- 1Department of Cardiac Surgery, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 2Chevidence Lab of Child & Adolescent Health, Department of Pediatric Research Institute, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Medicine IV, LMU University Hospital, Ludwig Maximilian University of Munich, Munich, Germany
Background: Valve-in-valve transcatheter mitral valve replacement (ViV-TMVR) is a minimally invasive option for patients with bioprosthetic mitral valve failure. Since January 2019, our center has been using a new innovative option, J-Valve, to treat patients with bioprosthetic mitral valve failure who were at high risk for open heart surgery. The aim of this study is to explore the effectiveness and safety of J-Valve and report the results from the four-year follow-up period of the innovative application of the transcatheter valve.
Methods: Patients who underwent the ViV-TMVR procedure between January 2019 and September 2022 in our center were included in the study. J-Valve™ system (JC Medical Inc., Suzhou, China) with three U-shape grippers was used for ViV-TMVR via transapical approach. Data on survival, complications, transthoracic echocardiographic results, New York Heart Association functional class in heart failure, and patient-reported health-related quality of life according to the Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) were collected during the four-year follow up.
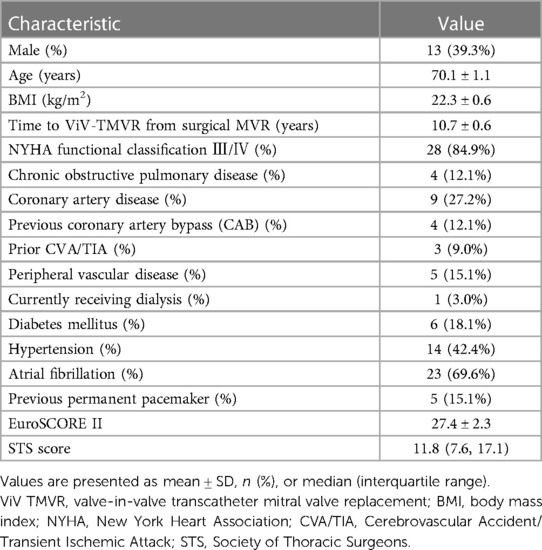
Results: Thirty-three patients (mean age 70.1 ± 1.1 years, 13 men) were included and received ViV-TMVR. The surgery success rate was 97%: only one patient was converted to open-heart surgery due to intraoperative valve embolization to the left ventricle. During the first 30 days all-cause mortality was 0%, risk of stroke 2.5% and risk of mild paravalvular leak 15.2%; mitral valve hemodynamics improved (179.7 ± 8.9 at 30 days vs. 269 ± 49 cm/s at baseline, p < 0.0001). Median time from operation to discharge was six days, and there were no readmissions within 30 days from operation. The median and maximum follow-up durations were 28 and 47 months, respectively; during the entire follow-up, all-cause mortality was 6.1%, and the risk of cerebral infarction 6.1%. Cox regression analysis did not identify any variables significantly associated with survival. The New York Heart Association functional class and the KCCQ-12 score improved significantly compared with their preoperative values.
Conclusion: The use of J-Valve for ViV-TMVR is safe and effective with a high success rate, low mortality and very few associated complications, representing an alternative surgical strategy for the elderly, high-risk patients with bioprosthetic mitral valve failure.
Introduction
The use of mechanical prostheses requires long-term anticoagulation, leading to an increased risk of bleeding in patients (1). In contrast, bioprosthetic valves are associated with a low rate of thrombosis and do not require lifetime anticoagulation. As a result, the preference for bioprosthetic valves has increased among patients with mitral valve disease over the past two decades (1). Guidelines for the treatment of valvular heart disease (2, 3) recommend patient preference as the primary criterion in selecting the type of prosthetic valve, further contributing towards the use of bioprosthetic valves. However, because of the limited durability, bioprosthetic valves need to be replaced over time. One study has shown that up to one-third of patients need to receive redo surgical treatment for mitral valve replacement (4). Since redo open-heart valve replacement surgery poses a risk of perioperative death (5), transcatheter valve-in-valve implantation technologies with less invasive alternatives for the treatment of bioprosthetic heart valve failure, which have been proven to be associated with lower risk of death, lower periprocedural morbidity, lower risk of complications, and lower need of resources, have gradually emerged since 2007 (4, 6).
However, valve-in-valve transcatheter mitral valve replacement (ViV-TMVR) still faces several challenges. Sapien 3 (Edwards Lifesciences Inc., Irvine, CA, USA) is the only transcatheter heart valve (THV) currently approved by the US Food and Drug Administration for ViV-TMVR and was also approved for the market by the Chinese National Medical Products Administration in 2020. Studies have also reported some disastrous complications associated with ViV-TMVR, such as valve migration or embolization and left ventricular outflow tract (LVOT) obstruction (7–11).
To avoid the above-mentioned complications, and also due to the fact that Sapien 3 was not available in China until 2019, we attempted to perform ViV-TMVR without changing the valve and transmitter structure using reverse-loaded J-Valve (JC Medical Inc., Suzhou, China), and concluded a standardized valve release process. J-Valve is a self-expanding transcatheter valve consisting of three U-shaped grippers, a crowned nitinol stent, porcine aortic valve leaflets, and an inner liner skirt. It was approved by China's Food and Drug Administration in 2017 with a dual indication for aortic stenosis and aortic regurgitation. Unlike cylindrical balloon-expanded valves, the anchoring of the J-Valve does not rely solely on radial forces. The three U-shaped grippers limit the movement of the valve towards the left atrium under left ventricular pressure, reducing the risk of valve migration or embolization. In addition, the three U-shaped grippers facilitate accurate commissural alignment, and the combined crowned stent and inner liner skirt both reduce the risk of left ventricular outflow tract obstruction and prevent the occurrence of paravalvular leaks.
In this study, we present the outcomes among elderly, high-risk patients with bioprosthetic mitral valve failure who were managed successfully by ViV-TMVR using J-Valve via transapical approach.
Methods
Patients
We included patients with failed surgical bioprosthetic valves who underwent ViV-TMVR in Beijing Anzhen Hospital (Capital Medical University, Beijing, China) between January 2019 and September 2022.
Preoperative electrocardiographic gated multislice computed tomographies (CT) were performed for all patients. Each patient was independently evaluated by at least two cardiac surgeons before the operation. We included patients aged ≥60 years for whom conventional redo valve surgery was associated with high risks (Society of Thoracic Surgery (STS) Risk Score or European System for Cardiac Operative Risk Evaluation (EuroSCORE) II of ≥8). Patients with the following conditions were excluded: (1) Combined moderate to severe mitral valve perivalvular leak (PVL); (2) History of stroke over the past three months; (3) Presence of left atrial or appendage thrombus; (4) Failed bioprosthetic valve type of ≤23 mm; (5) Presence of infective endocarditis; (6) Presence of LVOT obstruction; or (7) Combined multiple organ system failure or other diseases associated with a life expectancy of less than one year.
Written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki (2013 revision). The study design was approved by the Ethics Review Committee of Beijing Anzhen Hospital (No. 2022083X).
Procedure details
The procedure was performed in a hybrid operating room. Transesophageal echocardiography (TEE) was performed under general anesthesia to determine the presence of left atrial appendage thrombus and mitral valve PVL. All patients were treated with a transapical approach using the J-Valve™ System (Figure 1). The J-Valve was reverse loaded and sized according to the measured internal diameter of the failed bioprosthetic valve. The THVs' oversize ratio ranged from 5%–10%.
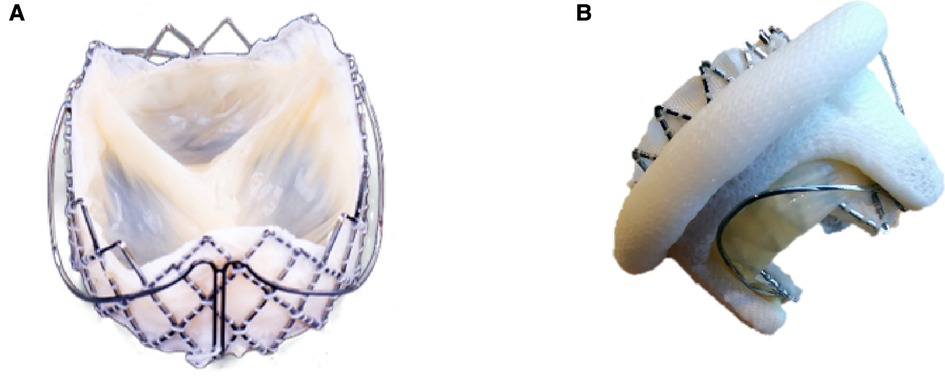
Figure 1. Features of J-Valve. The J-Valve is a self-expanding transcatheter valve with three U-shaped grippers and notches (A). The grippers help achieve commissure alignment (B) and further reduce the risk of left ventricular outflow tract obstruction.
The step-by-step procedure is shown in Figure 2 and Supplementary Video S1. After apical puncture, the failing bioprosthetic mitral valve can usually be crossed easily using J-tip guidewires. A transesophageal echocardiography was used to further confirm the guide wire into the left atrium. The wire was subsequently exchanged for an extra-stiff guide wire with curved tip. Conveyor curvature could be adjusted as needed to provide optimal coaxiality. After entering the left ventricle along the extra-stiff guidewire (Figure 2A), the conveyor first released three U-shaped grippers and subsequently staggered them between three struts of the bioprosthetic valve (Figure 2B). To improve the success and accuracy of this step, a preoperative computed tomography assessment was performed to calculate the C-arm angle at which the tips of the three struts are located at the same level. This is particularly important for the epic valve (St Jude Medical, Inc, St Paul, MN, USA) because its struts are radiolucent (Figure 3A). The valve was slowly released under rapid ventricular pacing (Figure 2C). Subsequently, the conveyor anchor device was controlled to de-load the valve (Figure 2D), the conveyer was withdrawn, and the guidewire retained (Figure 2E). Mitral flow velocity and paravalvular leak were explored using transesophageal echocardiography. Detection of mitral flow velocity and paravalvular leak using transesophageal echocardiography were utilized to determine whether to perform balloon valvuloplasty. The ideal implantation depth was considered to be 80% of the THV stent frame in the left ventricle and 20% in the left atrium (Figure 2F). Coincident native aortic valve disease or prosthetic bioprosthetic valve failure can also be managed concurrently (Figure 3B).
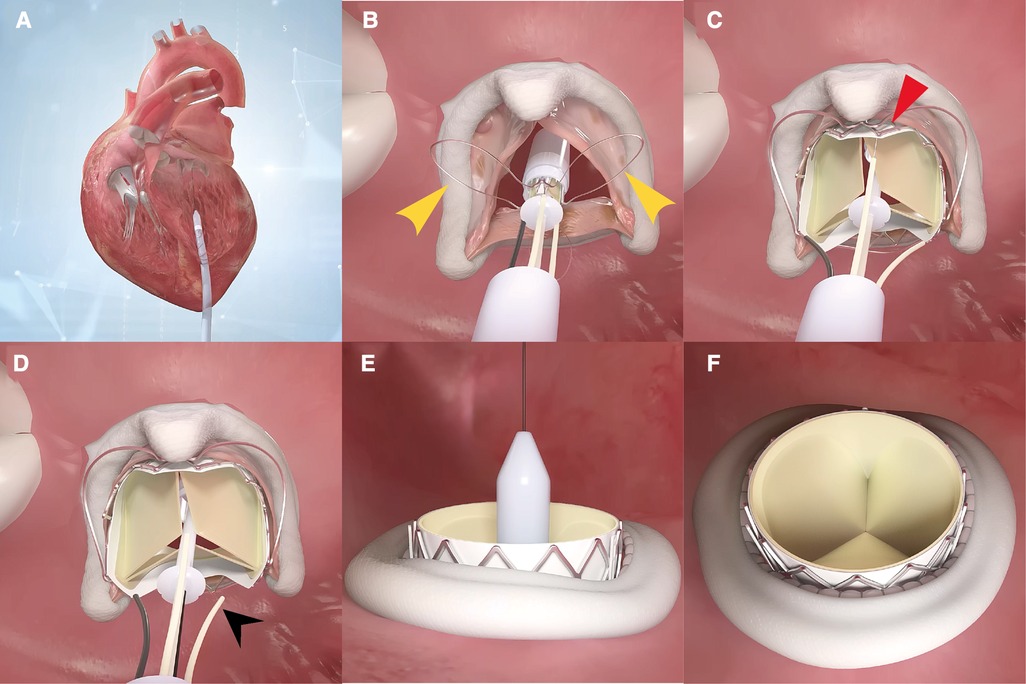
Figure 2. A step-by-step demonstration of the procedure of how J-valve functions. The conveyor enters the left ventricle along the extra-stiff guidewire (A), then the three U-shaped grippers are first released and subsequently staggered them between three struts of the bioprosthetic valve (B). The valve is then slowly released under rapid ventricular pacing (C). Subsequently, the conveyor anchor device is controlled to de-load the valve (D). The conveyer is withdrawn, and the guidewire retained (E). If balloon valvuloplasty is not required, the guidewire is withdrawn. The ideal implantation depth is 80% of the transcatheter heart valve stent frame in the left ventricle and 20% in the left atrium (F).
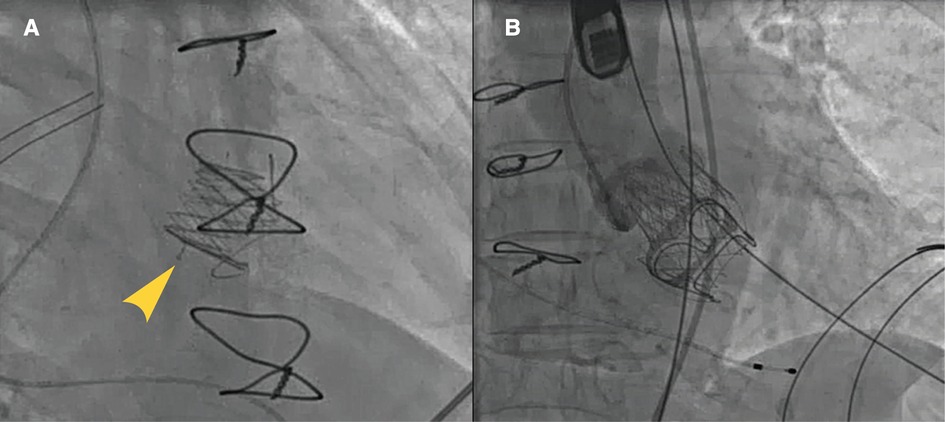
Figure 3. Typical cases of utilization of J-Valve. J-Valve applied to a strut-radiolucent epic valve (St Jude Medical) for ViVTMVR (A). Combined aortic valve disease with concomitant TAVR and ViV-TMVR procedures (B).
The strategy for postoperative anticoagulation is based on the current guidelines (2, 3) for the management of valvular heart disease and atrial fibrillation (12). Warfarin was administered on the first postoperative day and was continued for 3–6 months. The International Normalized Ratio value was maintained at 2.5. Anticoagulation or antiplatelet therapy was selected depending on the presence or absence of atrial fibrillation and the history of percutaneous coronary intervention with stent implantation or coronary artery bypass surgery.
Follow-up
All patients were followed up by four researchers (YL, JZ, KW and JS), including telephone interviews and in person visits. Follow-up data included complications reported according to the Valve Academic Research Consortium-2 definition (13), results of transthoracic echocardiography, NYHA functional class for heart failure, and patient-reported health-related quality of life outcome measured by the Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) score. The KCCQ-12 score quantitatively assesses the frequency of incident symptoms, physical limitations, social limitations, and quality of life in four areas through 12 questions. The scores can take values between 0 and 100, with higher scores meaning better health status (14).
Statistical analysis
Continuous variables were expressed as means ± standard deviations (SD) or medians with interquartile ranges, depending on whether they conformed to a normal distribution. Two-sample t-test or Wilcoxon rank sum test was used for comparisons between groups. Categorical variables were expressed as frequencies and percentages. Adverse event rates were based on Kaplan-Meier estimates, and all comparisons were made using the log-rank test. The data were analyzed using SPSS version 26.0 software (SPSS, Chicago, IL, USA). The Kaplan-Meier survival curve and bar chart with error bars were plotted using https://www.bioinformatics.com.cn (last accessed on 31 Oct. 31, 2022), an online platform for data analysis and visualization.
Results
Baseline characteristics
Baseline characteristics of the participants are shown in Table 1. Thirty-three consecutive patients underwent a ViV-TMVR procedure in the study cite, with a mean age of 70.1 ± 1.1 years. Thirteen (39.3%) patients were male. The mean time between surgical mitral valve replacement and ViV-TMVR was 10.7 ± 0.6 years. The New York Heart Association functional class was III/IV in 28 patients (84.9%). Surgical bioprosthetic valves included Carpentier-Edwards porcine and pericardial (Edwards Lifesciences, Inc., Irvine, CA, USA), Hancock II and Mosaic (Medtronic, Minneapolis, MN, USA), Epic heart valve (St Jude Medical, Inc, St Paul, MN, USA), and BalMedic bovine pericardial (Balance Medical, Beijing, China). In some patients we were unable to verify the valve type. All patients were subjected to surgical risk assessment, with a mean STS score of 11.8% and a mean (±SD) EuroSCORE II score of 27.4% ± 2.3%. All patients were considered as having high risks associated with conventional surgery.
Echocardiographic characteristics
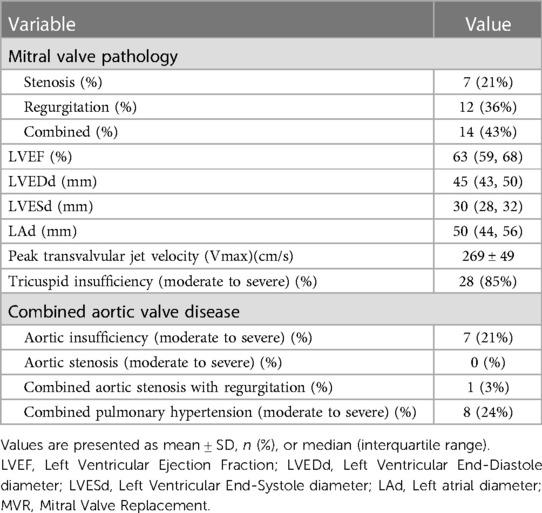
Echocardiographic characteristics are presented in Table 2. Preoperative echocardiographic evaluation results showed that seven patients (21%) had stenosis but no regurgitation, 12 patients (36%) had regurgitation but no stenosis, and 14 patients (43%) had both stenosis and regurgitation. The left ventricular ejection fraction and left ventricular size were in the normal range in 28 patients (85%). Mostly combined with moderate to severe tricuspid valve insufficiency. A total of eight patients (24%) had coexisting moderate to severe aortic valve disease. Eight patients (24%) had moderate to severe pulmonary hypertension.
Intraoperative outcomes
The surgery success rate was 97% according to the definition of the Mitral Valve Academic Research Consortium. One patient's procedure was converted to open-heart surgery due to intraoperative valve embolization to the left ventricle. Transapical access was used for all procedures with the J-Valve™ system. THV sizes ranged from 23 mm (n = 11, 33.3%) to 27 mm (n = 6, 18.2%), with 25 mm being the most utilized size in a total of 16 patients (48.5%). Pre-dilatation was performed in eight patients (24.2%) and post-dilatation in 10 patients (30.3%) due to a postoperative perivalvular leak. Perivalvular leak or concern about long-term migration due to suboptimal THV release position in two patients (5.1%) were resolved by implanting a second valve. Seven patients (21.2%) were concurrently treated for aortic valvular lesions. The valve in valve transcatheter aortic valve replacement (ViV-TAVR) was concurrently performed in four patients (12.1%) and transcatheter aortic valve replacement (TAVR) in three patients (9.1%).
Early outcomes
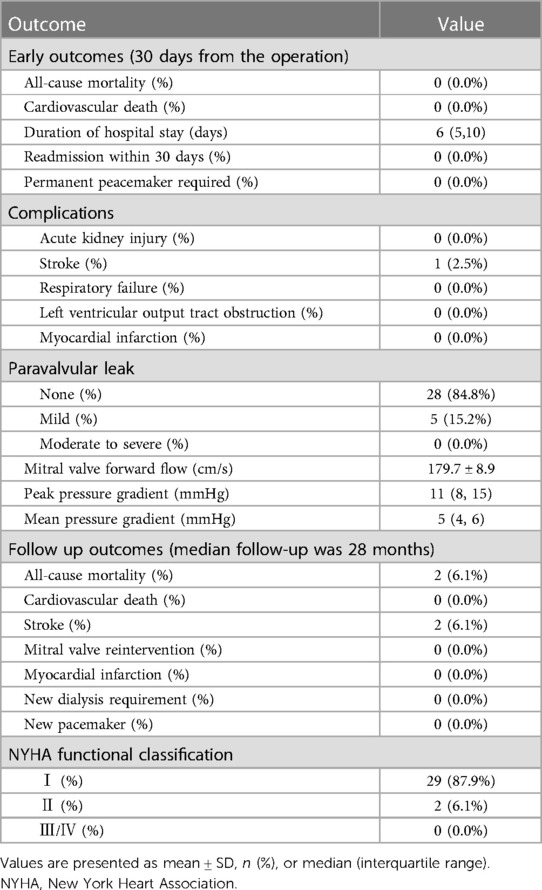
The early clinical outcomes (within 30 days from operation) are shown in Table 3. The median postoperative time to discharge was six days, with no hospital readmissions within 30 days from operation. There were no deaths or other serious complications, except for one patient (2.5%) who experienced stroke. Mild perivalvular leaks occurred in five patients (15.2%). Mitral valve hemodynamics improved postoperatively as demonstrated by the lower transvalvular flow velocity compared to the respective preoperative value (180 ± 9 vs. 269 ± 49 cm/s, p < 0.0001).
Follow-up outcomes
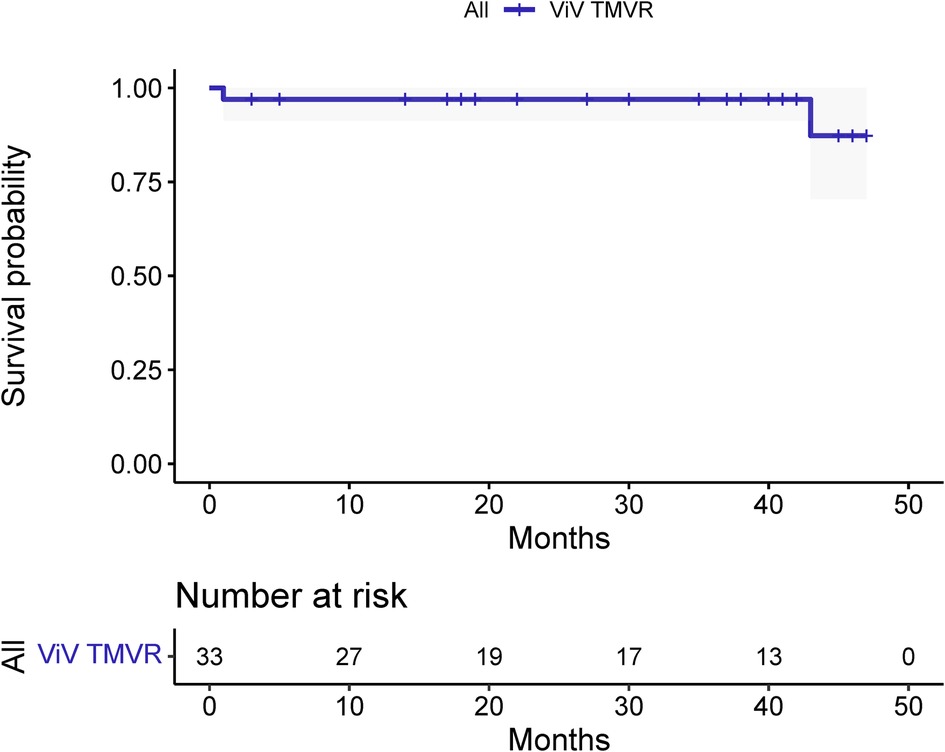
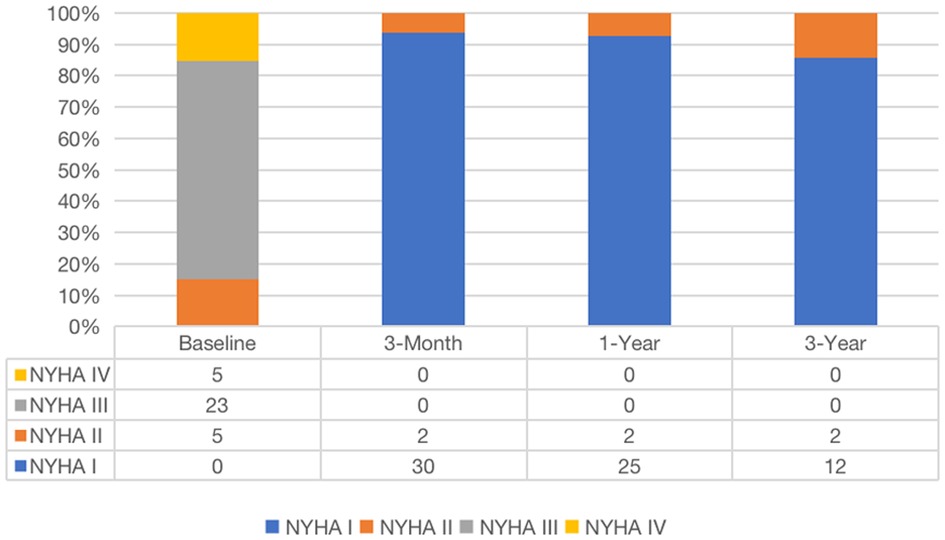
The median and maximum follow-up times were 28 and 45 months, respectively. Follow-up outcomes are shown in Table 3. The all-cause mortality was 6.1% (Figure 4): one patient died from pulmonary infection, and another experienced a sudden death for unknown reasons while sleeping at night. No cardiac deaths were recorded. Cerebral infarction occurred in two patients (6.1%): in one patient 10 months after surgery followed by left atrial appendage occlusion performed 7 months later; the other patient failed the ViV-TMVR due to a large left atrium and was converted to direct cardiac surgery with a mechanical prosthetic valve. There were no significant sequelae after thrombolytic therapy. Univariable Cox regression showed that only chronic obstructive pulmonary disease had a significant effect on survival. However, a multivariate Cox regression analysis did not identify any variables that significantly affected survival outcomes. The NYHA classification (Figure 5) and the KCCQ-12 score (Figure 6) were significantly improved when compared to their preoperative values. The mean changes in KCCQ-12 score from baseline three months and one year after the operation were 48.0 (95% confidence interval 46.0, 50.0) and 48.8 (95% confidence interval 47.1, 50.5), respectively (p < 0.001).
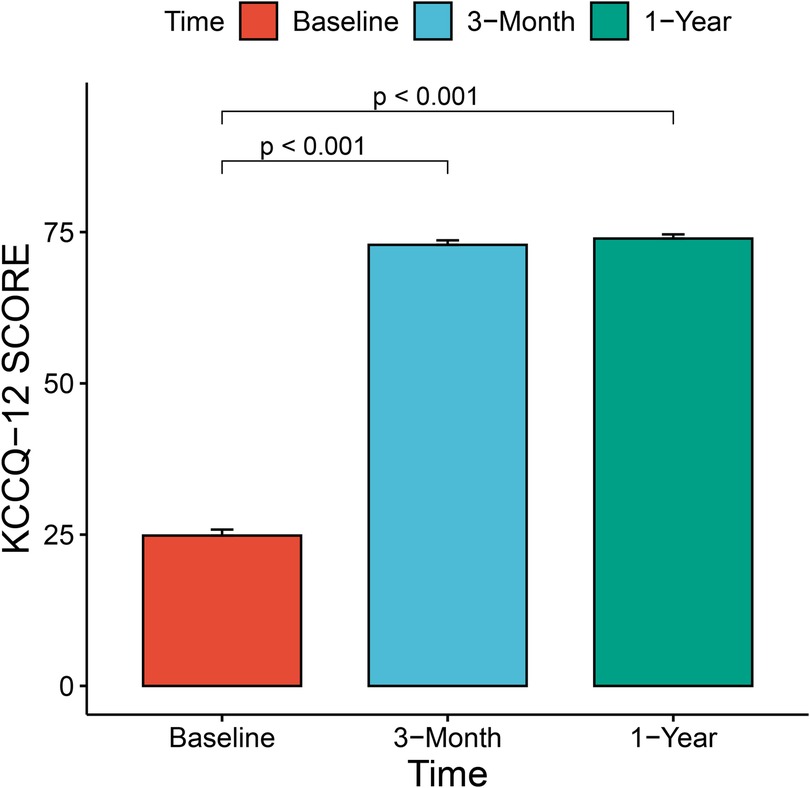
Figure 6. Change in patient health-related quality of life using the Kansas city cardiomyopathy questionnaire-12 score.
Discussion
The standard treatment for bioprosthetic valve failure is redo valve replacement (15, 16). However, studies (4, 6) have shown that ViV-TMVR is associated with lower in-hospital mortality, lower risk of complications, and lower need of resources. Previous studies have reported 30-day mortality rates between 3.2% and 7.5% and one-year mortality rates between 11.3% and 16.9% after ViV-TMVR (17–21). Outcomes from long-term follow-up are less commonly reported (22), with one study reporting a four-year mortality rate of 37.5%, a stroke incidence of less than 3%, and an LVOT obstruction incidence of 0% to 5% (18).
Our study demonstrated good mid-term clinical outcomes and health-related quality of life in patients who received ViV-TMVR, which is clearly better than reported by previous studies. In our study, no patients died immediately after the operation and the all-cause mortality during the follow-up with a median of 28 months was also low (6.1%). No LVOT obstructions were observed either. Mitral valve hemodynamics, NYHA classification and health-related quality of life were also significantly improved in patients after ViV-TMVR. This study used the KCCQ-12 score to reflect patients' health-related quality of life, which is a patient-reported outcome and more accurate than the NYHA classification for detecting changes in health status in patients with heart failure (23).
The advantages of J-Valve for ViV-TMVR
The J-Valve system consists of a self-expanding transcatheter valve and a transapical interventional device. Several studies (24–27) have confirmed its short- or medium-term safety and efficacy in the treatment of aortic valve disease.
Our center was the first to successfully complete ViV-TMVR using a reverse-loaded J-Valve in January 2019. ViV-TMVR was accomplished in an innovative way by changing the loading direction and release sequence without changing the structure of the J-Valve and conveyors. J-Valve has several advantages when applied to ViV-TMVR. First, its three grippers make leaflet-to-leaflet and commissure-to-commissure positioning simple, without the need to consider commissural misalignment (Figure 1B). The problem of misalignment due to ViV-TMVR has until now been largely ignored in clinical research. Correct orientation is mandatory for surgical bioprosthetic valve replacement (28), which means that commissural posts should not face the LVOT. The risk of LVOT obstruction may be increased if the THV commissure posts point toward the LVOT. There is however no way of preventing misalignment for balloon-expandable THV. Second, J-Valve also has U-shaped notches (Figure 1B) instead of a complete cylindrical metal stent, which minimizes the risk of LVOT obstruction and has particular advantages in patients with small left ventricular volumes (29), meaning that an evaluation of the neo-LVOT is not required (30). Third, because of the fixation of the grippers, the risk of the potentially fatal distant THV migration to the left atrial side (7–10) was reduced. Fourth, J-Valve can be used for both ViV-TAVR and ViV-TMVR, and even for tricuspid bioprosthetic valve failure, which allows valve-in-valve transcatheter tricuspid replacement (ViV-TTVR) using a right atrial approach. Fifth, the self-expanding THV can continuously apply a radial support force on the failed bioprosthetic valve's stents, so that the failed leaflets remain strongly anchored at the frame.
A CT imaging analysis should be considered during preoperative evaluation and planning, especially for evaluating the risk of LVOT obstruction. The two main risk factors for LVOT obstruction are the aortomitral angle and neo-LVOT area (31). The optimal size for the neo-LVOT is unknown, but a minimum of 200–250 mm2 has been suggested (32). In our study, LVOT obstruction has to our knowledge been never detected with postoperative TEE. Therefore, preoperative assessment of the risk of LVOT obstruction appeared to be unnecessary for J-Valve when ViV-TMVR was performed. The possibility to avoid CT imaging simplifies the pre-operative assessment procedure, demonstrating a further advantage of the J-Valve structure.
In addition, in our study, contrast agent were not needed to be used during the whole operation. This benefits patients with allergic asthma, abnormal thyroid function, and chronic renal insufficiency, and can reduce the risk of perioperative complications for such patients.
Surgical approach
The approach to ViV-TMVR can be divided into surgical access and complete percutaneous access. The corresponding approaches are transapical and transseptal, respectively. Currently, transapical approach is by far the most common way for transcatheter valve implantation in the mitral position. Updated data from the Valve-in-Valve-International-Data (VIVID) registry shows that the transapical approach is utilized in 81% of valve in valve cases and 68% in valve in ring cases (33). The proximity of the apex to the mitral valve allows for better control of the position of the delivery device with better coaxiality, does not require many guidewires and sheaths, and is suitable for surgeons with limited experience in performing this intervention. Attention should be drawn to the fact that transcatheter apical-related complications include not only the impairment of left ventricular apical function (34, 35), but also pleural effusion, bleeding, atrial fibrillation, and prolonged intubation time (36–39). Transseptal mitral valve implantations are becoming more common worldwide, and have the main advantage of being less invasive and not requiring open surgery or left ventricular trauma; unfortunately, the device has poor coaxiality with the mitral orifice plane. In addition, transseptal access requires puncture of the atrial septum and balloon atrial septostomy, which remains technically challenging and may present complications such as iatrogenic atrial septal defect (iASD), cardiac perforation and tamponade (40). Consequently, the transseptal approach is mainly suitable for surgeons with rich intervention experience.
In our study, only the transapical approach was used. The J-Valve system provides the most direct, shortest, and most coaxial access to the mitral valve. The transapical approach also enables treatment of aortic valve diseases or mitral perivalvular leak occlusion while performing ViV-TMVR. Seven patients in our study were treated for aortic valvular lesions and one patient for mitral perivalvular leak occlusion simultaneously while performing ViV-TMVR. From our experience, the transapical approach for simultaneous ViV-TMVR and ViV-TAVR appears to be operationally more convenient, allowing sequential release of both THVs from the same puncture site. In addition, none of the patients in this study experienced postoperative apical bleeding or complications such as guidewire-related cardiac injury, demonstrating the good safety of the transapical approach.
Limitations and future directions
The present investigation was a real-world, retrospective clinical study, which consequently comes with the limitations of an observational study. First, the study was conducted in a single center, so the selection of the patients may have been biased and the results are not necessarily generalizable for broader populations; however, the study population was enrolled consecutively to minimize selection bias. Second, considering that there are no long-term results of J-Valve for ViV-TMVR, we only performed this surgery on patients who are elderly, high-risk or surgically contraindicated, which resulted in a small sample size. Longer-term follow-up data are therefore needed. Third, in the absence of an echocardiographic core laboratory, echocardiographers were able to only determine the presence or absence of left ventricular outflow tract obstruction without recording the outflow tract flow velocities, making it impossible to track the values of the related variables or to compare differences in preoperative and postoperative outflow tract flow velocities. Fourth, some outcome measures were patient-reported, which may also cause bias.
Future multicenter clinical trials are needed to validate the safety and efficacy of the surgical approach addressed in our study. Studies that have sufficiently large sample sizes and long follow-up duration, and that include a control group, are needed also to identify the factors independently associated with survival.
Conclusion
This study demonstrates that J-Valve system is a safe and effective option for ViV-TMVR: it has a high success rate and low mortality, and resulted in very few complications. Mitral valve hemodynamics, NYHA classification and health-related quality of life also significantly improved in patients after ViV-TMVR with J-Valve. The innovative use of the J-Valve for ViV-TMVR is a promising alternative surgical option for the elderly, high-risk patients with bioprosthetic mitral valve failure. Future multicenter clinical trials with long-term follow-up are however needed to strengthen the evidence on the safety and efficacy of this surgical approach.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Beijing Anzhen Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL conducted the literature search and data acquisition, statistical analysis, manuscript preparation and revision; RL revised the paper, JZ, KW and JS helped with data collection and patient follow-up, ZZ edited the paper, JW revised the paper and provided administrative support, and HZ performed the surgery as the primary operator, drafted and revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Key R&D Program of China (2020YFC2008105).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1137663/full#supplementary-material
SUPPLEMENTARY VIDEO S1
Animated demonstration of applying J-Valve to ViV-TMVR surgical procedure.
References
1. Liew A, Eikelboom JW, O'Donnell M, Hart RG. Assessment of anticoagulation intensity and management of bleeding with old and new oral anticoagulants. Can J Cardiol. (2013) 29(7 Suppl):S34–44. doi: 10.1016/j.cjca.2013.04.013
2. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 Esc/eacts guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European society of cardiology (Esc) and the European association for cardio-thoracic surgery (eacts). Rev Esp Cardiol (Engl Ed). (2022) 75(6):524. doi: 10.1016/j.rec.2022.05.006
3. Writing Committee M, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, et al.. 2020 Acc/aha guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018
4. Khan M Z, Zahid S, Khan MU, Kichloo A, Jamal S, Mannan Khan Minhas A, et al. Redo surgical mitral valve replacement versus transcatheter mitral valve in valve from the national inpatient sample. J Am Heart Assoc. (2021) 10(17):e020948. doi: 10.1161/JAHA.121.020948
5. Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding corevalve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. (2007) 50(1):69–76. doi: 10.1016/j.jacc.2007.04.047
6. Zahid S, Ullah W, Hashem AM, Khan MZ, Gowda S, Vishnevsky A, et al. Transcatheter valve-in-valve implantation versus redo surgical mitral valve replacement in patients with failed mitral bioprostheses. EuroIntervention. (2022) 18(10):824–35. doi: 10.4244/EIJ-D-22-00437
7. Mick SL, Roselli EE, Kapadia S, Tuzcu EM, Krishnaswamy A, Svensson LG. Postoperative migration of an edwards-sapien Xt mitral valve-in-valve treated with direct vision implantation during beating-heart bypass. Ann Thorac Surg. (2016) 101(3):1182–5. doi: 10.1016/j.athoracsur.2015.04.145
8. Bapat VV, Khaliel F, Ihleberg L. Delayed migration of sapien valve following a transcatheter mitral valve-in-valve implantation. Catheter Cardiovasc Interv. (2014) 83(1):E150–4. doi: 10.1002/ccd.25076
9. Zegdi R, Allam B, Azarine A, Blanchard D. Late dislodgment of a prosthesis after mitral valve-in-valve implantation. J Thorac Cardiovasc Surg. (2014) 147(5):e59–61. doi: 10.1016/j.jtcvs.2013.11.067
10. Cheung A, Webb JG, Barbanti M, Freeman M, Binder RK, Thompson C, et al. 5-Year Experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol. (2013) 61(17):1759–66. doi: 10.1016/j.jacc.2013.01.058
11. Alperi A, Garcia S, Rodes-Cabau J. Transcatheter valve-in-valve implantation in degenerated surgical aortic and mitral bioprosthesis: current state and future perspectives. Prog Cardiovasc Dis. (2022) 72:54–65. doi: 10.1016/j.pcad.2021.10.001
12. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 Esc guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (Eacts): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (Esc) developed with the special contribution of the European heart rhythm association (Ehra) of the Esc. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
13. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document (varc-2). Eur J Cardiothorac Surg. (2012) 42(5):S45–60. doi: 10.1093/ejcts/ezs533
14. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas city cardiomyopathy questionnaire: a new health Status measure for heart failure. J Am Coll Cardiol. (2000) 35(5):1245–55. doi: 10.1016/s0735-1097(00)00531-3
15. Javadikasgari H, Chemtob RA, Gillinov AM, Pettersson GB, Lowry AM, Desai MY, et al. Outcomes of mitral valve Re-replacement for bioprosthetic structural valve deterioration. J Thorac Cardiovasc Surg. (2022) 163(5):1804–12.e5. doi: 10.1016/j.jtcvs.2020.08.067
16. Kilic A, Acker MA, Gleason TG, Sultan I, Vemulapalli S, Thibault D, et al. Clinical outcomes of mitral valve reoperations in the United States: an analysis of the society of thoracic surgeons national database. Ann Thorac Surg. (2019) 107(3):754–9. doi: 10.1016/j.athoracsur.2018.08.083
17. Hu J, Chen Y, Cheng S, Zhang S, Wu K, Wang W, et al. Transcatheter mitral valve implantation for degenerated mitral bioprostheses or failed surgical annuloplasty rings: a systematic review and meta-analysis. J Card Surg. (2018) 33(9):508–19. doi: 10.1111/jocs.13767
18. Simonato M, Whisenant B, Ribeiro HB, Webb JG, Kornowski R, Guerrero M, et al. Transcatheter mitral valve replacement after surgical repair or replacement: comprehensive midterm evaluation of valve-in-valve and valve-in-ring implantation from the vivid registry. Circulation. (2021) 143(2):104–16. doi: 10.1161/CIRCULATIONAHA.120.049088
19. Whisenant B, Kapadia SR, Eleid MF, Kodali SK, McCabe JM, Krishnaswamy A, et al. One-Year outcomes of mitral valve-in-valve using the sapien 3 transcatheter heart valve. JAMA Cardiol. (2020) 5(11):1245–52. doi: 10.1001/jamacardio.2020.2974
20. Yoon SH, Whisenant BK, Bleiziffer S, Delgado V, Schofer N, Eschenbach L, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol. (2017) 70(9):1121–31. doi: 10.1016/j.jacc.2017.07.714
21. Eleid MF, Whisenant BK, Cabalka AK, Williams MR, Nejjari M, Attias D, et al. Early outcomes of percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. (2017) 10(19):1932–42. doi: 10.1016/j.jcin.2017.08.014
22. Kamioka N, Babaliaros V, Morse MA, Frisoli T, Lerakis S, Iturbe JM, et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve-in-valve therapy. JACC Cardiovasc Interv. (2018) 11(12):1131–8. doi: 10.1016/j.jcin.2018.03.011
23. Greene SJ, Butler J, Spertus JA, Hellkamp AS, Vaduganathan M, DeVore AD, et al. Comparison of New York heart association class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. (2021) 6(5):522–31. doi: 10.1001/jamacardio.2021.0372
24. Luo X, Wang X, Li X, Wang X, Xu F, Liu M, et al. Transapical transcatheter aortic valve implantation using the J-valve system: a 1-year follow-up study. J Thorac Cardiovasc Surg. (2017) 154(1):46–55. doi: 10.1016/j.jtcvs.2017.03.054
25. Shi J, Wei L, Chen Y, Wang X, Ye J, Qin C, et al. Transcatheter aortic valve implantation with J-valve: 2-year outcomes from a multicenter study. Ann Thorac Surg. (2021) 111(5):1530–6. doi: 10.1016/j.athoracsur.2020.06.139
26. Xue Y, Zhou Q, Li S, Li J, Mu D, Luo X, et al. Transapical transcatheter valve replacement using J-valve for aortic valve diseases. Ann Thorac Surg. (2021) 112(4):1243–9. doi: 10.1016/j.athoracsur.2020.10.030
27. Zhu D, Chen Y, Guo Y, Hu J, Zhang J, Wei X, et al. Transapical transcatheter aortic valve implantation using a new second-generation tavi system—j-valve™ for high-risk patients with aortic valve diseases: initial results with 90-day follow-up. Int J Cardiol. (2015) 199:155–62. doi: 10.1016/j.ijcard.2015.07.037
28. Bortolotti U, Milano A, Tursi V, Minarini M, Thiene G, Mazzucco A. Fatal obstruction of the left ventricular outflow tract caused by low-profile bioprostheses in the mitral valve position. Chest. (1993) 103(4):1288–9. doi: 10.1378/chest.103.4.1288
29. Yoon SH, Bleiziffer S, Latib A, Eschenbach L, Ancona M, Vincent F, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv. (2019) 12(2):182–93. doi: 10.1016/j.jcin.2018.12.001
30. Anita W, Asgar AD, Messas N, Basmadjian A, Bouchard D, Pellerin M. Left ventricular outflow tract obstruction following mitral valve replacement: challenges for transcatheter mitral valve therapy. Structural Heart. (2018) 2(5):372–9. doi: 10.1080/24748706.2018.1494397
31. Blanke P, Naoum C, Dvir D, Bapat V, Ong K, Muller D, et al. Predicting lvot obstruction in transcatheter mitral valve implantation: concept of the neo-lvot. JACC Cardiovasc Imaging. (2017) 10(4):482–5. doi: 10.1016/j.jcmg.2016.01.005
32. Naoum C, Blanke P, Cavalcante JL, Leipsic J. Cardiac computed tomography and magnetic resonance imaging in the evaluation of mitral and tricuspid valve disease: implications for transcatheter interventions. Circ Cardiovasc Imaging. (2017) 10(3):e005331. doi: 10.1161/CIRCIMAGING.116.005331
33. Dvir D. Transseptal instead of transapical valve implantation: making mitral great again? JACC Cardiovasc Interv. (2016) 9(11):1175–7. doi: 10.1016/j.jcin.2016.04.006
34. Gutiérrez M, Rodés-Cabau J, Bagur R, Doyle D, DeLarochellière R, Bergeron S, et al. Electrocardiographic changes and clinical outcomes after transapical aortic valve implantation. Am Heart J. (2009) 158(2):302–8. doi: 10.1016/j.ahj.2009.05.029
35. Barbash IM, Dvir D, Ben-Dor I, Corso PJ, Goldstein SA, Wang Z, et al. Impact of transapical aortic valve replacement on apical wall motion. J Am Soc Echocardiogr. (2013) 26(3):255–60. doi: 10.1016/j.echo.2012.11.003
36. Biancari F, Rosato S, D'Errigo P, Ranucci M, Onorati F, Barbanti M, et al. Immediate and intermediate outcome after transapical versus transfemoral transcatheter aortic valve replacement. Am J Cardiol. (2016) 117(2):245–51. doi: 10.1016/j.amjcard.2015.10.036
37. Gada H, Kirtane AJ, Wang K, Lei Y, Magnuson E, Reynolds MR, et al. Temporal trends in quality of life outcomes after transapical transcatheter aortic valve replacement: a placement of aortic transcatheter valve (partner) trial substudy. Circ Cardiovasc Qual Outcomes. (2015) 8(4):338–46. doi: 10.1161/circoutcomes.114.001335
38. D'Onofrio A, Salizzoni S, Agrifoglio M, Lucchetti V, Musumeci F, Esposito G, et al. When does transapical aortic valve replacement become a futile procedure? An analysis from a national registry. J Thorac Cardiovasc Surg. (2014) 148(3):973–9; discussion 9-80. doi: 10.1016/j.jtcvs.2014.06.015
39. Ghatak A, Bavishi C, Cardoso RN, Macon C, Singh V, Badheka AO, et al. Complications and mortality in patients undergoing transcatheter aortic valve replacement with Edwards Sapien & Sapien Xt valves: a meta-analysis of world-wide studies and registries comparing the transapical and transfemoral accesses. J Interv Cardiol. (2015) 28(3):266–78. doi: 10.1111/joic.12201
Keywords: valve-in-valve transcatheter mitral valve replacement, failed mitral bioprosthetic valves, transapical approach, health-related quality of life outcomes, Kansas city cardiomyopathy questionnaire-12
Citation: Li Y, Lei R, Zhou J, Wu K, Shen J, Zhu Z, Wang J and Zhang H (2023) Innovative use of a self-expanding valve for valve-in-valve transcatheter mitral valve replacement: experience from a four-year single-center study. Front. Cardiovasc. Med. 10:1137663. doi: 10.3389/fcvm.2023.1137663
Received: 4 January 2023; Accepted: 31 May 2023;
Published: 12 June 2023.
Edited by:
Omar Chehab, St Thomas’ Hospital, United KingdomReviewed by:
Alberto Guido Pozzoli, Ospedale Regionale di Lugano, SwitzerlandPablo Avanzas, University of Oviedo, Spain
Salman Zahid, Rochester General Hospital, United States
© 2023 Li, Lei, Zhou, Wu, Shen, Zhu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangang Wang amlhbmdhbmd3YW5nQGNjbXUuZWR1LmNu Haibo Zhang emhhbmdoYjIzMThAMTYzLmNvbQ==
†ORCID Yuehuan Li orcid.org/0000-0001-8836-0198
 Yuehuan Li
Yuehuan Li Ruobing Lei2
Ruobing Lei2 Jiawei Zhou
Jiawei Zhou Kaisheng Wu
Kaisheng Wu Haibo Zhang
Haibo Zhang