- 1Department of Cardiology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India
- 2Department of Cardiology, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3Department of Cardiology, Hackensack Meridian Health, New Jersey, NJ, United States
- 4Department of Cardiology, Amsterdam University Medical Centres, Amsterdam, the Netherlands
- 5Department of Internal Medicine, Cleveland Clinic Akron General, Akron, OH, United States
- 6Department of Internal Medicine, New York Medical College, Metropolitan Hospital, New York, NY, United States
- 7Jindal Institute of Behavioral Sciences (JIBS), Jindal Global Institution of Eminence Deemed to Be University, Sonipat, India
- 8Department of Cardiovascular Medicine, Franciscan Health, Indiana, IN, USA; Co-CEO, Kalra Hospitals, New Delhi, India
- 9Professor of Cardiology, Norwich Medical School, University of East Anglia, Norwich, United Kingdom
Aim: Transfemoral Trans-catheter Aortic Valve Replacement (TF-TAVR) is a safe and effective therapy compared with surgical aortic valve replacement (SAVR) in patients across all risk profiles using balloon-expandable valves (BEV) and self-expanding valves (SEV). Our aim was to compare safety and efficacy of BEV vs. SEV in high-risk patients undergoing TF-TAVR.
Methods and results: We searched PubMed, EMBASE, Clinicaltrials.gov, Scopus, and Web of sciences for studies on patients with severe aortic stenosis undergoing TAVR. Primary outcome was 30-day all-cause mortality. Secondary outcomes defined by Valve Academic Research Consortium 2 (VARC-2) criteria were also examined. Six studies with 2,935 patients (1,439 to BEV and 1,496 to SEV) were included. BEV was associated with lower risk of all-cause mortality (2.2% vs. 4.5%; RR: 0.51; 95% CI: 0.31–0.82; p < 0.006) and cardiovascular mortality [(2.5% vs. 4.3%; RR: 0.54; 95% CI: 0.32–0.90; p = 0.01) at 30 days compared with SEV. Implantation of more than one valve per procedure (0.78% vs. 5.11%; RR: 0.15; 95% CI: 0.07–0.31; p < 0.00001), and moderate/severe AR/PVL (2.5% vs. 9.01%; RR: 0.3; 95% CI: 0.17–0.48); p < 0.00001) were also lower in the BEV arm.
Conclusion: BEV TAVR is associated with reduced all-cause mortality (High level of GRADE evidence), cardiovascular mortality (very low level) at 30 days compared with SEV TAVR in high surgical risk patients. Data are necessary to determine if the difference in outcomes persists in longer-term and if the same effects are seen in lower-risk patients.
Systematic Review Registration: identifier, CRD42020181190.
1. Introduction
Trans-catheter aortic valve replacement (TAVR) is an established therapy for patients with symptomatic severe aortic stenosis (AS) across all surgical risk profiles (1). Three different platforms of trans-catheter heart valves are currently available: balloon-expandable valve (BEV), self-expanding valve (SEV) and mechanically expandable valve (MEV) (2). The United States Food and Drug Administration (FDA) has approved BEV devices including Sapien, Sapien-XT, Sapien-3 and Sapien-3 Ultra (Edwards Lifesciences, Irvine, CA, USA), SEV devices including CoreValve, Evolut R, Evolut Pro and Evolut-Pro+ (Medtronic, Minneapolis, MN, USA) in all AS patients, and LOTUS Edge™ (Boston Scientific, Boston, MA, USA) in high or greater risk patients (3–5). Other commonly used self-expanding devices outside the United States include Conformitè Europëenne (CE) marked devices like Acurate-Neo (Boston Scientific, Boston, MA, USA), Portico (Abbott Structural Heart, Santa Clara, CA, USA), Jena Valve (Jena Valve Technologies, Irvine, CA, USA), and Allegra (New Valve Technologies, Germany), and China FDA approved Venus-A (Venus Meditech, China) (2). Recently, Lotus Edge has been retrieved from the market. TAVR has overcome SAVR in the United States. But studies comparing the outcomes of different transcatheter valve systems are limited. It is well known that BEV is associated with few pacemaker requirements than SEV. But data regarding other hard-end points are scare. We therefore performed a systematic review and meta-analysis of randomized studies to study the safety and efficacy of TAVR using BEV vs. SEV devices in high risk patients.
2. Methods
2.1. Study eligibility
Studies were included, if they fulfilled the following criteria.
a) Randomized controlled trials (RCTs) in patients with severe native AS undergoing TAVR.
b) RCTs or post hoc analysis of RCTs comparing valve platforms into BEV vs. SEV or an RCT with pre-specified analysis by valve platforms. If a trial included MEV platform in either study arm (SEV or BEV), then it had to be <5% for inclusion in the current study.
c) Study should report all-cause mortality at 30 days as either primary or secondary outcome.
2.2. Search strategy
We searched PubMed, EMBASE, Clinicaltrials.gov, Scopus, and Web of science for all studies on patients with severe aortic stenosis undergoing TAVR (since inception to April 17th 2020) without any language restriction. We used multiple posting suffix (.mp) to improve sensitivity of our search. In addition, we looked for cross-references in the screened studies, review articles, and meta-analyses to identify other potential studies to be included. Our detailed search strategy is provided in the Supplementary Material. The study protocol is registered with PROSPERO, International prospective register of systematic reviews (CRD42020181190).
2.3. Eligibility assessment, data extraction and validity assessment
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed during the development of this systematic review and meta-analysis (6). After eliminating duplicates, screening of manuscripts was done based on title and abstracts to remove irrelevant articles by two independent authors. Full text assessment of relevant, identified articles were scrutinized again by the above authors. Risk of bias assessment was done using Cochrane risk-of-bias tool for randomized trials version 2 (RoB 2) (7). Assessment of risk of bias, inconsistency, indirectness, imprecision, publication bias and effect size were assessed to calculate “certainty of evidence” using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach (8). Furthermore, the GRADEpro guideline development tool was used to create a “Summary of findings” table and a GRADE “Evidence profile”. Screening, full-text assessment, data extraction and validity assessment were independently performed by two authors (NBS and HB). Discrepancy was resolved by the third author (BC). We extracted baseline characteristics of patients, procedural details and clinical outcomes from included studies.
2.4. Outcomes
The primary outcome of our study was all-cause mortality at 30 days. Several endpoints on early safety, clinical efficacy and device success as defined by Valve Academic Research Consortium-2 (VARC-2) criteria were examined as secondary outcomes (9), including cardiovascular (CV) mortality, all stroke (disabling and non-disabling), life-threatening bleeding, major vascular complications, major bleeding, acute kidney injury (AKI) Stage 2 or 3 (including renal replacement therapy), myocardial infarction (MI), coronary artery obstruction requiring intervention, valve-related dysfunction requiring repeat procedure, moderate to severe aortic regurgitation (AR)/para-valvular leak (PVL), atrial fibrillation, rehospitalizations for valve-related symptoms or worsening congestive heart failure, permanent pacemaker implantation, prosthetic valve endocarditis, valve thrombosis, NYHA class III or IV, early valve-related dysfunction, implantation of more than one valve per procedure, valve malposition, and annular rupture. Whenever outcomes reported were too few in numbers (<5 events) or reported by a single study only, they were not included in the final analysis.
2.5. Statistical analysis
Data extracted from the studies were imported into Review Manager Version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration Copenhagen, Denmark) for analysis. Pairwise meta-analysis was performed for overall analysis. We used DerSimonian and Laird random effects model for our analysis to calculate pooled risk ratio (RR) and 95% confidence interval (CI) for all outcomes. We calculated between-study heterogeneity by using the Higgins I2 statistic. We defined low and high heterogeneity as I2 < 25% and >75% respectively. Publication bias was assessed visually by asymmetry in funnel plots. We performed sensitivity analyses utilizing various factors whenever statistically significant heterogeneity was found. This included an analysis after excluding studies one-by one that were considered an outlier based on methodological or interventional heterogeneity. A leave-one-out sensitivity analysis to remove the effect of one study at a time on our results was also performed. All tests were 2-tailed with a p value of <0.05 considered significant.
3. Results
We identified 2,207 studies through our databases search (Figure 1 and Supplementary Table S1). After removing 807 duplicate results, we selected 1,400 articles for title and abstract screening. We excluded 1,378 publications which were irrelevant. Twenty-two articles were studied for eligibility. Out of 22 studies, 15 were excluded and 6 studies were included for the final analysis. The reasons for exclusion of 16 studies are illustrated in Figure 1.
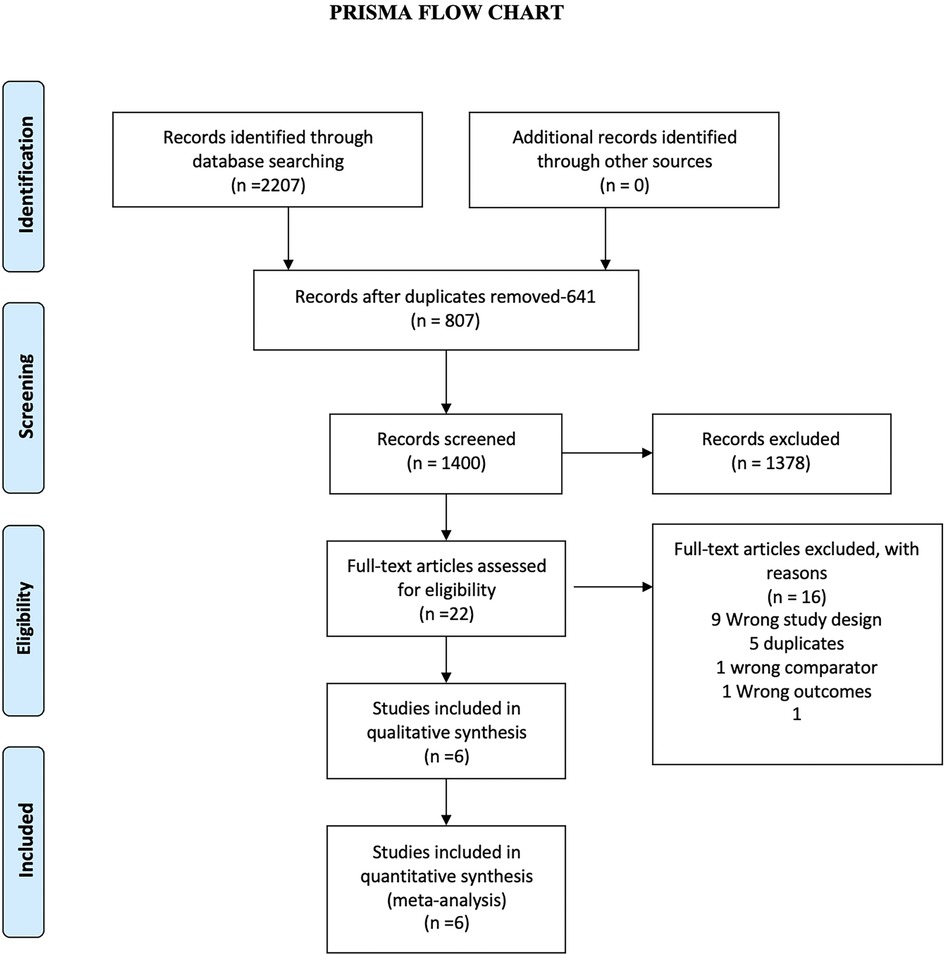
Figure 1. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) Chart. Electronic search from databases and study selection.
3.1. Characteristics of included studies
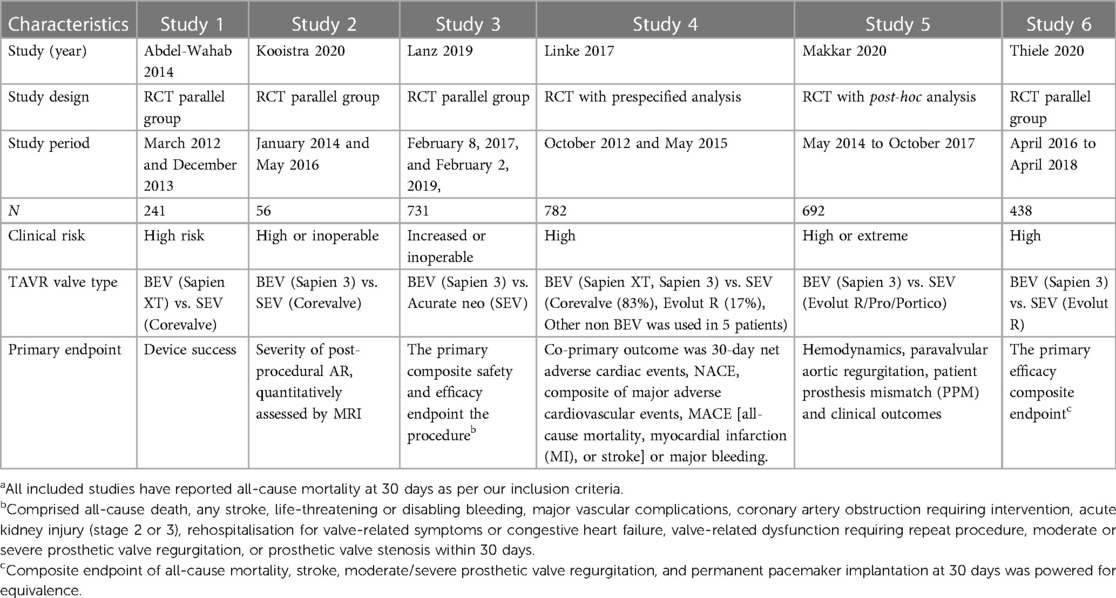
Six studies were included for final qualitative synthesis, of which four were RCTs (10–13), two were post hoc analysis of RCTs (14, 15) and one was a pre-specified device or valve type analysis of an RCT investigating the different peri-procedural antithrombotic strategies in patients undergoing TAVR (15) (Table 1). The post-hoc study belonged to the Portico-IDE (investigational device exemption) randomized trial, presented at international conferences (14, 15) but was not yet published in a peer-reviewed at the time of the databases search. Risk of-bias as assessed by RoB-2, showed low risk for 4 studies, some concern for 1 study and high risk for one study (Supplementary Table S2). BEV (Old generation-Sapien-XT and new generation- Sapien-3) was studied against four different types of SEVs (old generation SEV CoreValve and new generation SEVs that included Evolut R, Evolut PRO, Acurate neo, and Portico) and included a total of 3,141 patients (Figure 2). Out of 2,935 patients, 1,439 patients (49.0%) received BEV and 1,496 patients (51%) received SEV. The mean age of the patient population was 81.9 years and 53.3% were female. Baseline characteristics of included studies are summarized in the Supplementary Table S3.
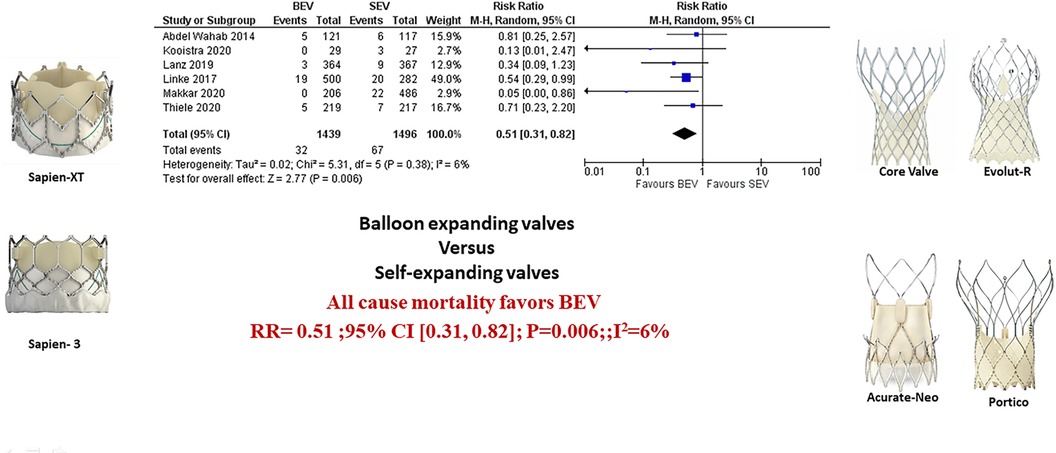
Figure 2. Comparison of balloon expandable platform vs. self-expanding platforms in high risk patients undergoing TAVR, BEV is associated with reduced risk of all-cause mortality at 30 days compared with SEV (A). The sub-group difference was not significant when the studies were stratified for the type of study (B). BEV, balloon expandable valve; SEV, self-expanding valve; TAVR, transcatheter aortic valve replacement; M-H, Mantel-Haenszel; CI, confidence interval.
3.2. Clinical outcomes in the patient population
Data regarding all-cause mortality was available from all six studies. Out of 1,439 patients who received BEV, 32 patients (2.2%) died at 30-day follow-up. In the SEV group, 67 patients (4.5%) died out of 1,496 patients. BEV was associated with significantly lower risk of death at 30 days compared with SEV [(2.2% vs. 4.5%; RR: 0.51; 95% CI: 0.31–0.82; p < 0.006, I2 = 6%); High level of GRADE Evidence); (Central Illustration, Figure 2)]. Sub-group analysis was performed showed no effect of new generation SEV (Evolut-R, Acurate-Neo and Portico) vs. old generation SEV (CoreValve) on the result (Supplementary Figure S1). A sensitivity analysis using Mantel-Haenszel methods using a fixed effect model showed similar result at 30 days (p < 0.0001) (Supplementary Figure S2). Further sensitivity analyses were performed by leave-one out statistical analyses showed similar results (Supplementary Figure S3). Additional analyses based on the role of study type (RCTs comparing BEV vs. SEV head to head with post-hoc and pre-specified analyses of RCTs), and the role of recapturable valves revealed no significant subgroup effects (Figure 3 and Supplementary Figure S4, respectively). There was no publication bias as assessed by funnel plot (Supplementary Figure S5).
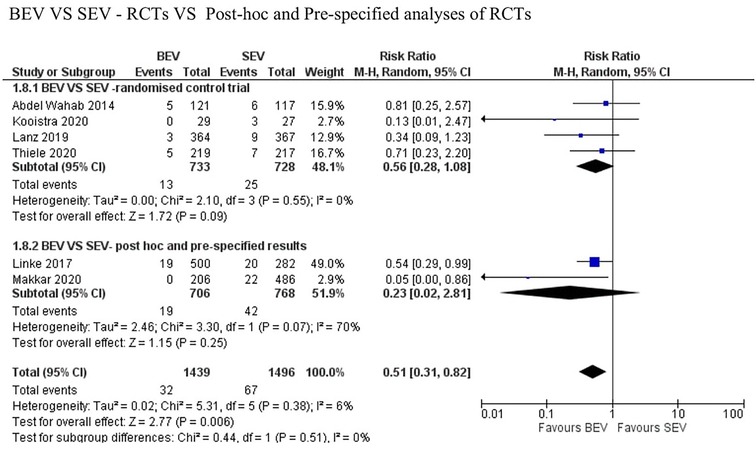
Figure 3. Comparison of balloon expandable platform with old generation self-expanding platforms for to assess the role of study type (RCTs comparing BEV vs. SEV head to head with post-hoc and pre-specified analyses of RCTs) showing no between-the-group difference on the result. BEV, balloon expandable valve; SEV, self-expanding valve; TAVR, trans-catheter aortic valve replacement; M-H, Mantel-Haenszel; CI, confidence interval.
CV mortality at 30 days was available in 5 studies. BEV was associated with lower 30-day CV mortality compared with SEV [(2.5% vs. 4.3%; RR: 0.54; 95% CI: 0.32–0.90; p = 0.02; I2 = 0%; Very low level of GRADE Evidence) (Figure 4A)]. Implantation of more than one valve per procedure (0.8% vs. 5.1%; RR: 0.15; 95% CI: 0.07–0.31); p < 0.00001; I2 = 0%; moderate level of GRADE Evidence), and moderate/severe AR/PVL (2.5% vs. 9.0%; RR: 0.29; 95% CI: 0.17–0.48); p < 0.00001; I2 = 0%; high level of GRADE Evidence) were also lower in the BEV arm (Figures 5A,B). A trend of reduced usage of pacemaker was observed with BEV as compared with SEV [(13.8% vs. 18.3%; RR: 0.73; 95% CI: 0.52–1.02; p = 0.06; I2 = 42%; very low level of GRADE Evidence) Figure 5C].
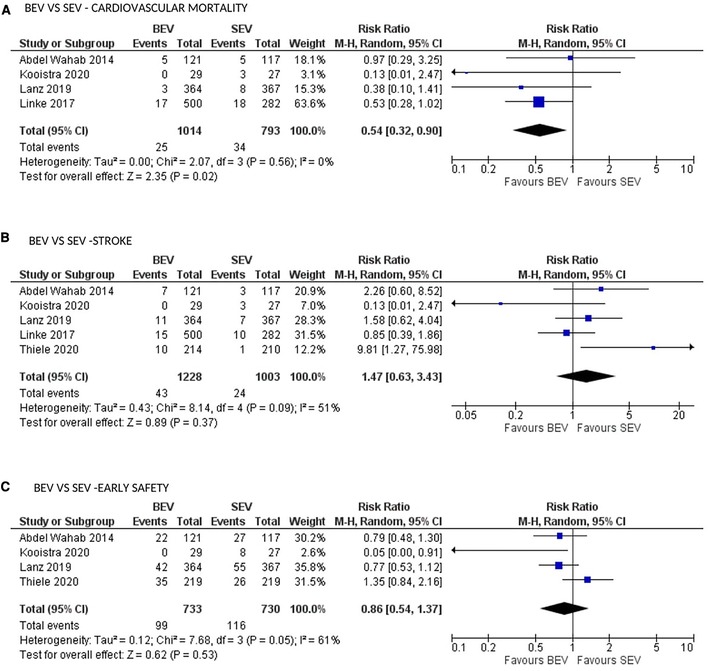
Figure 4. Comparison of balloon expandable platform vs. self-expanding platforms for cardiovascular mortality (A), stroke (B) and early safety (C) at 30 days in high risk patients undergoing TAVR. BEV, balloon expandable valve; SEV, self-expanding valve; TAVR, transcatheter aortic valve replacement; M-H, Mantel-Haenszel; CI, confidence interval.
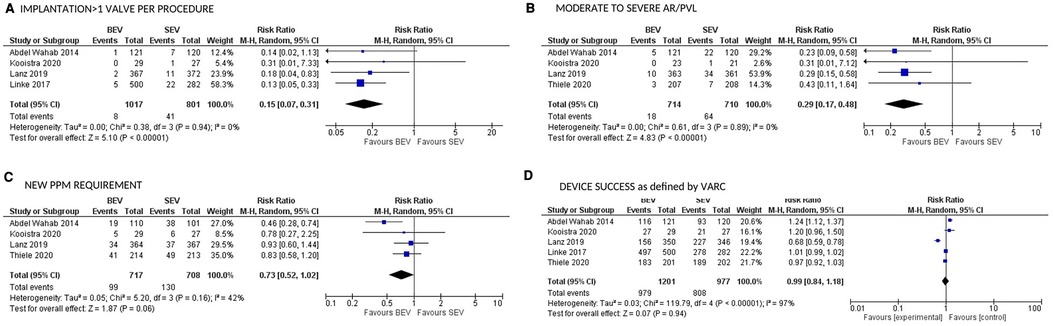
Figure 5. Comparison of balloon expandable platform vs. self-expanding platforms for implantation of >1 valve per procedure (A), moderate to severe AR/PVL (B), new PPM requirement (C), and device success as defined by VARC (D) at 30 days in high risk patients undergoing TAVR. BEV, balloon expandable valve; SEV, self-expanding valve; AR, aortic regurgitation; PVL, para valvular leak; PPM, permanent pacemaker; TAVR, transcatheter aortic valve replacement; VARC, valve academic research consortium; M-H, Mantel-Haenszel; CI, confidence interval.
All stroke (disabling and non-disabling) data were available in 6 studies. No significant difference in all stroke at 30 days was observed (Figure 4B). Similarly, no significant difference was noted between the 2 groups for early safety outcome at 30 days (Figure 4C), device success (Figure 5D), life-threatening bleeding, major vascular complications, AKI and atrial fibrillation (Figures 6A–D). In addition, no significant difference was found between the study arms for MI, major bleeding, rehospitalizations for valve-related symptoms or worsening congestive heart failure, valve related dysfunction requiring repeat procedure, valve malposition, and clinical efficacy (Supplementary Figures S6–S11). Sensitivity analysis using fixed effect model showed no difference in all the above parameters except AKI which was significantly lower in patients receiving BEV, with moderate heterogeneity (RR: 0.64; 95% CI: 0.40–1.01); p = 0.06; I2 = 48%) (Supplementary Table S4). Outcomes on cardiac tamponade, annular rupture, NYHA class improvement, NYHA status ≥class 3, and conversion to open heart surgery and valve related dysfunction were not analyzed as their reported numbers were very low.
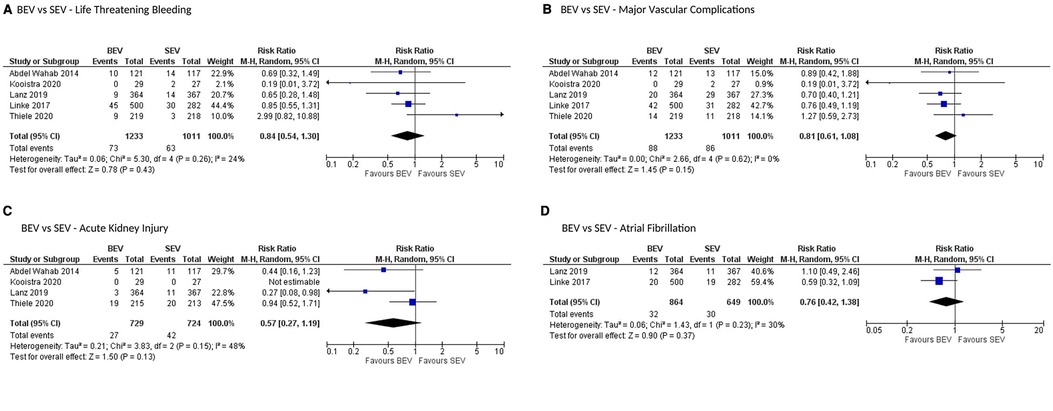
Figure 6. Comparison of balloon expandable platform vs. self-expanding platforms for life-threatening bleeding (A), major vascular complications (B), acute kidney injury (C), and atrial fibrillation (D) at 30 days in high risk patients undergoing TAVR. BEV, balloon expandable valve; SEV, self-expanding valve; TAVR, transcatheter aortic valve replacement; M-H, Mantel-Haenszel; CI, confidence interval.
3.3. Risk of bias assessment and quality of evidence
Four out of six studies had only low risk of bias assessment as assessed by Cochrane risk-of-bias tool for randomized trials version 2 (RoB 2). Two studies had some concern and one study was deemed to have risk of bias. GRADE system-based quality assessment was done for individual outcomes and a “Summary of findings” and GRADE “Evidence profile” are discussed in the Supplementary Tables S5, S6 respectively.
4. Discussion
In this meta-analysis of RCTs evaluating different THV platforms in high risk TAVR patients, BEV was associated with a lower risk of all-cause mortality at 30 days. In addition, BEV was found to be associated with lower CV mortality, reduced need for implantation of more than one valve per procedure, trend of decreased need for permanent pacemaker, and a lower incidence of moderate- severe AR/PVL at 30 days. There was no difference between BEV and SEV in terms of all stroke, MI, life-threatening bleeding, major vascular complications, rehospitalizations for valve-related symptoms or worsening congestive heart failure, valve related dysfunction requiring repeat procedure, valve malposition, AKI, atrial fibrillation, device success, early safety and clinical efficacy at 30 days. To our knowledge, this is the first systematic review and meta-analysis comparing BEV vs. all SEV platforms. The strengths of our systematic review include inclusion of RCTs only (including post hoc analysis or pre-specified analysis), detailed assessment of risk of-bias and rating the certainty of evidence utilizing the GRADE approach for all outcomes.
Only few studies have compared the safety and efficacy of BEV vs. SEV. The CENTER collaboration investigators studied 4,096 pairs of patients using propensity score matching from a pool of 12,381 TAVR patients (17). They observed lower in-hospital mortality in the BEV arm compared with the SEV arm (RR = 0.8; 95% CI: 0.6–0.9; p = 0.009); however, no difference was seen in 30 day-mortality [5.3% vs. 6.2%; relative risk, 0.9 (95% CI: 0.7–1.0); p = 0.10]. Similarly, reduced incidence of stroke (p = 0.03) and pacemaker requirement (p < 0.001) was noted in BEV. In contrast to our meta-analysis which included only RCTs, CENTER collaboration aggregated data from 9 registries and only one RCT. A recently published Bayesian meta-analysis comparing BEV vs. SEV found no difference in all-cause mortality, CV mortality, stroke, PVL, vascular complications but showed less pacemaker implantation with BEV (18). In contrast to our study, Osman et al. included 8 RCTs of all risk categories, out of which only one was a RCT comparing BEV with SEV in a head-to-head fashion. Four more studies have been published or presented comparing BEV with SEV using three different platforms after the above meta-analysis. Hence, an appropriately conducted pair-wise meta-analysis is warranted to compare the safety and efficacy of BEV vs. SEV.
Two RCTs comparing BEV vs. SEV were recently published. In SCOPE-1, 739 high risk patients were randomized to receive Acurate-Neo vs. Sapien 3. SEV failed to meet its non-inferiority for primary safety and clinical efficacy composite endpoint at 30 days (12). SOLVE-TAVI compared SEV (Evolut R) with BEV (Sapien-3) (13) among 447 high risk patients with aortic stenosis undergoing TF TAVR. No difference in all-cause mortality was observed at 30 days (p valve for equivalence <0.0001). A recently presented post-hoc analysis of PORTICO-IDE RCT (14, 15) comparing Portico SEV vs. commercially available Sapien-3 and Evolut R showed lower mortality in BEV at 30 days (Log-rank p = 0.005). The fourth RCT compared SEV with Lotus (MEV) (19), which is not within the scope of this meta-analysis.
Our study findings were also similar to those observed in two large registry analysis comparing BEV vs. SEV (20, 21). In the prospective FRANCE-TAVI registry, 3,910 matched pairs were studied from a pool of 12,141 patients. In-hospital mortality was higher in patients with SEV as compared with BEV (matched RR: 1.33; 95% CI: 1.06–1.16); p = 0.01). SEV was also associated with increase in moderate PVL, implantation of >1 device per procedure and permanent pacemaker implantation, although the predominant SEV used was the first-generation CoreValve. In another propensity score matched nationwide analysis from France, 10,459 patients who had received BEV (Sapien-3) or SEV (Evolut-R) were studied. Patients who had BEV had lower 1 year all-cause mortality (RR: 0.88; 95% CI: 0.82–0.95; p = 0.001), CV death (RR: 0.82; 95% CI: 0.73–0.92; p 0.0004), and rehospitalization for heart failure (RR: 0.84; 95% CI: 0.78–0.90; p < 0.0001) compared with SEV.
In our meta-analysis, TF-TAVR using BEV is associated with a 51% relative risk reduction in all-cause mortality and a 42% relative risk reduction in CV mortality compared with SEV arm. Test for sub-group difference was not significant based on the role of recapturable SEV or generation of SEV. The observed survival benefit with BEV could be due to their mode of deployment which is quicker, the utility of a flex-catheter in the BEV, decreased occurrence of moderate to severe AR/PVL, and reduced need for implantation of more than one valve per procedure. The use of flexible FlexNAV catheter in the parallel cohort of Portico-IDE showed similar results as compared with the BEV (15). Moderate or severe AR/PVL has been consistently shown to be associated with higher short-term and long-term mortality (22, 23). In our study, BEV was associated with a 29% relative risk reduction in moderate to severe AR/PVL. This could explain the lower 30-day mortality observed with BEV in our study. Use of a second valve was more common in SEV. However, its association with mortality could not be assessed due to non-availability of patient-level data. Increased need for permanent pacemaker observed in our study with SEV is consistent with current literature (24). The long-term implication of increased pacemaker requirement is yet to be studied and has to be considered as a significant factor, given expanded use of TAVR in low risk patients.
4.1. Limitations
Our limitations include being a study-level meta-analysis and not a patient-level meta-analysis. Comparison of all forms of SEV together might be a limitation in our study given different design characteristics, but all the studied SEV are made of nitinol. Two of them were recapturable (Portico and Evolut R). Sensitivity analyses investigating the impact of recapturable SEVs showed no significant effect on outcomes (Supplementary Figure S5) (25). Inclusion of post-hoc analyses and RCTs with pre-specified endpoints are possible limitations but sensitivity analysis showed no effect on our primary outcome. We limited our analysis to 30-day outcomes due to absence of data across trials. As the outcomes of intention to treat analyses were not available for the post hoc studies, we collected data from the utilized modified-as-treated analyses as reported by the authors. Some studies also excluded patients with heavy calcification in the aortic annulus, left ventricular outflow tract (LVOT) or sinotubular junction, limiting the interpretation of these findings to those subgroups. Being a study-level metanalysis, we could not calculate the outcomes based on recently published VARC-3 crtieria (26).
4.2. Conclusion
Balloon-expandable TAVR is associated with reduced all-cause mortality (High level of GRADE evidence), CV mortality (Very low level of GRADE evidence) at 30 days compared with self- expanding TAVR in high risk patients undergoing TF-TAVR. Longer-term data are necessary to determine if the difference in outcomes persist and if the same effects are seen in lower risk patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NS: planning, execution, data extraction, statistics, reporting, writing the first manuscript, review and editing. HB: planning, execution, data extraction, statistics. VB, PK, SG, RR, SL, AKu, MM, RJ, Aka, MF, GD, and VS: review and editing. PR: data extraction, review and editing. BC: planning, execution, review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all the fellows who helped us in the making of this manuscript. We thank Savithri in helping us in making the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1130354/full#supplementary-material
Abbreviations
AS, aortic stenosis; TAVR, trans-catheter aortic valve replacement; SAVR, surgical aortic valve replacement; BEV, balloon-expandable valve; SEV, self-expanding valve; FDA, food and drug adminstration; CE, Conformitè Europëenne.
References
1. Siontis GCM, Overtchouk P, Cahill TJ, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J. (2019) 40(38):3143–53. doi: 10.1093/eurheartj/ehz275
2. Bourantas CV, Modolo R, Baumbach A, et al. The evolution of device technology in transcatheter aortic valve implantation. EuroIntervention. (2019) 14(18):e1826–33. doi: 10.4244/EIJ-D-18-01048
3. Health C for D and R. Edwards SAPIEN 3 transcatheter heart valve system and Edwards SAPIEN 3 ultra transcatheter heart valve system - P140031/S085. FDA (2019) (Accessed April 29, 2020).
4. Health C for D and R. Medtronic CoreValve system; Medtronic CoreValve Evolut R system; medtronic CoreValve Evolut PRO system - P130021/S033. FDA (2019) (Accessed April 29, 2020).
6. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
7. Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 28(366):l4898. doi: 10.1136/bmj.l4898.
9. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document (VARC-2). Eur J Cardio-Thorac Surg. (2012) 42(5):S45–60. doi: 10.1093/ejcts/ezs533
10. Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. J Am Med Assoc. (2014) 311(15):1503–14. doi: 10.1001/jama.2014.3316
11. Kooistra NHM, Abawi M, Voskuil M, et al. Randomised comparison of a balloon-expandable and self-expandable valve with quantitative assessment of aortic regurgitation using magnetic resonance imaging. Neth Heart J. (2020) 28(5):253–65. doi: 10.1007/s12471-020-01414-0
12. Lanz J, Kim WK, Walther T, et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: a randomised non-inferiority trial. Lancet. (2019) 394(10209):1619–28. doi: 10.1016/S0140-6736(19)32220-2
13. Thiele H, Kurz T, Feistritzer H-J, et al. Comparison of newer b generation self-expandable vs. Balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J. (2020). doi: 10.1093/eurheartj/ehaa036
14. Maisano PF. PORTICO: a randomized trial of portico vs. commercially available transcatheter aortic valves in patients with severe aortic stenosis. TCTMD.com. (Accessed April 29, 2020).
15. Makkar RR, Waksman R, Groh M, et al. Comparison of valve performance of the intra-annular self-expanding Portico™ transcatheter aortic valve with contemporary supra-annular self-expanding and intra-annular balloon-expandable valves: insights from the PORTICO IDE trial. J Am Coll Cardiol Intv. (2020) 13(4 Supplement):S46. doi: 10.1016/j.jcin.2020.01.148
16. Linke A, Chandrasekhar J, Sartori S, et al. Effect of valve design and anticoagulation strategy on 30-day clinical outcomes in transcatheter aortic valve replacement: results from the BRAVO 3 randomized trial. Catheter Cardiovasc Interv. (2017) 90(6):1016–26. doi: 10.1002/ccd.27154
17. Vlastra W, Chandrasekhar J, Muñoz-Garcia AJ, et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transfemoral transcatheter aortic valve implantation: from the CENTER-collaboration. Eur Heart J. (2019) 40(5):456–65. doi: 10.1093/eurheartj/ehy805
18. Osman M, Ghaffar YA, Saleem M, et al. Meta-analysis comparing transcatheter aortic valve implantation with balloon versus self-expandable valves. Am J Cardiol. (2019) 124(8):1252–6. doi: 10.1016/j.amjcard.2019.07.028
19. Feldman TE, Reardon MJ, Rajagopal V, et al. Effect of mechanically expanded vs self-expanding transcatheter aortic valve replacement on mortality and major adverse clinical events in high-risk patients with aortic stenosis: the REPRISE III randomized clinical trial. J Am Med Assoc. (2018) 319(1):27–37. doi: 10.1001/jama.2017.19132
20. Van Belle E, Vincent F, Labreuche J, et al. Balloon-expandable versus self-expanding transcatheter aortic valve replacement: a propensity-matched comparison from the FRANCE-TAVI registry. Circulation. (2020) 141(4):243–59. doi: 10.1161/CIRCULATIONAHA.119.043785
21. Deharo P, Bisson A, Herbert J, et al. Impact of Sapien 3 balloon-expandable versus evolut R self-expandable transcatheter aortic valve implantation in patients with aortic stenosis: data from a nationwide analysis. Circulation. (2020) 141(4):260–8. doi: 10.1161/CIRCULATIONAHA.119.043971
22. Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. (2013) 61(15):1585–95. doi: 10.1016/j.jacc.2013.01.047
23. Kodali S, Pibarot P, Douglas PS, et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. (2015) 36(7):449–56. doi: 10.1093/eurheartj/ehu384
24. Rodés-Cabau J, Ellenbogen KA, Krahn AD, et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol. (2019) 74(8):1086–106. doi: 10.1016/j.jacc.2019.07.014
25. Hellhammer K, Piayda K, Afzal S, Kleinebrecht L, et al. The latest evolution of the medtronic CoreValve system in the era of transcatheter aortic valve replacement: matched comparison of the Evolut PRO and Evolut R. J Am Coll Cardiol Intv. (2018) 11(22):2314–22. doi: 10.1016/j.jcin.2018.07.023
Keywords: aortic stenosis, valve, balloon expandable, trans catheter aortic valve replacement, self-expanding
Citation: Senguttuvan NB, Bhatt H, Balakrishnan VK, Krishnamoorthy P, Goel S, Reddy PMK, Subramanian V, Claessen BE, Kumar A, Majmundar M, Ro R, Lerakis S, Jayaraj R, Kalra A, Flather M and Dangas G (2023) The safety and efficacy of balloon-expandable versus self-expanding trans-catheter aortic valve replacement in high-risk patients with severe symptomatic aortic stenosis. Front. Cardiovasc. Med. 10:1130354. doi: 10.3389/fcvm.2023.1130354
Received: 23 December 2022; Accepted: 2 May 2023;
Published: 25 May 2023.
Edited by:
Mamoo Nakamura, Cedars Sinai Medical Center, United StatesReviewed by:
Raymond McKay, Hartford HealthCare, United StatesMasaki Izumo, St. Marianna University School of Medicine, Japan
© 2023 Senguttuvan, Bhatt, Balakrishnan, Krishnamoorthy, Goel, Reddy, Subramanian, Claessen, Kumar, Majmundar, Ro, Lerakis, Jayaraj, Kalra, Flather and Dangas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nagendra Boopathy Senguttuvan bmFnZW5kcmFib29wYXRoeUBzcmlyYW1hY2hhbmRyYS5lZHUuaW4=, ZHJzbmJvb3BhdGh5QGdtYWlsLmNvbQ==
 Nagendra Boopathy Senguttuvan
Nagendra Boopathy Senguttuvan Hemal Bhatt2,3
Hemal Bhatt2,3 Vinod Kumar Balakrishnan
Vinod Kumar Balakrishnan Bimmer E. Claessen
Bimmer E. Claessen Ashish Kumar
Ashish Kumar Monil Majmundar
Monil Majmundar Richard Ro
Richard Ro Stamatios Lerakis
Stamatios Lerakis Ramamoorthi Jayaraj
Ramamoorthi Jayaraj Ankur Kalra
Ankur Kalra George Dangas
George Dangas