- 1Department of Cardiology, Lausanne University Hospital, Lausanne, Switzerland
- 2University of Lausanne, Lausanne, Switzerland
- 3Department of Cardiac Surgery, Lausanne University Hospital, Lausanne, Switzerland
Background: Paravalvular leak is one of the most common complications and is among the most important prognostic factors of short- and long-term mortality after transcatheter aortic valve implantation (TAVI). Percutaneous valvular leak repair constitutes a first-line treatment for paravalvular leaks and is associated with high success rates and few serious complications nowadays. To the best of our knowledge, this is the first case where placement of the device through the stenting of the bioprosthesis resulted in creating a new symptomatic stenosis that required surgery.
Case summary: We present a case of a patient with low-flow, low-gradient aortic stenosis treated with transfemoral implantation of a biological aortic prosthesis. One month after the procedure, the patient presented with acute pulmonary oedema and a paravalvular leak was discovered, which was corrected by percutaneous repair with a plug device. Five weeks after the valvular leak repair, the patient was readmitted for heart failure. At this time, a new aortic stenosis and paravalvular leak were diagnosed and the patient was referred for surgery. The new aortic mixed diseased was caused by the positioning of the plug device through the valve's metal stenting, which resulted in a paravalvular leak and pressed against the valve's leaflets, causing valvular stenosis. The patient was referred for surgical replacement and evolved well afterward.
Conclusion: This case illustrates a rare complication of a complex procedure, and it highlights the need for multidisciplinary decisions and good cooperation between the cardiology and cardiac surgery teams to develop better criteria in the selection of the appropriate technique for managing paravalvular leaks after TAVI.
Introduction
Despite the broadening of indications for transcatheter aortic valve implantation (TAVI) to include low-risk patients, as supported by subsequent studies (1, 2), some complications still undermine the use of this technique. Paravalvular leak (PVL) is one of the most common complications and is amongst the most important prognostic factors of mortality at short- and long-term after TAVI (3–5), being associated with a threefold increase in 30-day mortality (95% CI: 1.73–5.02) and a 2.3-fold increase in 1-year mortality (95% CI: −1.84 to 2.81) for moderate to severe leaks (6).
When comparing surgical to percutaneous aortic valve replacement, the incidence of moderate to severe paravalvular leaks between the percutaneous and the surgical series did not differ significantly in the PARTNER trial: the percutaneous group presenting 0.6% and the surgical group 0.5% at 1 year. By contrast, mild paravalvular leak at 1 year is still significantly higher in the percutaneous series, with 29.4% compared to 2.1% in the surgical one (2).
Here, we present the first case of paravalvular leak after TAVI treated percutaneously with an Amplatzer device where migration of the device resulted in severe aortic stenosis needing a surgical intervention.
Case presentation
A 79-year-old female with a history of hypertension, permanent atrial fibrillation, and progressing aortic stenosis presented with NYHA stage II dyspnoea and peripheral oedema. Echocardiography showed a tricuspid aortic valve with severe paradoxical low-flow, low-gradient aortic stenosis with a surface of 0.5 cm2 by planimetry, a mean gradient of 19 mmHg, a preserved ejection fraction at 60%, and a low left ventricular output of 25 ml/min/m2 due to moderate hypertrophy. A CT scan revealed a modified Agatston calcium score of 630, with a calcium volume of 197 mm3 and moderate calcifications of the valve with heterogenous peripheral distribution. The patient's surgical risk was characterised by an EuroSCORE II of 1.60% and an STS score of 3.4% of predicted mortality, and her frailty score was at class 5.
The case was discussed in a multidisciplinary Heart Team meeting, and due to the patient's persistent symptoms and recurring hospitalisations, despite the low calcium score, an invasive strategy was decided. The patient was strongly opposed to cardiac surgery, despite her relatively low surgical risk, which contributed to the decision to perform a percutaneous aortic valve implantation.
The patient underwent a transfemoral implantation of a biological aortic prosthesis type Edwards Sapien 3 of 23 mm, with a good echocardiographic result, mild paravalvular leak, and a mean valve gradient that came down to 8 mmHg.
Immediately after the procedure, a new left bundle branch block was noted (Figure 1) that motivated a His-ventricular (HV) exploration. A His-right ventricle conduction delay of 64 ms was found, which increased to 95 ms after Ajmaline provocation. Considering these results and given that the patient was in permanent atrial fibrillation with a difficult-to-control heart rate, despite bitherapy, the decision to implant a pacemaker was made. The patient was implanted with a single-chamber pacemaker SORIN, followed by an atrioventricular node ablation.

Figure 1. Left side: ECG before procedure showing atrial fibrillation, narrow QRS, and two premature ventricular contractions; right side: ECG after procedure showing a large QRS with a left bundle branch block.
One month after the procedure, the patient presented with acute pulmonary oedema. Physical examination revealed a new diastolic heart murmur, and the echocardiography confirmed a seemingly significant paravalvular leak with an ERO (Effective Regurgitant Orifice) by PISA method at 0.1 cm2 and a planimetry of 0.4 cm2.
Given the new findings, the patient was referred for percutaneous repair of the paravalvular leak and was successfully implanted with an Amplatzer Vascular Plug 4 of 8 mm (Figure 2B), resulting in a significant reduction of regurgitation and resolution of the heart murmur. Post-procedural echocardiography showed a residual paravalvular leak involving 1/5 of the valve perimeter, a calculated valve surface of 1 cm2, and a trans-aortic mean gradient of 14 mmHg.
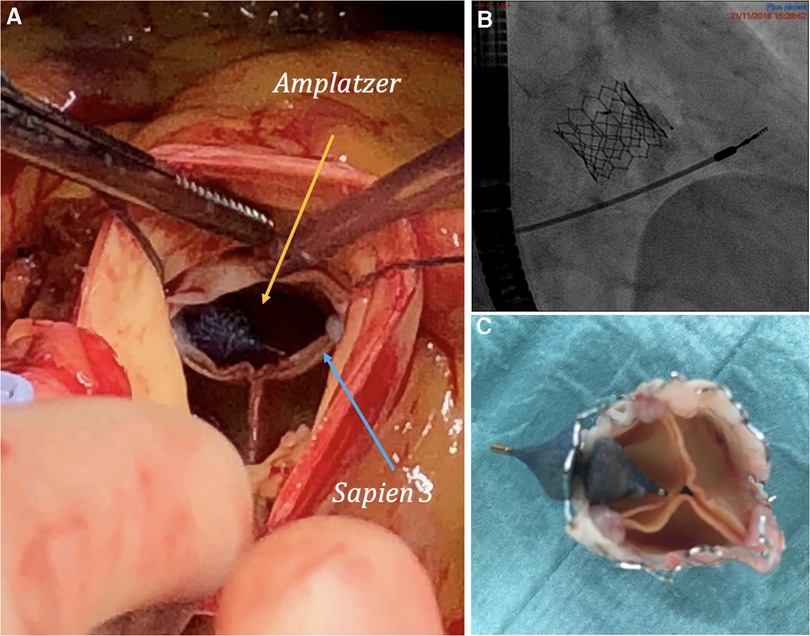
Figure 2. (A) Amplatzer vascular plug and bioprosthesis in vivo. (B) Angiography of initial implantation of Amplatzer vascular plug. (C) Bioprosthesis ex vivo with manual replacement of the Amplatzer vascular plug by the surgeon.
Five weeks after the valvular leak repair, the patient was readmitted for acute heart failure. At this time, echocardiography showed mixed aortic disease with aortic stenosis characterised by a surface of 1 cm2, a mean gradient of 23 mmHg, and a significant paravalvular aortic regurgitation involving 1/3 of the valve perimeter (Figure 3). The case was reconsidered by the Heart Team, and a surgical approach was proposed.
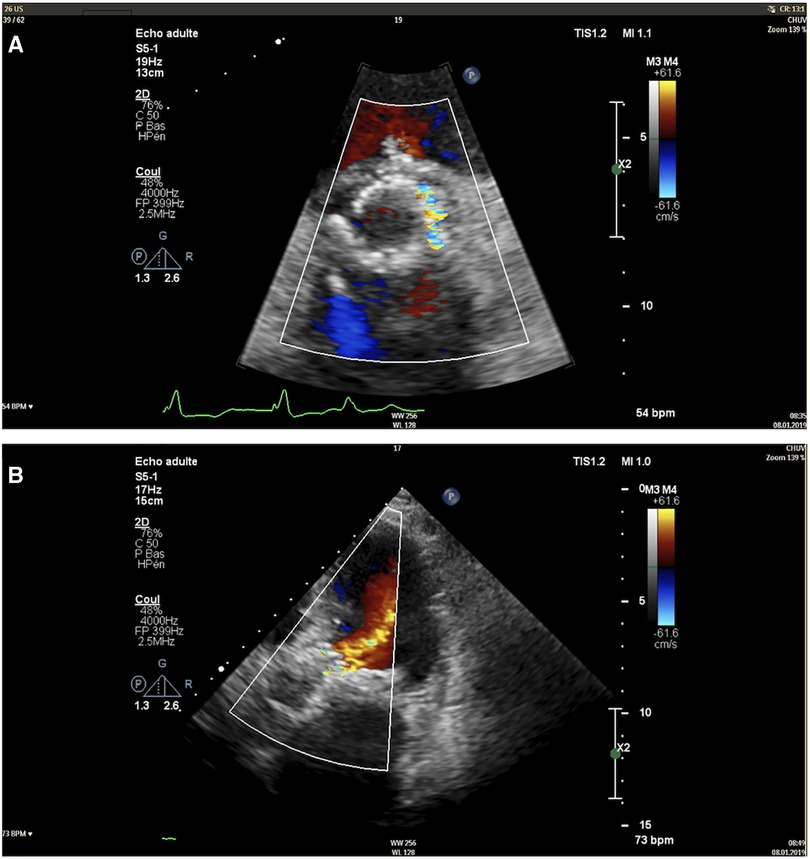
Figure 3. Transthoracic echocardiography image of the paravalvular leak in short-axis view (A) and apical five-chamber view (B).
During the surgical procedure, it was found that the Amplatzer had moved from its initial position and was oriented towards the leaflets of the bioprosthesis as shown in Figures 2 A,C. This contributed to the post-procedural aortic stenosis by impeding the opening of the right coronary leaflet and providing insufficient leak barrier. A surgical aortic valve replacement was performed using a sutureless LIVANOVA Perceval S bioprosthesis size M, which was completed without any complications. The postoperative echocardiography revealed a preserved left ventricle ejection fraction (62%) with grade 3 diastolic dysfunction, an aortic bioprosthesis with a calculated surface of 0.9 cm2, and a mean gradient of 18 mmHg with no regurgitation. The patient evolved well and was transferred to cardiac rehabilitation. The mid-term follow-up at 4 years showed a benefit of the procedures in terms of symptoms, with the patient now being in NYHA class I, and hospitalisations, with only one hospitalisation for acute heart failure since being discharged.
Discussion
This case highlights two common complications of TAVI procedures and, more importantly, a complication of percutaneous paravalvular leak repair.
In recent years, new data have emerged regarding electrical conduction complications, when comparing surgical to percutaneous aortic valve replacement. For instance, the incidence of a new left bundle branch block is found to be 10.5%–34.3% in TAVI procedures (7), compared to 4% in surgical aortic valve replacements (8). Additionally, 7.3% of the patients undergoing percutaneous aortic valve replacement end up needing a permanent pacemaker implantation (all causes combined), compared to 3.4% in the surgical series (p = 0.014) (9). New left bundle branch block and new pacemaker implantation are some of the few outcomes that favour surgical approach.
With regards to the mechanical complication, this patient initially presented with a mild paravalvular leak that later progressed to a more significant one. The mechanism of this progression is unknown to date. Progression of paravalvular leaks has been described by the PARTNER trial (10); however, it happened over years instead of months seen in our case. So far, no mechanism has been proposed to explain the improvement or worsening of PVL, and measurement methods may explain, in part, these findings. The calcium volume described in the CT scan was low, and there were no indicators of preferential deposition in the device landing zone that could help predict this outcome.
Percutaneous valvular leak repair is a first-line treatment for paravalvular leaks and is nowadays associated with high success rates and few serious complications (11). However, it is a delicate and complex procedure and the correct choice of device and placement is essential. The literature concerning percutaneous paravalvular leak closure after TAVI is relatively scarce, and only a few case series have been published. The number of closures is too low to provide a good understanding of the complications. In contrast, more cases of complications after percutaneous PVL closure have been described in surgical series, and a few larger studies have been published in the literature that describe the most frequent complications encountered (Table 1).
In our patient's case, the Amplatzer device appears to have moved and intertwined with the metallic stenting of the prosthesis, which not only caused a failure in completely resolving the leak but also restricted the valve's full opening and led to a reduction in the valve surface, resulting in a new aortic stenosis. The reason for the device's migration is unknown, but it could be related to its initial position during the procedure.
These complications highlight the ongoing debate on indications between TAVI and surgical approaches. Despite the patient's vehement objection to a surgical approach, in light of her Euroscore/STS score and frailty score, and considering the small size of the valve annulus, a surgical approach may have yielded better results in this case. Ultimately, the patient underwent surgery anyway.
Conclusion
To the best of our knowledge, this is the first case in which a TAVI paravalvular leak treated percutaneously with an Amplatzer device was complicated by the device's migration through the stenting of the bioprosthesis, resulting in a new symptomatic stenosis that required surgery. This highlights the need for better criteria for selecting the appropriate technique for managing paravalvular leaks after TAVI. Whether a baseline surgical approach or valve post-dilation would have been a better option in this patient remains unclear.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
BPG drafted the manuscript and was responsible for the patient follow-up. OR, EE, and MK provided critical review of the manuscript. EE performed the TAVI and the percutaneous valvular leak repair. MK performed the surgical valve replacement. All authors contributed to the article and approved the submitted version.
Funding
Open access funding was provided by University of Lausanne.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380(18):1695–705. doi: 10.1056/NEJMoa1814052
3. Détaint D, Lepage L, Himbert D, Brochet E, Messika-Zeitoun D, Lung B, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc Interv. (2009) 2(9):821–7. doi: 10.1016/j.jcin.2009.07.003
4. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363(17):1597–607. doi: 10.1056/NEJMoa1008232
5. Takagi H, Umemoto T. Impact of paravalvular aortic regurgitation after transcatheter aortic valve implantation on survival. Int J Cardiol. (2016) 221:46–51. doi: 10.1016/j.ijcard.2016.07.006
6. Athappan G, Patvardhan E, Tuzcu EM, Svensson LG, Lemos PA, Fraccaro C, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. (2013) 61(15):1585–95. doi: 10.1016/j.jacc.2013.01.047
7. Roten L, Meier B. Left bundle branch block after transcatheter aortic valve implantation: still a matter of concern? JACC Cardiovasc Interv. (2014) 7(2):137–9. doi: 10.1016/j.jcin.2013.11.006
8. Poels TT, Houthuizen P, Garsse LAFMV, Hamad MAS, Maessen JG, Prinzen FW, et al. Frequency and prognosis of new bundle branch block induced by surgical aortic valve replacement. Eur J Cardiothorac Surg. (2015) 47(2):e47–53. doi: 10.1093/ejcts/ezu435
9. Bagur R, Rodés-Cabau J, Gurvitch R, Dumont É, Velianou JL, Manazzoni J, et al. Need for permanent pacemaker as a complication of transcatheter aortic valve implantation and surgical aortic valve replacement in elderly patients with severe aortic stenosis and similar baseline electrocardiographic findings. JACC Cardiovasc Interv. (2012) 5(5):540–51. doi: 10.1016/j.jcin.2012.03.004
10. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. (2012) 366(18):1686–95. doi: 10.1056/NEJMoa1200384
11. Sorajja P, et al. Percutaneous repair of paravalvular prosthetic regurgitation: patient selection, techniques and outcomes. Heart. (2015) 101(9):665–73. doi: 10.1136/heartjnl-2014-306270
12. Arri SS, Poliacikova P, Hildick-Smith D. Percutaneous paravalvular leak closure for symptomatic aortic regurgitation after CoreValve transcatheter aortic valve implantation. Catheter Cardiovasc Interv. (2015) 85(4):657–64. doi: 10.1002/ccd.25730
13. Gérardin B, Champagnac D, Smolka G, Bouvaist H, Jakamy R, Ghostine S, et al. Fermeture des fuites para-prothétiques des TAVI. Ann Cardiol D'Angéiol. (2019) 68(6):453–61. doi: 10.1016/j.ancard.2019.09.027
14. Sorajja P, et al. Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgitation. J Am Coll Cardiol. (2011) 58(21):2218–24. doi: 10.1016/j.jacc.2011.07.041
15. Ruiz CE, Jelnin V, Kronzon I, Dudiy Y, Valle-Fernandez RD, Einhorn BN, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol. (2011) 58(21):2210–7. doi: 10.1016/j.jacc.2011.03.074
Keywords: percutaneous valve therapy, paravalvular leak repair, aortic valve disease percutaneous intervention, transcatheter aortic valve implantation (TAVI), case report
Citation: Pitta Gros B, Roux O, Eeckhout E and Kirsch M (2023) Case report: Stenosis turned leak … and turned stenosis—complications of paravalvular prosthetic leak closure with a plug device. Front. Cardiovasc. Med. 10:1132063. doi: 10.3389/fcvm.2023.1132063
Received: 26 December 2022; Accepted: 22 May 2023;
Published: 12 June 2023.
Edited by:
Fadi Sawaya, American University of Beirut, LebanonReviewed by:
Theodor (Teddy) Fischlein, Klinikum Nürnberg, GermanyVito Domenico Bruno, University of Bristol, United Kingdom
© 2023 Pitta Gros, Roux, Eeckhout and Kirsch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Pitta Gros QmFyYmFyYS5waXR0YS1ncm9zQGNodXYuY2g=
 Barbara Pitta Gros
Barbara Pitta Gros Olivier Roux1
Olivier Roux1 Matthias Kirsch
Matthias Kirsch