
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med., 12 April 2023
Sec. Heart Valve Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1134828
This article is part of the Research TopicComprehensive Insights into Mitral Valve Prolapse: From biology to future perspectives of treatment, passing through diagnostic tools, surgical techniques and transcatheter optionsView all 12 articles
 Myriam Carpenito1
Myriam Carpenito1 Martina Gelfusa1
Martina Gelfusa1 Simona Mega1
Simona Mega1 Valeria Cammalleri1
Valeria Cammalleri1 Giovanni Benfari2
Giovanni Benfari2 Domenico De Stefano3
Domenico De Stefano3 Gian Paolo Ussia1
Gian Paolo Ussia1 Christophe Tribouilloy4,5
Christophe Tribouilloy4,5 Maurice Enriquez-Sarano6
Maurice Enriquez-Sarano6 Francesco Grigioni1*
Francesco Grigioni1*
The most common organic etiology of mitral regurgitation is degenerative and consists of mitral valve prolapse (MVP). Volume overload because of mitral regurgitation is the most common complication of MVP. Advocating surgery before the consequences of volume overload become irreparable restores life expectancy, but carries a risk of mortality in patients who are often asymptomatic. On the other hand, the post-surgical outcome of symptomatic patients is dismal and life expectancy is impaired. In the present article, we aim to bridge the gap between these two therapeutic approaches, unifying the concepts of watchful waiting and early surgery in a “watchful surgery approach”.
Organic (primary) mitral regurgitation implies the presence of anatomic abnormalities affecting the leaflets or the sub-valvular apparatus. The most common etiology is degenerative (DMR) (1, 2), and consists of mitral valve prolapse (MVP). Less common etiologies include rheumatic heart disease, valve calcification because of aging, congenital diseases, and endocarditis (3).
On the histological ground, infiltrative or dysplastic tissue disorders characterize MVP, with/without chordal rupture, producing two different phenotypes (4). At one hand of the spectrum, a significant excess of tissue, multi-scallop prolapse, annular dilation, and diffuse thickening/elongation of the mitral tissue indicate Barlow's disease. At the other end, leaflets thinning, chordal elongation, and prolapse limited to a portion of the leaflets set up the phenotype of fibroblastic deficiency (Figure 1).
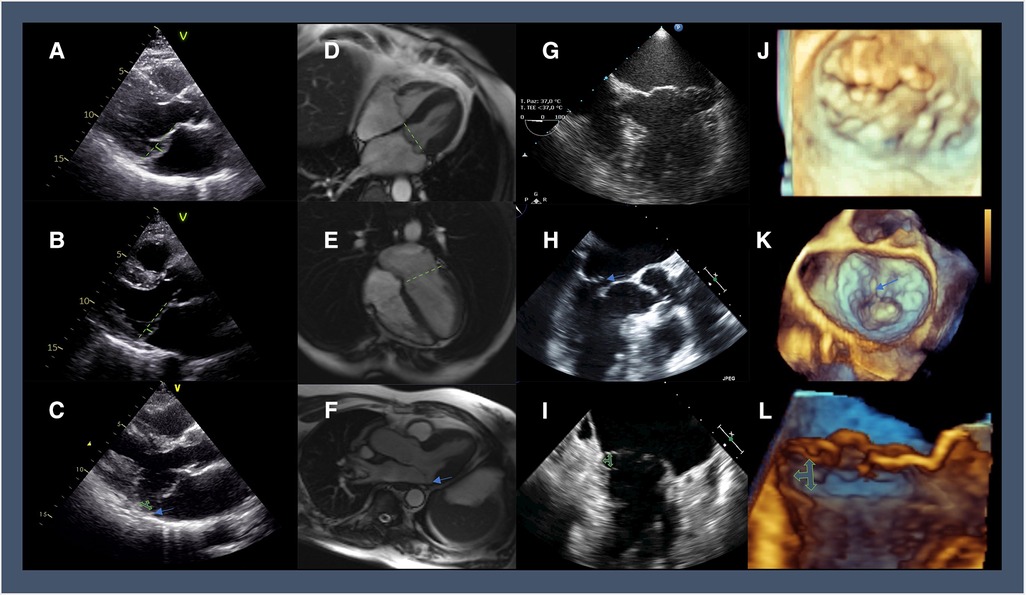
Figure 1. Multi-modality imaging highlighting the different features of MVP: 2D-TTE in parasternal long-axis view showing (A) diffuse thickening of the mitral tissue and the systolic displacement of posterior leaflets 3 mm beyond the plane of the annulus (green line); (B) leaflets thinning with a prolapse limited to posterior leaflets reaching cut-off of 2 mm (green line); (C) bileaflet prolapse and thickening of the mitral valve tissue with posterior MAD (green arrows; a blue arrow shows the detachment of postero-lateral annulus from posterolateral ventricular myocardium); Cine-CMR imaging in end-systolic 4-chamber view showing (D) Barlow's disease with bileaflet thickening of the mitral valve (green line representing the annulus plane); (E) fibroelastic deficiency with anterior leaflet thinning and prolapse in the left atrium; (F) MAD can be diagnosed in an SSFP three-chamber view in the systole (blue arrow); 2D-TEE (G) apical 4-chambers view showing Barlow's disease with a significantly enlarged mitral annulus and multisegmental prolapse and (H) 3-chamber of fibroelastic deficiency with P2 flail segment (blue arrow); (I) 2-chamber view of multiscallop prolapse with MAD (green arrow); 3D zoom aquisition of the mitral valve in surgical view showing Barlow's disease with multisegmental prolapse (J), and fibroelastic deficiency with P2 flail segment and ruptured chords (blue arrow) (K); cropped sagittal plane demonstrating a lateral view of the mitral valve with posterior MAD (green arrows) (L).
On the physio-pathological ground, a systolic displacement of one or both mitral leaflets more than 2 mm beyond the plane of the annulus in a long-axis view defines MVP, with or without leaflet thickening. Two-dimensional transthoracic echocardiography (2D-TTE) is the most common diagnostic tool (Figure 1) (5). Based on these criteria, MVP prevalence in the general population ranges from 0.6% to 3.1% (6) and is higher in females (6, 7).
MVP may be syndromic, familial, or isolated (sporadic). We encounter syndromic MVP in connective tissue disorders such as Marfan syndrome, Loeys–Dietz syndrome, Ehlers–Danlos syndrome, pseudoxanthoma elasticum, osteogenesis imperfecta, and aneurysms-osteoarthritis syndrome (8). Among familiar patterns, autosomal dominant inheritance with varying degrees of penetrance is more common than the X-linked (9). Gender and age influence gene expression, with high phenotypic variability even within the same family (9). Isolated MVP is more frequently associated with benign extra-cardiac manifestations.
Since MVP is associated with such a composed group of underlying conditions and histological characteristics, its outcome is heterogeneous (10). Besides rhythm disturbances and endocarditis, volume overload is the most common complication, mostly corrected by conventional surgery. Surgery performed before the complications of volume overload restores life expectancy but implies a mortality risk in an asymptomatic patient. On the other hand, when surgery is performed in symptomatic patients with irreparable consequences of volume overload, the post-surgical outcome is dismal, and life expectancy is impaired.
In the present article, we will try to bridge the gap among these two (perhaps only apparently) diverging therapeutic approaches, trying to unify the opposite attitudes of “watchful waiting” (11) and “early surgery” (12) in a “watchful surgery approach”.
In MVP, the classic auscultatory finding is a dynamic mid-to-late systolic click, frequently associated with a high-pitched, late systolic murmur (13). A very loud murmur carries a high probability of severe DMR, but patients often present with a medium-intensity murmur, which can either be generated by moderate or severe regurgitation (14). Similarly, symptoms and cardiac remodeling support the diagnosis of severe DMR, but imaging is key if we want to offer surgery before the consequences of volume overload become irreparable. Doppler echocardiography, by providing qualitative, semi-quantitative, and quantitative parameters, represents the standard tool to diagnose the etiology, mechanism, and severity of any valvular lesion, including DMR (15). Grading DMR should be comprehensive, using a combination of clues, signs, and measurements (Table 1) (16). A detailed review of the different echocardiographic methods to establish the severity of DMR is behind the scope of the present article. However, we will summarize a few concepts applying to MVP.
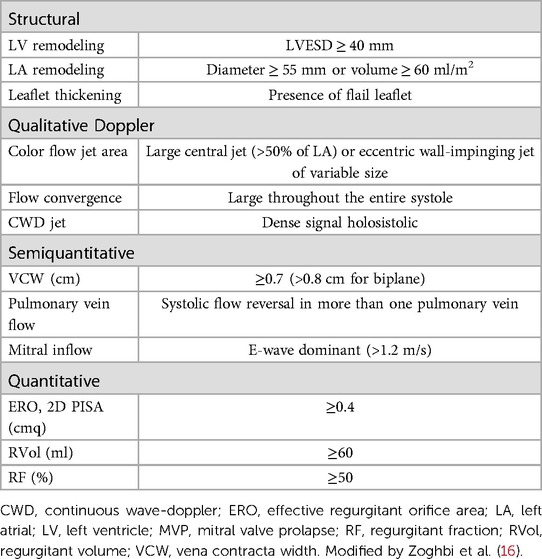
Table 1. Echocardiographic features encountered in MVP and indicators of severe chronic regurgitation.
Systolic flow reversal in more than one pulmonary vein is specific for severe DMR, although eccentric jets can alter flow patterns, even mild or moderate in severity when directed into a pulmonary vein. The jet area or jet area/left atrial area ratio by color flow imaging is valuable as a screening tool to confirm the presence of more than mild DMR, but it is imprecise. This happens particularly in eccentric, wall-impinging jets and in late-systolic ones, as it can occur in MVP (16).
The vena contracta (VC) is the narrowest portion of the regurgitant flow and its measurement represents an approximation of the anatomic regurgitant orifice. A VC width ≥0.7 cm is specific for severe MR and this cut-off can be applied both to central and eccentric jets (16). As for color flow imaging, VC in MVP can overestimate the severity of late-systolic jets.
Concerning quantitative approaches, all methods derive three measures. The effective regurgitant orifice area (EROA) (a measure of lesion severity), the regurgitant volume per beat (RVol) (the severity of volume overload), and the regurgitant fraction (the ratio of the RVol to the forward stroke volume). Measurements of EROA and RVol provide the strongest prognostic information (17). In mid-late systolic jets, EROA by flow convergence appears similar to holosystolic. However, a shorter duration of regurgitation results in a smaller RVol. Herein, Rvol, rather than EROA provides more valuable information in this setting (18). When different parameters are contradictory, we must explain discrepancies. If uncertainties persist, transesophageal echocardiography or cardiac magnetic resonance (CMR) can help. On CMR, diffuse interstitial or regional replacement fibrosis by T1 mapping and/or late gadolinium enhancement and edema by T2 mapping can help identify the optimal timing of surgery in MVP complicated by severe DMR (19).
Scientific guidelines recommend surgery (class I) in symptomatic severe DMR regardless of left ventricular (LV) function (15, 20) and the planned surgical procedure (e.g., repair/replacement). Before reviewing surgery in truly asymptomatic patients, it is worth noticing that several patients do not engage in a physical activity vigorous enough to reveal their symptoms.
To overcome this limitation, some studies analyzed the role of exercise as an additional prognostic indicator (21). Asymptomatic patients unable to exercise for at least 15 min on a treadmill using a modified Bruce protocol have a higher risk of adverse cardiac adverse events (22). Exercise echocardiography can provide additional information (20). A change in DMR severity (EROA ≥ 10 mm2, RVol ≥ 15 ml) by exercise echocardiography was associated with reduced symptom-free survival in more than moderate DMR (23). Similarly, exercise-induced right ventricular dysfunction (exercise TAPSE < 19 mm) and PASP > 55 mm Hg predict a worse outcome (24, 25).
A reduced peak oxygen consumption (<84% of expected) is common in DMR and is associated with adverse events (26). Although clinically appealing, the overall value of exercise-derived parameters in comprehensive DMR management remains to be properly tested. This is true particularly in the elderly, those with significant comorbidities, and—more in general- patients who cannot engage in intense physical activity.
The long-lasting physio-pathological dream in chronic DMR has been to identify the transition of the LV from the compensated phase (when LV is capable of managing the volume overload without permanent consequences if this is relieved by surgery) to the decompensated one (when the consequences of the volume overload are permanent) (27). In theory, the ability to diagnose this sweet spot would allow us to safely manage patients conservatively until the “real” need for surgery, without taking the risk of anticipating it when it is not necessary. Did this dream come through? Guidelines recommend (class I) surgery for DMR (regardless of symptoms and the planned surgical procedure e.g., repair or replacement) when the left LV ejection fraction (LVEF) is ≤60% or LV end-systolic diameter (LVESD) is ≥40 mm (15, 20). Although characterized by considerable interobserver variability of measurements (28), these two cut-offs should set the point in natural history when the risk of higher operative mortality and post-surgical ventricular dysfunction no longer justifies conservative management. Our group showed that patients with LVEF between 45% and 60% represent a large proportion of patients with DMR and, even if rarely symptomatic, they display a higher mortality rate and a worse post-surgical outcome as compared with LVEF > 60% (29).
LVESD is considered less loading dependent than LVEF, and a reliable indicator for surgery. Long-term multi-center studies in patients confirmed that LVESD > 40 mm (>22 mm/m2) represents a predictor of increased mortality under conservative management and after surgery (30). To further reduce the incidence of unexpected ventricular dysfunction after surgery, LVESD and LVEF have been combined, as a single indicator. Although this strategy is based on a valuable rationale, the occurrence of post-operative LV dysfunction remains notable (9% if LVEF ≥ 64% and LVESD < 37 mm, 21% if LVEF ≤ 64% or LVESD ≥ 37 mm, and 33% is LVEF ≥ 64% and LVESD ≥ 37 mm) (31).
Global longitudinal strain (GLS) of the LV is an early and sensitive method to detect dysfunction. In asymptomatic chronic DMR, the cutoffs to identify patients at high risk ranges from −17.9% to −21.7% (32). Further studies are needed to establish the clinical role of GLS in these patients. Similarly, three-dimensional echocardiography may represent a more reproducible and accurate method to assess ventricular size and function, but its incremental prognostic value over 2D-TTE in DMR needs confirmation (21).
Being ventricular function at rest modestly effective in predicting post-operative ventricular dysfunction, previous studies concentrated on exercise-derived parameters. In asymptomatic DMR, lack of contractile reserve (defined as an increase of 4% of LVEF) strongly predicts postoperative ventricular dysfunction (33). The absence of LV contractile reserve evaluated by GLS (exercise-induced increase less than 2%) may predict postoperative LV dysfunction (34).
In DMR, left atrial (LA) size is marginally affected by acute changes in preload and afterload, and its remodeling reflects the severity of volume/pressure overload over a longer period (35, 36). Our group showed LA size is a strong predictor of survival under non-surgical management and that LA volume ≥60 ml/m2 is a powerful prognostic indicator (37, 38). Accordingly, European guidelines indicate that a low-risk, effective, and durable mitral repair can be considered (Class II a) without any further risk factors when LA is ≥60 ml/m2 (diameter > 55 mm) (15, 39). LA function provides information (40). Among asymptomatic patients with preserved LVEF, both peaks of atrial longitudinal strain and reservoir strain may predict adverse outcomes (41). Left atrial coupling index (LACI) (represented by the ratio between LA volume index and tissue Doppler myocardial velocity during atrial contraction), emerged as a strong and independent determinant of the outcome under non-surgical treatment. LACI ≥ 5 was identified as a threshold for excess mortality (42). The novel finding that LA functional assessment is a meaningful marker of clinical outcomes underscores the need to standardize the atrial function assessment beyond morphology.
Besides being an indicator of overload severity, LA size negatively contributes to the outcome of DMR through the occurrence of atrial fibrillation (AFib). As the rhythm progress from sinus to paroxysmal to persistent AFib, the associated risk of mortality increases under non-surgical management, and the positive effects of surgery decrease in magnitude (43–45). Current European guidelines assign a Class IIa indication for surgery when Afib complicates DMR but a stronger strength of recommendation could be considered (15).
Pulmonary hypertension (PH) is a common complication of DMR and is related to increased LA pressure due to chronic volume overload. Abnormal pulmonary artery systolic pressure (PASP) independently affects prognosis, by causing right ventricular impairment (46), and subsequent functional tricuspid regurgitation (47). European guidelines recommend surgery when PASP at rest exceeds 50 mmHg (Class IIa) (15). A PASP value >50 mmHg is a powerful marker of poor prognosis in patients candidate for surgery (48). Recent data showed that an even milder increase in PASP (>35 mm Hg at rest) may predict early decompensation (49). In addition, the worsening of DMR during exercise and exercise-induced pulmonary hypertension is related to reduced symptom-free survival (23, 50). The presence of PH by Doppler echocardiography after correction of DMR is associated with late cardiac events and recurrence of symptoms (51). A more favorable postoperative outcome has been observed in patients who underwent surgery before the onset of PH (52, 53).
B-type natriuretic peptide (BNP) is released in response to increased myocardial wall stress (54, 55) and its amount of increase reflects DMR severity. Independent predictors of higher BNP plasma levels are LA and LV volumes, AFib, and PASP (56–61). A cut-off > 105 pg/ml of BNP may identify patients at higher risk (62). As BNP cut-off can vary significantly depending on patients' characteristics, a BNP ratio ≥1 (i.e., measured BNP to maximal expected normal value for age/gender and specific assay) is a powerful prognostic indicator in DMR independently of the other surgical triggers (63). BNP levels during exercise emerged as a marker of increased risk independently of baseline values and clinical/echocardiographic characteristics (64). When taken together, the pieces of evidence suggest that even if BNP is not yet included in guidelines, it may be considered in selected patients when the surgical triggers are contradictory and/or the timing of surgery uncertain.
Afib is not the only arrhythmic complication. MVP was identified as the cause of sudden cardiac death (SCD) in 4%–7% of young patients undergoing autopsy (65, 66). Arrhythmic mitral valve complex refers to MVP combined with frequent and/or complex LV arrhythmias in the absence of non-valvular pro-arrhythmic substrates (e.g., ventricular scar, channelopathy, etc..). The arrhythmic mitral valve complex includes patients with and without severe DMR (67). Risk factors for SCD in patients with MVP and severe MR are symptoms, Afib, and reduced LVEF (68). Mitral annular disjunction (MAD) is linked to LV arrhythmias in MVP regardless of DMR severity and is characterized by a systolic separation between the ventricular myocardium and the mitral annulus supporting the posterior leaflet (Figure 1) (69, 70). Depending on the diagnostic criteria, the prevalence of MAD in MVP varies between 20% and 58% (67). The origin of LV arrhythmias in MAD derives from the combination of the substrate (regional myocardial hypertrophy and fibrosis, Purkinje fibers) and the trigger (mechanical stretch) (66, 71, 72). In the first decade after diagnosis, MAD is not associated with increased mortality (73).
The stratification of SCD in MAD remains challenging and multiple indicators are currently under investigation. Late gadolinium enhancement localized on the LV infero-basal wall under the posterior leaflet, overlaps with myocardial fibrosis in the autoptic study in SCD victims (66). The prognostic significance of inducible arrhythmias in electrophysiological studies is currently unknown, and this test cannot be routinely recommended (71). While surgery is associated with a reduced risk of SCD in patients with severe DMR, its role in MAD without significant volume overload remains uncertain.
No randomized prospective data are yet available but a large body of evidence collected at multiple centers worldwide consistently indicates that in DMR an early, low-risk, effective and durable repair is associated with lower in-hospital mortality, better survival, and lower long-term morbidity (12, 74–76). The advantages of minimally invasive surgery over conventional sternotomy –although relevant- are behind the scope of this paper. While European guidelines are more conservative in accepting medical management in asymptomatic patients without any risk factors (15), American guidelines recommend surgery providing a probability of a durable repair >95% and an expected operative mortality <1% (Class IIa) (20). A fundamental question is how often all these surgical requirements are satisfied in MVP. DMR includes patients with a limited alteration of the mid portion of the posterior leaflets as well as severe bi-leaflets prolapse. Such a heterogeneous anatomical spectrum conditions the difficulty of surgical procedures.
In terms of prevalence, an isolated posterior leaflet involvement is present in more than 80% of all comers receiving a diagnosis of severe DMR, complicating MVP (77). Consequently, a simple surgical correction (resulting in a higher likelihood of effective and durable repair) can be achieved in more than 90% of patients referred for surgery (78).
Concerning operative mortality, this was 1.7% overall in consecutive patients referred at tertiary centers for severe DMR, and lower after MV repair (1.3%) than after MV replacement (4.7%) (74). Operative mortality in asymptomatic patients with normal ventricular function undergoing mitral repair has been described in multi-center registries approaching 0% (79).
Concerning durability, the results for anterior and bi-leaflets prolapse are less favorable regarding the recurrence rate of moderate or severe regurgitation, with this risk approaching 1%–2% per year (80–84). Nevertheless, mitral repair after 20 years from the operation is overall characterized by better survival and a similar rate of re-operation as compared to replacement (less than 10%) (74).
Recently, transcatheter edge-to-edge repair emerged as an alternative to conventional surgical repair in high-risk patients unsuitable for surgery. Comparing patients treated with transcatheter and non-operated showed a significant advantage of percutaneous treatment with a prolonged higher survival rate (85).
Yes, under few but stringent conditions.
The first condition is that DMR is truly severe. If uncertainties persist, we should take advantage of multiple diagnostic techniques or refer the patient to a heart valve center for a proper assessment.
The second condition is that an effective, low-risk, and durable repair can be achieved. If we cannot satisfy those requirements at our Institution, the referral of patients elsewhere represents the opportunity to improve and/or develop future internal dedicated surgical valve programs.
The third condition is that the procedure is not futile in light of a reduced life expectancy due to the presence of comorbidities. Percutaneous treatment is an option for these patients.
If multiple multi-center and single-center studies provided converging evidence that early surgical treatment is associated with a better long-term outcome (12, 76) data showing the overall safety and efficacy of a conservative approach taking into account post-surgical outcomes are lacking (11, 86).
DMR is a challenging yet tremendously exciting and evolving field of medicine. Thoughtful management of MVP demands a proper and comprehensive knowledge of genetics, hemodynamics, imaging, and arrhythmias substrates.
In the present article, we summarized old concepts, current orientations, and future perspectives applicable to everyday clinical practice.
MC and FG: conceptualization, writing and editing of the manuscript. MC, and MG: review of the literature and writing. SM, VC, DDS, GPU: critical revision to scientific content. GB, CT, MES: conceptual guidance, critical revision and editing of the manuscript. FG: conceptual guidance and editing of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACC, American college of cardiology; AFib, atrial fibrillation; AHA, American heart association; BNP, B-type natriuretic peptide; CMR, cardiac magnetic resonance; DMR, degenerative mitral regurgitation; EACTS, European association of cardiothoracic surgery; ESC, European society of cardiology; EROA, effective regurgitant orifice area; GLS, global longitudinal strain; LA, left atrial; LACI, left atrial coupling index; LV, left ventricle/ventricular; LVESD, LV end-systolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MAD, mitral annular disjunction; MVP, mitral valve prolapse; PASP, pulmonary artery systolic pressure; PH, pulmonary hypertension; RVol, regurgitant volume; SCD, sudden cardiac death; VC, vena contracta; 2D-TTE, two-dimensional transthoracic echocardiography.
1. Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R, et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. (2022) 23(5):e171–232. doi: 10.1093/ehjci/jeab253
2. Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. (2009) 373(9672):1382–94. doi: 10.1016/S0140-6736(09)60692-9
3. Dziadzko V, Dziadzko M, Medina-Inojosa JR, Benfari G, Michelena HI, Crestanello JA, et al. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J. (2019) 40(27):2194–202. doi: 10.1093/eurheartj/ehz314
4. Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. (2010) 31(16):1958–66. doi: 10.1093/eurheartj/ehq222
5. Levine RA, Hagége AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, et al. Mitral valve disease–morphology and mechanisms. Nat Rev Cardiol. (2015) 12(12):689–710. doi: 10.1038/nrcardio.2015.161
6. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. (1999) 341(1):1–7. doi: 10.1056/NEJM199907013410101
7. Flack JM, Kvasnicka JH, Gardin JM, Gidding SS, Manolio TA, Jacobs DR. Anthropometric and physiologic correlates of mitral valve prolapse in a biethnic cohort of young adults: the CARDIA study. Am Heart J. (1999) 138(3 Pt 1):486–92. doi: 10.1016/S0002-8703(99)70151-1
8. Le Tourneau T, Mérot J, Rimbert A, Le Scouarnec S, Probst V, Le Marec H, et al. Genetics of syndromic and non-syndromic mitral valve prolapse. Heart. (2018) 104(12):978–84. doi: 10.1136/heartjnl-2017-312420
9. Guicciardi NA, De Bonis M, Di Resta C, Ascione G, Alfieri O, Maisano F, et al. Genetic background of mitral valve prolapse. Rev Cardiovasc Med. (2022) 23(3):96. doi: 10.31083/j.rcm2303096
10. Avierinos JF, Gersh BJ, Melton LJ, Bailey KR, Shub C, Nishimura RA, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. (2002) 106(11):1355–61. doi: 10.1161/01.CIR.0000028933.34260.09
11. Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. (2006) 113(18):2238–44. doi: 10.1161/CIRCULATIONAHA.105.599175
12. Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. (2013) 310(6):609–16. doi: 10.1001/jama.2013.8643
13. Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. (2005) 365(9458):507–18. doi: 10.1016/S0140-6736(05)17869-6
14. Desjardins VA, Enriquez-Sarano M, Jamil Tajik A, Bailey KR, Seward JB. Intensity of murmurs correlates with severity of valvular regurgitation. Am J Med. (1996) 100(2):149–56. doi: 10.1016/S0002-9343(97)89452-1
15. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395. Erratum in: Eur Heart J. 2022 Feb 1834453165
16. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation. J Am Soc Echocardiogr. (2017) 30(4):303–71. doi: 10.1016/j.echo.2017.01.007
17. Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. (2005) 352(9):875–83. doi: 10.1056/NEJMoa041451
18. Topilsky Y, Michelena H, Bichara V, Maalouf J, Mahoney DW, Enriquez-Sarano M. Mitral valve prolapse with mid-late systolic mitral regurgitation: pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation. (2012) 125(13):1643–51. doi: 10.1161/CIRCULATIONAHA.111.055111
19. Delling FN, Noseworthy PA, Adams DH, Basso C, Borger M, Bouatia-Naji N, et al. Research opportunities in the treatment of mitral valve prolapse: jACC expert panel. J Am Coll Cardiol. (2022) 80(24):2331–47. doi: 10.1016/j.jacc.2022.09.044
20. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143(5):e35–71. doi: 10.1161/CIR.0000000000000932. Epub 2020 Dec 17. Erratum in: Circulation. (2021) 143(5):e228. Erratum in: Circulation. (2021) 143(10):e784.33332149
21. Flint N, Raschpichler M, Rader F, Shmueli H, Siegel RJ. Asymptomatic degenerative mitral regurgitation: a review. JAMA Cardiol. (2020) 5(3):346–55. doi: 10.1001/jamacardio.2019.5466
22. Supino PG, Borer JS, Schuleri K, Gupta A, Hochreiter C, Kligfield P, et al. Prognostic value of exercise tolerance testing in asymptomatic chronic nonischemic mitral regurgitation. Am J Cardiol. (2007) 100(8):1274–81. doi: 10.1016/j.amjcard.2007.05.046
23. Magne J, Lancellotti P, Piérard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol. (2010) 56(4):300–9. doi: 10.1016/j.jacc.2009.12.073
24. Coisne A, Aghezzaf S, Galli E, Mouton S, Richardson M, Dubois D, et al. Prognostic values of exercise echocardiography and cardiopulmonary exercise testing in patients with primary mitral regurgitation. Eur Heart J Cardiovasc Imaging. (2022) 23(11):1552–61. doi: 10.1093/ehjci/jeab231
25. Kusunose K, Popović ZB, Motoki H, Marwick TH. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovascular Imaging. (2013) 6(2):167–76. doi: 10.1161/CIRCIMAGING.112.000162
26. Messika-Zeitoun D, Johnson BD, Nkomo V, Avierinos JF, Allison TG, Scott C, et al. Cardiopulmonary exercise testing determination of functional capacity in mitral regurgitation: physiologic and outcome implications. J Am Coll Cardiol. (2006) 47(12):2521–7. doi: 10.1016/j.jacc.2006.02.043
27. Carabello BA, Crawford FA. Valvular heart disease. N Engl J Med. (1997) 337(1):32–41. doi: 10.1056/NEJM199707033370107
28. Thavendiranathan P, Popović ZB, Flamm SD, Dahiya A, Grimm RA, Marwick TH. Improved interobserver variability and accuracy of echocardiographic visual left ventricular ejection fraction assessment through a self-directed learning program using cardiac magnetic resonance images. J Am Soc Echocardiogr. (2013) 26(11):1267–73. doi: 10.1016/j.echo.2013.07.017
29. Tribouilloy C, Rusinaru D, Grigioni F, Michelena HI, Vanoverschelde JL, Avierinos JF, et al. Long-term mortality associated with left ventricular dysfunction in mitral regurgitation due to flail leaflets: a multicenter analysis. Circ Cardiovasc Imaging. (2014) 7(2):363–70. doi: 10.1161/CIRCIMAGING.113.001251
30. Tribouilloy C, Grigioni F, Avierinos JF, Barbieri A, Rusinaru D, Szymanski C, et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol. (2009) 54(21):1961–8. doi: 10.1016/j.jacc.2009.06.047
31. Tribouilloy C, Rusinaru D, Szymanski C, Mezghani S, Fournier A, Lévy F, et al. Predicting left ventricular dysfunction after valve repair for mitral regurgitation due to leaflet prolapse: additive value of left ventricular end-systolic dimension to ejection fraction. Eur J Echocardiogr. (2011) 12(9):702–10. doi: 10.1093/ejechocard/jer128
32. Bijvoet GP, Teske AJ, Chamuleau SaJ, Hart EA, Jansen R, Schaap J. Global longitudinal strain to predict left ventricular dysfunction in asymptomatic patients with severe mitral valve regurgitation: literature review. Neth Heart J. (2020) 28(2):63–72. doi: 10.1007/s12471-019-01318-8
33. Lee R. Functional and prognostic implications of left ventricular contractile reserve in patients with asymptomatic severe mitral regurgitation. Heart. (2005) 91(11):1407–12. doi: 10.1136/hrt.2004.047613
34. Lancellotti P, Cosyns B, Zacharakis D, Attena E, Van Camp G, Gach O, et al. Importance of left ventricular longitudinal function and functional reserve in patients with degenerative mitral regurgitation: assessment by two-dimensional speckle tracking. J Am Soc Echocardiogr. (2008) 21(12):1331–6. doi: 10.1016/j.echo.2008.09.023
35. Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. (2003) 42(7):1206–7. doi: 10.1016/S0735-1097(03)00956-2
36. Messika-Zeitoun D, Bellamy M, Avierinos JF, Breen J, Eusemann C, Rossi A, et al. Left atrial remodelling in mitral regurgitation–methodologic approach, physiological determinants, and outcome implications: a prospective quantitative Doppler-echocardiographic and electron beam-computed tomographic study. Eur Heart J. (2007) 28(14):1773–81. doi: 10.1093/eurheartj/ehm199
37. Rusinaru D, Tribouilloy C, Grigioni F, Avierinos JF, Suri RM, Barbieri A, et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging. (2011) 4(5):473–81. doi: 10.1161/CIRCIMAGING.110.961011
38. Le Tourneau T, Messika-Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. (2010) 56(7):570–8. doi: 10.1016/j.jacc.2010.02.059
39. Grigioni F, Clavel MA, Vanoverschelde JL, Tribouilloy C, Pizarro R, Huebner M, et al. The MIDA mortality risk score: development and external validation of a prognostic model for early and late death in degenerative mitral regurgitation. Eur Heart J. (2018) 39(15):1281–91. doi: 10.1093/eurheartj/ehx465
40. Carpenito M, Fanti D, Mega S, Benfari G, Bono MC, Rossi A, et al. The central role of left atrium in heart failure. Front Cardiovasc Med. (2021) 8:704762. doi: 10.3389/fcvm.2021.704762
41. Yang LT, Liu YW, Shih JY, Li YH, Tsai LM, Luo CY, et al. Predictive value of left atrial deformation on prognosis in severe primary mitral regurgitation. J Am Soc Echocardiogr. (2015) 28(11):1309–1317.e4. doi: 10.1016/j.echo.2015.07.004
42. Essayagh B, Benfari G, Antoine C, Maalouf J, Pislaru S, Thapa P, et al. Incremental prognosis by left atrial functional assessment: the left atrial coupling index in patients with floppy mitral valves. J Am Heart Assoc. (2022) 11(9):e024814. doi: 10.1161/JAHA.121.024814
43. Grigioni F, Avierinos JF, Ling LH, Scott CG, Bailey KR, Tajik AJ, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. (2002) 40(1):84–92. doi: 10.1016/S0735-1097(02)01922-8
44. Grigioni F, Benfari G, Vanoverschelde JL, Tribouilloy C, Avierinos JF, Bursi F, et al. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J Am Coll Cardiol. (2019) 73(3):264–74. doi: 10.1016/j.jacc.2018.10.067
45. Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation. (1999) 99(3):400–5. doi: 10.1161/01.CIR.99.3.400
46. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology. Clinical importance, and management of right ventricular failure. Circulation. (2008) 117(13):1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584
47. Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease. J Am Coll Cardiol. (2009) 53(5):401–8. doi: 10.1016/j.jacc.2008.09.048
48. Le Tourneau T, Richardson M, Juthier F, Modine T, Fayad G, Polge AS, et al. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart. (2010) 96(16):1311–7. doi: 10.1136/hrt.2009.186486
49. Mentias A, Patel K, Patel H, Gillinov AM, Sabik JF, Mihaljevic T, et al. Effect of pulmonary vascular pressures on long-term outcome in patients with primary mitral regurgitation. J Am Coll Cardiol. (2016) 67(25):2952–61. doi: 10.1016/j.jacc.2016.03.589
50. Suzuki K, Izumo M, Yoneyama K, Mizukoshi K, Kamijima R, Kou S, et al. Influence of exercise-induced pulmonary hypertension on exercise capacity in asymptomatic degenerative mitral regurgitation. J Cardiol. (2015) 66(3):246–52. doi: 10.1016/j.jjcc.2014.11.005
51. Murashita T, Okada Y, Kanemitsu H, Fukunaga N, Konishi Y, Nakamura K, et al. The impact of preoperative and postoperative pulmonary hypertension on long-term surgical outcome after mitral valve repair for degenerative mitral regurgitation. ATCS. (2015) 21(1):53–8. doi: 10.5761/atcs.oa.13-00364
52. Barbieri A, Bursi F, Grigioni F, Tribouilloy C, Avierinos JF, Michelena HI, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J. (2011) 32(6):751–9. doi: 10.1093/eurheartj/ehq294
53. Ghoreishi M, Evans CF, DeFilippi CR, Hobbs G, Young CA, Griffith BP, et al. Pulmonary hypertension adversely affects short- and long-term survival after mitral valve operation for mitral regurgitation: implications for timing of surgery. J Thorac Cardiovasc Surg. (2011) 142(6):1439–52. doi: 10.1016/j.jtcvs.2011.08.030
54. Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, McGregor CG, et al. Natriuretic peptide system in human heart failure. Circulation. (1993) 88(3):1004–9. doi: 10.1161/01.CIR.88.3.1004
55. Rossi A, Enriquez-Sarano M, Burnett JC, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation. J Am Coll Cardiol. (2000) 35(5):1256–62. doi: 10.1016/S0735-1097(00)00515-5
56. Sutton TM, Stewart RAH, Gerber IL, West TM, Richards AM, Yandle TG, et al. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. (2003) 41(12):2280–7. doi: 10.1016/S0735-1097(03)00486-8
57. Detaint D, Messika-Zeitoun D, Chen HH, Rossi A, Avierinos JF, Scott C, et al. Association of B-type natriuretic peptide activation to left ventricular end-systolic remodeling in organic and functional mitral regurgitation. Am J Cardiol. (2006) 97(7):1029–34. doi: 10.1016/j.amjcard.2005.10.061
58. Detaint D, Messika-Zeitoun D, Avierinos JF, Scott C, Chen H, Burnett JC, et al. B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome. Circulation. (2005) 111(18):2391–7. doi: 10.1161/01.CIR.0000164269.80908.9D
59. Shimamoto K, Kusumoto M, Sakai R, Watanabe H, Ihara S, Koike N, et al. Usefulness of the brain natriuretic peptide to atrial natriuretic peptide ratio in determining the severity of mitral regurgitation. Can J Cardiol. (2007) 23(4):295–300. doi: 10.1016/S0828-282X(07)70758-4
60. Bergler-Klein J, Gyöngyösi M, Maurer G. The role of biomarkers in valvular heart disease: focus on natriuretic peptides. Can J Cardiol. (2014) 30(9):1027–34. doi: 10.1016/j.cjca.2014.07.014
61. Klaar U, Gabriel H, Bergler-Klein J, Pernicka E, Heger M, Mascherbauer J, et al. Prognostic value of serial B-type natriuretic peptide measurement in asymptomatic organic mitral regurgitation. Eur J Heart Fail. (2011) 13(2):163–9. doi: 10.1093/eurjhf/hfq189
62. Pizarro R, Bazzino OO, Oberti PF, Falconi M, Achilli F, Arias A, et al. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol. (2009) 54(12):1099–106. doi: 10.1016/j.jacc.2009.06.013
63. Clavel MA, Tribouilloy C, Vanoverschelde JL, Pizarro R, Suri RM, Szymanski C, et al. Association of B-type natriuretic peptide with survival in patients with degenerative mitral regurgitation. J Am Coll Cardiol. (2016) 68(12):1297–307. doi: 10.1016/j.jacc.2016.06.047
64. Magne J, Mahjoub H, Pibarot P, Pirlet C, Pierard LA, Lancellotti P. Prognostic importance of exercise brain natriuretic peptide in asymptomatic degenerative mitral regurgitation. Eur J Heart Fail. (2012) 14(11):1293–302. doi: 10.1093/eurjhf/hfs114
65. Delling FN, Aung S, Vittinghoff E, Dave S, Lim LJ, Olgin JE, et al. Antemortem and post-mortem characteristics of lethal mitral valve prolapse among all countywide sudden deaths. JACC Clin Electrophysiol. (2021) 7(8):1025–34. doi: 10.1016/j.jacep.2021.01.007
66. Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. (2015) 132(7):556–66. doi: 10.1161/CIRCULATIONAHA.115.016291
67. Sabbag A, Essayagh B, Barrera JDR, Basso C, Berni A, Cosyns B, et al. EHRA Expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC council on valvular heart disease and the European association of cardiovascular imaging endorsed cby the heart rhythm society, by the Asia pacific heart rhythm society, and by the latin American heart rhythm society. Europace. (2022) 24(12):1981–2003. doi: 10.1093/europace/euac125
68. Grigioni F, Enriquez-Sarano M, Ling LH, Bailey KR, Seward JB, Tajik AJ, et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. (1999) 34(7):2078–85. doi: 10.1016/S0735-1097(99)00474-X
69. Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med. (1986) 314(9):535–40. doi: 10.1056/NEJM198602273140902
70. Faletra FF, Leo LA, Paiocchi VL, Caretta A, Viani GM, Schlossbauer SA, et al. Anatomy of mitral annulus insights from non-invasive imaging techniques. Eur Heart JCardiovasc Imaging. (2019) 20(8):843–57. doi: 10.1093/ehjci/jez153
71. Basso C, Iliceto S, Thiene G, Marra M P. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. (2019) 140(11):952–64. doi: 10.1161/CIRCULATIONAHA.118.034075
72. Fulton BL, Liang JJ, Enriquez A, Garcia FC, Supple GE, Riley MP, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. (2018) 29(1):146–53. doi: 10.1111/jce.13374
73. Essayagh B, Sabbag A, Antoine C, Benfari G, Batista R, Yang LT, et al. The mitral annular disjunction of mitral valve prolapse: presentation and outcome. JACC Cardiovasc Imaging. (2021) 14(11):2073–87. doi: 10.1016/j.jcmg.2021.04.029
74. Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. (2017) 135(5):410–22. doi: 10.1161/CIRCULATIONAHA.116.023340
75. Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation. (1995) 91(4):1022–8. doi: 10.1161/01.CIR.91.4.1022
76. Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. (2009) 119(6):797–804. doi: 10.1161/CIRCULATIONAHA.108.802314
77. Ling LH, Enriquez-Sarano M, Seward JB, Tajik AJ, Schaff HV, Bailey KR, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med. (1996) 335(19):1417–23. doi: 10.1056/NEJM199611073351902
78. Tamborini G, Muratori M, Maltagliati A, Galli CA, Naliato M, Zanobini M, et al. Pre-operative transthoracic real-time three-dimensional echocardiography in patients undergoing mitral valve repair: accuracy in cases with simple vs. complex prolapse lesions. Eur J Echocardiogr. (2010) 11(9):778–85. doi: 10.1093/ejechocard/jeq066
79. Grigioni F, Tribouilloy C, Avierinos JF, Barbieri A, Ferlito M, Trojette F, et al. Outcomes in mitral regurgitation due to flail leaflets a multicenter European study. JACC Cardiovasc Imaging. (2008) 1(2):133–41. doi: 10.1016/j.jcmg.2007.12.005
80. Tabata M, Kasegawa H, Fukui T, Shimizu A, Sato Y, Takanashi S. Long-term outcomes of artificial chordal replacement with tourniquet technique in mitral valve repair: a single-center experience of 700 cases. J Thorac Cardiovasc Surg. (2014) 148(5):2033–2038.e1. doi: 10.1016/j.jtcvs.2014.03.045
81. David TE, Armstrong S, Ivanov J. Chordal replacement with polytetrafluoroethylene sutures for mitral valve repair: a 25-year experience. J Thorac Cardiovasc Surg. (2013) 145(6):1563–9. doi: 10.1016/j.jtcvs.2012.05.030
82. Braunberger E, Deloche A, Berrebi A, Abdallah F, Celestin JA, Meimoun P, et al. Very long-term results (more than 20 years) of valve repair with carpentier's Techniques in nonrheumatic mitral valve insufficiency. Circulation. (2001) 104(12 Suppl 1):I8–11. doi: 10.1161/01.CIR.104.suppl_1.I-8
83. DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg. (2010) 139(1):76–83; discussion 83–84. doi: 10.1016/j.jtcvs.2009.08.058
84. Seeburger J, Borger MA, Falk V, Kuntze T, Czesla M, Walther T, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg. (2008) 34(4):760–5. doi: 10.1016/j.ejcts.2008.05.015
85. Benfari G, Sorajja P, Pedrazzini G, Taramasso M, Gavazzoni M, Biasco L, et al. Association of transcatheter edge-to-edge repair with improved survival in older patients with severe, symptomatic degenerative mitral regurgitation. Eur Heart J. (2022) 43(17):1626–35. doi: 10.1093/eurheartj/ehab910
Keywords: mitral valve prolapse, degenerative mitral regurgitation, mitral regurgitation, asymptomatic patient, mitral valve surgery
Citation: Carpenito M, Gelfusa M, Mega S, Cammalleri V, Benfari G, De Stefano D, Ussia GP, Tribouilloy C, Enriquez-Sarano M and Grigioni F (2023) Watchful surgery in asymptomatic mitral valve prolapse. Front. Cardiovasc. Med. 10:1134828. doi: 10.3389/fcvm.2023.1134828
Received: 30 December 2022; Accepted: 14 March 2023;
Published: 12 April 2023.
Edited by:
Payam Akhyari, University Hospital RWTH Aachen, GermanyReviewed by:
Augustin Coisne, Centre Hospitalier Regional et Universitaire de Lille, France© 2023 Carpenito, Gelfusa, Mega, Cammalleri, Benfari, De Stefano, Ussia, Tribouilloy, Enriquez-Sarano and Grigioni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Grigioni Zi5ncmlnaW9uaUBwb2xpY2xpbmljb2NhbXB1cy5pdA==
Specialty Section: This article was submitted to Heart Valve Disease, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.