
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 July 2023
Sec. Cardioneurology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1135069
 Yong Soo Kim1,2
Yong Soo Kim1,2 Han-Gil Jeong3*†
Han-Gil Jeong3*† In-Chang Hwang4
In-Chang Hwang4 Beom Joon Kim1
Beom Joon Kim1 Joon-Myung Kwon5
Joon-Myung Kwon5 Hee-Joon Bae1
Hee-Joon Bae1 Moon-Ku Han1
Moon-Ku Han1
Background and purpose: Tricuspid regurgitation (TR) is a common but overlooked valvular disease, and its association with the etiologic subtypes of ischemic stroke is unclear. We explored the relationship between TR and atrial fibrillation (AF) in patients with acute ischemic stroke.
Methods: This retrospective analysis of ongoing stroke registry assessed 6,886 consecutive acute ischemic stroke patients who underwent transthoracic echocardiography during their in-hospital care. Multivariable logistic regression models adjusted for age, sex, stroke characteristics, and echocardiographic indices were used to investigate the association between TR and total AF, and newly diagnosed AF during hospitalization and a 1-year follow-up period, respectively.
Results: TR was present in 877 (12.7%) patients (mild, 9.9%; moderate, 2.4%; severe, 0.5%). AF was identified in 24.1% (medical history, 11.1%; first detected in the emergency room, 6.6%; newly diagnosed after admission, 6.4%). TR was associated with AF [adjusted odds ratio (aOR) 4.87 (95% confidence interval (CI), 2.63–9.03)], compared with no/trivial TR. The association between TR and AF was consistent regardless of severity (aOR [95% CI], 4.57 [2.63–7.94] for mild and 7.05 [2.57–19.31] for moderate-to-severe TR) or subtype of TR (5.44 [2.91–10.14] for isolated and 3.81 [2.00–7.28] for non-isolated TR). Among the AF-naïve patients at admission, TR was associated with newly diagnosed AF during hospitalization and a 1-year follow-up period (aOR [95% CI], 2.68 [1.81–3.97]).
Conclusions: TR is associated with AF in acute ischemic stroke patients regardless of severity and subtypes of TR. TR is also associated with newly diagnosed AF after stroke.
More than 20% of ischemic stroke cases are caused by cardiac thromboembolism resulting from to atrial fibrillation (AF), and the prevalence of AF among stroke patients is increasing (1). AF-related strokes are more fatal or disabling compared to strokes caused by other etiologies and have the highest in-hospital mortality among ischemic stroke (2, 3). Because most AF-related strokes can be prevented by oral anticoagulants (OAC), echocardiographic or electrophysiologic biomarkers of AF have been studied to detect subclinical AF for effective prevention of stroke. However, previous predictors of hidden AF, including left atrial (LA) enlargement, have primarily focused on the left heart (4). Considering recent evidence linking right atrial (RA) structural remodeling to the development of incident AF, biomarkers associated with right heart may play a supportive role in estimating the risk of subclinical AF (5).
Tricuspid regurgitation (TR), a common valvular heart disease but long considered the “forgotten valve”, is one condition that may be associated with right heart remodeling. Over 90% of TR cases are functional and occurs secondary to various left-sided heart diseases or pulmonary hypertension (6, 7). Long-standing AF is another significant risk factor for TR, as it causes remodeling of the RA and tricuspid valve annulus (8). Moderate or severe TR occurs in approximately 30% of patients with newly diagnosed AF, and rhythm control of AF reduces the risk of TR (9). However, although it has recently been suggested that atrial cardiopathy, which can exist without overt AF, can potentially promote the development of AF and cause ischemic stroke, whether TR related right heart remodeling is a precursor to AF is not well defined (4, 10). Right-sided heart disease induces re-entrant activity in the RA through RA remodeling and can create a substrate for AF maintenance (11). Thus, TR could potentially serve as a surrogate marker or an accelerating factor of AF by causing atrial remodeling.
Understanding the association between TR and subclinical AF can help in the selection of appropriate candidates for extensive cardiac monitoring to detect AF in patients with ischemic stroke. In this study, our aim was to investigate the relationship between TR characteristics and AF in patients with acute ischemic stroke and determine whether TR is associated with newly diagnosed AF in AF-naïve stroke patients.
The 11,623 consecutive patients with stroke admitted to a single tertiary referral hospital between January 1, 2011 and December 31, 2020 were screened for this observational, retrospective, case–control study. We included individuals who had ischemic stroke or lesion-positive transient ischemic attack (TIA) confirmed by neuroimaging (n = 9,409), and further selected those who underwent transthoracic echocardiography (TTE) during their in-hospital care for acute stroke (n = 6,901). Lesion positive TIA was defined when the stroke symptom improved within 24 h and there was no evidence of stroke on initial brain imaging, but the ischemic lesion was identified on follow-up brain imaging. After excluding 15 patients without information on tricuspid valve function on TTE, 6,886 patients were finally included in this analysis.
This study involving human participants was reviewed and approved by the institutional review board of Seoul National University Bundang Hospital (approval number: B-2108/701-102). The requirement for written informed consent was waived by the institutional review board of Seoul National University Bundang Hospital due to the retrospective nature of the study.
Clinical information of patients, including demographics, vascular risk factors, and stroke characteristics were obtained from our prospectively collected stroke registry database. Data on the following vascular risk factors were retrieved for the study: history of stroke, hypertension, diabetes mellitus, hyperlipidemia, AF, smoking habits, and anemia. The initial severity of stroke upon arrival at the emergency department was measured using the National Institutes of Health Stroke Scale (NIHSS) score. Functional status before and after the stroke event was rated using the modified Rankin Scale (mRS).
AF was defined when a patient or family member self-reported that the patient had been diagnosed with AF prior to the stroke event, or when AF was diagnosed by 12-lead electrocardiogram (ECG), Holter monitoring, or implantable cardiac monitoring. When AF was suspected during the continuous ECG monitoring in a stroke unit or an intensive care unit, examination by 12-lead ECG was required to confirm AF diagnosis. AF-naïve patients were those with no diagnosis of AF at the time of admission by medical history or by the first 12-lead ECG in the emergency department. Paroxysmal AF was defined when normal sinus rhythm was restored within 7 days, while sustained AF was defined when the AF lasted more than 7 days (12). To investigate the presence of new onset AF in AF-naïve patients, the findings from 12-lead ECG, Holter monitoring, and implantable cardiac monitoring devices during hospitalization and over a 1-year follow-up period were reviewed.
The extent of diagnostic evaluation and acute treatment strategies were determined according to the institutional protocols, based on the guidelines of the American Heart Association/American Stroke Association, and were at the discretion of the stroke management physician (13).
TTE images obtained during admission were investigated. Echocardiographic assessments were performed according to the current guidelines of the American Society of Echocardiography and included M-mode, two-dimensional, and Doppler echocardiographic measurements (14). Mitral E velocity, septal mitral annular e′ velocity, E/e′ ratio, left ventricular (LV) ejection fraction, LA volume index, right ventricular (RV) systolic pressure, LV end-diastolic volume, LV end-systolic volume, LV mass index, severity of TR, aortic stenosis (AS), aortic regurgitation (AR), mitral stenosis (MS), and mitral regurgitation (MR) were assessed. Severity of TR was graded according to the structural findings (i.e., RA/RV size and TV morphology) of tricuspid valve, qualitative Doppler assessment, and semi-quantitative assessment. Regarding trivial TR as insignificant and due to small number of patients with severe TR, the severity of TR was divided into three categories: no or trivial TR, mild TR, and moderate-to-severe TR.
Isolated TR was diagnosed when the following conditions were met: (1) TR holosystolic and functional, (2) absence of pulmonary hypertension (RV systolic pressure <50 mmHg), (3) LV ejection fraction ≥50%, (4) absence of other moderate or severe valvular heart diseases, (5) absence of pacemaker or defibrillator wire across the tricuspid valve, (6) absence of congenital or pericardial disease, or (7) no history of valve surgery (15). Alternatively, TR was classified as non-isolated TR.
We compared the clinical and echocardiographic characteristics of patients according to the presence of TR and AF, using χ2 tests for categorical variables and t-tests for continuous variables, respectively. Multivariable logistic regression models were constructed to test the association of AF in acute ischemic stroke patients with the presence, severity, and subtype of TR. As a subgroup analysis, the association between TR and newly diagnosed AF during hospitalization and during the 1-year follow-up was assessed in patients who were AF-naïve at the time of admission. Adjustments for confounders were performed for variables with bivariate p-values <0.10 or for those that were considered clinically relevant. Multiple-imputation chained equations were used to impute the missing echocardiographic indices (16). The results of the regression models were reported using odds ratios (ORs) with 95% confidence interval (CIs), as appropriate.
Details of the multivariable models, data imputation, and complete case analysis are provided in Supplementary Methods. The levels of statistical significance were set at a p-value of 0.05 for two-tailed tests. The statistical analyses were performed using R version 4.0.3 (R Development Core Team, Vienna, Austria). The R packages used in this analysis were “MASS”, “Hmisc”, “tidyverse”, “descr”, “readxl”, “compareGroups”, “car” and “mice”.
Among the 11,623 patients who were screened for the study, 6,886 patients were finally eligible for this retrospective analysis (Supplementary Figure S1). A comparison of the eligible patients with the excluded patients (without TTE) is presented in Supplementary Table S1. The mean [SD] age of the eligible patients was 67.9 [13.4] years; 4,103 [59.6%] were male, and 1,409 [20.5%] had a history of stroke or TIA. Among the eligible patients, 6,859 [99.6%] were admitted to a stroke unit or an intensive care unit for a median duration of 20 h [interquartile range (IQR) 18–48 h]. At least one 12-lead ECG measurement was obtained in 99.0% of patients [2 (1–4) times per patient]. Holter and implantable cardiac monitoring were performed in 3,791 [55.1%] and 107 [1.6%] of the patients, respectively. The median [IQR] time from admission to TTE examination was 2.5 [1.4–4.3] days. AF was diagnosed in 1,658 [24.1%] of the patients, 1,216 [73.3%] of whom were diagnosed by medical history or first 12-lead ECG at the emergency department, while 442 [26.7%] were newly diagnosed during hospitalization or during the 1-year follow-up. Paroxysmal AF and sustained AF were detected in 518 [31.2%] and 1,140 [68.8%] of patients, respectively.
In the population with ischemic stroke, TR (≥mild) was observed in 877 [12.7%] of the patients, and the severity of TR was mild in 680 [9.9%], moderate in 164 [2.4%], and severe in 33 [0.5%]. Isolated TR and non-isolated TR was present in 498 [7.2%] and 364 [5.3%] of the patients, respectively. TR was due to primary tricuspid abnormalities in 3 patients, pacemaker/defibrillator in situ in 23 patients, abnormal right ventricular dilatation in 34 patients, previous valve surgery in 42 patients, and suspected congenital heart disease in 31 patients. Patients with TR were older and were more often dependent on others prior to stroke onset (mRS ≥ 3), had more severe neurological symptoms at onset, and had more multifocal acute ischemic lesions on cerebral imaging. Patients with TR more often had a history of stroke and hypertension, and less frequently had diabetes mellitus or smoked, compared with those without TR. AF was more frequently present in patients with TR than in patients without TR (594 [67.7%] vs. 1,064 [17.7%], p-value <0.01). Patients with TR had a lower LV ejection fraction, higher E/e′ ratio, higher LA volume index, and more often presented with left-sided valvular heart disease than their counterpart (Table 1). A comparison of the patient's characteristics according to the severity and subtype of TR is provided in Supplementary Table S2.
Patients with AF were older than those without AF and their neurological deficit at baseline was more severe (Supplementary Table S3). According to TTE, patients with AF had a lower LV ejection fraction, a higher E/e′ ratio, a higher LA volume index, a higher RV systolic pressure, and more left-sided valvular heart disease. TR was observed in 594 [35.8%] of patients with AF (mild TR, 434 [26.2%]; moderate-to-severe TR, 160 [9.7%]) and in 283 [5.4%] of patients without AF (mild TR, 246 [4.7%]; moderate-to-severe TR, 37 [0.7%]). Isolated TR accounted for 315 [19.4%] of the patients with AF and 183 [3.6%] of the patients without AF. The prevalence of AF increased with increasing severity of TR, in all strata of the LA volume index (Figure 1).
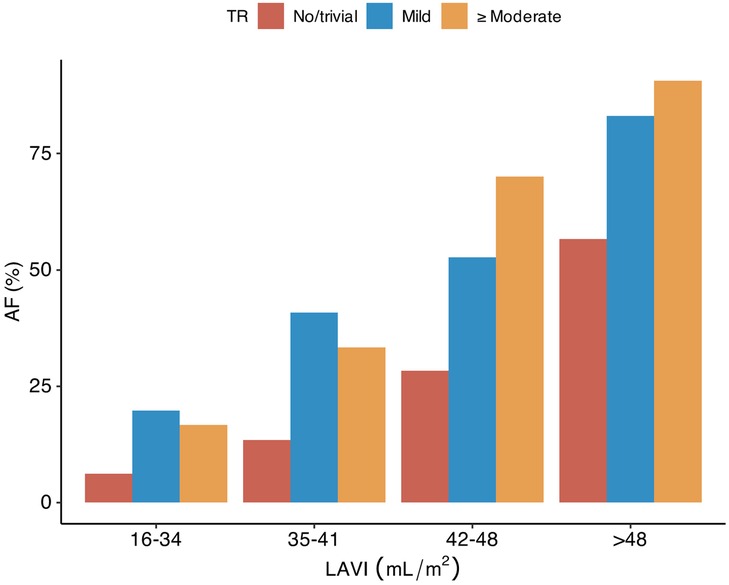
Figure 1. Tricuspid regurgitation, LA volume index, and atrial fibrillation in patients with acute ischemic stroke. TR, tricuspid regurgitation; LA, left atrium; LAVI, left atrial volume index; AF, atrial fibrillation.
The multivariable logistic regression model showed a significant association between the presence of any type of TR and AF (adjusted OR [aOR], 4.87 [95% CI 2.63–9.03]) (Table 2). Mild TR [aOR, 4.57 (2.63–7.94)], as well as moderate-to-severe TR [aOR 7.05 (2.57–19.31)], was associated with AF compared to no/trivial TR. Both isolated TR [aOR 5.44 (2.91–10.14)] and non-isolated TR [aOR 3.81 (2.00–7.28)] showed a significant association with AF. Details of the multivariable models are described in Supplementary Table S4. Complete case analyses without multiple imputations showed similar results (Supplementary Table S5).
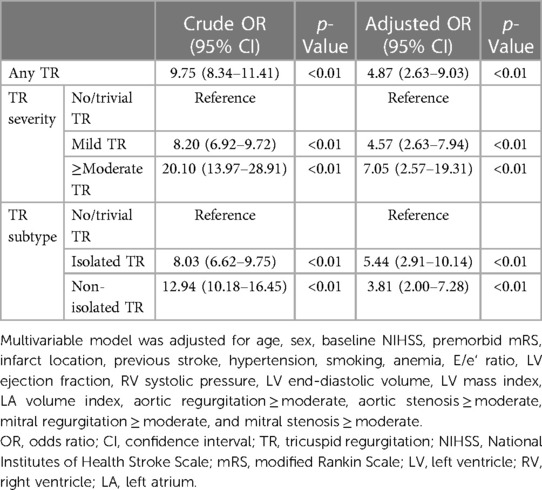
Table 2. Multivariable logistic regression analyses for atrial fibrillation in patients with acute ischemic stroke.
In subgroup of 5,576 AF-naïve patients at the time of admission, AF was newly diagnosed in 442 [7.9%] patients during admission or during the 1-year follow-up (Table 3): by 12-lead ECG in 330 [5.9%], by Holter monitoring in 86 [1.5%], and by an implantable cardiac monitoring device in 26 [0.5%]. The detection rate of newly diagnosed AF was higher in patients with TR than in patients without TR, in all strata of the LA volume index (Supplementary Figure S2). The presence of TR was associated with the detection of AF during admission and during the 1-year follow-up [aOR 2.68 (1.81–3.97)] (Table 4). In addition, in patients without AF at discharge, the presence of TR was also associated with newly diagnosed AF after discharge (Supplementary Table S6).
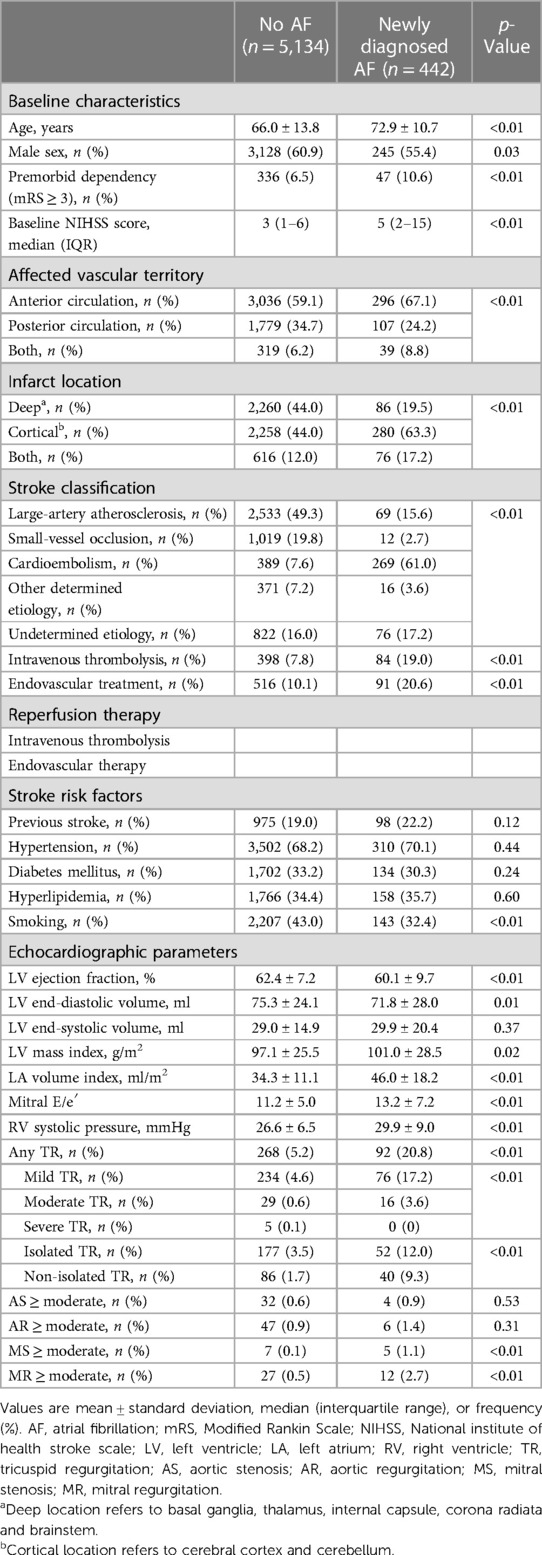
Table 3. Comparison of patients according to newly diagnosed atrial fibrillation after ischemic stroke in AF-naïve patients at admission.
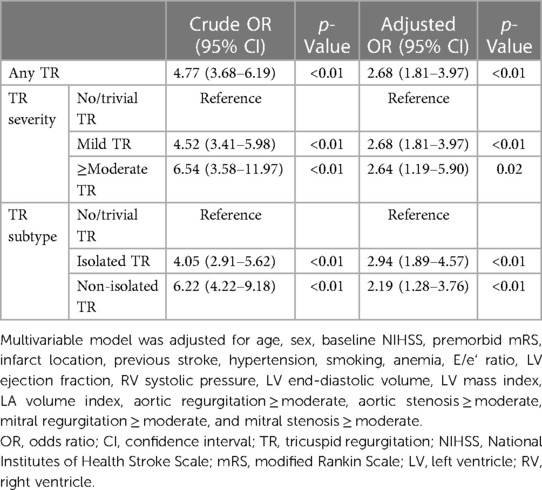
Table 4. Multivariable logistic regression analyses for newly diagnosed atrial fibrillation after ischemic stroke in AF-naïve patients at admission.
In our study, TR was significantly associated with AF in patients with acute ischemic stroke, and the association was evident even in those with mild TR and isolated functional TR. These results imply that TR may play a significant role in increasing susceptibility to AF. Newly diagnosed AF during hospitalization and the follow-up period was more frequently observed in patients with TR than in patients without TR. This finding suggests that TR needs to be considered as a potential risk factor for subclinical AF in the stroke population.
Cardiac evaluation in patients with ischemic stroke has focused on the left side of the heart because the thrombus causing cardioembolic stroke is mostly formed in the left heart chamber. However, as the heart is a single organ that has a connected tissue structure and electrical conduction system, both sides of the heart can interact in promoting atrial remodeling, which affects the initiation, maintenance, and progression of AF. Recently, an additional role of right-sided heart disease on the initiation or maintenance of AF has been attracting interest. Pulmonary artery hypertension or chronic obstructive lung disease are known to be related to AF (11, 17, 18). The resulting right heart remodeling can promote right atrial remodeling, which can act as a vulnerable intracardiac substrate for AF (11, 19). Our study investigated the association between TR, as a surrogate marker of right atrial remodeling, and AF in patients with acute ischemic stroke.
Chronic AF causes bilateral atrial enlargement (20). The LA volume index has previously been shown to have a positive relationship with AF in stroke patients, but the cut-off value of the LA volume index for defining a population with a high risk of AF is unclear (4, 21). AF-related dilatation of the atrium results in atrioventricular valve annulus dilation and enhances retrograde flow during systole (22). Due to the relatively thin right atrial wall and less developed fibrous skeleton of the annulus, the tricuspid valve is more prone to AF-related cardiac structural remodeling than the mitral valve (22–24). This phenomenon was also observed in this study, as AF was more frequently accompanied by TR than by MR, which implies that right atrial remodeling, reflected as TR, may be useful for identifying patients with a high risk of AF.
Previously, TR was considered as a consequence of tricuspid valve annulus dilatation due to long-standing AF-associated RA remodeling (6). However, in this study, approximately 24% of AF cases detected in patients with TR were paroxysmal. This suggests that TR may also serve to initiate and maintain paroxysmal AF that is sufficient to cause cardioembolic stroke. AF is typically thought to originate in the triggering foci located at the pulmonary vein myocardial sleeve (25). However, pulmonary vein isolation with catheter ablation was less effective in persistent AF cases, and several non-pulmonary vein triggers, such as those in the RA and coronary sinus, were suggested as additional ablation targets (26). The RA is one of the candidates for an AF substrate, but the factors causing RA remodeling remain unclear (27–30). TR may be a surrogate marker of RA remodeling that directly causes RA-related AF or secondary changes in LA-related AF, which can further aggravate RA remodeling and AF through a vicious cycle in patients with ischemic stroke.
In approximately 20% of patients with ischemic stroke, no probable cause of stroke can be identified after adequate diagnostic evaluation (31, 32). Subclinical AF accounts for 10%–15% of these undetermined causes of stroke, and identifying subclinical AF is crucial for appropriate secondary prevention of stroke (33, 34). Even if the index stroke was a non-AF related stroke, such as stroke due to large artery atherosclerosis or small vessel disease, AF detection after stroke usually compels physicians to initiate anticoagulation therapy. Atrial cardiopathy or atrial conduction abnormality including Bayes syndrome without overt AF is a high-risk cause of embolism in patients with an undetermined cause of stroke, and long-term monitoring of cardiac rhythm in this population is reasonable (10, 35, 36). The efficacy of anticoagulants in patients with evidence of atrial cardiopathy and cryptogenic stroke is being investigated in a randomized multicenter clinical trial (37). The association of TR and newly diagnosed AF in our study suggests that patients with TR may benefit from long-term cardiac monitoring to detect subclinical AF.
Moderate-to-severe TR, traditionally known as a bystander of left-sided heart disease, has recently been acknowledged to be associated with poor cardiovascular outcome (38). Although mild TR has been considered a benign echocardiographic finding due to its limited hemodynamic effect, recent reports have demonstrated that mild TR could progress and lead to cardiovascular morbidities, such as new onset AF (39). The association between mild TR and AF in our study suggests the potential role of mild TR in thromboembolic events and as a marker or promoter of atrial cardiopathy and AF.
This study had several limitations. First, this was a retrospective, single-center study with wide period of enrollment, implying that selection bias might have existed. Approximately 27% of patients with ischemic stroke or lesion positive TIA were excluded because an echocardiogram was not performed. Second, we did not have quantified measurements of right heart remodeling, including RA diameter or volume, RV systolic function, RV dimension, or TR severity at the time of AF detection. Additionally, unmeasured confounders, including the presence of thyroid disease, may have affected the relationship between TR and AF. Thus, this study does not provide sufficient evidence of a causal relationship between TR and AF, which is still speculative. Third, advanced cardiac images to assess tricuspid valve structure, including cardiac MRI, was not evaluated, which may underestimate the organic causes of TR. Fourth, the intensity of diagnostic work-up for AF was very heterogenous and all data from continuous ECG monitoring in the stroke units or intensive care units were not systemically reviewed. Fifth, we did not investigate the prevalence and risk of ischemic stroke in the general population with TR. Sixth, since we considered trivial TR as non-significant finding, hemodynamic effects of trivial TR may be underestimated. Lastly, we used multiple imputations, and the possible discrepancy between imputed data and real data should be considered.
In conclusion, our study found that TR was significantly associated with AF in patients with stroke, even in cases of mild or isolated TR. Furthermore, TR was associated with newly diagnosed AF during admission and during the 1-year follow-up period after ischemic stroke. TR, due to atrial remodeling or as a promoter of atrial cardiopathy, may be closely associated with the occurrence of cardioembolic stroke attributable to AF. Future studies investigating the causal relationship between preexisting TR and the development of new AF will help understanding atrial cardiopathy, which may be a potential treatment target in patients with ischemic stroke. Presence of significant TR and right heart dysfunction needs to be considered in future studies aiming to find hidden AF in patients with stroke.
The raw data supporting the conclusions of this article will be made available by the authors, upon request, to any qualified researcher.
This study was approved by the institutional review board of Seoul National University Bundang Hospital (approval number: B-2108/701-102). The requirement for written informed consent was waived by the institutional review board of Seoul National University Bundang Hospital due to the retrospective nature of the study.
H-GJ and YK contributed to conception, design of the study, performed statistical analysis, and drafted the manuscript. H-GJ, YK, I-CH, BK, H-JB, and M-KH contributed to analysis, or interpretation of data. I-CH, BK, J-MK, H-JB, and M-KH critically revised the manuscript for important intellectual consent. M-KH and H-GJ supervised the study. All authors contributed to the article and approved the submitted version.
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (grant number NRF-2020M3E5D9079768).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1135069/full#supplementary-material
1. Tsang TSM, Petty GW, Barnes ME, O’Fallon WM, Bailey KR, Wiebers DO, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota. J Am Coll Cardiol. (2003) 42(1):93–100. doi: 10.1016/S0735-1097(03)00500-X
2. Hayden DT, Hannon N, Callaly E, Ni Chroinin D, Horgan G, Kyne L, et al. Rates and determinants of 5-year outcomes after atrial fibrillation-related stroke: a population study. Stroke. (2015) 46(12):3488–93. doi: 10.1161/STROKEAHA.115.011139
3. Arboix A, Alio J. Acute cardioembolic stroke: an update. Expert Rev Cardiovasc Ther. (2011) 9(3):367–79. doi: 10.1586/erc.10.192
4. Jordan K, Yaghi S, Poppas A, Chang AD, Mac Grory B, Cutting S, et al. Left atrial volume index is associated with cardioembolic stroke and atrial fibrillation detection after embolic stroke of undetermined source. Stroke. (2019) 50(8):1997–2001. doi: 10.1161/STROKEAHA.119.025384
5. Ko KY, Jang JH, Choi SH, Baek YS, Kwon SW, Park SD, et al. Impact of right atrial enlargement on clinical outcome in patients with atrial fibrillation. Front Cardiovasc Med. (2022) 9:989012. doi: 10.3389/fcvm.2022.989012
6. Asmarats L, Taramasso M, Rodes-Cabau J. Tricuspid valve disease: diagnosis, prognosis and management of a rapidly evolving field. Nat Rev Cardiol. (2019) 16(9):538–54. doi: 10.1038/s41569-019-0186-1
7. Antunes MJ, Rodriguez-Palomares J, Prendergast B, De Bonis M, Rosenhek R, Al-Attar N, et al. Management of tricuspid valve regurgitation: position statement of the European society of cardiology working groups of cardiovascular surgery and valvular heart disease. Eur J Cardiothorac Surg. (2017) 52(6):1022–30. doi: 10.1093/ejcts/ezx279
8. Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography. (2012) 29(2):140–6. doi: 10.1111/j.1540-8175.2011.01565.x
9. Patlolla SH, Schaff HV, Nishimura RA, Stulak JM, Chamberlain AM, Pislaru SV, et al. Incidence and burden of tricuspid regurgitation in patients with atrial fibrillation. J Am Coll Cardiol. (2022) 80(24):2289–98. doi: 10.1016/j.jacc.2022.09.045
10. Jalini S, Rajalingam R, Nisenbaum R, Javier AD, Woo A, Pikula A. Atrial cardiopathy in patients with embolic strokes of unknown source and other stroke etiologies. Neurology. (2019) 92(4):e288–94. doi: 10.1212/WNL.0000000000006748
11. Hiram R, Naud P, Xiong F, Al-U'datt D, Algalarrondo V, Sirois MG, et al. Right atrial mechanisms of atrial fibrillation in a rat model of right heart disease. J Am Coll Cardiol. (2019) 74(10):1332–47. doi: 10.1016/j.jacc.2019.06.066
12. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2014) 64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022
13. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50(12):e344–418. doi: 10.1161/STR.0000000000000211
14. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
15. Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. (2014) 7(12):1185–94. doi: 10.1016/j.jcmg.2014.07.018
16. Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: Wiley-Interscience (2004). p. xxix, 287.
17. Goudis CA. Chronic obstructive pulmonary disease and atrial fibrillation: an unknown relationship. J Cardiol. (2017) 69(5):699–705. doi: 10.1016/j.jjcc.2016.12.013
18. Rajdev A, Garan H, Biviano A. Arrhythmias in pulmonary arterial hypertension. Prog Cardiovasc Dis. (2012) 55(2):180–6. doi: 10.1016/j.pcad.2012.06.002
19. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. (2014) 114(9):1483–99. doi: 10.1161/CIRCRESAHA.114.302226
20. Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. (1990) 82(3):792–7. doi: 10.1161/01.CIR.82.3.792
21. Tan BYQ, Ho JSY, Sia CH, Boi Y, Foo ASM, Dalakoti M, et al. Left atrial volume index predicts new-onset atrial fibrillation and stroke recurrence in patients with embolic stroke of undetermined source. Cerebrovasc Dis. (2020) 49(3):285–91. doi: 10.1159/000508211
22. Muraru D, Guta AC, Ochoa-Jimenez RC, Bartos D, Aruta P, Mihaila S, et al. Functional regurgitation of atrioventricular valves and atrial fibrillation: an elusive pathophysiological link deserving further attention. J Am Soc Echocardiogr. (2020) 33(1):42–53. doi: 10.1016/j.echo.2019.08.016
23. Utsunomiya H, Itabashi Y, Mihara H, Berdejo J, Kobayashi S, Siegel RJ, et al. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. (2017) 10(1):e004897. doi: 10.1161/CIRCIMAGING.116.004897
24. Zhou X, Otsuji Y, Yoshifuku S, Yuasa T, Zhang H, Takasaki K, et al. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ J. (2002) 66(10):913–6. doi: 10.1253/circj.66.913
25. Khan R. Identifying and understanding the role of pulmonary vein activity in atrial fibrillation. Cardiovasc Res. (2004) 64(3):387–94. doi: 10.1016/j.cardiores.2004.07.025
26. Parameswaran R, Al-Kaisey AM, Kalman JM. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol. (2021) 18(3):210–25. doi: 10.1038/s41569-020-00451-x
27. Lin YJ, Tai CT, Kao T, Tso HW, Huang JL, Higa S, et al. Electrophysiological characteristics and catheter ablation in patients with paroxysmal right atrial fibrillation. Circulation. (2005) 112(12):1692–700. doi: 10.1161/CIRCULATIONAHA.104.512731
28. Johner N, Namdar M, Shah DC. Right atrial complexity evolves with stepwise left-sided persistent atrial fibrillation substrate ablation and predicts outcomes. JACC Clin Electrophysiol. (2020) 6(13):1619–30. doi: 10.1016/j.jacep.2020.06.021
29. Al-Kaisey AM, Parameswaran R, Joseph SA, Kistler PM, Morton JB, Kalman JM. Extensive right atrial free wall low-voltage zone as the substrate for atrial fibrillation: successful ablation by scar homogenization. Europace. (2021) 23(1):59–64. doi: 10.1093/europace/euaa233
30. Ghannam M, Jame S, Jongnarangsin K, Cheng YW, Gunda S, Fadahunsi O, et al. Catheter ablation of the left and right atrial appendages without isolation in persistent atrial fibrillation. Heart Rhythm. (2021) 18(5):694–701. doi: 10.1016/j.hrthm.2021.01.006
31. Saver JL. CLINICAL PRACTICE. Cryptogenic stroke. N Engl J Med. (2016) 374(21):2065–74. doi: 10.1056/NEJMcp1503946
32. Veltkamp R, Pearce LA, Korompoki E, Sharma M, Kasner SE, Toni D, et al. Characteristics of recurrent ischemic stroke after embolic stroke of undetermined source: secondary analysis of a randomized clinical trial. JAMA Neurol. (2020) 77(10):1233–40. doi: 10.1001/jamaneurol.2020.1995
33. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. (2014) 370(26):2478–86. doi: 10.1056/NEJMoa1313600
34. Wachter R, Gröschel K, Gelbrich G, Hamann GF, Kermer P, Liman J, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (find-AF RANDOMISED): an open-label randomised controlled trial. Lancet Neurol. (2017) 16(4):282–90. doi: 10.1016/S1474-4422(17)30002-9
35. Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci. (2019) 4(5):640–54. doi: 10.1016/j.jacbts.2019.05.005
36. Arboix A, Marti L, Dorison S, Sanchez MJ. Bayes syndrome and acute cardioembolic ischemic stroke. World J Clin Cases. (2017) 5(3):93–101. doi: 10.12998/wjcc.v5.i3.93
37. Kamel H, Longstreth WT Jr., Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, et al. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. (2019) 14(2):207–14. doi: 10.1177/1747493018799981
38. Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv-Baran T, Richert E, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. (2020) 21(2):157–65. doi: 10.1093/ehjci/jez216
Keywords: atrial fibrillation, atrial cardiopathy, ischemic stroke, stroke subtype, tricuspid regurgitation
Citation: Kim YS, Jeong H-G, Hwang I-C, Kim BJ, Kwon J-M, Bae H-J and Han M-K (2023) Tricuspid regurgitation: a hidden risk factor for atrial fibrillation related stroke? Front. Cardiovasc. Med. 10:1135069. doi: 10.3389/fcvm.2023.1135069
Received: 31 December 2022; Accepted: 29 June 2023;
Published: 18 July 2023.
Edited by:
Gani Bajraktari, Umeå University, SwedenReviewed by:
Adria Arboix, Sacred Heart University Hospital, Spain© 2023 Kim, Jeong, Hwang, Kim, Kwon, Bae and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han-Gil Jeong aGFuLmcuamVvbmdAZ21haWwuY29t
†ORCID Han-Gil Jeong orcid.org/0000-0002-1418-1768
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.