- 1Department of Cardiology, College of Medicine, Tanta University, Tanta, Egypt
- 2Heart Health Center, King Saud medical city, Riyadh, Saudi Arabia
- 3Division of Cardiology, Department of Medicine, London Health Sciences Centre, Western University London, Ontario, ON, Canada
- 4King Fahad Medical City, Riyadh, Saudi Arabia
- 5Clinical Pharmacy & Pharmacy Practice Department, Faculty of Pharmacy, Damanhour University, Damanhour, Egypt
- 6Infection Control Administration, King Saud Medical City, Riyadh, Saudi Arabia
- 7College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
- 8Internal Medicine Department, King Faisal University, Alahsa, Saudi Arabia
Background: Respiratory infections are one of the most common comorbidities identified in hospitalized patients. The coronavirus disease 2019 (COVID-19) pandemic greatly impacted healthcare systems, including acute cardiac services.
Aim: This study aimed to describe the echocardiographic findings of patients with COVID-19 infections and their correlations with inflammatory biomarkers, disease severity, and clinical outcomes.
Methods: This observational study was conducted between June 2021 and July 2022. The analysis included all patients diagnosed with COVID-19 who had transthoracic echocardiographic (TTE) scans within 72 h of admission.
Results: The enrolled patients had a mean age of 55.6 ± 14.7 years, and 66.1% were male. Of the 490 enrolled patients, 203 (41.4%) were admitted to the intensive care unit (ICU). Pre-ICU TTE findings showed significantly higher incidence right ventricular dysfunction (28 [13.8%] vs. 23 [8.0%]; P = 0.04) and left ventricular (LV) regional wall motion abnormalities (55 [27.1%] vs. 29 [10.1%]; p < 0.001) in ICU patients compared to non-ICU patients. In-hospital mortality was 11 (2.2%), all deaths of ICU patients. The most sensitive predictors of ICU admission (p < 0.05): cardiac troponin I level (area under the curve [AUC] = 0.733), followed by hs-CRP (AUC = 0.620), creatine kinase-MB (AUC = 0.617), D-dimer (AUC = 0.599), and lactate dehydrogenase (AUC = 0.567). Binary logistic regression showed that reduced LV ejection fraction (LVEF), elevated pulmonary artery systolic pressure, and dilated right ventricle were echocardiographic predictors of poor outcomes (p < 0.05).
Conclusion: Echocardiography is a valuable tool in assessing admitted patients with COVID-19. Lower LVEF, pulmonary hypertension, higher D-dimer, C-reactive protein, and B-type natriuretic peptide levels were predictors of poor outcomes.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic of 2021–2022 has resulted in >664 million confirmed cases and >6 million deaths globally by January 2023 (1). While the respiratory system has been the most directly affected, there is growing evidence that COVID-related heart disease is critical in disease severity and clinical outcomes (2).
The heart muscle is damaged by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and its immunopathological cardiac inflammation consequences (3). It is associated with several direct or indirect cardiovascular complications, such as myocardial damage, myocarditis, heart failure (HF), arrhythmia, and venous thromboembolism (4–6). Myocardial ischemia and necrosis are associated with impaired ventricular function, increasing the risk of mortality in these patients (7). The cause of cardiac involvement may include endothelial dysfunction, cytokine-mediated systemic damage, or stress-induced cardiomyopathy (8, 9). Poor outcomes are associated with higher mortality and cardiac involvement, as indicated by increased troponin T and brain-type-natriuretic peptide (BNP) levels or decreased left ventricular (LV) ejection fraction (EF) (5, 6, 10). Elevated serum troponin levels are present in 17%–36% of COVID-19 patients, indicating myocardial damage (11).
Transthoracic echocardiography (TTE) is a common, low-cost technology for assessing heart anatomy and function. A targeted evaluation provides essential information that can influence clinical decisions in critically ill patients (12, 13).
TTE may provide clues for diagnosing other clinical conditions, such as acute respiratory distress syndrome (ARDS), pulmonary embolism (PE), cardiogenic/non-cardiogenic shock, myocardial infarction, and myocarditis (14–16). Therefore, it is necessary to describe the echocardiographic features of COVID-19 patients and its relationship with biomarkers and poor outcomes. This study aimed to evaluate the possible correlations of echocardiographic findings and inflammatory biomarkers with disease outcomes in hospital-admitted patients with confirmed COVID-19 infections.
Methods
Study population
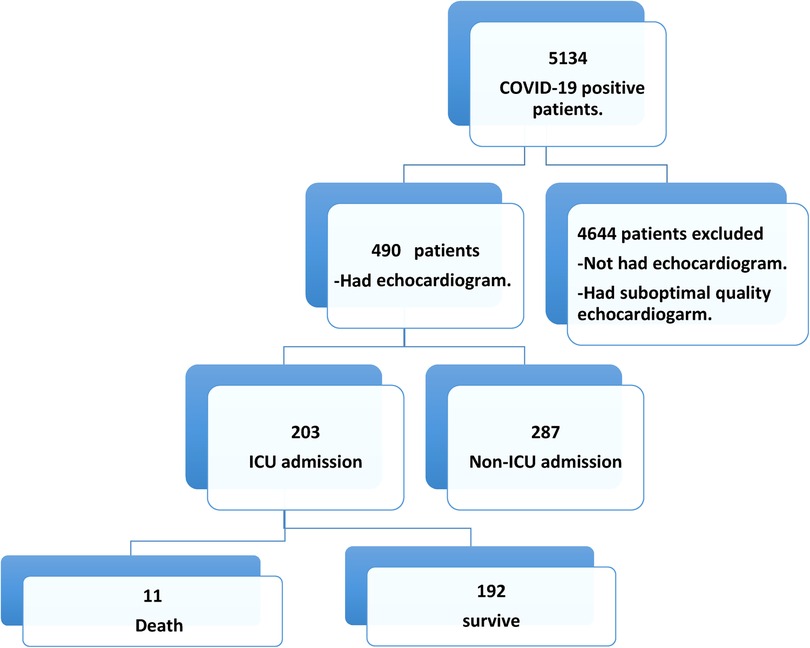
We conducted an observational study that included 490 hospitalized patients. It included all patients aged ≥18 years admitted with confirmed COVID-19 infections who underwent echocardiography at admission between June 2021 and July 2022. Patients aged <18 years, who were COVID-19 negative, patients who have not done echocardiogram and those with suboptimal quality echocardiogram were excluded. All patients were recruited at the Heart Health Center, King Saud Medical City, Riyadh, Kingdom of Saudi Arabia (KSA).
TTE acquisition
TTE orders from the in-patient service with specific clinical indications were subjected to an additional screening process by cardiologists to assess whether appropriate echocardiographic findings affected the management plan. As recommended by the Saudi Arabian Society of Echocardiography for COVID-19 (17), we followed a simplified protocol that enabled rapid bedside TTE assessments to reduce exposure time (17).
TTEs were performed using a Vivid S70 or Vivid q ultrasound system with an M5 or M5-S phased array probe (GE Healthcare Vingmed Ultrasound AS, Horten, Norway).
LV dimensions, volumes, EF (biplane LV planimetry by the modified Simpson's rule), and mass were assessed. According to the Guidelines and Standards for Cardiac Chamber Quantification by Echocardiography in Adults recommendations, an LVEF of <52% for men and <54% for women suggested abnormal LV systolic function (18).
LV diastolic function was also assessed, according to Nagueh et al. (19), by left atrial volume index (LAVI) and tricuspid regurgitation velocity (TRV), the average ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity (E/e'), and the average ratio between E-wave and A-wave (E/A). In patients with depressed EFs and in patients with normal EFs and myocardial disease, if E/A ratio is <0.8 along with a peak E velocity of <50 cm/sec, then mean left atrial pressure (LAP) is either normal or low and patient has grade I diastolic dysfunction.
If E/A ratio is > 2, LA mean pressure is elevated, grade III diastolic dysfunction is present. Deceleration time (DT) is usually short in patients with heart failure with reduced ejection fraction (HFrEF) and restrictive filling pattern (<160 msec). However, in patients with heart failure with preserved ejection fraction (HFpEF), DT can be normal despite elevated LV filling pressures. If E/A ratio <0.8 along with a peak E velocity of >50 cm/sec, or an E/A ratio > 0.8 but < 2, additional parameters are needed. These include peak TR velocity, E/è ratio and LA maximum volume index. Their cutoff values to conclude elevated LAP are peak velocity of TR jet >2.8 m/sec, average E/è ratio > 14, and LA maximum volume index > 34 ml/m2. If more than half or all of the variables meet the cutoff values, then LAP is elevated, and grade II diastolic dysfunction is present. If only one of three available variables meet the cutoff value, then LAP is normal and grade I diastolic dysfunction is present. In case of 50% discordance or with only one available variable, findings are inconclusive to estimate LAP.
In patients with depressed LVEF, pulmonary vein systolic/diastolic (S/D) ratio may be used if one of the three main parameters are not available. A ratio < 1 is consistent with increased LAP.
Right ventricular (RV) systolic function was evaluated using a tricuspid annular plane systolic excursion (TAPSE) of <17 mm, pulsed tissue Doppler S wave of <9.5 cm/sec and RV fractional area change (RV-FAC) <35% (18). Pulmonary hypertension (PH) was diagnosed by using the peak tricuspid regurgitation velocity (TRV) as the key variable for assigning the echocardiographic probability of PH. A peak TRV equal to 2.8 m/s may suggest PH. Then other echocardiographic parameters suggesting PH (including pulmonary flow acceleration time) must be used to assign the probability of PH.
If the TRV is >3.4 m/s then the echocardiographic probability of PH is high. If the TRV is ≤3.4 m/s, then other echocardiographic parameters suggesting PH must be used to assign the probability of PH. These parameters are split into three categories (A: the ventricles; B: the pulmonary artery; C: the inferior vena cava (IVC) and right atrium). Parameters from at least two different categories are needed to determine the probability of PH (Supplementary Material Table S1 and Figure S1). Following the guideline protocol from the British Society of Echocardiography 2018 (20) and the 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension (21).
All TTE scans were reviewed manually by an expert echocardiographer unaware of the patient's clinical history.
Specimen collection and serum biomarker measurements
Venous blood samples were collected in serum vacutainer test tubes from each patient between 8 and 9 am after a 30 min rest in the supine position. Blood samples were allowed to clot for 15–30 min and then centrifuged at 3000 rpm for 15 min using a Hettich Zentrifugen EBA 20 centrifuge (Merck, Germany). Blood glucose was measured using the glucose oxidase method. The remaining serum sample was stored at 8°C until measurement of D-dimer, high sensitive C-reactive protein (hs-CRP), BNP, cardiac troponin I, creatine kinase, creatine kinase-myocardial band (MB), and lactate dehydrogenase using commercially available enzyme-linked immunosorbent assays (Sunred Biological Technology, Shanghai, China) according to manufacturer`s instructions.
Clinical outcomes data collection
The patients' medical history, comorbidities, laboratory data, treatments, and outcomes were extracted from the hospital's electronic medical records. Patients with in-hospital poor outcomes, including PE, HF, ARDS, septic shock, respiratory failure (RF), myocarditis, acute kidney injury (AKI) and death, were assessed. The study evaluating the association between echo-cardiographic features and inflammatory biomarkers with ICU admission and poor outcomes in the enrolled COVID-19 Patients.
Ethics approval
This study was conducted according to the World Medical Association Declaration of Helsinki and received ethics approval from the Institutional Review Boards of the US Department of Health and Human Services (IORG: IORG0010374) and the King Abdulaziz City for Science and Technology, KSA (registration number: H-01-R-053). The study protocols were approved by the Saudi Arabian Society of Echocardiography for COVID-19 (17).
Statistical analysis
The data were statistically analyzed using Microsoft Excel 2016 (Seattle, WA, USA) and SPSS Statistics for Windows (version 26.0; IBM Corp., Armonk, NY, USA). The normality of each variable's data was checked using Kolmogorov–Smirnov and Shapiro–Wilk tests. Continuous, normally distributed variables are expressed as Means ± Standard Deviations, and 95% confidence intervals (CIs) and were compared between groups using the Student's t-test. Non-normally distributed variables are expressed as median (25th and 75th percentiles) and were compared using the Mann-Whitney U test. Whereas, the categorical variables are expressed as frequency (percent) and were compared using the Chi-squared test. The significance level was set at a p-value of 0.05. Predictors of poor outcomes (defined as number of patients who have one or more complications including PE, HF, ARDS, septic shock, RF, myocarditis, AKI and death) were assessed using binary logistic regression analysis. Receiver operator characteristic (ROC) curves were used to assess the inflammatory biomarkers and cardiac enzymes for predicting of Intensive Care Unit (ICU) admission.
Results
Baseline characteristics
Over 12 months, 5134 patients were confirmed as COVID-19 positive, of which 490 (9.54%) had TTE scans (Figure 1). Their mean age was 55.6 ± 14.7 years, and 66.1% were male. Nearly half of the patients had diabetes (49.2%) and/or hypertension (52.2%). Their further clinical features are presented in Table 1.
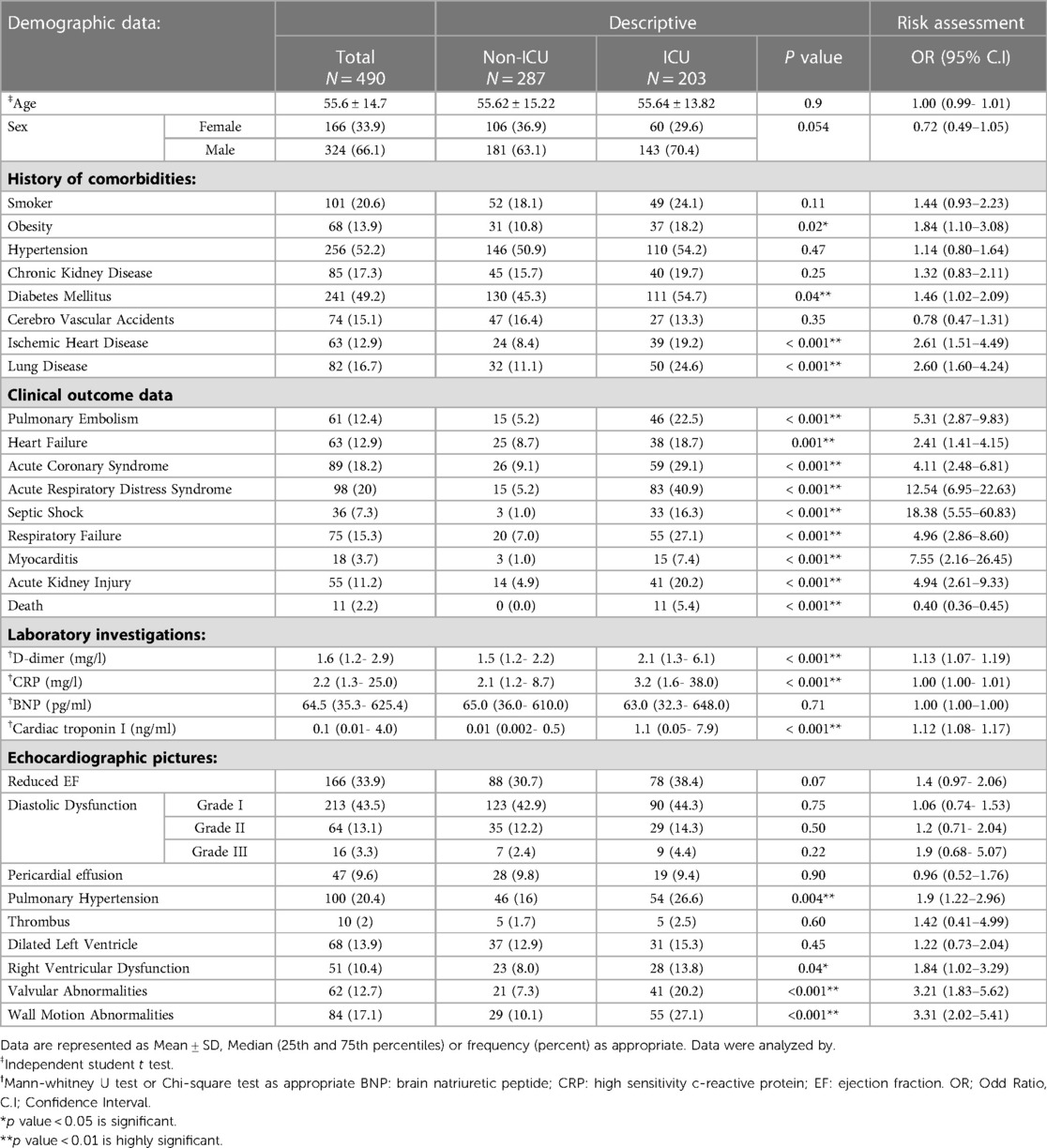
Table 1. Patients’ demographics and clinical data outcomes with the risk assessment for ICU admission.
Clinical presentation and associated outcomes
Patients' clinical presentations varied. One or more of the following symptoms were reported: shortness of breath (42.4%), fever (31.6%), cough (35.3%) and 188 (38.4%) had pneumonia (Supplementary Material Table S2). In-hospital mortality was 11 (2.2%) among ICU-admitted patients. Patient outcomes were reported as follows: 61 (12.4%) had PE, 63 (12.9%) had heart failure (HF), 89 (18.2%) had acute coronary syndrome (ACS), 98 (20%) had ARDS, 36 (7.3%) had septic shock, 75 (15.3%) had RF, 18 (3.7%) had myocarditis, and 55 (11.2%) had AKI (Table 1).
Laboratory results
Hemoglobin level, white blood cell (WBC) count, leukopenia, platelet count, international normalized ratio (INR), creatinine, urea, blood sugar, and liver enzymes did not differ significantly between ICU and non-ICU patients (Supplementary Material Table S2).
However, ICU patients had significantly higher D-dimer (2.1 vs. 1.5 mg/l, p = 0.001), hs-CRP (3.2 vs. 2.1 mg/l, p = 0.001), and cardiac troponin I (1.1 vs. 0.01 ng/ml, p = 0.001) levels compared to non-ICU patients. In contrast, BNP, and creatine kinase levels did not differ significantly between ICU and non-ICU patients (Table 1) and (Supplementary Material Table S2).
Electrocardiographic and echocardiographic measurements
Electrocardiograms (ECGs) showed sinus rhythm in 398 (81.2%) patients, atrial fibrillation in 18 (3.7%) patients, ACS in 59 (12%) patients and, arrythmia in 15 (3.1%) patients (Supplementary Material Table S2). LVEF was preserved in 324 (66.1%) patients and reduced (<52% for men and <54% for women) in 166 (33.9%). Diastolic function was normal in 197 (40.2%) patients, while grade 1 diastolic dysfunction was detected in 213 (43.5%), grade II in 64 (13.1%), and grade III in 16 (3.3%). Increased pulmonary artery pressure was observed in 100 patients (20.4%). Other laboratory investigations are presented in Table 1.
Pre-ICU TTE findings showed significantly higher incidence RV dysfunction (28 [13.8%] vs. 23 [8.0%] patients; p = 0.04) and LV regional wall motion abnormalities (55 [27.1%] vs. 29 [0.1%]; p < 0.001] in ICU compared to non-ICU patients (Table 1).
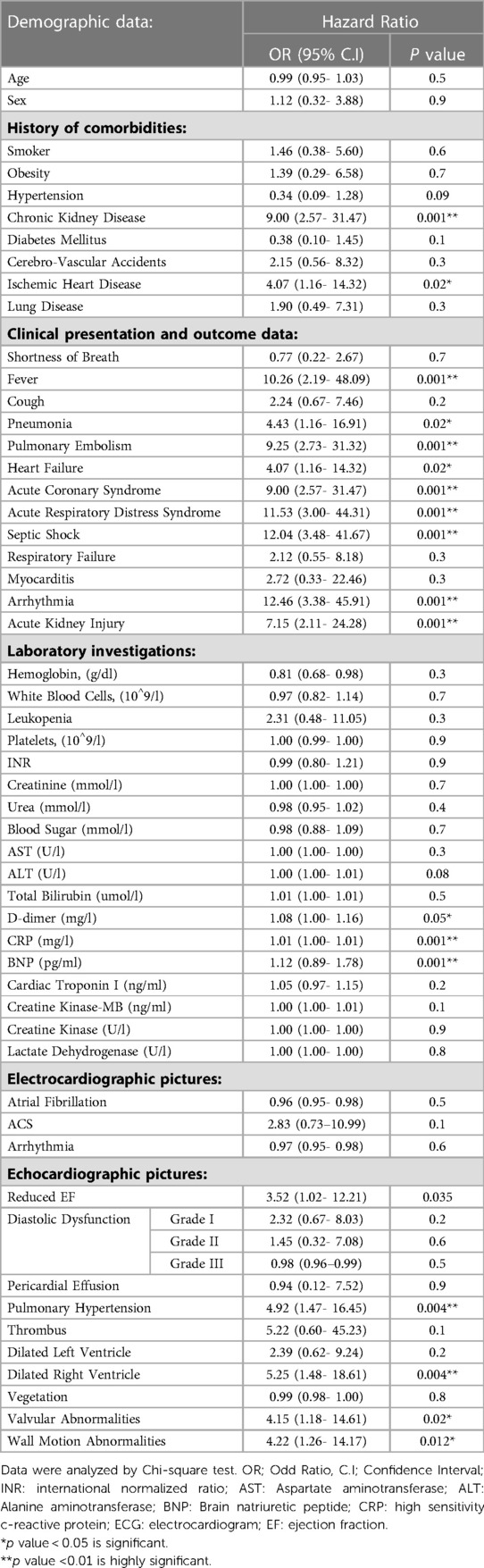
Table 2 shows predictors of mortality in the studied COVID-19 patients. Comorbidities such as chronic kidney diseases and medical history of ischemic heart disease were predictors for mortality. Moreover, clinical presentation and other factors including fever, pneumonia, PE, heart failure, acute coronary syndrome, ARDS, septic shock, elevated levels of D-dimer, CRP, BNP, pulmonary hypertension and dilated right ventricle were also predictors of mortality. Table 3 shows Association between echo-cardiographic features and inflammatory biomarkers with poor outcomes in the studied COVID-19 Patients by binary logistic regression. Echocardiographic predictors of poor outcomes were reduced LVEF (p = 0.037), pulmonary hypertension (p = 0.037), dilated right ventricle (p = 0.006), valvular abnormalities (p = 0.021), wall motion abnormalities (p = 0.000), D-dimer (p = 0.000), hs-CRP (p = 0.002) and BNP (p = 0.017) as shown in Table 3. The area under receiver operator characteristic (ROC) curves shows the most sensitive predictors of ICU admission: cardiac troponin I level (area under the curve [AUC] = 0.733, p = 0.000), followed by hs-CRP (AUC = 0.620, p = 0.000), creatine kinase-MB (AUC = 0.617, p = 0.000), D-dimer (AUC = 0.599, p = 0.000), lactate dehydrogenase (AUC = 0.567, p = 0.012), creatine kinase (AUC = 0.543, p = 0.105), and BNP (AUC = 0.488, p = 0.661) level as shown in Figure 2.
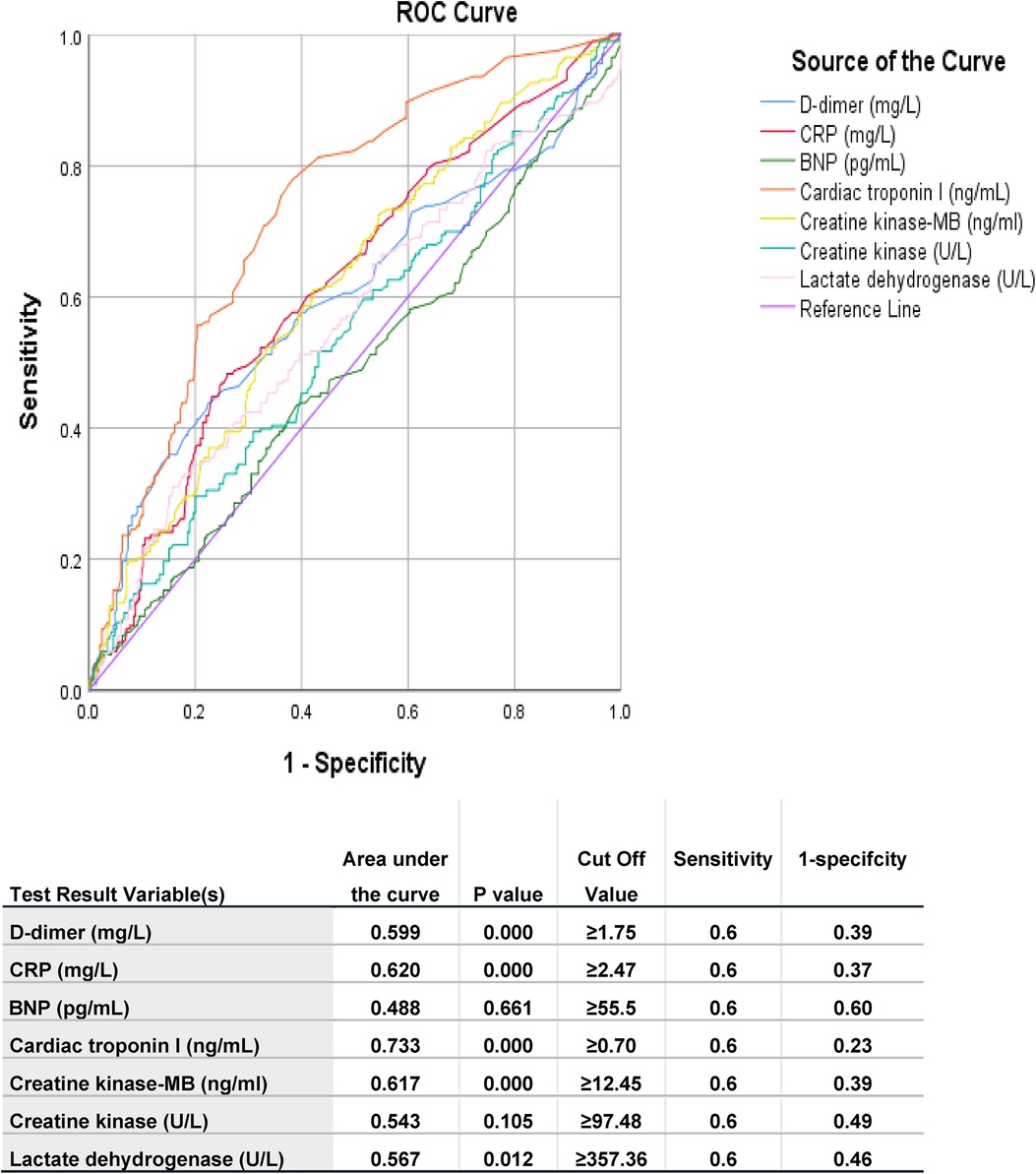
Figure 2. Area under ROC curve of different measured parameters of studied populations predicting ICU admission. The test result variable(s): D-dimer, CRP, BNP, Cardiac troponin I, Creatine kinase-MB, Creatine kinase, Lactate dehydrogenase has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased. BNP: brain natriuretic peptide; CRP: high sensitive c-reactive protein.
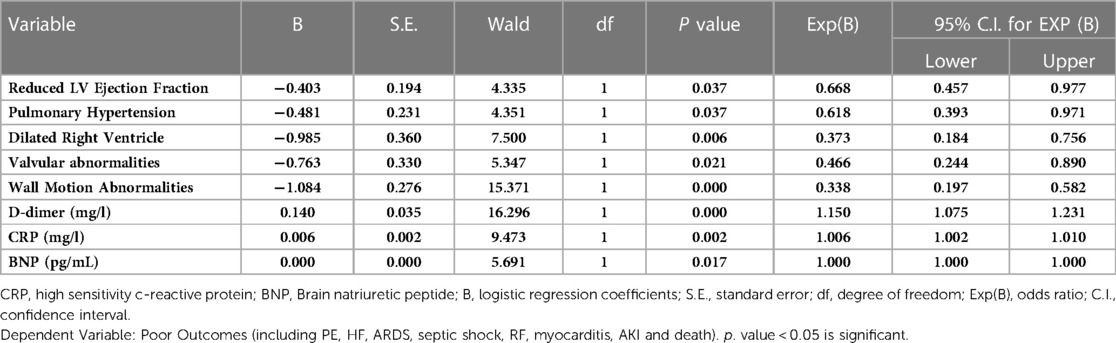
Table 3. Association between echo-cardiographic features and inflammatory biomarkers with poor outcomes in the studied COVID-19 patients by binary logistic regression (N = 263; 93 Non-ICU patients and 170 ICU patients).
The Supplementary Video shows a representative example of echocardiographic images from COVID-19 patients with poor outcomes (thrombus in the RV).
Discussion
Our study enrolled 490 COVID-19-infected patients admitted to King Saud Medical City, of which 41.4% were admitted to the ICU. Pre-ICU TTE findings showed that patients admitted to the ICU had significantly higher incidence RV dysfunction and LV regional wall motion abnormalities than non-ICU patients. In-hospital mortality was 2.2%, with all deaths occurring in the ICU. Reduced LVEF and elevated pulmonary artery systolic pressure were found to be echocardiographic predictors of death among our studied patients.
Consistently, a recent study found that COVID-19 infection was associated with various echocardiographic abnormalities, including wall motion abnormalities, impaired LV and RV systolic and diastolic function, and pericardial effusions (22).
Our study's first key finding was the higher incidence of RV dysfunction in ICU patients, with higher pulmonary systolic pressure associated with increased mortality. This finding is consistent with Kim et al. (23), who found that 172/510 (34%) of their participants had an abnormal RV size, and Gomez et al., who reported that one-third of their participants had RV dysfunction, which was associated with 60-day mortality (odds ratio = 1.93, 95% CI: 1.13–3.3; p = 0.016) (24). Hypoxia caused by COVID-19 and increased pulmonary circulation demands enhanced RV afterload. The thin RV walls make it highly vulnerable to dilatation and dysfunction, with an abrupt increase in pulmonary vascular resistance and pressure secondary to hypoxia, hypercapnia, and pulmonary vascular remodeling with ARDS (24, 25).
Our study's second key finding was impaired LV systolic function in 38.4% of ICU patients. This finding is consistent with Díaz et al., who reported that LVEF was the most predictive of mortality (HR = 0.94) (26). Additionally, Jain et al. retrospectively analyzed the echocardiographic data of 72 COVID-19-positive patients, observed that about one-third had impaired LV systolic function (27). Several mechanisms have been suggested that the cause is COVD-19-related cardiac injury (28). It has been associated with increased inflammatory markers during a “cytokine storm” (29). Other studies found an association between LV systolic dysfunction and elevated inflammation biomarkers, such as CRP and troponin levels and lymphocyte percentages (CD31+, CD41+, CD81+, and T-cell) (30–32).
The pathogenesis of COVID-19 is thought to be connected to direct cardiac involvement via the angiotensin converting enzyme-2 (ACE2) signaling pathway, though the precise cause of cardiac involvement in COVID-19 is still unknown (7). The substantially elevated risk of death in this population of patients may also be explained by increased ACE2 secretion in those with cardiovascular diseases as underlying conditions (30, 33). Other theoretical explanations include cytokine-mediated harm, an imbalance between oxygen supply and demand, ischemic injury brought on by the creation of microvascular thrombi, and direct viral invasion of the myocardium (28, 34). In addition, the risk of coronary thrombotic events from atherosclerotic plaque rupture has previously been shown to be increased during viral infections (35, 36).
TTE can detect variable cardiovascular abnormalities in the clinical settings of severe COVID-19 infection. For example, TTE measurements might be crucial to distinguish underlying cardiac and non-cardiac causes in patients with RF, shock, and greatly elevated cardiac biomarkers. In addition, more abnormal echocardiogram findings were seen in patients with myocardial injury compared to those without myocardial injury. COVID-19 has a wide range of echocardiographic abnormalities, including LV dysfunction, abnormal wall motion, diastolic dysfunction, RV dysfunction, and pericardial effusions (24, 37).
Our findings showed that the inflammatory biomarkers D-dimer, hs-CRP, and BNP were associated with worse outcomes among hospitalized COVID-19 patients.
Some prognostic indicators of poor prognosis have already been identified, particularly elevated high-sensitivity troponin I levels (38). Additionally, biomarkers of worse prognosis, such as troponin I and D-dimer, are associated with RV dysfunction markers and have been proposed as independent predictors of all-cause death (39, 40). Therefore, TAPSE, which correlated with acute-phase indicators, including troponins and D-dimer (40), was the RV variable independently associated with adverse outcomes, even after adjusting for D-dimer levels (41), RV dysfunction in COVID-19 patients remained an independent predictor of death. The association between it and high-sensitivity troponin, one of the best predictors of in-hospital mortality, is supported by recent data (42). Analysis of longitudinal strain should be used to assess RV function in patients with HFrEF to enhance identifying those at risk for adverse outcomes (43). Another recent study found that right ventricular longitudinal strain (RVLS) was a strong predictor of increased mortality in patients with coronavirus illness (14, 44).
Our study highlighted the new and additive predictive significance of RV measures in patients with COVID-19 infection and underlying cardiovascular disease, in addition to confirming the function of these previously described risk predictors.
This study had a few limitations, primarily the issue of referral bias and possible confounding. TTE scans were collected using criteria implemented at our hospital during the COVID-19 outbreak (17). To reduce unnecessary exposure to healthcare professionals, TTEs were limited to ill patients (45). While this limit the generalizability of our findings to the full spectrum of COVID-19 infections, they generally represent unvaccinated, symptomatic, and hospitalized COVID-19 patients. Another limitation was that TTEs were performed as, focused scans to acquire the most critical and clinically relevant information to guide decision-making. Therefore, additional quantitative assessment techniques, such as three-dimensional scans, were unavailable. The study design, a retrospective observational study that did not include control, was another limitation of our study. Further prospective randomized controlled studies are needed and further extension of this work is warranted.
In conclusion, our study showed the likely effect of COVID-19 infection on the heart and highlighted the various echocardiographic findings of hospitalized patients. Despite the potential risk of personnel infection during the COVID-19 pandemic, TTE could be a valuable tool for initial assessment, risk stratification, and guiding patient management. Moreover, we found that lower LVEF, Dilated RV and higher pulmonary systolic pressure were echocardiographic predictor of poor outcomes that could be used as imaging markers along with elevated D-dimer, hs-CRP, and BNP levels for predicting worse outcomes and ICU admission among hospitalized COVID-19 patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by King Saud Medical City, Ministry of Health, Kingdom of Saudi Arabia. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Authors contribution
SA, RA, MA, AA, MA, LK, RW, SA, AA and EE shared in participants selection, and enrolment, data analysis, literature reviewing, study design construction, wrote and revised the manuscript. HA, YA, AM, MA, OA and HG performed data collection. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are thankful to our participants and to the medical staff at Heart Health Center, King Saud medical city, Riyadh, Saudi Arabia, for their honest assistance and recommendations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1134601/full#supplementary-material.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports. Coronavirus disease (COVID-2019) situation reports. 2020;2020).
2. Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, Savardashtaki A, et al. COVID-19 and cardiac injury: clinical manifestation, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther. (2021) 19(3):345–57. doi: 10.1080/14787210.2020.1822737
3. Yao L, Lu L, Ma W. Immunopathological changes, complications, sequelae and immunological memory in COVID-19 patients. Heliyon. (2022) 8(4):e09302. doi: 10.1016/j.heliyon.2022.e09302
4. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. (2020) 109(5):531–8. doi: 10.1007/s00392-020-01626-9
5. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China. JAMA Cardiol. (2020) 5(7):802–10. 8. doi: 10.1001/jamacardio.2020.0950
6. Labbé V, Ederhy S, Lapidus N, Salem JE, Trinh-Duc A, Cohen A, et al. Characterization and outcomes of acute myocardial injury in COVID-19 intensive care patients. Infection. (2021) 49(3):563–6. doi: 10.1007/s15010-020-01560-y
7. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17(5):259–60. doi: 10.1038/s41569-020-0360-5
8. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5
9. Abdel Moneim A, Radwan MA, Yousef AI. COVID-19 and cardiovascular disease: manifestations, pathophysiology, vaccination, and long-term implication. Curr Med Res Opin. (2022) 38(7):1071–9. doi: 10.1080/03007995.2022.2078081
10. Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19. Eur Heart J. (2020) 41(19):1801–3. doi: 10.1093/eurheartj/ehaa235
11. AlQassas I, Hassan W, Sunni N, Lhmdi M, Nazzal A, Mohamed MJ, et al. The prognostic significance of elevated cardiac troponin in non-cardiac medical disorders. Pilot study. Int J Clin Cardiol. (2019) 6:136. doi: 10.23937/2378-2951/1410136
12. Carrizales-Sepúlveda EF, Vera-Pineda R, Flores-Ramírez R, Hernández-Guajardo DA, Pérez-Contreras E, Lozano-Ibarra MM, et al. Echocardiographic manifestations in COVID-19: a review. Heart Lung Circ. (2021) 30(8):1117–29. doi: 10.1016/j.hlc.2021.02.004
13. Cresti A, Barchitta A, Barbieri A, Monte IP, Trocino G, Ciampi Q, et al. Echocardiography and multimodality cardiac imaging in COVID-19 patients. J Cardiovasc Echogr. (2020) 30(Suppl 2):S18–24. doi: 10.4103/jcecho.jcecho_58_20
14. Li Y, Li H, Zhu S, Xie Y, Wang B, He L, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. (2020) 13(11):2287–99. doi: 10.1016/j.jcmg.2020.04.014
15. Mahmoud-Elsayed HM, Moody WE, Bradlow WM, Khan-Kheil AM, Senior J, Hudsmith LE, et al. Echocardiographic findings in patients with COVID-19 pneumonia. Can J Cardiol. (2020) 36(8):1203–7. doi: 10.1016/j.cjca.2020.05.030
16. Ródenas-Alesina E, Rodríguez-Palomares J, Bach-Oller M, Jordán P, Badia C, Herrador L, et al. Echocardiographic assessment of COVID19 sequelae in survivors with elevated cardiac biomarkers. Int J Cardiol. (2022) 360:104–10. doi: 10.1016/j.ijcard.2022.04.070
17. Ghazal S, Qaddoura F, Kinsara A, Omran A, Atiyah M, Al Refae M, et al. Saudi Arabian society of echocardiography recommendations for echocardiography service during Corona virus disease 2019 (COVID-19) outbreak. J Saudi Heart Assoc. (2020) 32(5):1–5. doi: 10.37616/2212-5043.1038
18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
19. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29(4):277–314. doi: 10.1016/j.echo.2016.01.011
20. Augustine DX, Coates-Bradshaw LD, Willis J, Harkness A, Ring L, Grapsa J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British society of echocardiography. Echo Res Pract. (2018) 5(3):G11–24. doi: 10.1530/ERP-17-0071
21. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. (2022) 43(38):3618–731. doi: 10.1093/eurheartj/ehac237
22. Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. (2020) 76(18):2043–55. doi: 10.1016/j.jacc.2020.08.069
23. Kim J, Volodarskiy A, Sultana R, Pollie MP, Yum B, Nambiar L, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. (2020) 76(17):1965–77. doi: 10.1016/j.jacc.2020.08.066
24. Gomez JMD, Zimmerman AC, du Fay de Lavallaz J, Wagner J, Tung L, Bouroukas A, et al. Echocardiographic predictors of mortality and morbidity in COVID-19 disease using focused cardiovascular ultrasound. Int J Cardiol Heart Vasc. (2022) 39:100982. doi: 10.1016/j.ijcha.2022.100982
25. Isgro G, Yusuff HO, Zochios V. The right ventricle in COVID-19 lung injury: proposed mechanisms, management, and research gaps. J Cardiothorac Vasc Anesth. (2021) 35(6):1568–72. doi: 10.1053/j.jvca.2021.01.014
26. Díaz JJS, Rincon JM, López MAR, Zuleta MB, Castellanos N, Saavedra ZS, et al. Echocardiographic 60-day mortality markers in patients hospitalized in intensive care for COVID-19. Heart Lung. (2022) 52:123–9. doi: 10.1016/j.hrtlng.2021.12.007
27. Jain SS, Liu Q, Raikhelkar J, Fried J, Elias P, Poterucha TJ, et al. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr. (2020) 33(10):1278–84. doi: 10.1016/j.echo.2020.06.009
28. Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Special article—acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis. (2020) 63(5):682–9. doi: 10.1016/j.pcad.2020.05.013
29. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. (2020) 7(6):998–1002. doi: 10.1093/nsr/nwaa041
30. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5(7):811–8. doi: 10.1001/jamacardio.2020.1017
31. Li D, Chen Y, Jia Y, Tong L, Tong J, Wang W, et al. SARS-CoV-2-Induced immune dysregulation and myocardial injury risk in China: insights from the ERS-COVID-19 study. Circ Res. (2020) 127(3):397–9. doi: 10.1161/CIRCRESAHA.120.317070
32. Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. (2015) 3:48. doi: 10.1186/s40560-015-0112-5
33. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. Erratum in: Lancet. (2020) 395(10229):1038. PMID: 32171076; PMCID: PMC7270627
34. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. (2020) 63:390–1. doi: 10.1016/j.pcad.2020.03.001
35. Kwong JC, Schwartz KL, Campitelli MA. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. (2018) 378:2540–1. doi: 10.1056/NEJMoa1702090
36. Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. (2020) 141(25):2113–6. doi: 10.1161/CIRCULATIONAHA.120.047525
37. Zhang L, Wang B, Zhou J, Kirkpatrick J, Xie M, Johri AM. Bedside focused cardiac ultrasound in COVID-19 from the Wuhan epicenter: the role of cardiac point-of-care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J Am Soc Echocardiogr. (2020) 33(6):676–82. doi: 10.1016/j.echo.2020.04.004
38. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Br Med J. (2020) 368:m1091. doi: 10.1136/bmj.m1091
39. Capone V, Cuomo V, Esposito R, Canonico ME, Ilardi F, Prastaro M, et al. Epidemiology, prognosis, and clinical manifestation of cardiovascular disease in COVID-19. Expert Rev Cardiovasc Ther. (2020) 18:531–9. doi: 10.1080/14779072.2020.1797491
40. Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. (2020) 13:2459–61. doi: 10.1016/j.jcmg.2020.05.010
41. Moody WE, Mahmoud-Elsayed HM, Senior J, Gul U, Khan-Kheil AM, Horne S, et al. Impact of right ventricular dysfunction on mortality in patients hospitalized with COVID-19. According to Race. CJC Open. (2021) 3(1):91–100. doi: 10.1016/j.cjco.2020.09.016
42. Gonzalez-Fernandez O, Ponz de Antonio I, Rosillo Rodriguez SO, Ruiz Cantador J, Figueira Iglesias JC, Lopez-Sendon Hentschel JL. D-dimer and right ventricular abnormalities as prognostic factors in critically ill COVID-19 patients. Rev Esp Cardiol (Engl Ed. (2020) 73(11):966–8. doi: 10.1016/j.recesp.2020.07.015
43. Carluccio E, Biagioli P, Alunni G, Murrone A, Zuchi C, Coiro S, et al. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ Cardiovasc Imaging. (2018) 11(1):e006894. doi: 10.1161/CIRCIMAGING.117.006894
44. Stockenhuber A, Vrettos A, Androschuck V, George M, Robertson C, Bowers N, et al. A pilot study on right ventricular longitudinal strain as a predictor of outcome in COVID-19 patients with evidence of cardiac involvement. Echocardiography. (2021) 38(2):222–9. doi: 10.1111/echo.14966
Keywords: echocardiography, COVID-19, outcome, biomarker, thrombus, mortality, VEF, pulmonary hypertension
Citation: Abohamr SI, Abazid RM, Alhumaid MK, Abdulrahim AE, Aldossari MA, Khedr L, Werida RH, Alkheledan HS, Aleid YS, Abdelhamid SW, Al Mefarrej A, Abdelhamid AW, Alaboud MH, Alhasan OT, Gomaa HM and Elsheikh E (2023) Association between echocardiographic features and inflammatory biomarkers with clinical outcomes in COVID-19 patients in Saudi Arabia. Front. Cardiovasc. Med. 10:1134601. doi: 10.3389/fcvm.2023.1134601
Received: 30 December 2022; Accepted: 2 May 2023;
Published: 26 May 2023.
Edited by:
Sanjeev Bhattacharyya, Barts Health NHS Trust, United KingdomReviewed by:
Aliki Tsagkridi, Barts Heart Centre, United KingdomApostolos Vrettos, Barts Health NHS Trust, United Kingdom
© 2023 Abohamr, Abazid, Alhumaid, Abdulrahim, Aldossari, Khedr, Werida, Alkheledan, Aleid, Abdelhamid, AI Mefarrej, Abdelhamid, Alaboud, Alhasan, Gomaa and Elsheikh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samah I Abohamr cy5hYm9oYW1hckBrc21jLm1lZC5zYQ== Rehab H. Werida cmVoYWJ3cmllZGFAcGhhcm0uZG11LmVkdS5lZw== Eman Elsheikh ZWVsc2hlaWtoQGtmdS5lZHUuc2E=
 Samah I. Abohamr1,2*
Samah I. Abohamr1,2* Lamiaa Khedr
Lamiaa Khedr Rehab H. Werida
Rehab H. Werida Abdulmohsen Al Mefarrej
Abdulmohsen Al Mefarrej Omar T. Alhasan
Omar T. Alhasan Eman Elsheikh
Eman Elsheikh