A, Sommerkamp C, May B. Increased production of vascular endothelial growth factor by peripheral blood mononuclear cells in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. (1999) 11:175–9. doi: 10.1097/00042737-199902000-00019
16. Kamperidis N, Kamperidis V, Zegkos T, Kostourou I, Nikolaidou O, Arebi N, et al. Atherosclerosis and inflammatory bowel disease-shared pathogenesis and implications for treatment. Angiology. (2021) 72:303–14. doi: 10.1177/0003319720974552
17. Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. (2010) 127:295–314. doi: 10.1016/j.pharmthera.2010.05.002
18. Yu J, Liu Y, Peng W, Xu Z. Serum VCAM-1 and ICAM-1 measurement assists for MACE risk estimation in ST-segment elevation myocardial infarction patients. J Clin Lab Anal. (2022) 36:e24685. doi: 10.1002/jcla.24685
19. Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, et al. Adhesion molecules in inflammatory bowel disease. Gut. (1995) 36:724–30. doi: 10.1136/gut.36.5.724
20. Nevulis MG, Baker C, Lebovics E, Frishman WH. Overview of link between inflammatory bowel disease and cardiovascular disease. Cardiol Rev. (2018) 26:287–93. doi: 10.1097/CRD.0000000000000214
21. Esteve E, Castro A, López-Bermejo A, Vendrell J, Ricart W, Fernández-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. (2007) 30:939–45. doi: 10.2337/dc06-1793
22. Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arter Thromb Vasc Biol. (1999) 19:2364–7. doi: 10.1161/01.ATV.19.10.2364
23. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. (2004) 351:2599–610. doi: 10.1056/NEJMoa040967
24. Wu P, Jia F, Zhang B, Zhang P. Risk of cardiovascular disease in inflammatory bowel disease. Exp Ther Med. (2017) 13:395–400. doi: 10.3892/etm.2016.3966
25. Violi F, Cammisotto V, Bartimoccia S, Pignatelli P, Carnevale R, Nocella C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat Rev Cardiol. (2023) 20:24–37. doi: 10.1038/s41569-022-00737-2
26. Koboziev I, Karlsson F, Grisham MB. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann N Y Acad Sci. (2010) 1207(Suppl):E86–93. doi: 10.1111/j.1749-6632.2010.05711.x
27. Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, Nurmohamed MT. Is atherosclerosis an autoimmune disease? BMC Med. (2014) 12:47. doi: 10.1186/1741-7015-12-47
28. Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. (2001) 411:599–603. doi: 10.1038/35079107
29. Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. (2001) 411:603–6. doi: 10.1038/35079114
30. Galluzzo S, Patti G, Dicuonzo G, Di Sciascio G, Tonini G, Ferraro E, et al. Association between NOD2/CARD15 polymorphisms and coronary artery disease: a case-control study. Hum Immunol. (2011) 72:636–40. doi: 10.1016/j.humimm.2011.04.005
31. Zhang M, Bai Y, Wang Y, Cui H, Tang M, Wang L, et al. Cumulative evidence for associations between genetic variants in interleukin 6 receptor gene and human diseases and phenotypes. Front Immunol. (2022) 13:860703. doi: 10.3389/fimmu.2022.860703
32. Massironi S, Mulinacci G, Gallo C, Viganò C, Fichera M, Villatore A, et al. The oft-overlooked cardiovascular complications of inflammatory bowel disease. Expert Rev Clin Immunol. (2023) 8:1–17. doi: 10.1080/1744666X.2023.2174971
33. Khorsand B, Asadzadeh Aghdaei H, Nazemalhosseini-Mojarad E, Nadalian B, Nadalian B, Houri H. Overrepresentation of Enterobacteriaceae and Escherichia coli is the major gut microbiome signature in Crohn’s disease and ulcerative colitis; a comprehensive metagenomic analysis of IBDMDB datasets. Front Cell Infect Microbiol. (2022) 12:1015890. doi: 10.3389/fcimb.2022.1015890
34. Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. (2017) 8:845. doi: 10.1038/s41467-017-00900-1
35. Amiri P, Hosseini SA, Ghaffari S, Tutunchi H, Ghaffari S, Mosharkesh E, et al. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: a comprehensive narrative review. Front Pharmacol. (2022) 12:837509. doi: 10.3389/fphar.2021.837509
36. Ferrer-Picón E, Dotti I, Corraliza AM, Mayorgas A, Esteller M, Perales JC, et al. Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2020) 26:43–55. doi: 10.1093/ibd/izz119
37. Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. (2014) 63:1275–83. doi: 10.1136/gutjnl-2013-304833
38. Osterman MT, Yang YX, Brensinger C, Forde KA, Lichtenstein GR, Lewis JD. No increased risk of myocardial infarction among patients with ulcerative colitis or Crohn’s disease. Clin Gastroenterol Hepatol. (2011) 9:875–80. doi: 10.1016/j.cgh.2011.06.032
39. Barnes EL, Beery RM, Schulman AR, McCarthy EP, Korzenik JR, Winter RW. Hospitalizations for acute myocardial infarction are decreased among patients with inflammatory bowel disease using a nationwide inpatient database. Inflamm Bowel Dis. (2016) 22:2229–37. doi: 10.1097/MIB.0000000000000899
40. Gill GS, Fernandez SJ, Malhotra N, Mete M, Garcia-Garcia HM. Major acute cardiovascular events in patients with inflammatory bowel disease. Coron Artery Dis. (2021) 32:73–7. doi: 10.1097/MCA.0000000000000899
41. Setyawan J, Mu F, Zichlin ML, Billmyer E, Downes N, Yang H, et al. Risk of thromboembolic events and associated healthcare costs in patients with inflammatory bowel disease. Adv Ther. (2022) 39:738–53. doi: 10.1007/s12325-021-01973-7
42. Sun HH, Tian F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: a meta-analysis. Eur J Prev Cardiol. (2018) 25:1623–31. doi: 10.1177/2047487318792952
43. Li Z, Qiao L, Yun X, Du F, Xing S, Yang M. Increased risk of ischemic heart disease and diabetes in inflammatory bowel disease. Z Gastroenterol. (2021) 59:117–24. doi: 10.1055/a-1283-6966
44. Feng W, Chen G, Cai D, Zhao S, Cheng J, Shen H. Inflammatory bowel disease and risk of ischemic heart disease: an updated meta-analysis of cohort studies. J Am Hear Assoc. (2017) 6:e005892. doi: 10.1161/JAHA.117.005892
45. Bernstein CN, Wajda A, Blanchard JF. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: a population-based study. Clin Gastroenterol Hepatol. (2008) 6:41–5. doi: 10.1016/j.cgh.2007.09.016
46. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide danish cohort study. Gut. (2013) 62:689–94. doi: 10.1136/gutjnl-2012-303285
47. Panhwar MS, Mansoor E, Al-Kindi SG, Sinh P, Katz J, Oliveira GH, et al. Risk of myocardial infarction in inflammatory bowel disease: a population-based national study. Inflamm Bowel Dis. (2019) 25:1080–7. doi: 10.1093/ibd/izy354
48. Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. (2011) 106:741–7. doi: 10.1038/ajg.2011.63
49. Aniwan S, Pardi DS, Tremaine WJ, Lofus EV Jr. Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2018) 16:1607–15.e1. doi: 10.1016/j.cgh.2018.04.031
50. Aarestrup J, Jess T, Kobylecki CJ, Nordestgaard BG, Allin KH. Cardiovascular risk profile among patients with inflammatory bowel disease: a population-based study of more than 100 000 individuals. J Crohns Colitis. (2019) 13:319–23. doi: 10.1093/ecco-jcc/jjy164
51. Aggarwal A, Atreja A, Kapadia S, Lopez R, Achkar JP. Conventional risk factors and cardiovascular outcomes of patients with inflammatory bowel disease with confirmed coronary artery disease. Inflamm Bowel Dis. (2014) 20:1593–601. doi: 10.1097/MIB.0000000000000109
52. Haapamäki J, Roine RP, Turunen U, Färkkilä MA, Arkkila P. Increased risk for coronary heart disease, asthma, and connective tissue diseases in inflammatory bowel disease. J Crohns Colitis. (2011) 5:41–7. doi: 10.1016/j.crohns.2010.09.008
53. Choi YJ, Lee DH, Shin DW, Han KD, Yoon H, Shin CM, et al. Patients with inflammatory bowel disease have an increased risk of myocardial infarction: a nationwide study. Aliment Pharmacol Ther. (2019) 50:769–79. doi: 10.1111/apt.15446
54. Nasir K, Acquah I, Dey AK, Agrawal T, Hassan SZ, Glassner K, et al. Inflammatory bowel disease and atherosclerotic cardiovascular disease in U.S. adults-A population-level analysis in the national health interview survey. Am J Prev Cardiol. (2022) 9:100316. doi: 10.1016/j.ajpc.2022.100316
55. Fang L, Gao H, Gao X, Wu W, Miao Y, Zhang H, et al. Risks of cardiovascular events in patients with inflammatory bowel disease in China: a retrospective multicenter cohort study. Inflamm Bowel Dis. (2022) 28:S52–8. doi: 10.1093/ibd/izab326
56. Alayo Q, Loftus EV Jr, Yarur A, Alvarado D, Ciorba MA, de Las Fuentes L, et al. Inflammatory bowel disease is associated with an increased risk of incident acute arterial events: analysis of the United Kingdom biobank. Clin Gastroenterol Hepatol. (2023) 21:761–70.e13. doi: 10.1016/j.cgh.2022.08.035
57. Kirchgesner J, Beaugerie L, Carrat F, Andersen NN, Jess T, Schwarzinger M, et al. Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut. (2018) 67:1261–8. doi: 10.1136/gutjnl-2017-314015
58. Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, et al. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death–a Danish nationwide cohort study. PLoS One. (2013) 8:e56944. doi: 10.1371/journal.pone.0056944
59. Tsai MS, Lin CL, Chen HP, Lee PH, Sung FC, Kao CH. Long-term risk of acute coronary syndrome in patients with inflammatory bowel disease: a 13-year nationwide cohort study in an Asian population. Inflamm Bowel Dis. (2014) 20:502–7. doi: 10.1097/01.MIB.0000441200.10454.4f
60. Le Gall G, Kirchgesner J, Bejaoui M, Landman C, Nion-Larmurier I, Bourrier A, et al. Clinical activity is an independent risk factor of ischemic heart and cerebrovascular arterial disease in patients with inflammatory bowel disease. PLoS One. (2018) 13:e0201991. doi: 10.1371/journal.pone.0201991
61. Card TR, Zittan E, Nguyen GC, Grainge MJ. Disease activity in inflammatory bowel disease is associated with arterial vascular disease. Inflamm Bowel Dis. (2021) 27:629–38. doi: 10.1093/ibd/izaa156
62. Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. (2009) 54:1561–75. doi: 10.1016/j.jacc.2009.04.098
63. Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. (2006) 145:21–9. doi: 10.7326/0003-4819-145-1-200607040-00128
64. Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. (2021) 97:S6–16. doi: 10.1212/WNL.0000000000012781
65. Koh SH, Park HH. Neurogenesis in stroke recovery. Transl Stroke Res. (2017) 8:3–13. doi: 10.1007/s12975-016-0460-z
66. Raaby L, Ahlehoff O, de Thurah A. Psoriasis and cardiovascular events: updating the evidence. Arch Dermatol Res. (2017) 309:225–8. doi: 10.1007/s00403-016-1712-1
67. Gu MM, Wang XP, Cheng QY, Zhao YL, Zhang TP, Li BZ, et al. A meta-analysis of cardiovascular events in systemic lupus erythematosus. Immunol Invest. (2019) 48:505–20. doi: 10.1080/08820139.2019.1567534
68. Emerging Risk Factors Collaboration; Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. (2010) 375:132–40. doi: 10.1016/S0140-6736(09)61717-7
69. Biondi RB, Salmazo PS, Bazan SGZ, Hueb JC, de Paiva SAR, Sassaki LY. Cardiovascular risk in individuals with inflammatory bowel disease. Clin Exp Gastroenterol. (2020) 13:107–13. doi: 10.2147/CEG.S243478
70. Ghoneim S, Shah A, Dhorepatil A, Butt MU, Waghray N. The risk of cerebrovascular accidents in inflammatory bowel disease in the United States: a population-based national study. Clin Exp Gastroenterol. (2020) 13:123–9. doi: 10.2147/CEG.S250182
71. Huang WS, Tseng CH, Chen PC, Tsai CH, Lin CL, Sung FC, et al. Inflammatory bowel diseases increase future ischemic stroke risk: a Taiwanese population-based retrospective cohort study. Eur J Intern Med. (2014) 25:561–5. doi: 10.1016/j.ejim.2014.05.009
72. Keller JJ, Wang J, Hwang YL, Chou CC, Wang LH, Hsu JL, et al. Increased risk of stroke among patients with Crohn’s disease: a population-based matched cohort study. Int J Color Dis. (2015) 30:645–53. doi: 10.1007/s00384-015-2132-y
73. Tanislav C, Trommer K, Labenz C, Kostev K. Inflammatory bowel disease as a precondition for stroke or TIA: a matter of Crohn’s disease rather than ulcerative colitis. J Stroke Cerebrovasc Dis. (2021) 30:105787. doi: 10.1016/j.jstrokecerebrovasdis.2021.105787
74. Chen Y, Wang X. Increased risk of stroke among patients with inflammatory bowel disease: a PRISMA-compliant meta-analysis. Brain Behav. (2021) 11:e02159. doi: 10.1002/brb3.2159
75. Andersohn F, Waring M, Garbe E. Risk of ischemic stroke in patients with Crohn’s disease: a population-based nested case-control study. Inflamm Bowel Dis. (2010) 16:1387–92. doi: 10.1002/ibd.21187
76. Xiao Z, Pei Z, Yuan M, Li X, Chen S, Xu L. Risk of stroke in patients with inflammatory bowel disease: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2015) 24:2774–80. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.008
77. Kristensen SL, Lindhardsen J, Ahlehoff O, Erichsen R, Lamberts M, Khalid U, et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. (2014) 16:477–84. doi: 10.1093/europace/eut312
78. Yuan M, Zhou HY, Xiao XL, Wang ZQ, Yao-Zhi YX. Inflammatory bowel disease and risk of stroke: a meta-analysis of cohort studies. Int J Cardiol. (2016) 202:106–9. doi: 10.1016/j.ijcard.2015.08.190
79. Desreumaux P, Ghosh S. Review article: mode of action and delivery of 5-aminosalicylic acid—new evidence. Aliment Pharmacol Ther. (2006) 24(Suppl 1):2–9. doi: 10.1111/j.1365-2036.2006.03069.x
80. Carty E, MacEy M, Rampton D. Inhibition of platelet activation by 5-aminosalicylic acid in inflammatory bowel disease. Aliment Pharmacol Ther. (2000) 14:1169–79. doi: 10.1046/j.1365-2036.2000.00824.x
81. Lewis JD, Scott FI, Brensinger CM, Roy JA, Osterman MT, Mamtani R, et al. Increased mortality rates with prolonged corticosteroid therapy when compared with antitumor necrosis factor-α-directed therapy for inflammatory bowel disease. Am J Gastroenterol. (2018) 113:405–17. doi: 10.1038/ajg.2017.479
82. Schinzari F, Armuzzi A, De Pascalis B, Mores N, Tesauro M, Melina D, et al. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn’s disease. Clin Pharmacol Ther. (2008) 83:70–6. doi: 10.1038/sj.clpt.6100229
83. Voloshyna I, Seshadri S, Anwar K, Littlefield MJ, Belilos E, Carsons SE, et al. Infliximab reverses suppression of cholesterol efflux proteins by TNF-α: a possible mechanism for modulation of atherogenesis. Biomed Res Int. (2014) 2014:312647. doi: 10.1155/2014/312647
84. Kirchgesner J, Nyboe Andersen N, Carrat F, Jess T, Beaugerie L, BERENICE study group . Risk of acute arterial events associated with treatment of inflammatory bowel diseases: nationwide French cohort study. Gut. (2020) 69:852–8. doi: 10.1136/gutjnl-2019-318932
85. Dheyriat L, Ward D, Beaugerie L, Jess T, Kirchgesner J. Risk of recurrent acute arterial events associated with thiopurines and anti-tumor necrosis factor in inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2023) 21:164–72.e11. doi: 10.1016/j.cgh.2022.06.011
86. dos Santos LC, Costa AV, Lopes LG, Leonel AJ, Aguilar EC, Noviello Mde L, et al. Combination of azathioprine and aminosalicylate treatment prevent risk of cardiovascular disease in women with ulcerative colitis by reducing inflammation. Med Sci Monit. (2015) 21:2305–15. doi: 10.12659/MSM.893865
87. Cohen RD, Bhayat F, Blake A, Travis S. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. (2020) 14:192–204. doi: 10.1093/ecco-jcc/jjz137
88. Panaccione R, Danese S, Sandborn WJ, O’Brien CD, Zhou Y, Zhang H, et al. Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy. Aliment Pharmacol Ther. (2020) 52:1658–75. doi: 10.1111/apt.16119
89. Abreu MT, Rowbotham DS, Danese S, Sandborn WJ, Miao Y, Zhang H, et al. Efficacy and safety of maintenance ustekinumab for ulcerative colitis through 3 years: UNIFI long-term extension. J Crohns Colitis. (2022) 16:1222–34. doi: 10.1093/ecco-jcc/jjac030
90. Ghosh S, Ott E, Gasink C, Miao Y, Colombel JF. 131 Safety of ustekinumab in ibd: a comprehensive analysis of major cardiovascular events (mace) through 5 years in CD and 2 years in UC. Gastroenterology. (2021) 160:37. doi: 10.1016/S0016-5085(21)00834-9
91. Singh S, Iversen AT, Allin KH, Jess T. Comparative outcomes and safety of vedolizumab vs tumor necrosis factor antagonists for older adults with inflammatory bowel diseases. JAMA Netw Open. (2022) 5:e2234200. doi: 10.1001/jamanetworkopen.2022.34200
92. Sleutjes JAM, Roeters van Lennep JE, van der Woude CJ, de Vries AC. Lipid changes after induction therapy in patients with inflammatory bowel disease: effect of different drug classes and inflammation. Inflamm Bowel Dis. (2023) 29:531–8. doi: 10.1093/ibd/izac100
93. Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2017) 376:1723–36. doi: 10.1056/NEJMoa1606910
94. Feagan BG, Danese S, Loftus EV Jr, Vermeire S, Schreiber S, Ritter T, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. (2021) 397:2372–84. doi: 10.1016/S0140-6736(21)00666-8
95. Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. (2022) 399:2113–28. doi: 10.1016/S0140-6736(22)00581-5
96. Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, Boy M, Zuckerman A, Soma K, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. (2016) 46:261–71. doi: 10.1016/j.semarthrit.2016.05.014
97. Gladman DD, Charles-Schoeman C, McInnes IB, Veale DJ, Thiers B, Nurmohamed M, et al. Changes in lipid levels and incidence of cardiovascular events following tofacitinib treatment in patients with psoriatic arthritis: a pooled analysis across phase III and long-term extension studies. Arthritis Care Res. (2019) 71:1387–95. doi: 10.1002/acr.23930
98. Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. (2017) 377:1537–50. doi: 10.1056/NEJMoa1615975
99. Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. (2012) 367:616–24. doi: 10.1056/NEJMoa1112168
100. Wolk R, Armstrong EJ, Hansen PR, Thiers B, Lan S, Tallman AM, et al. Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J Clin Lipidol. (2017) 11:1243–56. doi: 10.1016/j.jacl.2017.06.012
101. Sands BE, Taub PR, Armuzzi A, Friedman GS, Moscariello M, Lawendy N, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. (2020) 18:123–32.e3. doi: 10.1016/j.cgh.2019.04.059
102. Sands BE, Colombel JF, Ha C, Farnier M, Armuzzi A, Quirk D, et al. Lipid profiles in patients with ulcerative colitis receiving tofacitinib-implications for cardiovascular risk and patient management. Inflamm Bowel Dis. (2021) 27:797–808. doi: 10.1093/ibd/izaa227
103. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. (2022) 386:316–26. doi: 10.1056/NEJMoa2109927
104. European Medicines Agency. (2022). Available at: https://www.ema.europa.eu/en/news/ema-confirms-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic (Accessed December 19, 2022).
105. Sandborn WJ, Lawendy N, Danese S, Su C, Loftus EV Jr, Hart A, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. (2022) 55:464–78. doi: 10.1111/apt.16712
106. Kochar BD, Cheng D, Cai T, Ananthakrishnan AN. Comparative risk of thrombotic and cardiovascular events with tofacitinib and anti-TNF agents in patients with inflammatory bowel diseases. Dig Dis Sci. (2022) 67:5206–12. doi: 10.1007/s10620-022-07404-z
107. Seo GH, Jung SH. The comparative risk of serious adverse events with tofacitinib and TNF inhibitors in patients with ulcerative colitis: the Korean experience as revealed by a national database. J Korean Med Sci. (2022) 37:e123. doi: 10.3346/jkms.2022.37.e123
108. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. (2020) 158:1554–73.e12. doi: 10.1053/j.gastro.2020.01.001
109. Wu H, Hu T, Hao H, Hill MA, Xu C, Liu Z. Inflammatory bowel disease and cardiovascular diseases: a concise review. Eur Hear J Open. (2021) 2:oeab029. doi: 10.1093/ehjopen/oeab029
110. Sinh P, Cross R. Cardiovascular risk assessment and impact of medications on cardiovascular disease in inflammatory bowel disease. Inflamm Bowel Dis. (2021) 27:1107–15. doi: 10.1093/ibd/izaa258
111. Peppas S, Piovani D, Peyrin-Biroulet L, Danese S, Bonovas S. Statins and inflammatory bowel disease: where do we stand? Eur J Intern Med. (2020) 75:10–4. doi: 10.1016/j.ejim.2020.02.017
112. Naito Y, Katada K, Takagi T, Tsuboi H, Isozaki Y, Handa O, et al. Rosuvastatin, a new HMG-CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. Int J Mol Med. (2006) 17:997–1004. doi: 10.3892/ijmm.17.6.997
113. Crockett SD, Hansen RA, Stürmer T, Schectman R, Darter J, Sandler RS, et al. Statins are associated with reduced use of steroids in inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. (2012) 18:1048–56. doi: 10.1002/ibd.21822
Keywords: inflammatory bowel disease, atherosclerotic cardiovascular disease, ischemic heart disease, ischemic stroke, IBD therapy
Citation: Gabbiadini R, Dal Buono A, Mastrorocco E, Solitano V, Repici A, Spinelli A, Condorelli G and Armuzzi A (2023) Atherosclerotic cardiovascular diseases in inflammatory bowel diseases: to the heart of the issue. Front. Cardiovasc. Med. 10:1143293. doi: 10.3389/fcvm.2023.1143293
Received: 12 January 2023; Accepted: 4 May 2023;
Published: 16 May 2023.
Edited by:
Bernhard Wernly, Paracelsus Medical University, AustriaReviewed by:
Yun Qiu, The First Affiliated Hospital of Sun Yat-sen University, ChinaTommaso Bucci, Sapienza University of Rome, Italy
© 2023 Gabbiadini, Dal Buono, Mastrorocco, Solitano, Repici, Spinelli, Condorelli and Armuzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Armuzzi YWxlc3NhbmRyby5hcm11enppQGh1bmltZWQuZXU=
 Roberto Gabbiadini
Roberto Gabbiadini Arianna Dal Buono1
Arianna Dal Buono1 Elisabetta Mastrorocco
Elisabetta Mastrorocco Virginia Solitano
Virginia Solitano Gianluigi Condorelli
Gianluigi Condorelli