- 1Department of Cardiology, Teine Keijinkai Hospital, Sapporo, Japan
- 2Department of Cardiology, Hakodate Goryokaku Hospital, Hakodate, Japan
- 3Department of Cardiology, Renal and Metabolic Medicine, Sapporo Medical University, Sapporo, Japan
Aims: Cardiac mortality in patients with heart failure (HF) is likely to be aggravated by malnutrition, assessed by serum cholinesterase (ChE) level, as well as by kidney dysfunction or impairment of cardiac sympathetic denervation. Their prognostic interactions, however, have not been determined.
Methods: A total of 991 systolic HF patients were enrolled in our HF database following clinical evaluation including evaluation of the nutrition state and assessment of standardized heart-to-mediastinum ratio (sHMR) of iodine-123-labeled meta-iodobenzylguanidine activity. Patients were followed up for an average of 43 months with the primary endpoint of fatal cardiac events (CEs).
Results: The CE patient group had a lower level of ChE, lower estimated glomerular filtration rate (eGFR) and lower late sHMR than those in the non-CE patient group. A five-parameter model with the addition of serum ChE selected in the multivariate logistic analysis (model 2) significantly increased the AUC predicting risk of cardiac events compared with a four-parameter model without serum ChE (model 1), and net reclassification analysis also suggested that the model with the addition of serum cholinesterase significantly improved cardiac event prediction. Moreover, in overall multivariate Cox hazard analysis, serum ChE, eGFR and late sHMR were identified to be significant prognostic determinants. HF patients with two or all of the prognostic variables of serum ChE < 230 U/L, eGFR < 48.8 ml/min/1.73 m2 and late sHMR < 1.90 had significantly and incrementally increased CE rates compared to those in HF patients with none or only one of the prognostic variables.
Conclusion: Decreases in cholinesterase level and kidney function further increase cardiac mortality risk in HF patients with impairment of cardiac sympathetic innervation.
Introduction
Obesity is well known to be an independent risk factor for metabolic, endocrine and cardiovascular diseases (1). On the other hand, it has been shown that heart failure (HF) patients who are malnourished or experiencing rapid weight loss may have an increased prognostic risk independent of age, New York Heart Association (NYHA) functional class, or cardiac functions (2–4). These findings clearly demonstrate the importance of controlling energy intake and body weight for better prognosis, as indicated by many prophylactic or treatment guidelines for atherosclerotic disorders. The dichotomic aspects have emphasized the necessity to appropriately select a nutritional intervention strategy in association with the nutrition state, patient activity, baseline disease, comorbidities and cardiac function. Although body mass index (BMI) is conventionally used for the assessment of obesity or calorie intake in relatively heathy subjects, the usefulness of BMI may be limited for identifying a malnutrition state or sub-clinical cachexia in patients with pathologic conditions. This is because a pathological undernutrition state is related to many factors such as protein catabolism, lipolysis, bone loss, kidney dysfunction and anemia together with increases in sympathetic nerve activity, inflammatory cytokines and/or insulin resistance (5). It has recently been shown that serum cholinesterase can be an indicator of hepatic function and nutrition, and several investigations have revealed its close correlation with prognosis in HF patients (6, 7). Although these biomarkers are speculated to increase cardiac mortality by the development of a vicious cycle, there are few reports on the prognostic interactions in HF patients.
With reference to previous studies on the prognosis of HF (8–13), the aim of the present study was to determine prognostic incremental values for risk stratification of HF patients with reduced left ventricular ejection fraction (LVEF), with particular focus on the prognostic interactions of serum cholinesterase levels, renal function and cardiac sympathetic innervation as nutritional parameters.
Materials and methods
Study design and study subjects
A total of 991 consecutive patients with symptomatic HF and echocardiographic LVEF <50% who were admitted to our hospital between April 2010 and December 2016 and underwent myocardial scintigraphy of 123I-labeled meta-iodobenzylguanidine (MIBG) for prognostic evaluation after compensatory and optimal medical therapy were retrospectively enrolled in our HF database.
The inclusion criteria for this retrospective study were symptomatic HF requiring hospitalization, LVEF <50% determined by echocardiography, and age >20 years. Patients who refused resuscitation, patients who had obvious malignancy or hemorrhagic disease, and patients who were younger than 20 years of age were excluded. The patients included 738 males (74.5%). The mean age of the patients was 67.5 ± 12.9 years and the mean LVEF was 32.7 ± 11.3%. The diagnosis of HF was made by the Framingham criteria including typical symptoms of jugular venous distention, peripheral edema, pulmonary rales, S3 or S4 gallop sounds, and tachycardia. A chest x-ray was taken and two-dimensional echocardiography was performed to corroborate the diagnosis and rule out other diseases with similar symptoms and signs. In addition to a history of myocardial infarction or coronary revascularization, the etiology of HF, such as ischemic or nonischemic, was differentiated by electrocardiography, echocardiography, scintigraphy, or a combination of these when necessary as well as by coronary vascular information obtained by computed tomography, magnetic resonance imaging, and invasive selective coronary angiography. Just before discharge, blood tests were performed to measure the levels of hemoglobin (Hb), albumin, lipid profiles, glutamic-oxaloacetic transferase (GOT), glutamic-pyruvic transferase (GPT), lactate dehydrogenase (LDH), serum ChE, creatinine and brain natriuretic peptide (BNP). Renal function was evaluated by estimated glomerular filtration rate (eGFR) using the standard formula. The baseline geriatric nutrition risk index (GNRI) was calculated from serum albumin and BMI using the following formula: GNRI = 14.89 × serum albumin (g/dl) + 41.7 × present body weight/[height2 (m2) × 22] = 14.89 × serum albumin (g/dl) + 41.7 × BMI/22. Plasma BNP levels were measured in 598 patients (60.3%) and NT-pro BNP levels were measured in the remaining 393 patients (39.6%) before discharge when heart failure was stable. To evaluate BNP and NT-pro BNP in an integrated manner, all of the HF patients were classified into four groups according to the following values based on ESC HF guidelines: 0–40 pg/ml and 0–125 pg/ml for stage 1, 41–100 pg/ml and 126–400 pg/ml for stage 2, 101–200 pg/ml and 401–900 pg/ml for stage 3, and 201 pg/ml and 901 pg/ml for stage 4, respectively.
Echocardiographic assessment
Two-dimensional echocardiography was performed by echocardiographic technicians with no knowledge of the patients' clinical data in the left lateral recumbent position using a commercially available ultrasound machine equipped with a 2.5 MHz variable frequency transducer to measure the parameters from views of the paracentral long axis and short axis and apical 4, 3, and 2 chambers. The following echocardiographic parameters were measured at the time of compensated heart failure prior to discharge: left atrium diameter (LAD; mm), left ventricular end-diastolic diameter (LVDd; mm), LVEF (%) calculated using the biplane modified Simpson's method, left ventricular volume at end-diastole (EDV; ml), left ventricular volume at end-systole (ESV; ml) and septal E/e’ (14, 15).
Measurement of sympathetic denervation of the left ventricle
Cardiac sympathetic innervation by 123I-labeled MIBG of 111 MBq was quantitatively assessed in patients with compensated heart failure in a fasting and resting state by cardiac imaging using a gamma camera with a low-energy generic collimator at 15–30 min (early images) and 4 h (late images) after an intravenous tracer injection, as previously described (9–11).
Cardiac 123I-MIBG activity was measured as heart-to-mediastinum ratio (HMR) using dedicated MIBG software (Smart MIBG Software, Tokyo, Japan) operated by experienced nuclear medicine technicians with no knowledge of the patients' clinical data, automatically setting the region of interest to the upper mediastinum and the entire heart on a planar anterior image. 123I-MIBG washout kinetics from the left ventricle were calculated from early and late cardiac 123I-MIBG activities as washout rate (WR). The HMR of cardiac 123I-MIBG was measured using a late image and standardized as sHMR for medium energy collimator conditions by a mathematical method established in a cross-calibration phantom experiment (12, 16). This method not only minimizes the variability of 123I-MIBG HMR but also makes it possible to compare HMR data from sHMR regardless of differences in data acquisition methods such as data obtained using low- and medium-energy collimators or data obtained at different research facilities.
Follow-up protocol
HF patients enrolled in our hospital HF database were regularly followed up by experienced cardiologists in the out-patient clinic for one year for a longer period if the patients survived. The cardiac events (CEs) that were considered as the primary endpoints of the present study were as follows: fatal CEs such as sudden cardiac death, death due to refractory and progressive pump failure, fatal sustained ventricular tachyarrhythmias, including ventricular tachycardia and ventricular fibrillation, and appropriate implantable cardioverter defibrillator (ICD) therapy against fatal ventricular arrhythmias. Clinical findings were confirmed by reviewing the patients' medical records and outcome analysis was retrospectively performed. In HF patients enrolled in the present study, sudden cardiac death was defined as witnessed cardiac arrest and death within one hour of acute onset or unexpected death in patients who had survived for the previous 24 h. The present study was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by our institutional ethics committee for enrollment in our database and use of data for clinical research. The present study was approved by the Ethics Committee of Obihiro Kosei Hospital. Its approval number was 2016-015. Due to the retrospective, non-interventional, observational nature of the study as well as the fact that the clinical research was publicly disclosed on the institution's Web site and the fact that comprehensive consent was determined by the institution's ethics committee, the need for each patient's written informed consent was waived.
Statistical analysis
Statistical values are presented as means ± SD. Means of values in the two groups were compared by the unpaired t-test and categorical variables were compared by the χ2 test. New parameters were added to the conventional risk factors for patients with HF, and area under the curve (AUC) and net reclassification index (NRI) were used to assess whether the prognostic evaluation was significantly improved. Reclassification was additionally used to re-evaluate the results.
A time-dependent cumulative event-free curve was generated by Kaplan-Meier analysis using the key parameters identified in the present study, and log-rank tests were also used to compare the curves as needed. The number of significant variables identified in the univariate analysis was statistically appropriate for the number of cardiac events in the present study, and multivariate analysis with the Cox proportional hazards model was performed to calculate hazard ratios and 95% confidence intervals (CIs) for the significant variables. Receiver operating characteristic (ROC) analysis was performed to determine the optimal cut-off values for the independent significant parameters of CEs. Statistical analysis in the present study was performed using the computer software SAS for Windows (version 9.4, SAS Institute, Cary, North Carolina, USA) and Mathematica software for Windows (version 12.3, Wolfram Research Inc., Champaign, IL. USA). P values less than 0.05 were considered significant.
Results
Following measurement of serum ChE and calculation of eGFR, cardiac sympathetic innervation was quantified as standardized HMR of MIBG activity as shown by two typical cases (Figure 1). Despite having almost identical levels of cardiac dysfunction, Case 1 with almost normal values of serum ChE, eGFR and late sHMR (2.37) had no CEs, while Case 2 with apparent decreases in serum ChE, eGFR and late sHMR (1.34) died of progressive pump failure during the observation period. In 313 HF patients (31.5%), CEs recorded during a mean follow-up period of 43 months were as follows: sudden cardiac death in 29 patients, cardiac death due to refractory and progressive pump failure in 240 patients, lethal ventricular tachyarrhythmias in 22 patients and appropriate ICD treatment against lethal ventricular tachyarrhythmias in 22 patients.
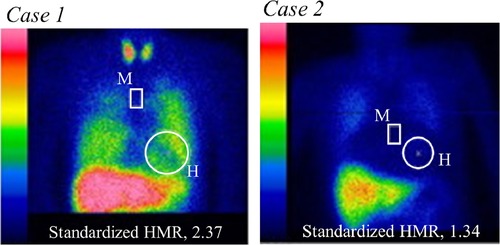
Figure 1. Measurements of heart (H)-to-mediastinum (M) ratio (HMR) of 123I-MIBG activity using a late planar anterior image standardized with dedicated MIBG software (smart MIBG software, Tokyo, Japan). Case 1 (left panel): In a 51-year-old male, LVEF was reduced (21%) due to ischemic cardiomyopathy, but eGFR (67 ml/min/1.73 m2), serum cholinesterase (330 U/L) and late standardized HMR (2.37) were nearly normal. No fatal cardiac events occurred during the observation period. Case 2 (right panel): A 71-year-old woman underwent cardiac resynchronization therapy after refractory heart failure management with optimal medical therapy alone. She had significantly decreased values of LVEF (28%) due to non-ischemic cardiomyopathy, eGFR (23 ml/min/1.73 m2), serum cholinesterase (136 U/L), and late standardized HMR (1.34). The patient died of progressive pump failure during the observation period.
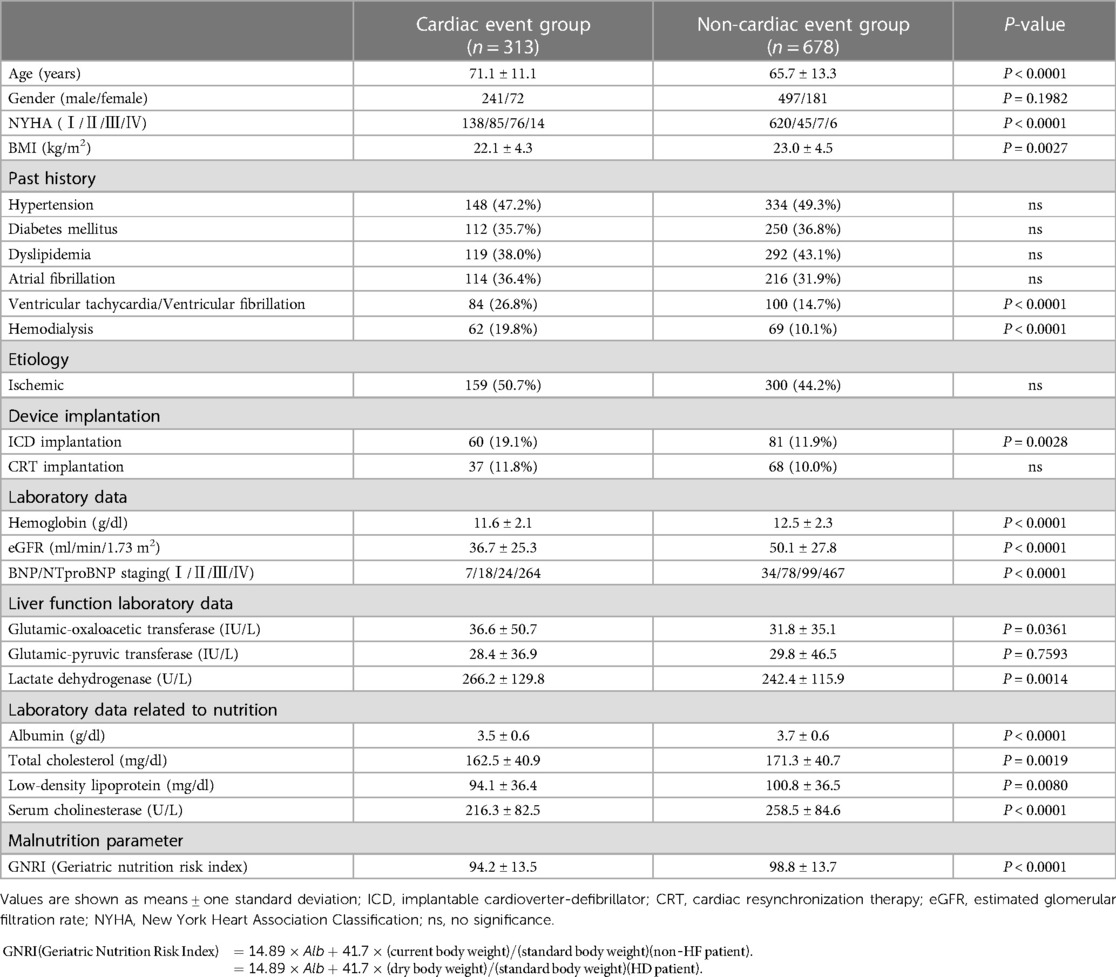
HF patients in the CE group were older than those in the non-CE group, and HF patients in the CE group had a greater NYHA functional class, lower eGFR (36.7 ± 25.3 vs. 50.1 ± 27.8 ml/min/1.73 m2, P < 0.0001), lower BMI (22.1 ± 4.3 vs. 23.0 ± 4.5, P = 0.0027) and lower serum ChE (216.3 ± 82.5 vs. 258.5 ± 84.6 U/L, P < 0.0001) than those in patients in the group without CEs (Table 1). Patients in the CE group had a significantly lower late sHMR than that in patients in the non-CE group: 1.72 ± 0.41 vs. 2.00 ± 0.48, P < 0.0001 (Table 2).
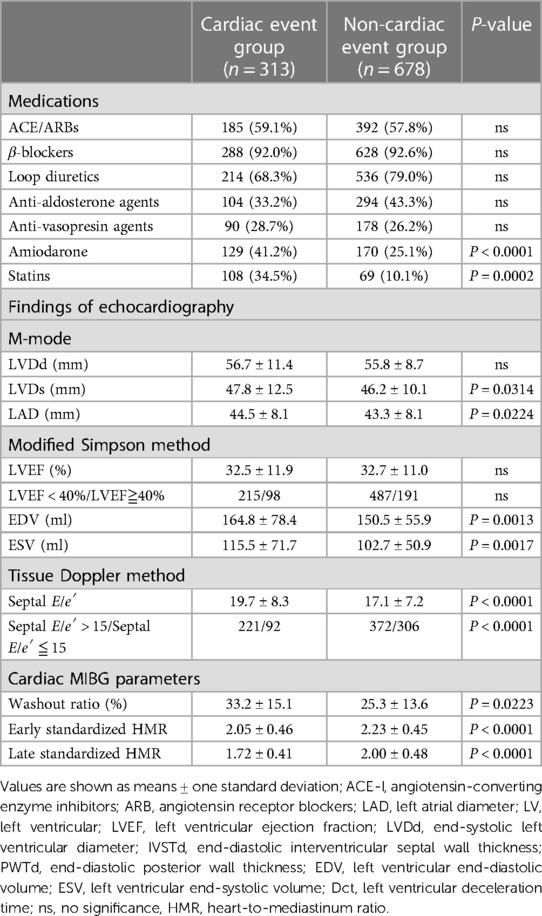
Table 2. Comparison of medications and two-dimensional echocardiographic parameters and scintigraphic parameters in patients with and those without cardiac events.
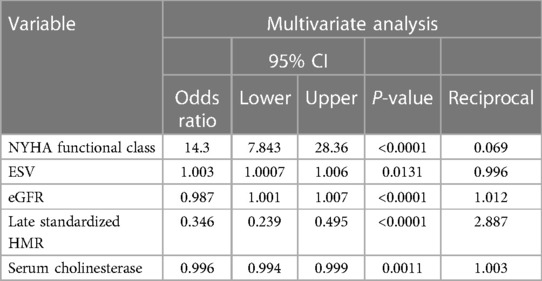
In the multivariate logistic regression analysis excluding serum ChE (model 1), significant variables were NYHA functional class (OR: 10.1, χ2 = 67.1, P < 0.0001), ESV (OR: 1.002, χ2 = 4.74, P = 0.0295), eGFR (OR: 0.985, χ2 = 23.6, P < 0.0001) and late sHMR (OR: 0.333, χ2 = 38.7, P < 0.0001). The ROC AUC of model 1 was 0.751. In the logistic regression analysis with serum ChE (model 2), significant variables were NYHA functional class (OR: 14.3, χ2 = 48.7, P < 0.0001), ESV (OR: 1.003, χ2 = 6.15, P = 0.0131), eGFR (OR: 0.987, χ2 = 16.4, P < 0.0001), late sHMR (OR: 0.346, χ2 = 35.1, P < 0.0001), and serum ChE (OR: 0.996, χ2 = 10.72, P = 0.0011) (Table 3). The ROC-AUC of model 2 was 0.814. Of the two curves, the model including serum ChE had a significantly larger AUC (P < 0.0001).
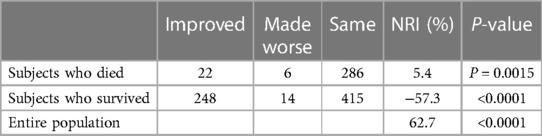
Net reclassification analysis was performed using the four-parameter logistic model created by the combination of NYHA functional class, ESV, eGFR and late sHMR (model 1) and by the five-parameter model that included ChE (model 2) [Table 4A]. Of the 314 patients who died of a cardiac event, classification was improved in 22 patients and was made worse in 6 patients using the five-parameter model. The net gain in reclassification proportion was 5.4% (P = 0.0015). Of the 677 patients who did not die of a cardiac event, 14 were reclassified upward and 248 patients were reclassified downward, with a net gain in reclassification of −57.4% (P < 0.0001). The NRI in all subjects was 62.7% (P < 0.0001) [Table 4B]. Thus, model 2 significantly improved the identification of patients at low risk of cardiac death when compared to model 1. Based on logistic regression analysis, an equation with five variables was used to estimate the prediction of CEs (model 2):
where the variables bintercep, bNYHA, bESV, beGFR, blate sHMR, and bChE derived from the parameter estimates of the model were 1.875, 2.655, 0.003, −0.012, −1.060, and −0.003 (χ2 = 233.3, P < 0.0001), respectively.
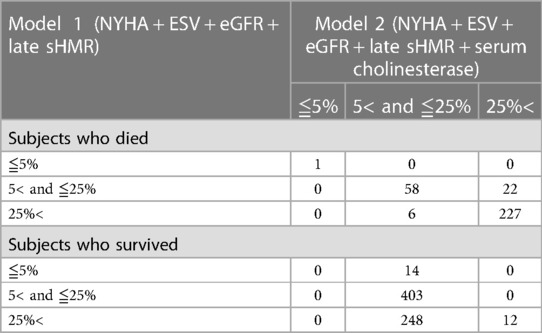
Table 4A. Reclassification analysis in patients who died and those who did not die of a cardiac event.
In the present formula, NYHA functional class (class Ⅰ/1, class Ⅱ/2, class Ⅲ/3, class Ⅳ/4) was a categorical variable, and ESV, eGFR, late sHMR and ChE were continuous variables.
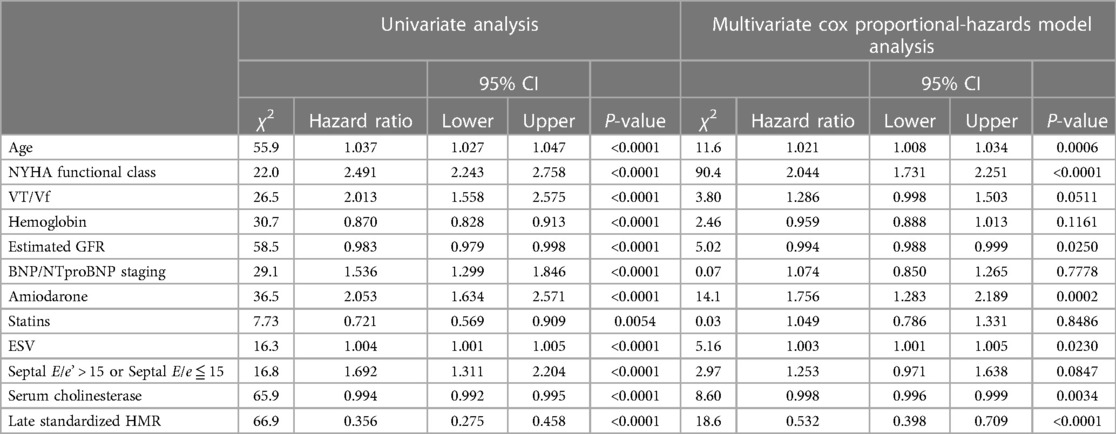
Among the significant univariate variables (Table 5), serum ChE, eGFR and late sHMR were identified as significant independent prognostic determinants in multivariate Cox analysis together with NYHA functional class, ESV and amiodarone use, with hazard ratios and 95% CIs of 0.998, 0.996–0.999 (P = 0.0034), 0.994, 0.988–0.999 (P = 0.0250) and 0.532, 0.398–0.709 (P < 0.0001), respectively.
The respective cut-off values for serum ChE, eGFR and late sHMR were calculated from the ROC curve. By using cut-off values of the three parameters, patients were clearly categorized into high-risk and low-risk sub-groups (Figure 2). Patients who had a serum ChE level of less than 230 U/L, eGFR of less than 48.8 ml/min/1.73 m2 or late sHMR of less than 1.90 had significantly lower cardiac event-free rates than did their counterparts (Figure 2). The combination of two of the three parameters (ChE, eGFR and late sHMR) more clearly discriminated HF patients in three risk categories: low, intermediate, and high-risk groups (Figure 3). Further accumulation of the three parameters (i.e., serum ChE < 230 U/L, eGFR < 48.8 ml/min/1.73 m2 and late sHMR < 1.90) was demonstrated to increase CE risks in HF patients (Figure 4).
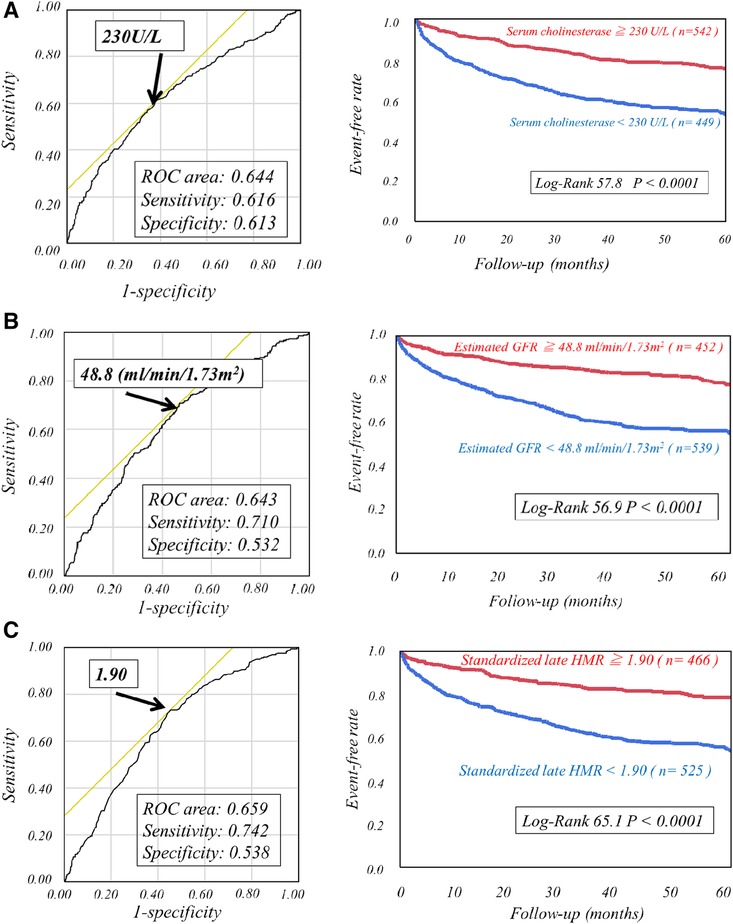
Figure 2. Kaplan-Meier event-free curves with ROC analysis cutoff values of serum cholinesterase of 230 U/L (A), estimated glomerular filtration rate of 48.8 ml/min/1.73 m2 (B), and late standardized MIBG heart-to-mediastinum ratio (sHMR) of 1.90 (C) clearly identified high-risk patients from low-risk patients.
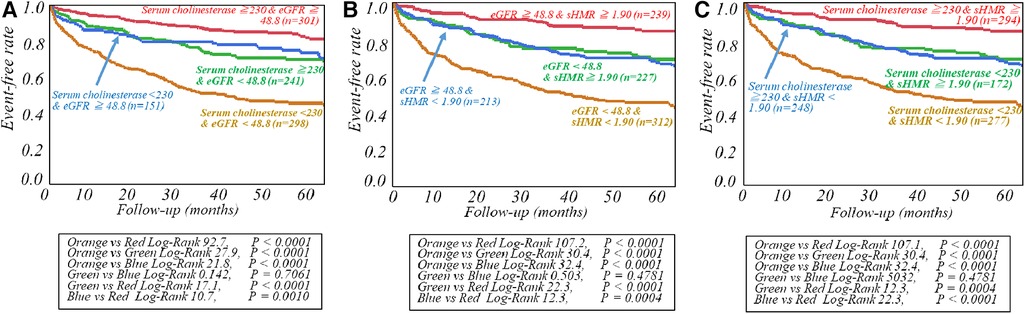
Figure 3. Kaplan-Meier event-free curves generated with two of the three prognostic variables, including serum cholinesterase, estimated glomerular filtration rate (eGFR), and late standardized MIBG heart-to-mediastinum ratio (sHMR), were used to clearly classify patients into low-, intermediate-, and high-risk groups. Risk stratification of heart failure patients was performed by combining serum cholinesterase and estimated glomerular filtration rate (eGFR) in A, estimated glomerular filtration rate (eGFR) and late standardized MIBG heart-to-mediastinum ratio (sHMR)in B and serum cholinesterase and late standardized MIBG heart-to-mediastinum ratio (sHMR)in C, respectively.
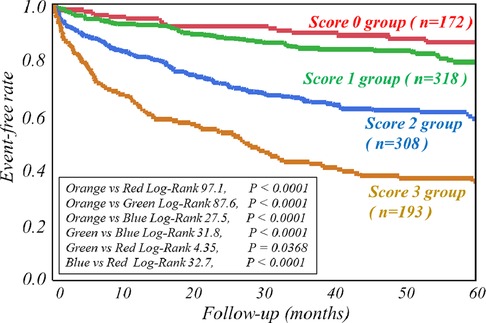
Figure 4. Kaplan-Meier event-free curves clearly show that accumulation of the three abnormal parameters (i.e., serum cholinesterase <230 U/L, estimated glomerular filtration rate (eGFR) < 48.8 ml/min/1.73 m2 and late standardized heart-to-mediastinum ratio of MIBG activity (sHMR) < 1.90) synergistically decreased event-free rates. The score category (0–3) indicates the number of the three abnormal parameters.
Discussion
The present study revealed a synergistic role of serum ChE as a malnutritional parameter in risk stratification of patients with systolic HF, conventionally assessed by renal dysfunction and cardiac sympathetic denervation.
Cardiac sympathetic nerve denervation and malnutrition
A low nutritional status worsens the prognosis of HF patients (2, 17). In HF patients, impaired absorption and increased permeability due to liver dysfunction, intestinal edema and decreased appetite due to right HF are possible causes of a malnutritional state. In elderly HF patients, furthermore, inadequate energy intake, increased energy expenditure, and impaired anabolism can combine to form a malnutritional state that is prone to water retention and infection. In addition, the parasympathetic nervous system is predominantly involved in the secretion of digestive enzymes such as glucosidase, peptidase, and lipase, and digestion and absorption are inhibited in a hyper-sympathetic state, which likely contributes to malnutrition in HF patients. In the present study, levels of liver enzymes were also significantly higher in the CE group than in the non-CE group. Serum ChE level, an indicator of liver dysfunction and malnutrition, was also an independent prognostic factor together with an indicator of cardiac sympathetic dysfunction. Moreover, the combination of these two parameters suggests that further risk stratification of HF patients is possible.
Renal dysfunction and malnutrition
Recently, the concept of protein energy wasting (PEW), defined as a depletion of protein energy sources, has been used to define malnutrition in patients with renal dysfunction. The causes of PEW include inadequate nutritional intake, systemic inflammatory response syndrome due to a cytokine storm including HF, uremia, endocrine abnormalities (increased insulin resistance, overproduction of catabolic hormones, decreased production of anabolic hormones), and metabolic acidosis (18, 19).
The vicious cycle leads to hypoproteinemia and albuminemia.
In addition, hyperglycemia and increased insulin resistance in patients with renal dysfunction lead to abnormal glucose metabolism. In the liver, this leads to accelerated glycogen breakdown, increased glycogenesis, and inhibition of sugar and amino acid oxidation (18). Furthermore, for adipose tissue, renal dysfunction induces insulin-dependent suppression of glucose oxidation, increased lactate production and fatty acid release. Levels of total cholesterol, HDL and LDL are decreased (19).
In the present study, the synthesis of lipids and proteins was also suppressed in the CE group compared to that in the non-CE group, with significantly lower blood levels of lipid profile and protein/albumin in the CE group. Serum ChE level, a surrogate parameter for lipid and protein-albumin synthesis in the liver, was also significantly lower in the CE group. Serum ChE was an independent prognostic factor, as was eGFR, a surrogate parameter for renal dysfunction, suggesting that the combination of these two parameters might enable synergistic risk stratification of HF patients.
Cardiac sympathetic nerve denervation and renal dysfunction
When hypoperfusion is sustained due to HF, the renin-angiotensin-aldosterone system, cardiac sympathetic nerve function, arginine vasopressin, endothelin I, and atrial natriuretic peptide are constantly activated (8). Long-lasting HF leads to increased cardiac sympathetic function, contraction of import and export arteries, and decreased GFR, which further contribute to renal dysfunction.
Decreased GFR leads to anemia due to impaired erythropoietin production and other causes. Anemia worsens heart failure, increases cardiac sympathetic function and leads to refractory HF (8, 20).
This study also showed significant cardiac sympathetic dysfunction and renal dysfunction in the CE group compared to those in the non-CE group. Moreover, cardiac sympathetic denervation and renal dysfunction were selected as independent prognostic factors, indicating that the combination of these two parameters might enable clearer risk stratification in HF patients.
Limitations
The present study was designed as an observational and non-interventional cohort study of patients with irreversible systolic HF at a single facility.
A large multicenter intervention study based on the results of our study could contribute to the development of better prevention and treatment strategies, including diet and cardiac rehabilitation, using appropriate indications for malnutrition in HF patients with comorbid cardiac cachexia who are at high risk of death. In the present study, there was no evaluation of the Prognostic Nutrition Index (PNI) or Controlling Nutritional Status (CONUT), which have been reported to have a prognostic value for malnutrition in HF patients (21, 22).
In HF patients at risk for nutritional impairment, approaches to improving nutritional status have been reaffirmed as part of the role of cardiac rehabilitation. Improvement of nutritional status is expected to be effective for improving exercise capacity. It has also been reported that cardiac rehabilitation increases muscle mass, prevents muscle hypercatabolism, and improves nutritional status (23, 24).
There are two main types of therapy for HF patients: pharmacological and non-pharmacological. The use of percutaneous coronary intervention, catheter ablation, ICDs and biventricular pacemakers, transcatheter aortic valve implantation, MitraClip ® implantation, and other non-pharmacologic therapies have received attention, and the importance of nutritional management, including diet and exercise rehabilitation, should be further discussed.
Conclusion
A lower level of serum ChE can independently and synergistically predict the risk of cardiac mortality in systolic HF patients with cardiac sympathetic innervation and renal dysfunction.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Obihiro Kosei Hospital (Approval No. 2016-015). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin due to the retrospective, non-interventional, observational nature of the study, as well as the fact that the clinical research was publicly disclosed on the institution's website and the fact that comprehensive consent was determined by the institution's Ethics Committee.
Author contributions
TD, a principal investigator of this study, participated in study design, collection of patient data and analysis of data and drafted the initial version of this manuscript. TT, TM, DN, AH, and SY participated in study design and collection and analysis of clinical data. TN, a principal investigator and supervisor of this study, participated in study design, data analysis and final manuscript preparation. All authors contributed to the article and approved the submitted version.
Acknowledgments
All authors sincerely appreciate the clinical services and technical assistance of the staff of the Nuclear Medicine Laboratory at Obihiro Kosei Hospital (Obihiro, Japan).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. (2002) 347:305–13. doi: 10.1056/NEJMoa020245
2. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. (1997) 349:1050–53. doi: 10.1016/S0140-6736(96)07015-8
3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1016/j.jacc.2017.04.025
4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. Authors/task force members; document reviewers. ESC scientifc document group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
5. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. (2008) 27:793–9. doi: 10.1016/j.clnu.2008.06.013
6. Seo M, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, et al. Prognostic significance of serum cholinesterase in patients with acute decompensated heart failure: a prospective comparative study with other nutritional indices. Am J Clin Nutr. (2019) 110:330–9. doi: 10.1093/ajcn/nqz103
7. Shiba M, Kato T, Morimoto T, Yaku H, Inuzuka Y, Tamaki Y, et al. Serum cholinesterase as a prognostic biomarker for acute heart failure. Eur Heart J Acute Cardiovascular Care. (2021) 10:335–42. doi: 10.1093/ehjacc/zuaa043
8. Doi T, Nakata T, Hashimoto A, Yuda S, Wakabayashi T, Kouzu H, et al. Cardiac mortality assessment improved by evaluation of cardiac sympathetic nerve activity in combination with hemoglobin and kidney function in chronic heart failure patients. J Nucl Med. (2012) 53:731–40. doi: 10.2967/jnumed.111.095786
9. Doi T, Nakata T, Yuda S, Hashimoto A. Synergistic prognostic implications of left ventricular mechanical dyssynchrony and impaired cardiac sympathetic nerve activity in heart failure patients with reduced left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. (2018) 19:74–83. doi: 10.1093/ehjci/jew334
10. Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. (2013) 6:772–84. doi: 10.1016/j.jcmg.2013.02.007
11. Nakajima K, Nakata T, Doi T, Kadokami T, Matsuo S, Konno T, et al. Validation of 2-year 123I-meta-iodobenzylguanidine-based cardiac mortality risk model in chronic heart failure. Eur Heart J Cardiovasc Imaging. (2018) 19:749–56. doi: 10.1093/ehjci/jey016
12. Nakajima K, Nakata T, Doi T, Tada H, Maruyama K. Machine learning-based risk model using 123I-metaiodobenzylguanidine to differentially predict modes of cardiac death in heart failure. J Nucl Caridiol. (2022) 29:190–201. doi: 10.1007/s12350-020-02173-6
13. Doi T, Nakata T, Noto T, Mita T, Nagahara D, Yuda S, et al. Impaired cardiac sympathetic innervation increases the risk of cardiac events in heart failure patients with left ventricular hypertrophy and mechanical dyssynchrony. J Clin Med. (2021) 10:5047. doi: 10.3390/jcm10215047
14. Daimon M, Iwano H, Onishi T, Ohara T, Tanaka H, Hirano Y, et al. Guideline from the Japanese society of echocardiography: guidance for the management and maintenance of echocardiography equipment: 2022 focused update. J Echocardiogr. (2022) 2:1–6. doi: 10.1007/s12574-022-00588-3 Online ahead of print.
15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
16. Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol. (2014) 21:970–8. doi: 10.1007/s12350-014-9916-2
17. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. (2013) 77:705–11. doi: 10.1253/circj.CJ-12-1091
18. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. (2008) 73:391–8. doi: 10.1038/sj.ki.5002585
19. Fiaccadori E, Cremaschi E. Nutritional assessment and support in acute kidney injury. Curr Opin Crit Care. (2009) 15:474–80. doi: 10.1097/MCC.0b013e328332f6b2
20. Palazzuoli A, Silverberg DS, Iovine F, Calabrò A, Campagna MS, Gallotta M, et al. Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. (2007) 154:e9–15. doi: 10.1016/j.ahj.2007.07.022
21. Sargento L, Longo S, Lousada N, dos Reis RP. The importance of assessing nutritional status in elderly patients with heart failure. Curr Heart Fail Rep. (2014) 11:220–6. doi: 10.1007/s11897-014-0189-5
22. Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev. (2016) 21:549–65. doi: 10.1007/s10741-016-9540-0
23. Yokota J, Takahashi R, Matsukawa Y, Matsushima K. Examination of independent predictors of discharge disposition in acute phase hospitalized heart failure patients undergoing phase I cardiac rehabilitation. Eur J Phys Rehabil Med. (2020) 56:780–6. doi: 10.23736/S1973-9087.20.06347-9
24. European Association of Cardiovascular Prevention and Rehabilitation Committee for Science Guidelines; EACPR, Corrà U, Piepoli MF, Carré F, Heuschmann P, Hoffmann U, et al. Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training: key components of the position paper from the cardiac rehabilitation section of the European association of cardiovascular prevention and rehabilitation. Eur Heart J. (2010) 31:1967–74. doi: 10.1093/eurheartj/ehq236
Keywords: heart failure, prognosis, malnutrition, serum cholinesterase, risk stratification
Citation: Doi T, Nakata T, Tsuzuki T, Mita T, Nagahara D, Yuda S and Hashimoto A (2023) Decreased cholinesterase level combined with renal dysfunction and sympathetic denervation associated with increased cardiac mortality in systolic heart failure. Front. Cardiovasc. Med. 10:1131282. doi: 10.3389/fcvm.2023.1131282
Received: 24 December 2022; Accepted: 13 September 2023;
Published: 29 September 2023.
Edited by:
Jonathan Vigne, Centre Hospitalier Universitaire de Caen, FranceReviewed by:
Alex Parker, University of Florida, United StatesHilmi Alnsasra, Soroka Medical Center, Israel
Eisuke Amiya, The University of Tokyo Hospital, Japan
© 2023 Doi, Nakata, Tsuzuki, Mita, Nagahara, Yuda and Hashimoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takahiro Doi ZG9pdGFrYTUxOEB5YWhvby5jby5qcA==
 Takahiro Doi
Takahiro Doi Tomoaki Nakata2
Tomoaki Nakata2