Abstract
Introduction:
Among 28 cancer types, bladder cancer (BC) patients have the highest risk of dying from cardiovascular disease (CVD). We aimed to identify the independent risk factors and develop a novel nomogram for predicting long-term cardiovascular mortality in patients with BC.
Methods:
We extracted data from the Surveillance, Epidemiology, and End Results (SEER) database for patients diagnosed with bladder cancer (BC) between 2000 and 2017. The cumulative incidence function (CIF) was computed for both CVD-related death and other causes of death. Then we performed univariate and multivariate analyses to explore the independent risk factors and further develop a novel nomogram to predict cardiovascular mortality at 5- and 10-year for patients with BC by using the Fine-Gray competing risk model. The efficacy of the developed nomogram was assessed by the concordance index (C-index), receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA).
Results:
A total of 12,9765 patients were randomly divided into training (n = 90,835, 70%), and validation (n = 38,930, 30%) cohorts. During the follow-up period, 31,862 (46.4%) patients died from BC, and 36793 (53.6%) patients died from non-BC, of which CVD-related death accounted for 17,165 (46.7%), being the major cause of non-cancer deaths. The multivariate analysis showed that age, sex, race, marital status, histologic type, tumor grade, summary stage, and chemotherapy were independent risk factors of CVD-related death in BC patients. The nomogram based on the above eight factors showed good discrimination power, excellent consistency, and clinical practicability: (1) the areas under the curve of the ROC for 5- and 10-year CVD-related death of 0.725 and 0.732 in the training cohort and 0.726 and 0.734 in the validation cohort; (2) the calibration curves showed that the prediction probabilities were basically consistent with the observed probabilities; (3) the DCA curves revealed that the nomogram had high positive net benefits.
Discussion:
To our knowledge, this was the first study to identify the independent risk factors and develop a novel nomogram for predicting long-term cardiovascular mortality in patients with BC based on the competing risk model. Our results could help clinicians comprehensively and effectively manage the co-patient of BC and CVD, thereby reducing the risk of cardiovascular mortality in BC survivors.
1. Introduction
Bladder cancer (BC) is the most frequently diagnosed malignant tumor in the urinary system and ranks among the top ten most prevalent malignancies worldwide. As estimated by GLOBOCAN in 2020, this disease is projected to account for 573,278 new cases and 212,536 BC-related deaths, resulting in a significant disease burden, increased healthcare costs, and diminished health-related quality of life (1, 2). With the improvement of modern medical care, the life expectancy of cancer patients has increased. The current study reports a higher proportion of non-cancer deaths among all BC patients, with cardiovascular death being one of the primary causes of non-cancer deaths (3–8).
Cardiovascular disease (CVD) represents the leading cause of mortality worldwide (9, 10). More and more evidences indicate that tumors and CVD share several high-risk factors, such as obesity, hyperglycemia, inflammation, hormone replacement therapy, smoking, and lower socioeconomic status, requiring common target interventions to prevent the onset and progression of tumors and CVD (11–14). Many cancer therapies, including chest irradiation, chemotherapy, and immunotherapy, exhibit varying degrees of cardiovascular toxicity (15, 16). Therefore, the incidence of concomitant or secondary CVD in cancer survivors is increasing every year, leading to the formation of a new discipline: cardio-oncology.
A previous observational study of Surveillance, Epidemiology, and End Results (SEER) data reported that BC patients have the highest risk of dying from CVD among 28 cancer types, with 19.4% of BC patients had died from CVD (4). In addition, elderly patients with BC demonstrated an elevated risk of CVD-related death as compared to the general population (17). Approximately 5–10 years following diagnosis, CVD-related death superseded BC as the primary cause of death among older BC patients, particularly among subgroups of patients with localized and low-grade tumors (8).
In our study, we aimed to conduct a population-based analysis of a cohort of BC patients in the SEER database to identify the independent risk factors associated with CVD-related death, and subsequently, to build and validate a scoring system to predict the long-term cardiovascular mortality risk of BC patients. Cardio-oncology research studies often necessitate consideration of potential competing risks, wherein the incidence of other events (e.g., cancer-related death) may preclude the primary event of interest (e.g., cardiovascular outcome) (18). As such, our work is all based on the method of the competing risk model. Our results could help clinicians comprehensively and effectively manage the co-patient of BC and CVD and reduce the risk of cardiovascular mortality in BC survivors.
2. Materials and methods
2.1. Data source and patient selection
Data from the Surveillance, Epidemiology, and End Results (SEER) database (http://seer.cancer.gov/) were used in our study.The SEER program of the National Cancer Institute is a network of population-based incident tumour registries, covering 28% of the US population, including incidence, survival, and treatment (19). Given that our study solely utilized identified data from the SEER database and did not involve the direct participation of patients, institutional review board approval and informed consent were deemed unnecessary for this investigation. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were adhered to whilst conducting this research (20).
Patients diagnosed with BC from 2000 to 2017 from 17 population-based cancer registries were included in our study. The inclusion criteria for our study were as follows: (1) case selection based on site and morphology, with primary site labeled as “C67.0–9”; (2) availability of active follow-up information and defined causes of mortalities; (3) BC was the only one and primary cancer. Next, we collected the following informations of included patients: age, sex, race, marital status, primary site, histologic type, tumor grade, summary stage, surgery, radiotherapy, chemotherapy, survival months, and causes of death. Patients with incomplete data about any of the aforementioned variables were excluded.
2.2. Study outcomes
The primary outcome was death from CVD. As recorded in the SEER database, CVD-related death was defined by the following six causes: (1) disease of heart, (2) hypertension without heart disease, (3) cerebrovascular disease, (4) atherosclerosis, (5) aortic aneurysm and dissection, (6) other diseases of arteries, arterioles, and capillaries. Competing events against cardiovascular death in our study consisted of deaths from BC and other non-CVD related causes. Follow-up time was calculated from the initial diagnosis of BC until the patient was lost to follow-up, date of death, or final follow-up date.
2.3. Statistical analysis
The patients included in this study were randomly assigned to either training or validation cohort in a 7:3 ratio. Differences in baseline characteristics between the two groups were assessed using the chi-squared test. To assess the interaction between BC and CVD-related deaths, we calculated the cumulative incidence function (CIF) to determine the probabilities of each event at 5- and 10-year. Fine and Gray's subdistribution proportional hazards model was conducted on the training cohort to identify the independent risk factors of death from CVD and then construct a novel competing risk model to predict the probabilities of CVD-related death at 5- and 10-year for patients with BC. Then to measure the discriminative performance of the model, we calculated the bootstrap-corrected concordance index (C-index) and constructed the receiver operating characteristic (ROC) curves based on the area under the curve (AUC) values of the corresponding variables. The calibration curves and decision curve analysis (DCA) were performed to verify the consistency and investigate the clinical utilities and net benefits of this model.
All analyses were performed using SEER*Stat software (version 8.4.0.1, National Cancer Institute, Bethesda, MD, USA), Stata/MP software (version 16.0, Stata Corp, College Station, TX, USA), and R software (version 4.2.1; The R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value < 0.05 was considered statistically significant.
3. Results
3.1. Patients characteristics
In this study, a total of 129,765 eligible patients diagnosed with BC from the SEER database were randomly assigned to either training (n = 90,835, 70%) or validation (n = 38,930, 30%) cohort. Most of the patients were over 65 years old (67.5%), male (73.8%), white (89.7%), and married (62.6%). A larger proportion of patients were diagnosed with transitional cell carcinoma (95.8%), grade Ⅳ (32.7%), and in situ (47.3%). With regard to treatment, the majority of patients had undergone surgery (96.2%) and only a small number of patients had received radiotherapy (5.9%) or chemotherapy (21.1%). As for the causes of death, 31862 (46.4%) patients died due to BC, and 36793 (53.6%) patients died due to non-BC, of which CVD-related death accounted for 17165 (46.7%), being the major cause of non-cancer deaths.
No statistically significant differences in any of the analyzed variables, including age, sex, race, marital status, primary site, histologic type, tumor grade, summary stage, surgery, radiotherapy, chemotherapy, and causes of death, were observed between the training and validation cohorts (Table 1).
Table 1
| Variables | Training cohort | Validation cohort | Total | P value | |||
|---|---|---|---|---|---|---|---|
| 90,835 | 70% | 38930 | 30% | 1,29,765 | 100% | ||
| Age (years) | 0.924 | ||||||
| <64 | 29499 | 32.5% | 12686 | 32.6% | 42185 | 32.5% | |
| 65–74 | 25605 | 28.2% | 10919 | 28.0% | 36524 | 28.1% | |
| 75–84 | 24676 | 27.2% | 10555 | 27.1% | 35231 | 27.1% | |
| >85 | 11055 | 12.2% | 4770 | 12.3% | 15825 | 12.2% | |
| Sex | 0.377 | ||||||
| Male | 67,130 | 73.9% | 28,679 | 73.7% | 95,809 | 73.8% | |
| Female | 23,705 | 26.1% | 10,251 | 26.3% | 33,956 | 26.2% | |
| Race | 0.323 | ||||||
| White | 81,494 | 89.7% | 34,926 | 89.7% | 1,16,420 | 89.7% | |
| Black | 4,818 | 5.3% | 2,122 | 5.5% | 6,940 | 5.3% | |
| Others | 4,523 | 5.0% | 1,882 | 4.8% | 6,405 | 4.9% | |
| Marital status | 0.676 | ||||||
| Married | 56,786 | 62.5% | 24,385 | 62.6% | 81,171 | 62.6% | |
| Single/other | 34,049 | 37.5% | 14,545 | 37.4% | 48,594 | 37.4% | |
| Primary site | 0.868 | ||||||
| C67.0-Trigone of bladder | 6,048 | 6.7% | 2,522 | 6.5% | 8,570 | 6.6% | |
| C67.1-Dome of bladder | 3,264 | 3.6% | 1,416 | 3.6% | 4,680 | 3.6% | |
| C67.2-Lateral wall of bladder | 20,004 | 22.0% | 8,698 | 22.3% | 28,702 | 22.1% | |
| C67.3-Anterior wall of bladder | 2,033 | 2.2% | 883 | 2.3% | 2,916 | 2.2% | |
| C67.4-Posterior wall of bladder | 8,599 | 9.5% | 3,677 | 9.4% | 12,276 | 9.5% | |
| C67.5-Bladder neck | 2,543 | 2.8% | 1,122 | 2.9% | 3,665 | 2.8% | |
| C67.6-Ureteric orifice | 3,660 | 4.0% | 1,560 | 4.0% | 5,210 | 4.0% | |
| C67.7-Urachus | 153 | 0.2% | 72 | 0.2% | 225 | 0.2% | |
| C67.8-Overlapping lesion of bladder | 10,905 | 12.0% | 4,678 | 12.0% | 15,583 | 12.0% | |
| C67.9-Bladder, NOS | 33,636 | 37.0% | 14,302 | 36.7% | 47,938 | 36.9% | |
| Histologic type | 0.729 | ||||||
| Transitional cell carcinoma | 87,039 | 95.8% | 37,308 | 95.8% | 1,24,347 | 95.8% | |
| Squamous cell carcinoma | 1,686 | 1.9% | 705 | 1.8% | 2,391 | 1.8% | |
| Adenocarcinoma | 695 | 0.8% | 318 | 0.8% | 1,013 | 0.8% | |
| Other and unclassified carcinoma | 1,415 | 1.6% | 599 | 1.5% | 2,014 | 1.6% | |
| Tumor grade | 0.935 | ||||||
| I | 12,587 | 13.9% | 5,443 | 14.0% | 18,030 | 13.9% | |
| II | 28,506 | 31.4% | 12,183 | 31.1% | 40,699 | 31.4% | |
| III | 20,014 | 22.0% | 8,553 | 22.0% | 28,567 | 22.0% | |
| IV | 29,728 | 32.7% | 12,751 | 32.8% | 42,479 | 32.7% | |
| Summary stage | 0.278 | ||||||
| In situ | 42,890 | 47.2% | 18,424 | 47.3% | 61,314 | 47.3% | |
| Localized | 35,837 | 39.5% | 15,191 | 39.0% | 51,028 | 39.3% | |
| Regional | 7,679 | 8.5% | 3,345 | 8.6% | 11,024 | 8.5% | |
| Distant | 4,429 | 4.9% | 1,970 | 5.1% | 6,399 | 4.9% | |
| Surgery | 0.257 | ||||||
| No | 3,395 | 3.7% | 1,506 | 3.9% | 4,901 | 3.8% | |
| Yes | 87,440 | 96.3% | 37,424 | 96.1% | 1,24,864 | 96.2% | |
| Radiotherapy | 0.068 | ||||||
| No | 85,526 | 94.2% | 36,553 | 93.9% | 1,22,079 | 94.1% | |
| Yes | 5,309 | 5.8% | 2,377 | 6.1% | 7,686 | 5.9% | |
| Chemotherapy | 0.638 | ||||||
| No | 71,657 | 78.9% | 30,756 | 79.0% | 1,02,413 | 78.9% | |
| Yes | 19,178 | 21.1% | 8,174 | 21.0% | 27,352 | 21.1% | |
| Causes of death | 0.948 | ||||||
| Death from BC | 22,298 | 24.5% | 9,564 | 24.6% | 31,862 | 24.6% | |
| Death from CVD | 11,997 | 13.2% | 5,168 | 13.3% | 17,165 | 13.2% | |
| Death from other causes | 13,772 | 15.2% | 5,856 | 15.0% | 19,628 | 15.1% | |
| Total death | 48,067 | 52.9% | 20,588 | 52.9% | 68,655 | 52.9% | |
The Baseline demographic and clinicopathological characteristics of the included BC patients.
3.2. Cumulative incidence function survival analysis
The results of cumulative incidence analysis showed that the 5-, and 10-year CIF of death due to BC were 21.3 and 24.8%, the 5-, and 10-year CIF of death due to CVD were 7.8 and 13.5%, and the 5-, and 10-year CIF of death due to non-CVD were 8.3 and 15.1%, respectively (Table 2). In the subgroup analysis stratified by characteristics of CVD mortality, only the difference in surgical status was not significant (P = 0.08), while other characteristics showed significant intergroup differences (P < 0.001). The CIF of death due to BC gradually slowed down with survival time, while the CIF of death due to CVD steadily increased. Among non-BC causes of death, cardiovascular deaths are almost comparable to those from all other causes (Figure 1). In a subsequent subgroup analysis stratified by patients’ characteristics, we observed that a high CIF of death due to CVD occurred predominantly in patients aged ≥65 years, who were white, unmarried, and had grade I-II of the tumor, in situ or localized summary stage, and who were not receiving radiotherapy or chemotherapy (Figure 2).
Table 2
| Characteristics | Death from CVD (%) | P | Death from BC (%) | P | Death from non-CVD (%) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| 5-years | 10-years | 5-years | 10-years | 5-years | 10-years | ||||
| Total | 7.8 | 13.5 | 21.3 | 24.8 | 8.3 | 15.1 | |||
| Age (years) | <0.001 | <0.001 | <0.001 | ||||||
| <65 | 2.0 | 3.9 | 15.9 | 18.2 | 3.1 | 5.8 | |||
| 65–74 | 5.6 | 11.0 | 18.5 | 22.2 | 7.0 | 14.1 | |||
| 75–84 | 11.7 | 20.8 | 24.8 | 29.3 | 12.0 | 22.9 | |||
| ≥85 | 20.4 | 29.4 | 35.4 | 38.7 | 17.5 | 25.4 | |||
| Sex | <0.001 | <0.001 | <0.001 | ||||||
| Male | 8.3 | 14.3 | 19.8 | 23.3 | 8.6 | 15.5 | |||
| Female | 6.4 | 11.3 | 25.8 | 28.8 | 7.5 | 13.9 | |||
| Race | <0.001 | <0.001 | <0.001 | ||||||
| White | 8.0 | 13.8 | 20.7 | 24.1 | 8.4 | 15.3 | |||
| Black | 6.9 | 11.0 | 33.3 | 37.1 | 7.7 | 12.7 | |||
| Others | 5.6 | 10.1 | 20.7 | 23.9 | 7.2 | 13.0 | |||
| Marital status | <0.001 | <0.001 | <0.001 | ||||||
| Married | 7.0 | 12.4 | 17.9 | 21.3 | 7.2 | 13.7 | |||
| Single/other | 9.4 | 15.3 | 27.1 | 30.6 | 10.3 | 17.4 | |||
| Primary site | <0.001 | <0.001 | <0.001 | ||||||
| C67.0-Trigone of bladder | 7.5 | 13.7 | 20.8 | 23.8 | 7.7 | 14.0 | |||
| C67.1-Dome of bladder | 8.4 | 14.2 | 27.1 | 30.7 | 8.5 | 15.6 | |||
| C67.2-Lateral wall of bladder | 7.4 | 13.7 | 14.9 | 17.8 | 8.3 | 15.0 | |||
| C67.3-Anterior wall of bladder | 9.0 | 15.4 | 26.2 | 29.5 | 9.6 | 16.1 | |||
| C67.4-Posterior wall of bladder | 8.6 | 15.1 | 16.4 | 19.6 | 8.7 | 16.4 | |||
| C67.5-Bladder neck | 8.8 | 14.6 | 24.3 | 27.2 | 8.1 | 14.1 | |||
| C67.6-Ureteric orifice | 6.9 | 13.0 | 10.3 | 13.1 | 7.3 | 14.4 | |||
| C67.7-Urachus | 2.4 | 3.2 | 37.3 | 45.5 | 4.1 | 6.4 | |||
| C67.8-Overlapping lesion of bladder | 8.2 | 12.9 | 32.5 | 36.3 | 8.7 | 14.8 | |||
| C67.9-Bladder, NOS | 7.8 | 12.9 | 23.0 | 26.9 | 8.3 | 15.2 | |||
| Histologic type | <0.001 | <0.001 | <0.001 | ||||||
| Transitional cell carcinoma | 8.0 | 13.7 | 19.9 | 23.4 | 8.4 | 15.3 | |||
| Squamous cell carcinoma | 5.6 | 8.7 | 51.6 | 53.5 | 8.0 | 12.2 | |||
| Adenocarcinoma | 5.6 | 7.8 | 52.1 | 57.1 | 7.0 | 8.3 | |||
| Other and Unclassified carcinoma | 5.1 | 7.2 | 60.1 | 63.4 | 6.6 | 9.8 | |||
| Tumor grade | <0.001 | <0.001 | <0.001 | ||||||
| I | 7.4 | 14.2 | 4.3 | 6.7 | 8.1 | 16.4 | |||
| II | 7.8 | 14.6 | 6.8 | 9.9 | 8.1 | 16.1 | |||
| III | 8.4 | 13.3 | 34.9 | 38.8 | 8.8 | 14.5 | |||
| IV | 7.7 | 12.2 | 33.9 | 38.2 | 8.3 | 13.9 | |||
| Summary stage | <0.001 | <0.001 | <0.001 | ||||||
| In situ | 7.8 | 14.9 | 3.6 | 6.1 | 8.2 | 16.6 | |||
| Localized | 8.9 | 14.2 | 26.8 | 31.6 | 9.4 | 15.7 | |||
| Regional | 5.4 | 7.6 | 60.1 | 63.9 | 6.5 | 9.2 | |||
| Distant | 3.1 | 3.4 | 87.4 | 88.5 | 4.3 | 4.9 | |||
| Surgery | 0.08 | <0.001 | 0.03 | ||||||
| No | 8.3 | 12.7 | 34.7 | 36.8 | 8.4 | 13.6 | |||
| Yes | 7.8 | 13.5 | 20.8 | 24.3 | 8.3 | 15.2 | |||
| Radiotherapy | <0.001 | <0.001 | <0.001 | ||||||
| No | 7.8 | 13.6 | 18.7 | 22.1 | 8.3 | 15.3 | |||
| Yes | 8.8 | 11.5 | 63.0 | 66.9 | 8.8 | 11.9 | |||
| Chemotherapy | <0.001 | <0.001 | <0.001 | ||||||
| No | 8.5 | 14.4 | 17.9 | 21.2 | 8.8 | 15.9 | |||
| Yes | 5.4 | 9.8 | 34.2 | 38.6 | 6.4 | 11.7 | |||
Cumulative incidence of cause-specific death and Gray's test in the whole cohort.
Figure 1
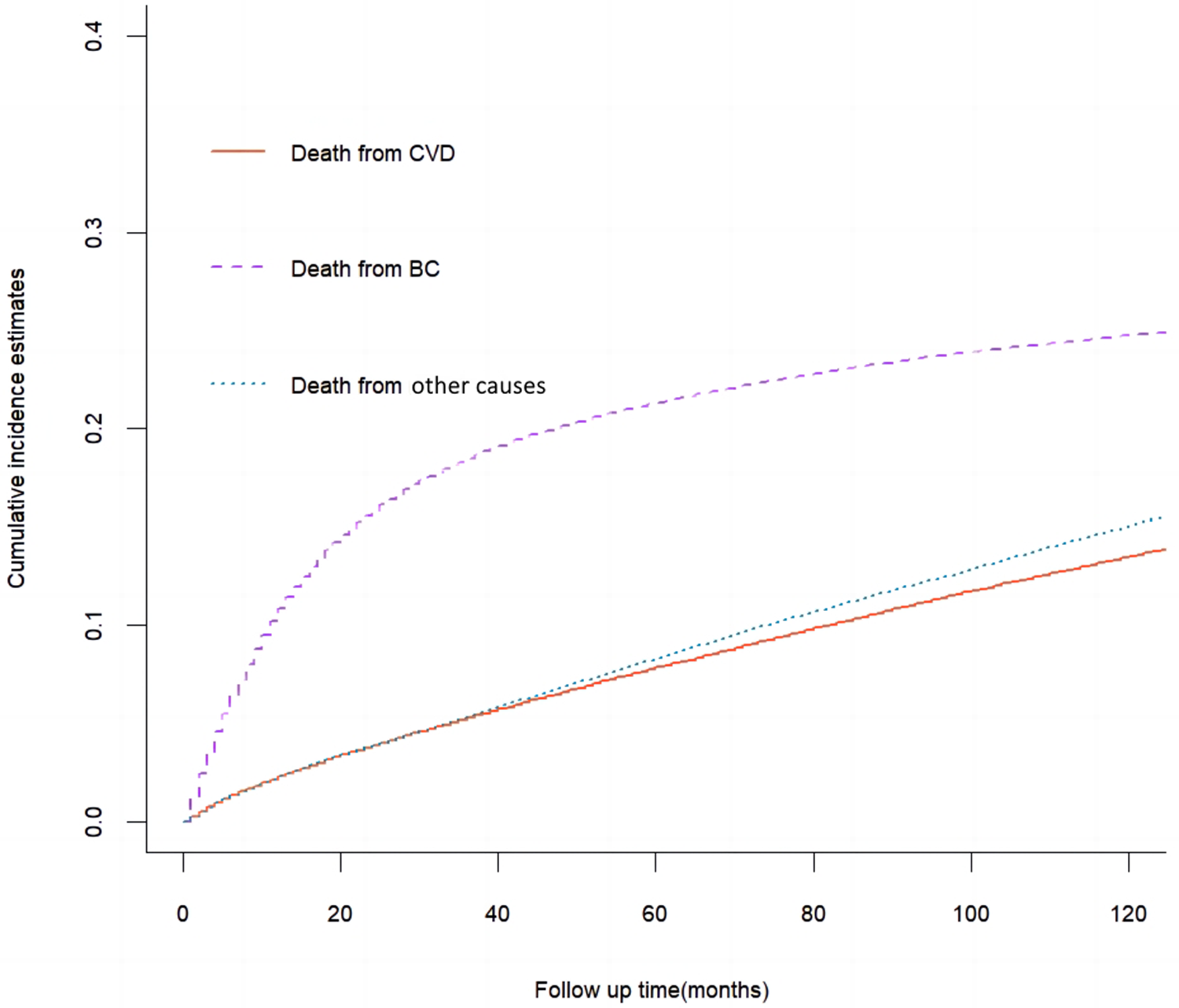
Cumulative incidence estimates of death causes among patients with bladder cancer (BC) in the whole cohort.
Figure 2
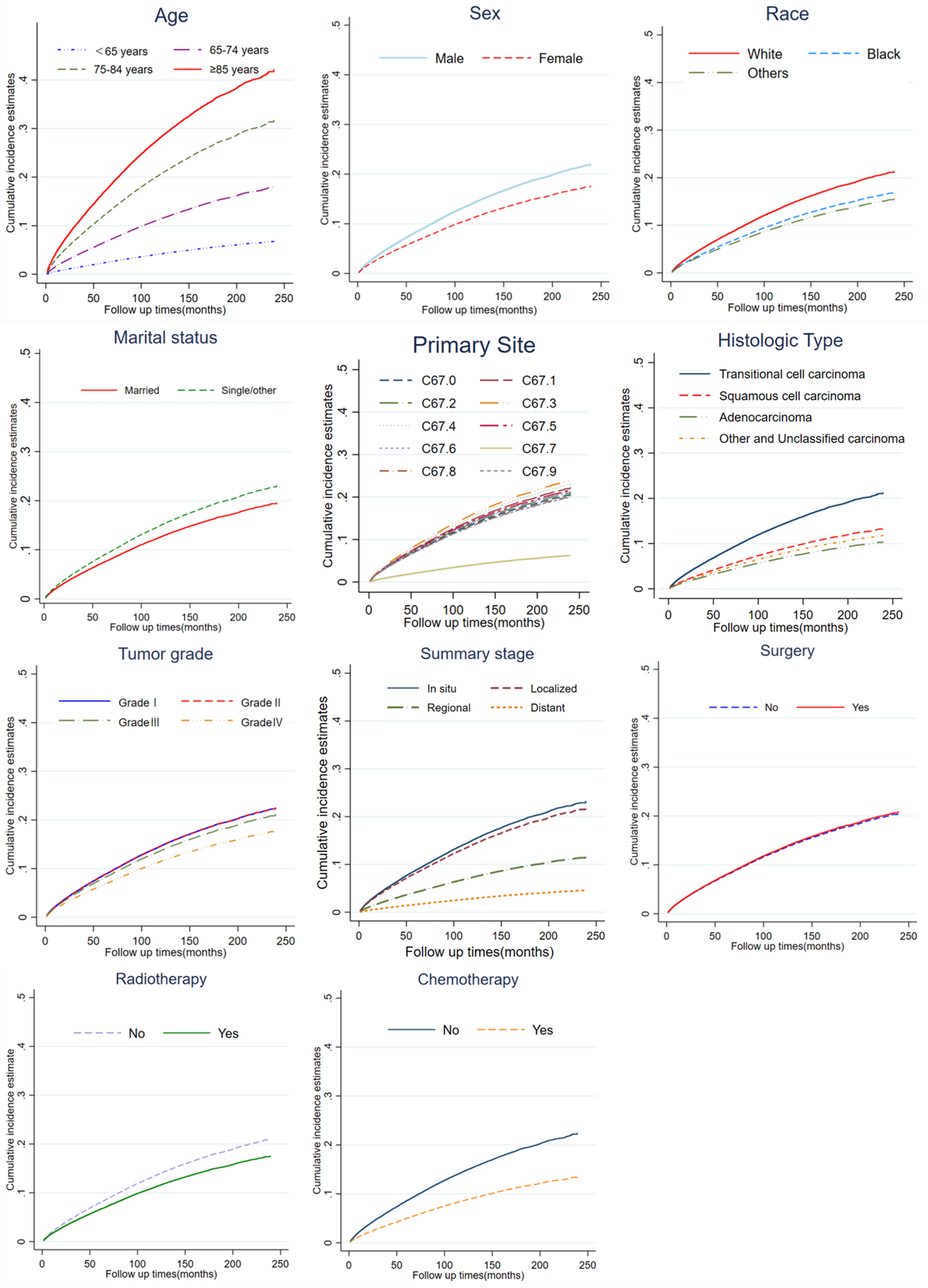
Cumulative incidence estimates of CVD-related death among patients with BC according to age, sex, race, marital status, primary site, histologic type, tumor grade, summary stage, surgery, radiotherapy and chemotherapy.
3.3. Independent risk factors of death from CVD
According to the results of univariate analysis based on the Fine-Gray hazard model in the training cohort, age, sex, race, marital status, primary site, histologic type, tumor grade, summary stage, radiotherapy, and chemotherapy were significantly associated with CVD-related death (p < 0.05). Then, variables with a p-value < 0.05 during univariate analysis were selected for multivariate competing risk analysis to minimize the risks of producing false positive results. The analysis revealed that the following eight factors were independently associated with an elevated risk of cardiovascular death: age, sex, race, marital status, histologic type, tumor grade, summary stage, and chemotherapy (Table 3).
Table 3
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | ||||
| <65 | Reference | Reference | ||
| 65–74 | 2.82 (2.64–3.02) | <0.001 | 2.89 (2.70–3.10) | <0.001 |
| 75–84 | 5.40 (5.07–5.74) | <0.001 | 5.55 (5.22–5.91) | <0.001 |
| ≥85 | 7.73 (7.23–8.27) | <0.001 | 8.00 (7.45–8.55) | <0.001 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.79 (0.76–0.83) | <0.001 | 0.68 (0.65–0.71) | <0.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.79 (0.71–0.85) | <0.001 | 0.97 (0.88–1.06) | 0.527 |
| Others | 0.76 (0.69–0.84) | <0.001 | 0.78 (0.71–0.86) | <0.001 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Single/other | 1.25 (1.20–1.29) | <0.001 | 1.22 (1.17–1.27) | <0.001 |
| Primary site | ||||
| C67.0-Trigone of bladder | Reference | Reference | ||
| C67.1-Dome of bladder | 1.08 (0.96,1.22) | 0.185 | 1.02 (0.90–1.14) | 0.789 |
| C67.2-Lateral wall of bladder | 1.07 (0.98,1.15) | 0.123 | 1.04 (0.96–1.12) | 0.392 |
| C67.3-Anterior wall of bladder | 1.17 (1.02,1.34) | 0.021 | 1.09 (0.95–1.25) | 0.213 |
| C67.4-Posterior wall of bladder | 1.14 (1.04,1.25) | 0.005 | 1.05 (0.96–1.15) | 0.318 |
| C67.5-Bladder neck | 1.08 (0.95,1.23) | 0.237 | 1.03 (0.90–1.17) | 0.695 |
| C67.6-Ureteric orifice | 1.03 (0.92,1.15) | 0.613 | 1.03 (0.92–1.15) | 0.656 |
| C67.7-Urachus | 0.21 (0.10,0.56) | 0.002 | 0.73 (0.27–1.94) | 0.524 |
| C67.8-Overlapping lesion of bladder | 1.00 (0.91,1.09) | 0.996 | 1.01 (0.92–1.10) | 0.891 |
| C67.9-Bladder, NOS | 0.99 (0.91,1.07) | 0.757 | 0.96 (0.89–1.04) | 0.311 |
| Histologic type | ||||
| Transitional cell carcinoma | Reference | Reference | ||
| Squamous cell carcinoma | 0.60 (0.51,0.71) | <0.001 | 0.73 (0.61–0.86) | <0.001 |
| Adenocarcinoma | 0.46 (0.34,0.62) | <0.001 | 0.71 (0.52–0.96) | 0.028 |
| Other and unclassified carcinoma | 0.53 (0.43,0.65) | <0.001 | 0.82 (0.67–1.01) | 0.064 |
| Tumor grade | ||||
| I | Reference | Reference | ||
| II | 1.02 (0.97,1.08) | 0.459 | 0.99 (0.94–1.04) | 0.705 |
| III | 0.95 (0.89,1.00) | 0.071 | 0.92 (0.86–0.98) | 0.009 |
| IV | 0.79 (0.75,0.84) | <0.001 | 0.77 (0.72–0.82) | <0.001 |
| Summary stage | ||||
| In situ | Reference | Reference | ||
| Localized | 0.95 (0.91,0.98) | 0.004 | 0.90 (0.86–0.94) | <0.001 |
| Regional | 0.49 (0.45,0.53) | <0.001 | 0.60 (0.55–0.66) | <0.001 |
| Distant | 0.22 (0.19,0.27) | <0.001 | 0.29 (0.24–0.35) | <0.001 |
| Surgery | ||||
| No | Reference | |||
| Yes | 1.07 (0.97,1.19) | 0.183 | ||
| Radiotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.82 (0.76,0.90) | <0.001 | 0.96 (0.87–1.05) | 0.344 |
| Chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.57 (0.54,0.60) | <0.001 | 0.77 (0.73–0.82) | <0.001 |
Univariate and multivariable competing risk analysis for CVD-related death among patients with BC in the training cohort.
3.4. Construction of the competing risk model
Utilizing the eight identified factors, a nomogram was established for predicting long-term CVD-related death in BC patients based on the Fine-Gray competing risk model. As illustrated in Figure 3, the corresponding point value of each independent risk factor in the nomogram was obtained by drawing a straight line to the top point row and then summed to obtain the total point. By drawing vertical lines from the total point row to the bottom timeline, it is possible to estimate the likelihood of cardiovascular mortality in patients with BC based on their individual characteristics.
Figure 3
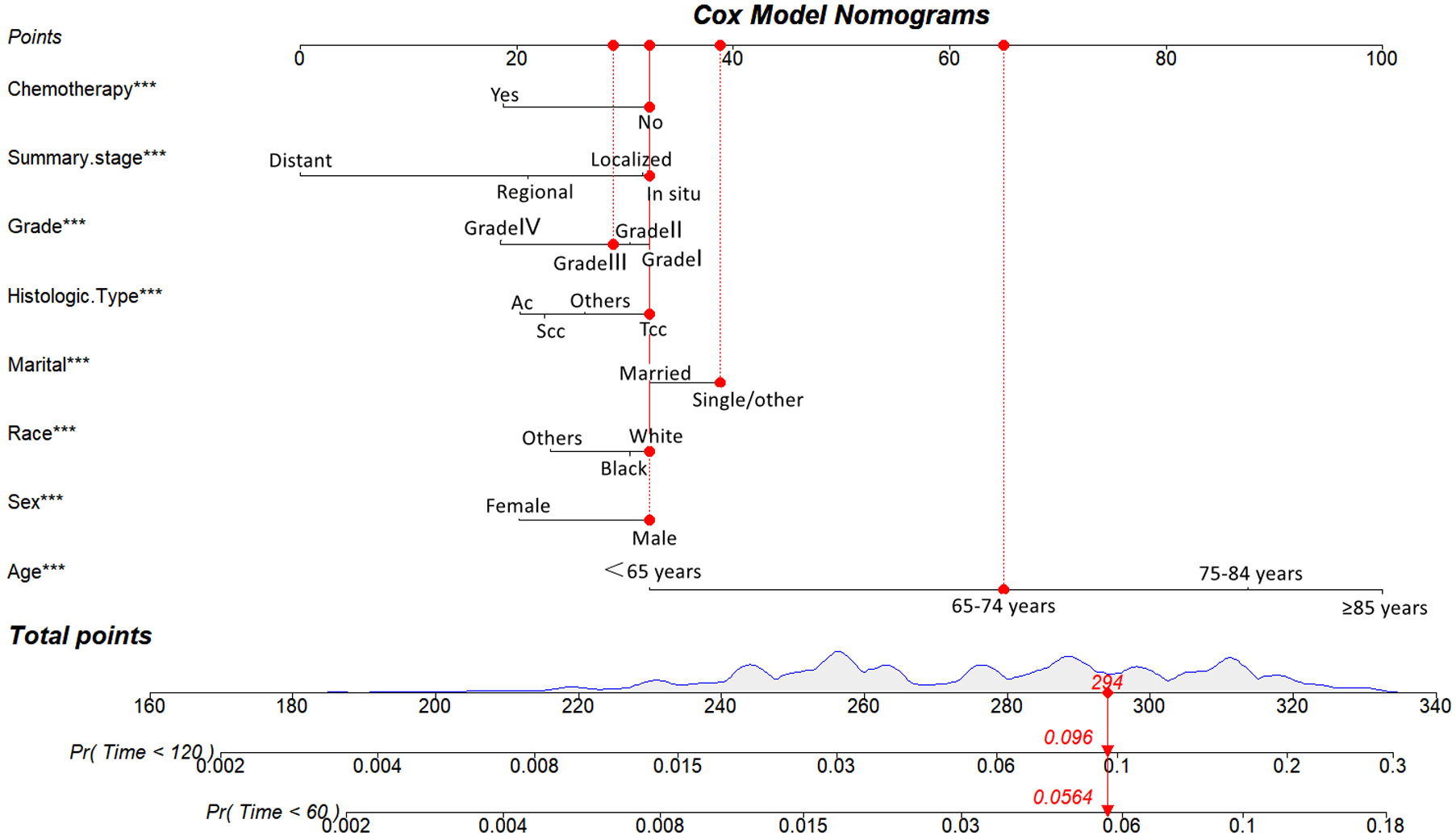
Competing risk model for predicting the 5-, and 10-year probabilities of CVD-related death among patients with BC. For example, for an 68-year-old married white race male patient with grade III, Tcc of histologic type, in situ of summary stage of BC that did not undergo chemotherapy, the total score is 65(68 years old) + 32(male) + 32 (white race) + 39 (single/other) + 32(Tcc) + 30(Grade III) + 32 (in situ) + 32(no chemothaoy)= 294, and the corresponding risk of CVD-related death at 5- and 10-year are 0.0564 and 0.096.
3.5. Validation of the competing risk model
To assess the discriminative performance of the model, we calculated the bootstrap-corrected C-index, which was 0.716 and 0.709 in the training cohort and validation cohort, respectively. While the AUCs for the 5-year probabilities of CVD in the training and validation cohorts were 0.725 and 0.732, respectively. Consistently, the AUCs for the 10-year probabilities of CVD in the training and validation cohorts were 0.726 and 0.734, respectively (Figure 4). These results showed that our model had a great discrimination ability in predicting CVD-related death in BC patients. Furthermore, we utilized the calibration curves to evaluate the accuracy of the nomogram and observed excellent consistency between the predicted probability of CVD-related death at 5- and 10-years and the actual observations in both the training and validation cohorts (Figure 5). Then, the DCA curves also revealed that the nomogram had high positive net benefits, indicating greater clinical application value and prospects in predicting CVD-related death in BC patients (Figure 6).
Figure 4
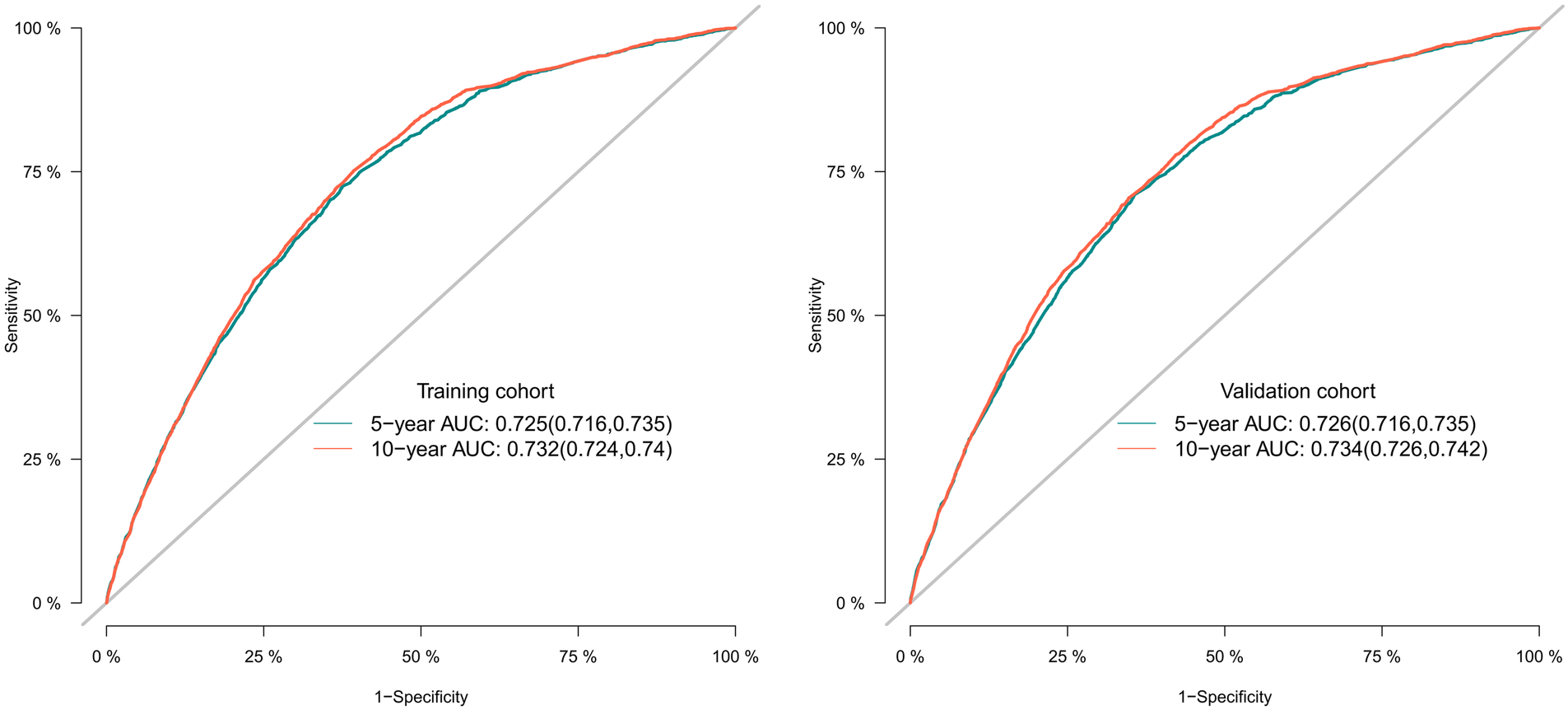
The receiver operating characteristic curves for predicting the 5-, and 10-year probabilities of CVD-related death in the training and the validation cohort, respectively.
Figure 5
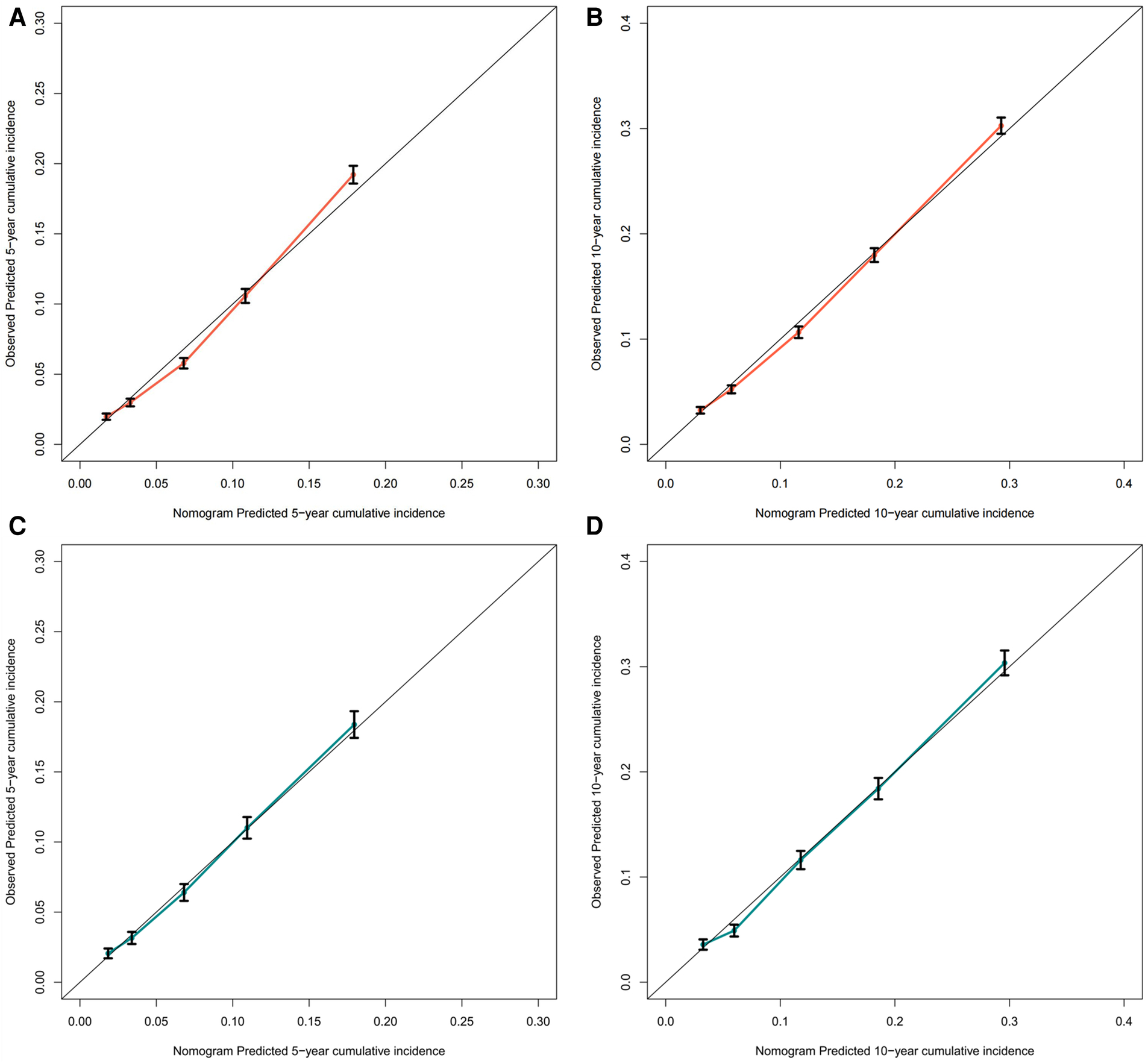
The calibration curve for predicting the 5-, and 10-year probabilities of CVD-related death in the training (A,B) and the validation cohort (C,D), respectively.
Figure 6
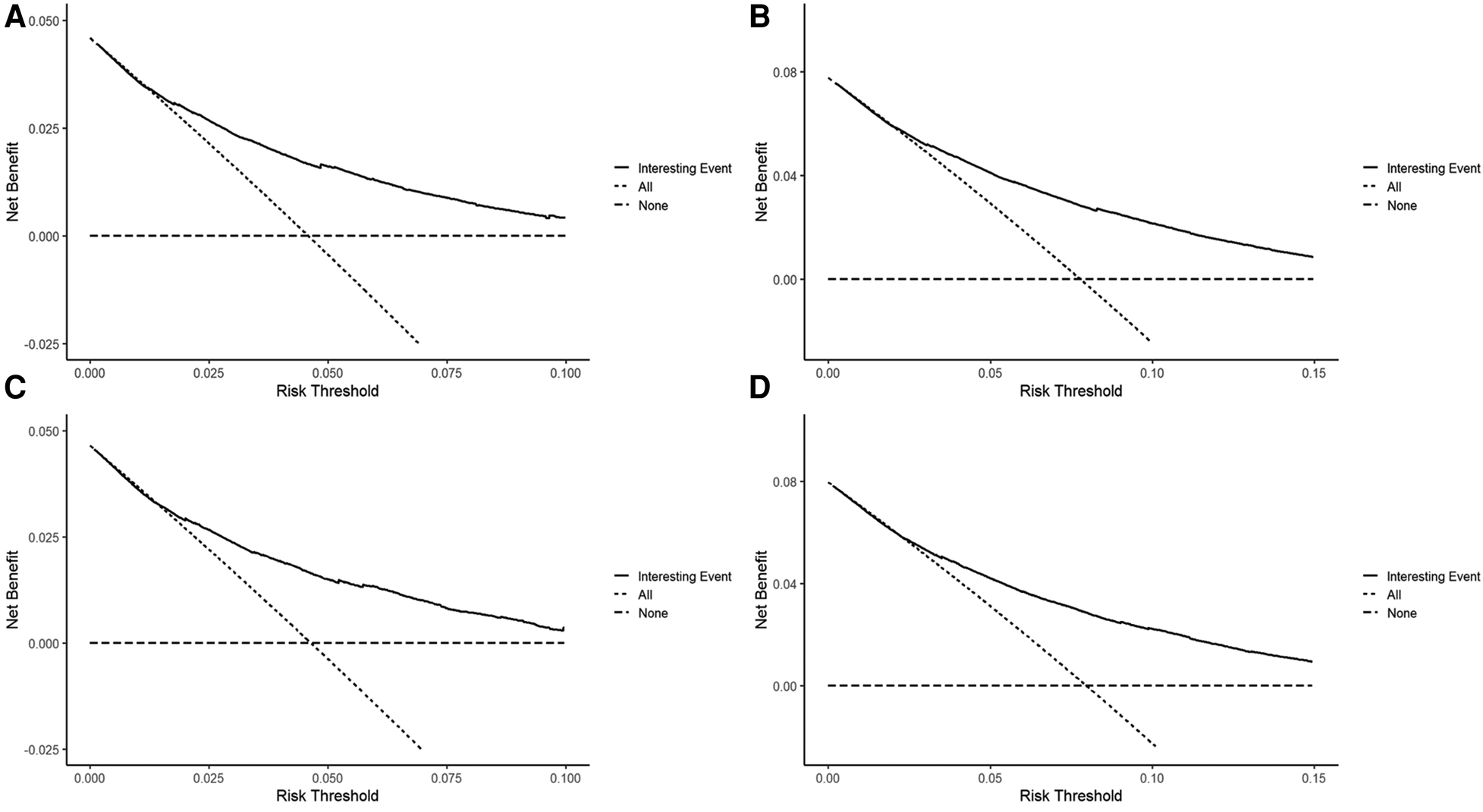
The decision curves for predicting the 5-, and 10-year probabilities of CVD-related death in the training (A,B) and the validation cohort (C,D), respectively.
4. Discussion
In this large-scale, population-based study, we conducted a comprehensive assessment of cardiovascular mortality risk among 129765 patients with BC based on the SEER database. Overall 68,655 BC patients died during the follow-up period, and more than half of them (53.6%) died from non-tumor causes, with CVD-related death accounting for 46.7%. Furthermore, we identified age, sex, race, marital status, histologic type, tumor grade, summary stage, and chemotherapy as independent risk factors that were used to establish and validate a nomogram for predicting the 5-, and 10-year probabilities of CVD-related death in BC patients. To the best of our knowledge, this was the first study to develop a novel nomogram using the Fine-Gray competing risk model for predicting long-term cardiovascular mortality risk in BC patients.
The risk of cancer and CVD advance with age (21). Indeed, a prior investigation has demonstrated that BC patients ≥65 years may face a greater risk of CVD-related mortality compared to mortality attributed directly to BC (8). In addition, it is widely acknowledged that older patients generally have a worse prognosis, which is often attributed to a range of factors including reduced physical function, cognitive impairment, and comorbidities such as hypertension, hyperlipidemia, and diabetes. These factors can also contribute to an increased risk of CVD-related mortality in this patient population. Furthermore, we demonstrated that marital status can also potentially influence CVD-related death among BC survivors. Marriage offers a direct form of social support and greater financial resources, which can help to reduce the likelihood of engaging in unhealthy behaviors such as poor diet or alcohol use, and increase the probability of receiving timely screening facilities and medical care (22–24). Conversely, unmarried individuals may have less access to emotional, financial, and companionship support, and may even experience more sub-clinical symptoms of depression, anxiety, and major mental disorders, all of which can contribute to an increased risk of cardiovascular mortality when compared to their married counterparts (23, 25–27).
Recent efforts have identified sex and racial disparities in BC (28, 29). It is also worth mentioning that BC is one of the top nonreproductive cancers exhibiting stark male and female differences: men are at a 3–5 times greater risk of developing BC, while females are more likely to be diagnosed with advanced-stage disease (30). Our results also show that the 5-, and 10-year CIF of BC were 19.8 and 23.3% in males, 25.8 and 28.8% in females. While, the 5-, and 10-year CIF of CVD were 8.3 and 14.3% in males, and 6.4 and 11.3% in females, indicating that female patients may be more likely to die from BC and male patients may be more likely to die from CVD. The observed sex disparities in mortality rates may be partially explained by unequal smoking patterns between males and females (31–33). Interestingly, this difference is also observed across ethnic groups. Despite BC incidence rates being two times higher in White people than in Black people, the latter experience a higher tumor stage at presentation and worse cancer-specific survival (34, 35). Possible contributing factors may include delayed diagnosis, surgical treatment at low-volume hospitals, and poor adherence to evidence-based care among Black people (36, 37).
Another independent risk factor of CVD-related death in BC patients is transitional cell carcinoma (TCC) of histologic type (17). TCC is the most prevalent histological subtype of BC and approximately 80% are non-muscle invasive at the time of diagnosis (38). The other histological subtype of BC, such as squamous cell carcinoma (SCC) and adenocarcinoma (AC), are much less common, all of which often present at an advanced stage and have shown a poor prognosis (39). A previous study showed that risk factors for SCC include schistosomiasis, chronic inflammation, recurrent urinary tract infections, and prior exposure to cyclophosphamide chemotherapy (40). While environmental exposures such as cigarette smoke and occupational exposures are the most well-established risk factors for TCC. Thus smoking has been shown to further contribute to the development of CVD in patients with TCC, through increased heart rate and myocardial contraction, inflammation, endothelial damage, thrombosis, and decreased serum HDL cholesterol levels (41–44).
Moreover, we observed that the CVD-related death of BC patients with Grade I/II and in situ or localized stage was higher than those with Grade III/IV and regional or distant stage. On the one hand, low-grade tumors have a low progression rate and can be initially treated with endoscopic approaches and surveillance but are generally not life-threatening to the patient. On the other hand, high-grade tumors have a high malignant potential and are associated with significant rates of progression and cancer-related mortality (45). The 5-, and 10-year CIF of BC were 3.6 and 6.1% in the stage of in situ, 87.4 and 88.5% in the stage of distant, providing compelling evidence for the critical need to improve early diagnosis rates of BC.
In terms of treatment, chemotherapy is widely used for BC which included systemic chemotherapy, neoadjuvant chemotherapy, and local perfusion chemotherapy. The current first-line chemotherapy drugs recommended by multiple treatment guidelines, such as cisplatin, anthracycline, mitomycin, and epirubicin magnitude, all come with varying degrees of cardiovascular toxicity, which significantly increases the risk of cardiovascular mortality (46). However, our study yielded a seemingly contradictory finding that the BC patients with chemotherapy were at a lower risk of CVD compared with those without chemotherapy. Notably, this result is consistent with previous studies conducted on other tumor types, such as colorectal cancer (47) and primary central nervous system lymphoma (48). The reasons for this association could be related to the following factors. Firstly, there were several instances of missing records for treatment data in the SEER database, including the information on patient's chemotherapy regimen and duration, as well as the presence of co-existing CVD at the time of diagnosis. Individuals with baseline CVD have contraindications to chemotherapy, and subjects with better cardiovascular status are more likely to receive chemotherapy (49). Therefore, a higher risk of cardiovascular death was observed in those not receiving chemotherapy. Secondly, chemotherapy is generally used for muscle-invasive BC with a relatively high stage, so limited life expectancy and a greater likelihood of death from BC. The underlying mechanism remains unclear and further investigation is needed. Therefore, these results should be interpreted with caution. However, it is noteworthy that some potential new strategies, such as gliflozins, have been shown to possess anti-inflammatory and cardioprotective effects against anthracycline-induced cardiotoxicity, thus may offering a new treatment option for chemotherapy patients (50).
The strengths of our study were that this was the first study to develop a novel nomogram for predicting long-term cardiovascular mortality risk in patients with BC based on the competing risk model. Furthermore, our study benefited from a large enough sample size and long-term follow-up based on the SEER database, which covers a significant proportion of the US population (approximately one-third). Then, given the potential presence of competing events in the analysis of CVD through the Cox regression model, we utilized a competing risk model to investigate risk factors and develop a novel nomogram. Moreover, our nomogram showed good discrimination power, excellent consistency, and clinical practicability.
There are still some limitations in our study. First of all, even though our analysis is computer-based, the underlying data is sourced from the SEER database which records real-world patients from high-quality cancer registries and relies on systematic, standardized, and regular data collection procedures for quality assurance and the avoidance of surveillance bias (51). Second, given that our study is a retrospective cohort study based on the SEER database, selection bias is inevitable. Third, the SEER database did not provide detailed information on cardiovascular comorbidities or other relevant cardiovascular-related factors, such as blood pressure, blood glucose, smoking, alcohol consumption, and so on. This limitation precluded a more comprehensive investigation into their potential impact on the risk of CVD mortality. Fourth, data on specific regimens and duration of chemotherapy or immunotherapy were not recorded in the SEER database, despite these factors being closely linked to the development of cardiotoxicity. Lastly, it is important to acknowledge that our competing risk model was developed using risk factors that were solely recorded in the SEER database and did not account for other potentially relevant risk factors that were not recorded. Therefore, while the model demonstrated promising results, external validation is still necessary to further confirm its utility and reliability in clinical practice.
5. Conclusion
Given that BC patients have the highest risk of dying from CVD among 28 cancer types, it is imperative to enhance the management of CVD in this patient population. Our study has identified several independent risk factors and built a novel nomogram using a competing risk model to accurately predict the probabilities of long-term cardiovascular mortality in BC patients. With the help of this well-established nomogram, urologists and cardiologists could work together to comprehensively assess the potential risks of treatment, providing early monitoring and prevention of heart damage, thereby minimizing factors that are harmful to a patient's cardiovascular prognosis.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ZZ conceived and supervised the study. JL performed the literature review, extracted the data, analyzed the pooled data, and carried out the first draft of the manuscript. ZZ revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to all patients and institutions involved in this study and the National Cancer Institute's SEER database (http://seer.cancer.gov/) that provide cancer research data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660
2.
Lobo N Afferi L Moschini M Mostafid H Porten S Psutka SP et al Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol. (2022) 5(6):628–39. 10.1016/j.euo.2022.10.003
3.
Zaorsky NG Churilla TM Egleston BL Fisher SG Ridge JA Horwitz EM et al Causes of death among cancer patients. Ann Oncol. (2017) 28:400–7. 10.1093/annonc/mdw604
4.
Sturgeon KM Deng L Bluethmann SM Zhou S Trifiletti DM Jiang C et al A population-based study of cardiovascular disease mortality risk in us cancer patients. Eur Heart J. (2019) 40:3889–97. 10.1093/eurheartj/ehz766
5.
Stoltzfus KC Zhang Y Sturgeon K Sinoway LI Trifiletti DM Chinchilli VM et al Fatal heart disease among cancer patients. Nat Commun. (2020) 11:2011. 10.1038/s41467-020-15639-5
6.
Kong J Diao X Diao F Fan X Zheng J Yan D et al Causes of death in long-term bladder cancer survivors: a population-based study. Asia Pac J Clin Oncol. (2019) 15:e167–74. 10.1111/ajco.13156
7.
Zhai M Tang C Li M Chen X Jin Y Ying X et al Short-term mortality risks among patients with non-metastatic bladder cancer. Bmc Cancer. (2020) 20:1148. 10.1186/s12885-020-07655-x
8.
Guan T Su M Luo Z Peng W Zhou R Lu Z et al Long-term cardiovascular mortality among 80,042 older patients with bladder cancer. Cancers (Basel). (2022) 14(19):4572. 10.3390/cancers14194572
9.
Roth GA Forouzanfar MH Moran AE Barber R Nguyen G Feigin VL et al Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. (2015) 372:1333–41. 10.1056/NEJMoa1406656
10.
Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet (London, England). (2018) 392:1736–88. 10.1016/S0140-6736(18)32203-7
11.
Cardinale D Colombo A Lamantia G Colombo N Civelli M De Giacomi G et al Cardio-oncology: a new medical issue. Ecancermedicalscience. (2008) 2:126. 10.3332/ecancer.2008.126
12.
Mehta LS Watson KE Barac A Beckie TM Bittner V Cruz-Flores S et al Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American heart association. Circulation. (2018) 137:e30–66. 10.1161/CIR.0000000000000556
13.
Barone B Finati M Cinelli F Fanelli A Del GF De Berardinis E et al Bladder cancer and risk factors: data from a multi-institutional long-term analysis on cardiovascular disease and cancer incidence. J Pers Med. (2023) 13(3):512. 10.3390/jpm13030512
14.
Quagliariello V De Laurentiis M Cocco S Rea G Bonelli A Caronna A et al Nlrp3 as putative marker of ipilimumab-induced cardiotoxicity in the presence of hyperglycemia in estrogen-responsive and triple-negative breast cancer cells. Int J Mol Sci. (2020) 21(20):7802. 10.3390/ijms21207802
15.
Chhabra N Kennedy J . A review of cancer immunotherapy toxicity: immune checkpoint inhibitors. J Med Toxicol. (2021) 17:411–24. 10.1007/s13181-021-00833-8
16.
Cadeddu Dessalvi C Deidda M Noto A Madeddu C Cugusi L Santoro C et al Antioxidant approach as a cardioprotective strategy in chemotherapy-induced cardiotoxicity. Antioxid Redox Signal. (2021) 34:572–88. 10.1089/ars.2020.8055
17.
Wang S Ge C Zhang J . Cardiovascular mortality risk in patients with bladder cancer: a population-based study. J Cardiovasc Dev Dis. (2022) 9(8):255. 10.3390/jcdd9080255
18.
Li Y Sun L Burstein DS Getz KD . Jacc: cardiooncologyconsiderations of competing risks analysis in cardio-oncology studies: state-of-the-art review. JACC Cardiooncol. (2022) 4:287–301. 10.1016/j.jaccao.2022.08.002
19.
Warren JL Klabunde CN Schrag D Bach PB Riley GF . Overview of the seer-medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. (2002) 40:3–18. 10.1097/01.MLR.0000020942.47004.03
20.
Ghaferi AA Schwartz TA Pawlik TM . Strobe reporting guidelines for observational studies. Jama Surg. (2021) 156:577–8. 10.1001/jamasurg.2021.0528
21.
Zamorano JL Lancellotti P Rodriguez Muñoz D Aboyans V Asteggiano R Galderisi M et al 2016 Esc position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the esc committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European society of cardiology (esc). Eur Heart J. (2016) 37:2768–801. 10.1093/eurheartj/ehw211
22.
Haley WE . Family caregivers of elderly patients with cancer: understanding and minimizing the burden of care. J Support Oncol. (2003) 1:25–9. PMID: .
23.
Wang Y Jiao Y Nie J O'Neil A Huang W Zhang L et al Sex differences in the association between marital status and the risk of cardiovascular, cancer, and all-cause mortality: a systematic review and meta-analysis of 7,881,040 individuals. Glob Health Res Policy. (2020) 5:4. 10.1186/s41256-020-00133-8
24.
Simeonova E . Marriage, bereavement and mortality: the role of health care utilization. J Health Econ. (2013) 32:33–50. 10.1016/j.jhealeco.2012.10.010
25.
Robles TF Kiecolt-Glaser JK . The physiology of marriage: pathways to health. Physiol Behav. (2003) 79:409–16. 10.1016/s0031-9384(03)00160-4
26.
Yan XY Huang SM Huang CQ Wu WH Qin Y . Marital status and risk for late life depression: a meta-analysis of the published literature. J Int Med Res. (2011) 39:1142–54. 10.1177/147323001103900402
27.
Huang M Yen C Lung F . Moderators and mediators among panic, agoraphobia symptoms, and suicidal ideation in patients with panic disorder. Compr Psychiatry. (2010) 51:243–9. 10.1016/j.comppsych.2009.07.005
28.
Danforth KN Luong TQ Yi DK Yamamoto A Kawatkar AA Kim PH et al Disparities in stage at diagnosis in an equal-access integrated delivery system: a retrospective cohort study of 7244 patients with bladder cancer. Clin Genitourin Cancer. (2020) 18:e91–102. 10.1016/j.clgc.2019.09.002
29.
Jacobs BL Montgomery JS Zhang Y Skolarus TA Weizer AZ Hollenbeck BK . Disparities in bladder cancer. Urol Oncol. (2012) 30(1):81–8. 10.1016/j.urolonc.2011.08.011
30.
Lam CM Li Z Theodorescu D Li X . Mechanism of sex differences in bladder cancer: evident and elusive sex-biasing factors. Bl Cancer. (2022) 8:241–54. 10.3233/BLC-211658
31.
Cumberbatch M Jubber I Black PC Esperto F Figueroa JD Kamat AM et al Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. (2018) 74:784–95. 10.1016/j.eururo.2018.09.001
32.
Vollset SE Tverdal A Gjessing HK . Smoking and deaths between 40 and 70 years of age in women and men. Ann Intern Med. (2006) 144:381–9. 10.7326/0003-4819-144-6-200603210-00004
33.
Hjellvik V Selmer R Gjessing HK Tverdal A Vollset SE . Body mass index, smoking, and risk of death between 40 and 70 years of age in a Norwegian cohort of 32,727 women and 33,475 men. Eur J Epidemiol. (2013) 28:35–43. 10.1007/s10654-012-9758-7
34.
Lee CT Dunn RL Williams C Underwood W . Racial disparity in bladder cancer: trends in tumor presentation at diagnosis. J Urol. (2006) 176:927–33. 10.1016/j.juro.2006.04.074
35.
Mahran A Miller A Calaway A Prunty M Arenas-Gallo C Isali I et al The impact of race and sex on metastatic bladder cancer survival. Urology. (2022) 165:98–105. 10.1016/j.urology.2021.08.049
36.
Barocas DA Alvarez J Koyama T Anderson CB Gray DT Fowke JH et al Racial variation in the quality of surgical care for bladder cancer. Cancer. (2014) 120:1018–25. 10.1002/cncr.28520
37.
Casey MF Gross T Wisnivesky J Stensland KD Oh WK Galsky MD . The impact of regionalization of cystectomy on racial disparities in bladder cancer care. J Urol. (2015) 194:36–41. 10.1016/j.juro.2015.01.076
38.
Martyn-Hemphill C Mak D Khan MS Challacombe BJ Bishop CV . Recent advances in diagnosis and treatment of transitional cell carcinoma of the bladder. Int J Surg. (2013) 11:749–52. 10.1016/j.ijsu.2013.08.018
39.
Chalasani V Chin JL Izawa JI . Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J. (2009) 3:S193–8. 10.5489/cuaj.1195
40.
El-Sebaie M Zaghloul MS Howard G Mokhtar A . Squamous cell carcinoma of the bilharzial and non-bilharzial urinary bladder: a review of etiological features, natural history, and management. Int J Clin Oncol. (2005) 10:20–5. 10.1007/s10147-004-0457-6
41.
Dikalov S Itani H Richmond B Vergeade A Rahman S Boutaud O et al Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am J Physiol. (2019) 316:H639–46. 10.1152/ajpheart.00595.2018
42.
Kondo T Nakano Y Adachi S Murohara T . Effects of tobacco smoking on cardiovascular disease. Circ J. (2019) 83:1980–5. 10.1253/circj.CJ-19-0323
43.
Barua RS Ambrose JA . Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler, Thromb, Vasc Biol. (2013) 33:1460–7. 10.1161/ATVBAHA.112.300154
44.
Zaid M Miura K Okayama A Nakagawa H Sakata K Saitoh S et al Associations of high-density lipoprotein particle and high-density lipoprotein cholesterol with alcohol intake, smoking, and body mass index- the interlipid study. Circ J. (2018) 82:2557–65. 10.1253/circj.CJ-18-0341
45.
Kirkali Z Chan T Manoharan M Algaba F Busch C Cheng L et al Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. (2005) 66:4–34. 10.1016/j.urology.2005.07.062
46.
Lyon AR López-Fernández T Couch LS Asteggiano R Aznar MC Bergler-Klein J et al 2022 Esc guidelines on cardio-oncology developed in collaboration with the European hematology association (eha), the European society for therapeutic radiology and oncology (estro) and the international cardio-oncology society (ic-os). Eur Heart J. (2022) 43:4229–361. 10.1093/eurheartj/ehac244
47.
Zhang S Wang Y Zhang P Ai L Liu T . Cardiovascular outcomes in the patients with colorectal cancer: a multi-registry-based cohort study of 197,699 cases in the real world. Front Cardiovasc Med. (2022) 9:851833. 10.3389/fcvm.2022.851833
48.
Guan T Qiu Z Su M Yang J Tang Y Jiang Y et al Cardiovascular death risk in primary central nervous system lymphoma patients treated with chemotherapy: a registry-based cohort study. Front Oncol. (2021) 11:641955. 10.3389/fonc.2021.641955
49.
Weberpals J Jansen L Müller OJ Brenner H . Long-term heart-specific mortality among 347 476 breast cancer patients treated with radiotherapy or chemotherapy: a registry-based cohort study. Eur Heart J. (2018) 39:3896–903. 10.1093/eurheartj/ehy167
50.
Quagliariello V De Laurentiis M Rea D Barbieri A Monti MG Carbone A et al The sglt-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. (2021) 20:150. 10.1186/s12933-021-01346-y
51.
Park HS Lloyd S Decker RH Wilson LD Yu JB . Overview of the surveillance, epidemiology, and end results database: evolution, data variables, and quality assurance. Curr Probl Cancer. (2012) 36:183–90. 10.1016/j.currproblcancer.2012.03.007
Summary
Keywords
bladder cancer, cardiovascular mortality, cardio-oncology, SEER database, competing risk model, nomogram
Citation
Liao J and Zhou Z (2023) Long-term cardiovascular mortality risk in patients with bladder cancer: a real-world retrospective study of 129,765 cases based on the SEER database. Front. Cardiovasc. Med. 10:1142417. doi: 10.3389/fcvm.2023.1142417
Received
11 January 2023
Accepted
30 October 2023
Published
09 November 2023
Volume
10 - 2023
Edited by
Jiandong Zhou, University of Warwick, United Kingdom
Reviewed by
Wilhelm Mistiaen, University of Antwerp, Belgium Muni Rubens, Baptist Hospital of Miami, United States
Updates
Copyright
© 2023 Liao and Zhou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Zihua Zhou zzhua2001@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.