
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 07 June 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1140234
This article is part of the Research Topic Cardiotoxicity Monitoring and Prevention in Pediatric Acute Myeloid Leukemia View all articles
 Yichan Zhang1,2,3,†
Yichan Zhang1,2,3,† Yuting Tan1,2,3,†
Yuting Tan1,2,3,† Tianshu Liu1,2,3
Tianshu Liu1,2,3 Yanan Fu1,2,3
Yanan Fu1,2,3 Yixia Lin1,2,3
Yixia Lin1,2,3 Jiawei Shi1,2,3
Jiawei Shi1,2,3 Yanting Zhang1,2,3
Yanting Zhang1,2,3 Wenhui Deng1,2,3
Wenhui Deng1,2,3 Shukun He1,2,3
Shukun He1,2,3 Yali Yang1,2,3
Yali Yang1,2,3 Qing Lv1,2,3
Qing Lv1,2,3 Li Zhang1,2,3
Li Zhang1,2,3 Mingxing Xie1,2,3*
Mingxing Xie1,2,3* Jing Wang1,2,3*
Jing Wang1,2,3*
Background: The relationship between acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) and cardiac function is not well established. This study aimed to evaluate whether AML patients exist early myocardial damages prior to chemotherapy and to investigate its association with cardiovascular biomarkers.
Methods: Conventional echocardiography and three-dimensional speckle-tracking strain analysis were performed prospectively in 72 acute leukemia (AL) patients before any chemotherapy therapy (of whom 44 were AML patients, 28 ALL patients). The results were compared with those from 58 control group matched for age and gender.
Results: There were no significant differences in conventional biventricular systolic function parameters between AL patients and controls. The left ventricular global longitudinal strain (LVGLS) and right ventricular free wall longitudinal strain (RVFWLS) were significantly lower in AL patients (−23.0 ± 1.4% vs. −24.1 ± 1.3% and −27.9 ± 7.1% vs. −33.0 ± 4.6%, respectively, P < 0.001 for all). Compared with ALL patients, AML patients had lower LVGLS and RVFWLS (−22.7 ± 1.3% vs. −23.5 ± 1.6% and −26.2 ± 7.6% vs. −30.4 ± 5.5%, respectively, P < 0.05 for all). LVGLS was lower in ALL patients compared with controls (−23.5 ± 1.6% vs. −24.7 ± 1.4%, P < 0.05), however, there was no difference in right ventricular systolic function parameters between the two groups. LVGLS in AL patients was independently correlated with left ventricular ejection fraction (LVEF) and the absolute number of circulating lymphocytes.
Conclusions: Our findings suggest that baseline myocardial systolic function is lower in AL patients than controls. AML patients had lower baseline LVGLS and RVFWLS than controls and ALL patients. The decreased LVGLS is correlated with LVEF and the absolute number of circulating lymphocytes.
For decades, anticancer therapies, such as anthracyclines, cyclophosphamide and trastuzumab, have found broad applications in hematological malignancies and solid tumors, and greatly benefited many patients. However, cardiac injury is the major adverse effect of anticancer treatment (1–3). Many cancer survivors experience not only congestive heart failure, but also myocardial ischemia, thromboembolism, hypertension and arrhythmias that affect their quality of life (1, 4). In a large retrospective study, 3.5% cancer patients developed cardiac toxicity during 10-year period (5). It is worth noting that, higher rates of cardiotoxicity after anthracycline chemotherapy (13%) have been reported in patients with acute leukemia (AL) compared with other types of cancer (6, 7). Patients with acute myeloid leukemia (AML) had an increased incidence of heart failure after anthracycline than patients with acute lymphoblastic leukemia (ALL) (8, 9). After adjustment for age and cumulative anthracycline dose, the presence of AML was still associated with symptomatic HF (9).
AL per se may be associated with cardiac abnormalities due to a robust systemic inflammatory response and direct myocardial infiltration by leukemic cells before chemotherapy (10–12). Furthermore, AML and ALL patients may also have different myocardial systolic patterns due to their differences in pathophysiology (13). Current cardiotoxicity studies are mainly focus on the changes in cardiac function during and after chemotherapy (14–17). However, baseline myocardial function in tumor patients is associated with symptomatic heart failure and cardiac death in patients treated with anthracycline (6). A baseline risk assessment is mandatory in all AML patients before initiation of therapy, focusing on early, preclinical detection of cardiotoxicity, providing a basis for early clinical identification of patients with high risk of heart failure and guiding cardioprotective medications. Few studies have been performed in this area.
Abnormalities in strain are reported to occur prior to changes in conventional echo parameters, likely left ventricular ejection fraction (LVEF) in different clinical settings (18, 19). Three-dimensional speckle-tracking echocardiography (3D-STE) is an advanced imaging technique with capabilities to track out-of-plane motion of speckles, higher feasibility and reproducibility over 2D-STE (20, 21). Accordingly, this prospective study was designed to evaluate the application of 3D-STE in identifying subclinical myocardial damages before initiation of chemotherapy.
We prospectively enrolled consecutive AL patients (≥18 years of age) who were referred to Wuhan Union Hospital before any chemotherapy between March 2021 and April 2022. Patients with reduced LVEF (<52% for male and <53% for female) (22), more than mild valvular heart disease, atrial fibrillation, congenital heart disease, previous chemotherapy history and inadequate echocardiographic images were excluded from this study. The AL cohort consisted of a total of 72 patients, of whom 44 were diagnosed with AML and 28 with ALL. All of them had an echocardiogram performed before initiation of chemotherapy. 72 AL patients and 58 cases of control group were matched for age and gender.
This study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from all patients.
Transthoracic echocardiographic examinations were performed by an experienced echocardiographic doctor using Philip EPIQ 7C (Philips Medical Systems, Andover, USA) equipped with S5-1 and X5-1. All measurements were performed according to the current recommendations of the American Society of Echocardiography (23). 2D echocardiographic acquisitions were recorded from the basal, middle and apical levels of parasternal short axis views and the apical 4-chamber (Ch), 2-Ch and 3-Ch views with 4 consecutive cardiac cycles. The frame rate was set between 55 and 90 frames per second. LVEF was calculated by biplane Simpson's method from apical 4-Ch and 2-Ch views (24). Tricuspid annular plane systolic excursion (TAPSE) was measured as the systolic displacement of the tricuspid lateral annulus, recorded on M-mode imaging. Right ventricular fractional area change (RVFAC) was calculated as: (RV end-diastolic area−RV end-systolic area)/end-diastolic area × 100%. Tricuspid lateral annular systolic velocity (S') was assessed using tissue Doppler imaging from the apical 4-ch view.
3D echocardiographic acquisitions were recorded from the four-chamber apical view in heart model mode with volume rate of 19–25 volumes/s, and were gathered over four cardiac cycles, during a breath-hold lasting for a few seconds. Left ventricular end-systolic volume (LVESV), end-diastolic volume (LVEDV), right ventricular end-systolic volume (RVESV), end-diastolic volume (RVEDV) and RV ejection fraction (RVEF) were measured by 3D echocardiography. The 2D and 3D echocardiographic datasets were stored digitally for offline analysis.
The off-line 3D-STE analyses were performed using the vendor-independent software TomTec (4D LV-Analysis 3.1 for 3D-STE Tom Tec Imaging Systems, Unterschleissheim, Germany) by an experienced researcher who was blinded to the groups. The software semi-automatically aligned and displayed the LV in the short axis view at level of the aortic valve and 3 standard apical views (25). To avoid foreshortening, manual adjustment was necessary to obtain the maximal LV long axis view. The endocardial surface of the ventricle in end-diastolic and end-systolic frames was identified automatically for initial contour detection. The operator could manually adjust the ventricular endocardial surface if necessary. Subsequently, the software automatically tracked the endocardium throughout the entire cardiac cycle, then the software provides the time-volume and time-strain curves (Figure 1, Supplementary Figure S1).

Figure 1. Rv three-dimensional image. (A) Three-dimensional image of RV-focused apical 4-chamber view. (B) Reference points setting. (C) RV endocardial border identification and tracking at end-systole and end-diastole. (D) Longitudinal strain of RV free wall and septum. RV, right ventricular.
The 3D LV global longitudinal strain (LVGLS) and global circumferential strain (LVGCS) were quantified as the mean of the strain values from all 16 segments of LV. The 3D RV free wall longitudinal strain (RVFWLS), RV septal wall longitudinal strain (RVSWLS) and RV global longitudinal strain (RVGLS) were acquired simultaneously. The patients with persistent inadequate tracking were excluded from the study.
Intraobserver and interobserver variability of the 3D-STE parameters were analyzed in 20 randomly selected patients from AL group. Intraobserver reproducibility was assessed by the same observer performed 4 weeks later again. Interobserver reproducibility was assessed by the second observer who was blinded to the measurements of the first observer in the same 20 patients.
Continuous variables are presented as mean ± standard deviation or as median (interquartile range), and categorical variables as number (%). Comparisons of clinical and echocardiographic parameters between AL patients and control group were performed using the Student's t test or the Wilcoxon rank sum tests for continuous variables and the χ2 or Fisher's exact test for categorical variables. Comparisons of echocardiographic parameters between AML and ALL patients were performed using one-way analysis of covariance. Correlations between LVGLS and clinical, laboratory, and echocardiographic variables were estimated using Pearson correlation coefficients or Spearman ρ test for nonparametric values. A multivariable linear regression was also performed to explore the independent clinical and echocardiographic parameters associated with GLS in patients with AL. All variables reaching P values of < 0.1 on univariate analysis were included in the multivariate analyses. The intraobserver and interobserver variability of 3D-STE parameters were assessed by the intraclass correlation coefficients (ICCs) and the Bland–Altman analyses in a random sample of 20 patients. All statistical analyses were performed on SPSS version 26.0 (Statistical Package for the Social Sciences, Chicago, Illinois) and GraphPad Prism version 8.0.1. A 2-sided P value of < 0.05 was considered as statistically significant.
There was no difference in age, sex distribution, BMI, diabetes, prevalence of hypertension, dyslipidemia, smoking, prior coronary artery disease (CAD) and chronic kidney disease (CKD) between AL patients and controls. The history of medications did not differ between the two groups (Table 1).
LV volume and LV volume indexed to body surface area were slightly higher in patients with AL (P < 0.05). Systolic dyssynchrony index (SDI) was similar between the two groups. Although LVEF was similar between AL patients and controls (61.1 ± 4.2% vs. 62.4 ± 4.9%, P > 0.05), a statistically significant difference was noted with regard to LVGLS (−23.0 ± 1.4% vs. −24.1 ± 1.3%, P < 0.001). There was no significant difference in LVGCS (Table 2).
Conventional parameters of RV systolic function (FAC, TAPSE and S’) were similar between two groups. Compared with controls, the RVGLS and RVFWLS were lower in AL patients (−25.9 ± 4.7% vs. −28.2 ± 4.3%, P < 0.05 and −27.9 ± 7.1% vs. −33.0 ± 4.6%, P < 0.001, respectively) (Table 2). There was no significant difference in RVSWLS.
After adjustment for hemoglobin counts, only LVGLS and RVFWLS were lower in patients with AML (−22.7 ± 1.3% vs. −23.5 ± 1.6% and −26.2 ± 7.6% vs. −30.4 ± 5.5%, respectively, P < 0.05 for all) (Table 3). LVGLS was lower in ALL patients compared with controls (−23.5 ± 1.6% vs. −24.7 ± 1.4%, P < 0.05). However, there was no difference in RV systolic function parameters between the two groups (Figure 2).

Figure 2. LVGLS and RVFWLS in patients with ALL (n = 28) and controls (n = 20). (A) Comparison of LVGLS between patients with ALL and controls. (B) Comparison of RVFWLS between patients with ALL and controls. LVGLS, left ventricle global longitudinal strain; RVFWLS, right ventricular free wall longitudinal strain; ALL, acute lymphoblastic leukemia; LVGLS and RVFWLS values are absolute values; *P < 0.05 compared with controls; ns, not statistically different.
Among all AL patients, LVGLS was correlated with LVEF (r = −0.430, P < 0.001), BMI (r = 0.263, P = 0.026), age, (r = 0.272, P = 0.021), the absolute number of C reactive protein (CRP) (r = 0.298, P = 0.011), the absolute number of circulating lymphocytes (r = −0.524, P < 0.001) and hemoglobin counts (r = 0.304, P = 0.009; Figure 3). Therefore, a decrease in LVGLS was associated with a reduction in the absolute number of lymphocytes and LVEF and with an increase in age, BMI, the absolute number of CRP and hemoglobin counts. In a multivariable linear regression analysis, the factors independently associated with LVGLS were LVEF (β = −0.085, P = 0.019) and the absolute number of circulating lymphocytes (β = −0.814, P = 0.004). However, RVFWLS was only correlated with BMI (r = 0.323, P = 0.005) and hemoglobin counts (r = 0.267, P = 0.021) (Supplementary Table S1).
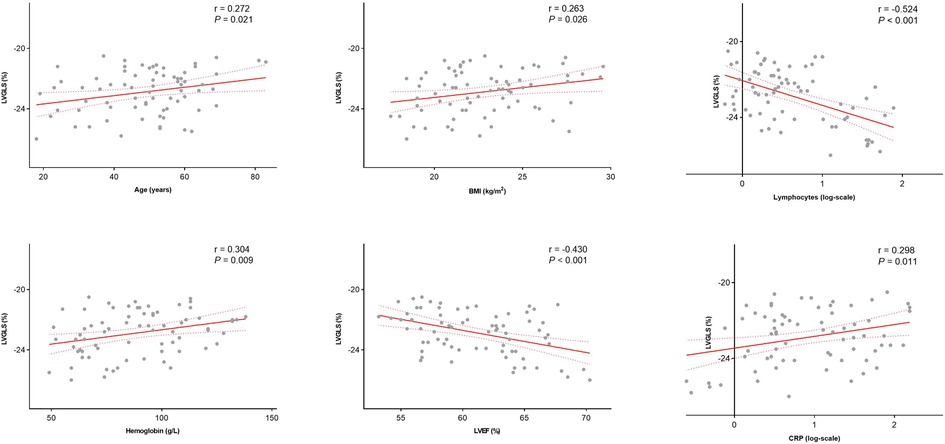
Figure 3. Correlation between LVGLS and age, BMI, absolute number of lymphocytes, hemoglobin counts, LVEF and absolute number of CRP among AL patient. LVEF, left ventricular ejection fraction; CRP, C reactive protein.
Intraobserver and interobserver variability of the 3D-STE parameters are presented in Table 4. Strain parameters measured by 3D-STE showed excellent reproducibility, as evidenced by the high ICC, small bias, and narrow limits of agreement.
To the best of our knowledge, this is the first study to evaluate the myocardial systolic function before chemotherapy in AML and ALL patients by 3D-STE. The present investigation revealed several interesting findings that deserve comment. (1) AL patients had lower baseline LVGLS and RVGLS. (2) AML patients had lower baseline LVGLS and RVFWLS. (3) Among AL patients, LVGLS was independently associated with LVEF and the absolute number of circulating lymphocytes.
The majority of studies have focused on LV dysfunction induced by different chemotherapeutic agents — anthracyclines and trastuzumab in patients with cancer. Previous studies (26, 27) have showed that AL by itself may be associated with cardiac alterations. Several factors may explain the prechemotherapy myocardial remodeling in AL patients. It had been confirmed that the imbalance between pro-inflammatory and anti-inflammatory cytokines is an important reason for the formation of leukemia blasts. Elevated plasma CRP levels were found in most AL patients (28). The activation of inflammation in the heart causes LV remodeling and dysfunction and is associated with adverse outcomes in patients with heart failure. Another possible mechanism to explain cardiac alterations in patients with AL is direct infiltration of the heart by leukemic cells, which has been extensively described in previous autopsy studies (10, 29).
Our research demonstrates that LVGLS is attenuated in AML patients compared with ALL patients. Plasma TNF-a, interleukin-6 (IL-6) and IL-10 levels were higher in AML patients compared with ALL patients and control group and plasma levels of IL-6 and IL-10 were associated with patient survival and event-free survival. A significantly higher incidence of cardiac infiltration has been found in AML patients than in ALL patients revealed by autopsy findings (10), which may be the reason for lower LVGLS in AML patients (11, 12). Some evidence has shown an association between somatic mutations in AML patients and an increased risk for coronary artery disease, thus being a potential mechanism pathway (30). Furthermore, mutations in isocitrate dehydrogenase 1 and 2, with an overall frequency of approximately 9% to 16% in patients with AML, have been implicated in cardiac dysfunction (31). This is important, because AML patients, a population with many senior citizens, show increased cardiac-specific mortality (8). Ali et al. (6) showed that prechemotherapy LVGLS is an effective tool to stratify patients at high risk for cardiac events after anthracycline therapy and may help tailor treatments to decrease anthracycline-induced cardiotoxicity. More attention should be paid to cardiac function of AML patients during and after chemotherapy.
RVFWLS was decreased in AML patients compared to ALL patients despite normal RVSWLS and RVGLS, which may reflect early systolic function changes of RV myocardium. Longitudinal RV function is substantial, accounting for >60% of the ejection fraction and might be reflected by RVFWLS (32). Global RV systolic parameters, for example RVGLS, the average strain value obtained by tracking six segments of the RV, may be affected by LV systolic function owing to the fact that the interventricular septum is conventionally regarded as one part of LV (33). RVFWLS may serve as an early marker of altered RV myocardial function. Considering the fact that the reduction of RVFWLS was significantly correlated with dyspnea severity and heart failure, RVFWLS may play an important role in the follow up of AML patients (34).
Our study did not show significant change in echocardiographic parameters of ventricular structure and function between ALL patients and controls except for LVGLS. Baseline myocardial contraction alterations need to be demonstrated in a longer course.
In AL patients, decreased LVGLS was correlated with reduced LVEF and the absolute number of circulating lymphocytes. Cardiac dysfunction represents a serious complication in patients with cancer. Although cardiotoxicity is generally related to chemotherapy and other anticancer therapies, the impact, molecular mechanisms and biological basis of the effects induced by tumor growth on cardiac functions, regardless of therapy, still remain unclear and little investigated (12). Previous studies have shown that the systemic inflammatory response may result in cardiac damage (35). In our study, the increased number of CRP and decreased number of circulating lymphocytes were associated with LVGLS reduction. We speculate that cancer growth could exert per se, independently from chemotherapy effects, cardiotoxicity, and inflammatory molecules could be responsible for promoting LV systolic dysfunction (36).
This study has several limitations. First, this was a single-center study and the sample size was relatively small. Second, we did not acquire all the laboratory biomarker tests of our patients, such as high-sensitivity troponin I (hs-TNI) and IL-6, which reflect the level of inflammation in the body. Third, our study lacks histological confirmation of interstitial inflammatory infiltrates of myocardial tissue. Fourth, 3D-STE technique was dependent on image quality, and the value of strain in our study may not apply to other software algorithms due to inter-vendor variability. Therefore, future studies with multicenter involvement may strengthen the study power.
Our findings suggest that baseline myocardial systolic function is lower in AL patients than controls. AML patients had lower baseline LVGLS and RVFWLS than controls and ALL patients. The decreased LVGLS is correlated with LVEF and the absolute number of circulating lymphocytes. These findings suggest that AL by itself maybe associated with cardiac alterations and may help understand the higher cardiovascular risk and cardiotoxicity after chemotherapy in AL patients compared to other types of cancer. Further studies are needed to investigate the pathophysiologic basis of preexisting cardiac alterations.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: YicZ, LZ, MX and JW; methodology: LZ, MX and JW; software: YicZ, YT and TL; formal analysis: YicZ and YL; investigation: YicZ and YF; resources: JS and YanZ; data curation: YicZ, LZ, JW and MX; writing—original draft preparation: YicZ, YT, TL, YL, YF, JS, YanZ, WD, SH, YY, QL, LZ, MX and JW; writing—review and editing: YicZ, YT, TL, YL, YF, JS, YanZ, WD, SH, YY, QL, LZ, MX and JW; visualization: YicZ; supervision: LZ, MX and JW; project administration: MX; funding acquisition: JW. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. Grant Nos. 82171961, 82211530116).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1140234/full#supplementary-material
Supplementary Figure
LV three-dimensional image. (A) Three-dimensional image of LV apical 4-chamber view. (B) Reference points setting. (C) LV endocardial border identification and tracking at end-systole and end-diastole. (D) Longitudinal strain of LV. LV, left ventricular.
1. Alexandre J, Cautela J, Ederhy S, Damaj GL, Salem JE, Barlesi F, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. J Am Heart Assoc. (2020) 9(18):e018403. doi: 10.1161/jaha.120.018403
2. Rotz SJ, Ryan TD, Hayek SS. Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation. J Thromb Thrombolysis. (2021) 51(4):854–69. doi: 10.1007/s11239-020-02344-9
3. Wang Y, Wang Y, Han X, Sun J, Li C, Adhikari BK, et al. Cardio-Oncology: a myriad of relationships between cardiovascular disease and cancer. Front Cardiovasc Med. (2022) 9:727487. doi: 10.3389/fcvm.2022.727487
4. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. (2020) 17(8):474–502. doi: 10.1038/s41569-020-0348-1
5. Shah CP, Moreb JS. Cardiotoxicity due to targeted anticancer agents: a growing challenge. Ther Adv Cardiovasc Dis. (2019) 13:1753944719843435. doi: 10.1177/1753944719843435
6. Ali MT, Yucel E, Bouras S, Wang L, Fei HW, Halpern EF, et al. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J Am Soc Echocardiogr. (2016) 29(6):522–7e3. doi: 10.1016/j.echo.2016.02.018
7. Wang L, Tan TC, Halpern EF, Neilan TG, Francis SA, Picard MH, et al. Major cardiac events and the value of echocardiographic evaluation in patients receiving anthracycline-based chemotherapy. Am J Cardiol. (2015) 116(3):442–6. doi: 10.1016/j.amjcard.2015.04.064
8. Li G, Zhou Z, Yang W, Yang H, Fan X, Yin Y, et al. Long-term cardiac-specific mortality among 44,292 acute myeloid leukemia patients treated with chemotherapy: a population-based analysis. J Cancer. (2019) 10(24):6161–9. doi: 10.7150/jca.36948
9. Kang Y, Assuncao BL, Denduluri S, McCurdy S, Luger S, Lefebvre B, et al. Symptomatic heart failure in acute leukemia patients treated with anthracyclines. JACC: CardioOncology. (2019) 1(2):208–17. doi: 10.1016/j.jaccao.2019.10.008
10. Sumners WWJ JE, Ainger LE. Childhood leukemic heart disease. A study of 116 hearts of children dying of leukemia. Circulation. (1969) 40(4):575–81. doi: 10.1161/01.cir.40.4.575
11. Sanchez-Correa B, Bergua JM, Campos C, Gayoso I, Arcos MJ, Bañas H, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. (2013) 61(3):885–91. doi: 10.1016/j.cyto.2012.12.023
12. Buoncervello M, Maccari S, Ascione B, Gambardella L, Marconi M, Spada M, et al. Inflammatory cytokines associated with cancer growth induce mitochondria and cytoskeleton alterations in cardiomyocytes. J Cell Physiol. (2019) 234(11):20453–68. doi: 10.1002/jcp.28647
13. Horacek JM, Kupsa T, Vasatova M, Jebavy L, Zak P. Serum cytokine and adhesion molecule profile differs in newly diagnosed acute myeloid and lymphoblastic leukemia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2015) 159(2):299–301. doi: 10.5507/bp.2014.051
14. Kang Y, Xiao F, Chen H, Wang W, Shen L, Zhao H, et al. Subclinical anthracycline-induced cardiotoxicity in the long—term follow-up of lymphoma survivors: a multi-layer speckle tracking analysis. Arq Bras Cardiol. (2018) 110(3):219–28. doi: 10.5935/abc.20180042
15. Laufer-Perl M, Perelman-Gvili M, Sirota Dorfman S, Baruch G, Rothschild E, Beer G, et al. Prevalence of right ventricle strain changes following anthracycline therapy. Life. (2022) 12(2):291. doi: 10.3390/life12020291
16. Wang B, Yu Y, Zhang Y, Hao X, Zhao H, Yang S, et al. Speckle tracking echocardiography in the early detection and prediction of anthracycline cardiotoxicity in diffuse large B-cell lymphoma treated with (R)-CHOP regimen. Echocardiography. (2020) 37(3):421–8. doi: 10.1111/echo.14622
17. Zhao R, Shu F, Zhang C, Song F, Xu Y, Guo Y, et al. Early detection and prediction of anthracycline-induced right ventricular cardiotoxicity by 3-dimensional echocardiography. JACC CardioOncol. (2020) 2(1):13–22. doi: 10.1016/j.jaccao.2020.01.007
18. Bárczi A, Lakatos BK, Szilágyi M, Kis É, Cseprekál O, Fábián A, et al. Subclinical cardiac dysfunction in pediatric kidney transplant recipients identified by speckle-tracking echocardiography. Pediatr Nephrol. (2022) 37(10):2489–501. doi: 10.1007/s00467-022-05422-7
19. Azak E, Uçaktürk SA, Çetin İ, Gürsu HA, Mengen E, Pamuk U. Subclinical myocardial dysfunction demonstrated by speckle tracking echocardiography in children with euthyroid Hashimoto's thyroiditis. J Clin Res Pediatr Endocrinol. (2019) 11(4):410–8. doi: 10.4274/jcrpe.galenos.2019.2018.0273
20. Wang J, Zhang Y, Zhang L, Tian F, Wang B, Xie Y, et al. Assessment of myocardial fibrosis using two-dimensional and three-dimensional speckle tracking echocardiography in dilated cardiomyopathy with advanced heart failure. J Card Fail. (2021) 27(6):651–61. doi: 10.1016/j.cardfail.2021.01.003
21. Cameli M, Mandoli GE, Sciaccaluga C, Mondillo S. More than 10 years of speckle tracking echocardiography: still a novel technique or a definite tool for clinical practice? Echocardiography. (2019) 36(5):958–70. doi: 10.1111/echo.14339
22. Yao GH, Deng Y, Liu Y, Xu MJ, Zhang C, Deng YB, et al. Echocardiographic measurements in normal Chinese adults focusing on cardiac chambers and great arteries: a prospective, nationwide, and multicenter study. J Am Soc Echocardiogr. (2015) 28(5):570–9. doi: 10.1016/j.echo.2015.01.022
23. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. (2019) 32(1):1–64. doi: 10.1016/j.echo.2018.06.004
24. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39e14. doi: 10.1016/j.echo.2014.10.003
25. Nabeshima Y, Seo Y, Takeuchi M. A review of current trends in three-dimensional analysis of left ventricular myocardial strain. Cardiovasc Ultrasound. (2020) 18(1):23. doi: 10.1186/s12947-020-00204-3
26. Assuncao B, Handschumacher MD, Brunner AM, Yucel E, Bartko PE, Cheng KH, et al. Acute leukemia is associated with cardiac alterations before chemotherapy. J Am Soc Echocardiogr. (2017) 30(11):1111–8. doi: 10.1016/j.echo.2017.07.016
27. Tadic M, Genger M, Baudisch A, Kelle S, Cuspidi C, Belyavskiy E, et al. Left ventricular strain in chemotherapy-naive and radiotherapy-naive patients with cancer. Can J Cardiol. (2018) 34(3):281–7. doi: 10.1016/j.cjca.2017.11.018
28. Bruserud Ø, Aarstad HH, Tvedt THA. Combined C-reactive protein and novel inflammatory parameters as a predictor in cancer-what can we learn from the hematological experience? Cancers. (2020) 12(7):1966. doi: 10.3390/cancers12071966
29. Roberts GPB WC, Wertlake PT. The heart in acute leukemia. A study of 420 autopsy cases. Am J Cardiol. (1968) 21(3):388–412. doi: 10.1016/0002-9149(68)90143-4
30. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. (2014) 371(26):2488–98. doi: 10.1056/NEJMoa1408617
31. Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH, et al. Oncometabolite d-2-hydroxyglutarate impairs α-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A. (2016) 113(37):10436–41. doi: 10.1073/pnas.1601650113
32. Keller M, Lang T, Schilling A, Nowak-Machen M, Rosenberger P, Magunia H. Novel mesh-derived right ventricular free wall longitudinal strain analysis by intraoperative three-dimensional transoesophageal speckle-tracking echocardiography: a comparison with conventional parameters. Int J Cardiovasc Imaging. (2019) 35(12):2177–88. doi: 10.1007/s10554-019-01669-8
33. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29(4):277–314. doi: 10.1016/j.echo.2016.01.011
34. Carluccio E, Biagioli P, Lauciello R, Zuchi C, Mengoni A, Bardelli G, et al. Superior prognostic value of right ventricular free wall compared to global longitudinal strain in patients with heart failure. J Am Soc Echocardiogr. (2019) 32(7):836–44.e1. doi: 10.1016/j.echo.2019.02.011
35. Sun W, Zhang Y, Wu C, Xie Y, Peng L, Nie X, et al. Incremental prognostic value of biventricular longitudinal strain and high-sensitivity troponin I in COVID-19 patients. Echocardiography. (2021) 38(8):1272–81. doi: 10.1111/echo.15133
Keywords: acute myeloid leukemia, three-dimensional speckle-tracking strain, ventricular systolic function, oncology cardiology, echocardiography
Citation: Zhang Y, Tan Y, Liu T, Fu Y, Lin Y, Shi J, Zhang Y, Deng W, He S, Yang Y, Lv Q, Zhang L, Xie M and Wang J (2023) Decreased ventricular systolic function in chemotherapy-naive patients with acute myeloid leukemia: a three-dimensional speckle-tracking echocardiography study. Front. Cardiovasc. Med. 10:1140234. doi: 10.3389/fcvm.2023.1140234
Received: 8 January 2023; Accepted: 19 May 2023;
Published: 7 June 2023.
Edited by:
Andreas M. Beyer, Medical College of Wisconsin, United StatesReviewed by:
Kalliopi Keramida, National and Kapodistrian University of Athens, Greece© 2023 Zhang, Tan, Liu, Fu, Lin, Shi, Zhang, Deng, He, Yang, Lv, Zhang, Xie and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Wang amluZ3dhbmcyMDA0QGh1c3QuZWR1LmNu Mingxing Xie eGllbXhAaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.