- 1Department of Cardiology, Amiens University Hospital, Amiens, France
- 2EA 7517, Jules Verne University of Picardie, Amiens, France
- 3Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, United States
- 4Department of Echocardiography, Cardio X Clinic, France
Patients with mitral valve prolapse (MVP) may develop ventricular arrhythmias, ranging from premature ventricular contractions through more complex non-sustained ventricular tachycardia to sustained life-threatening ventricular arrhythmias. The prevalence of MVP in autopsy series of young adults who died suddenly has been estimated to be between 4% and 7%. Thus, “arrhythmic MVP” has been reported as an underappreciated cause of sudden cardiac death, leading to a renewed interest in the study of this association. The term “arrhythmic MVP” refers to a small subset of patients who have, in the absence of any other arrhythmic substrate, MVP, with or without mitral annular disjunction, and frequent or complex ventricular arrhythmias. Our understanding of their coexistence in terms of contemporary management and prognosis is still incomplete. While literature regarding the arrhythmic MVP may be contrasting despite recent consensus document, the present review summarizes the relevant evidence concerning the diagnostic approach, prognostic implications, and targeted therapies for MVP-related ventricular arrhythmias. We also summarize recent data supporting left ventricular remodeling, which complicates the coexistence of MVP with ventricular arrhythmias. As the evidence for a putative link between MVP-associated ventricular arrhythmias and sudden cardiac death is scarce and based on scant and retrospective data, risk prediction remains a challenge. Thus, we aimed at listing potential risk factors from available seminal reports for further use in a more reliable prediction model that requires additional prospective data. Finally, we summarize evidence and guidelines on targeted therapies of ventricular arrhythmias in the setting of MVP, including implantable cardioverter defibrillators and catheter ablation. Our review highlights current knowledge gaps and provides an action plan for structured research on the pathophysiological genesis, diagnosis, prognostic impact, and optimal management of patients with arrhythmic MVP.
Introduction
The association of mitral valve prolapse (MVP) with life-threatening ventricular arrhythmias (VA) has baffled cardiologists since the early 1980 s. There is currently a renewed interest in the study of this association, which has led to the development of new concepts. Mitral valve prolapse often presents with premature ventricular contractions (PVCs) (1) but only a small subset of patients with MVP are considered to be at high risk of malignant VA. “Malignant MVP” has been reported to be an underappreciated cause of sudden cardiac death (SCD) in young adults, as the prevalence of MVP in autopsy series has been estimated to be between 4% and 7% (2–4). The rate of SCD among patients with MVP has been prospectively estimated to range from 0.2% to 0.4% per year and to be 1.8% per year for patients with severe mitral regurgitation (MR) due to leaflet flail (5–7). Yet, the identification of subgroups of MVP patients with higher arrhythmic risk is still a challenge. This review focuses on current knowledge of clinical data, electrocardiographic aspects, and the specific imaging presentation of arrhythmic MVP, and summarizes evidence on risk stratification and management. In addition, the main knowledge gaps concerning diagnostic approaches and therapeutic options of arrhythmic MVP are highlighted.
Clinical and ECG presentation
Clinical presentation of the arrhythmic MVP may vary in severity, ranging from asymptomatic patients, through palpitations to presyncope/syncope (3). Syncope was shown to be a frequent preceding symptom for patients who developed cardiac arrest (8). While the initial focus was on bileaflet MVP as the main culprit, the arrhythmic MVP phenotype is multidimensional and characterized by severe myxomatous disease with marked leaflet redundancy and thickening, inferolateral mitral annular disjunction (MAD) (Figure 1) with frequent inverted T-waves in the inferior leads, frequent PVCs by ECG, more non-sustained ventricular tachycardia (VT) and a higher PVC burden by Holter monitoring than those with normal mitral valves (2, 8, 9). Phenotypic presentations of MVP with an increased risk of VA and/or SCD are shown in Table 1.
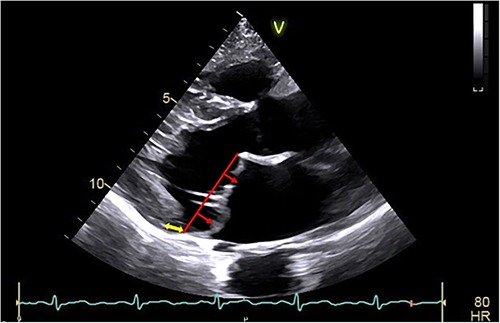
Figure 1. Arrhythmic mitral valve prolapse by transthoracic-echocardiography. Transthoracic-echocardiographic long-axis view in end-systole with a displacement of both leaflet >2 mm (red arrow) above the plane of the annulus (red line) defining a bileaflet mitral valve prolapse with thick leaflets linked to myxomatous degeneration. Note the presence of the detachment of the posterior leaflet from the left-ventricular myocardium (mitral annular disjunction, yellow arrow) to be assessed in dynamic analysis (that is a frame by frame analysis during the entire cardiac cycle) to better ascertain the position of the posterior mitral annulus.
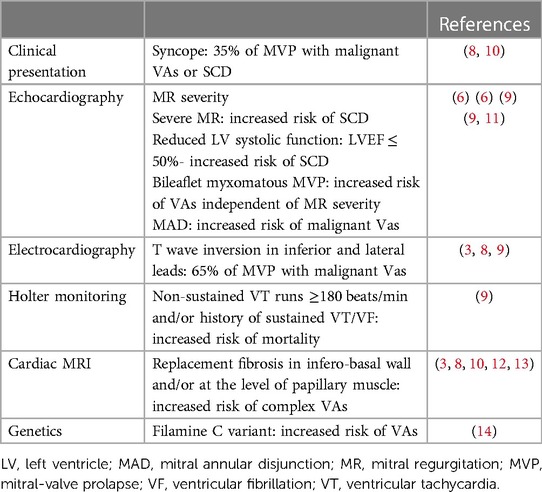
Table 1. Mitral valve prolapse phenotypes with increased risk of ventricular arrhythmias and/or sudden cardiac death.
Early data showed a high prevalence of PVCs in MVP patients, ranging from 58% to 89% (1). A more recent prospective study on a large population of patients with different stages of MVP showed the utility of the exercise test to reveal PVCs and their potential site of origin for more than two-thirds of patients with MVP (15). Patients with identified isolated PVCs were older, presented more non-sustained VT during the exercise test, and more often had MR of stage >II, with “deeper” valve prolapse and longer MV leaflets. In this study, patients with PVCs also showed elevated T1 values for the postero-medial papillary muscle (PPM) and left ventricular infero-latero-basal wall by contrast-enhanced cardiac magnetic resonance (MRI) imaging. The most common PVC and non-sustained VT morphology was associated with the PPM. Other PVC morphologies suggestive of the mitral valve apparatus as the origin have been described in the setting of patients with arrhythmic MVP, including the antero-lateral papillary muscle, aorto-mitral continuity, or peri-mitral valve area, as well as more distant sites, such as the outflow tract, moderator band, or fascicular sites (Figure 2) (2, 15, 16).
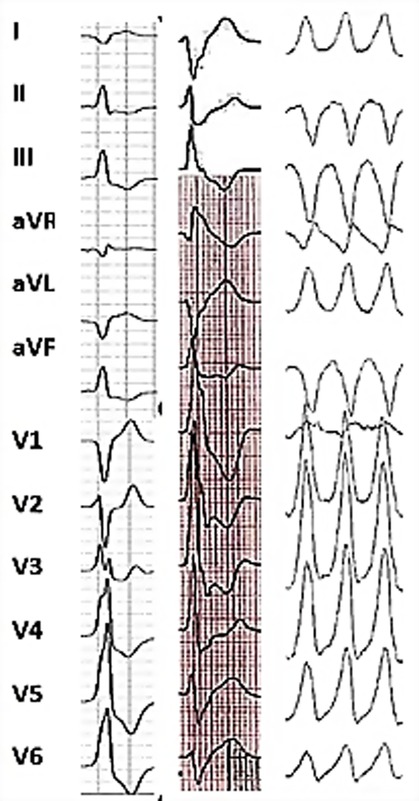
Figure 2. Representative 12-lead surface ECG illustrating multifocal ventricular arrhythmias in the same patient. Frequent premature ventricular complexes of 2 different morphologies were recorded: the first one showing left bundle branch block configuration, V3 transition and inferior axis consistent with an outflow tract site and the second one with right bundle branch block morphology, V5 transition and D2/D3 negative/positive discordance (meaning an opposite depolarization vector along bipolar limb leads II and III being equivalent to a frontal axis of +150 to +210°) suggesting the origin from the anterolateral papillary muscle. Sustained monomorphic ventricular tachycardia (on the right) with a right bundle branch block configuration and superior axis pointing towards the postero-basal left ventricular wall origin was induced with programmed electrical stimulation.
MRI findings
Cardiac MRI may help in the diagnosis of MAD as a part of the arrhythmic MVP complex as well as phenotypic markers of higher risk as fibrosis (17, 18). Indeed fibrosis localized to the PPM and left ventricular (LV) segments adjacent to the PPM, identified by MRI and in pathological studies, has been highlighted as a potential myocardial source of VA that complicates MVP (3, 10, 19, 20). A higher prevalence of replacement fibrosis in the LV infero-basal wall was identified in patients with MVP and complex VAs than those without VAs (73% vs. 7%) (3). In another study, LV fibrosis was not only associated with more frequent VAs but also with a more dilated LV and, interestingly, with MR severity, increasing from 13% of patients with mild MR to 37% of those with severe MR (12).
Contrast-enhanced cardiac magnetic resonance data of a large cohort of patients with primary MR showed that replacement fibrosis affected 36% of patients with MVP specifically in the basal segments, as well as extending to the mid inferolateral LV wall (10). In this study, the presence of replacement fibrosis was related to increased symptomatic ventricular arrhythmic events affecting 4.5% patients with MVP vs. 0.6% of patients in the non-MVP cohort. Furthermore, cases with MVP-SCD matched to control cases with noncardiac death showed increased LV anterior, lateral, posterior, and interventricular septum fibrosis, characterized by an endocardial-to-epicardial gradient (13). Left ventricular fibrosis was also found to be associated with systolic curling, described as an atypical motion of the posterior mitral ring (21).
Left ventricular remodeling
The outcome in MVP is determined by both MR severity and its left-atrial and left-ventricular consequences (22, 23). Disproportionate LV dilation has been reported for 16% of patients without significant MR not exposed to significant volume overload, in addition to excess annular enlargement in systole, suggestive of an MVP-associated myocardial disease (12, 24). Larger LV dimensions were independent of the degree of replacement fibrosis but were linked to more frequent PVCs. A study by Yang et al. also showed that patients with less-than-moderate degenerative MR due to MVP exhibited early LV remodeling independent of MR progression but strongly associated with more frequent PVCs (25). The question of whether such myocardial changes are linked to the MVP phenotype itself or rather the consequence of PVC-induced cardiomyopathy remains unanswered.
Sudden cardiac death
In previous studies, severe VAs were rarely documented before SCD (6), possibly due to the scarcity of Holter-monitoring performed. However, patients who present with VA by monitoring show progressive but significant excess mortality (9).
The yearly incidence of SCD in patients with MVP has been estimated to be 0.2%–0.4% and up to 1.8% per year for those with severe MR due to leaflet flail (5–7, 26, 27). Arrhythmic MVP without severe MR has been suggested to be an underestimated cause of SCD among young adults (2, 3). However, patients with MVP and proven VA are often in their 60 s (9). Severe VA, defined as non-sustained VT ≥ 180 beats/min or a proven history of sustained VT/VF, is infrequent (9%) but associated with excess mortality subsequent to its diagnosis (9).
Mitral annular disjunction in the context of MVP diagnosed by cardiac MRI or by standard transthoracic echocardiography after cardiac arrest (3, 8, 18) has been suggested to be a harbinger of SCD. The MAD-VA link is hypothesized to be subsequent to local fibrosis induced by the tension generated by such an ample prolapse (21). MVP patients with MAD developed within the first 10 years more frequent VA than those without MAD (11). However, recent outcomes data in MVP patients demonstrate that MAD may be associated with the secondary development of arrhythmias but is not associated with excess mortality during the first 10 years and should not, per se, lead to risky electrophysiological interventions (11). Of note, MAD is a common finding in the general population and only rare inferolateral MAD is associated with MVP and myxomatous MV disease (28). MVP is frequent and MAD is identified in up to 40% of patients with connective tissue diseases (29, 30). In patients with Marfan syndrome and Loeys–Dietz syndrome MAD was associated with increased risk of mitral surgery, but was not linked to higher risk of life-threatening VA (29). In addition, no association has been found between MAD and the PVC site of origin (15). Therefore, although MAD is a component of the multifaceted arrhythmic MVP phenotype, it does not appear by itself, to be a direct harbinger of SCD. Thus, in the presence of MAD careful monitoring is recommended and MAD should not, by itself, lead to risky electrophysiological interventions (17). Notably, some authors challenge the currently used definition of MAD based only on its systolic description that can be partly biased by including cases of juxtaposition of posterior leaflet and atrial wall (31). They identify a less prevalent “true MAD” defined as the insertion of the leaflet on the atrial wall observed in diastole with the presence of a separating sub-valvular membrane. These uncertainties could lead to false stratification of arrhythmic risk in MVP patients and need more clarification. Possible inheritable proarrhythmic genetic substrate in the form of cardiomyopathy-causative variants of FLNC, encoding filamin C, has been reported in patients with arrhythmic MVP (14).
Additional research is needed to establish the optimal approach for risk stratification for patients with MVP, including the prognostic role of cardiac MRI. Severity and the prognostic implication of arrhythmias in patients with MVP will require large registries to provide sufficient power to define clinical determinants and outcomes accounted for by well-defined covariates. The utility of treadmill tests and loop recorders with remote monitoring for stratification of the risk of SCD in arrhythmic MVP needs to be defined by prospective cohorts with long-term follow-up. Additional research is also needed to determine the precise role of advanced electrophysiological testing, including programmed ventricular stimulation in the setting of MVP associated VAs.
Management of MVP with ventricular arrhythmia
Most cases of severe organic MR are treated by surgery. Surgical cryoablation for VA during cardiac surgery has been reported but data on long-term results are lacking (32, 33). Nonetheless, the correction of flail leaflet is possibly associated with a lower risk of SCD (6, 9) and some authors suggest that VA-excess mortality tends to disappear post MV surgery with MAD disappearance (9, 24). However, non-sustained VT by Holter-monitoring following MR repair or replacement may be a long-term predictor of SCD (34). After surgical MR repair or replacement, an implantable cardioverter-defibrillator (ICD) is indicated as a class I recommendation for patients who satisfy implantation criteria for secondary prevention of SCD according to current ESC Guidelines (35).
If MVP is associated with PVCs or non-sustained VT, therapies that prevent SCD and provide symptomatic benefits should be considered. A history of palpitations or syncope or the detection of the phenotypic characteristics of arrhythmic MVP (MAD, leaflet redundancy, and T-wave inversion) should prompt Holter monitoring to identify VA (9, 17). In addition, cardiac MRI should be considered for risk stratification (3, 11). A predictive role of programmed ventricular stimulation to guide therapy for patients with valvular heart disease referred for syncope or VT has been suggested, but uncertainty remains (36). An electrophysiological study is reasonable for patients with syncope if sustained VT is suspected based on symptoms or non-invasive assessment (37). Risk stratification in patients with arrhythmic MVP using clinical and imaging criteria is represented by a modified EHRA statement scheme (Figure 3) (17).
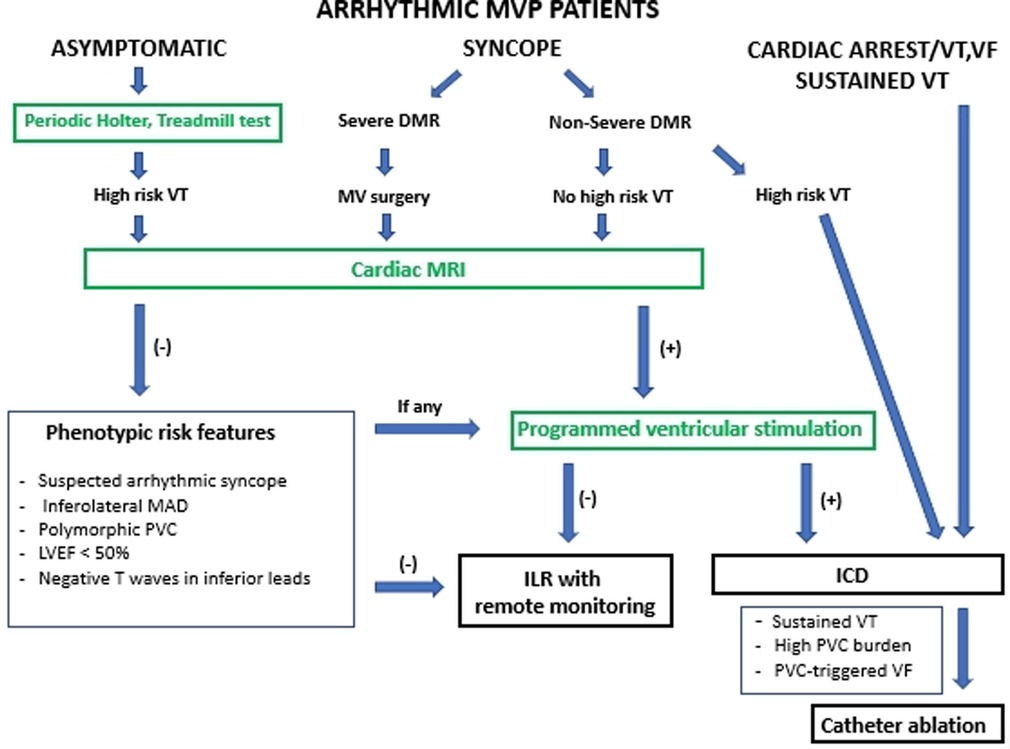
Figure 3. Risk stratification in patients with arrhythmic MVP using clinical and imaging criteria represented by modified EHRA risk stratification scheme (17). Arrhythmic MVP: the presence of MVP with or without MAD, frequent ventricular ectopy (≥5% of total beats), complex ectopy or sustained VAs in the absence of any other well-defined arrhythmic substrate (e.g. active ischemia, ventricular scar due to another defined etiology, primary cardiomyopathy or channelopathy); High risk VT: fast (>180 bpm) non-sustained VT, polymorphic non-sustained VT, Cardiac MRI: only LGE within the mitral apparatus (papillary muscles and peri-annular region) has a clear pathophysiological relevance. DMR, degenerative mitral regurgitation; ICD, implantable cardioverter defibrillator; ILR, implantable loop recorder; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MAD, mitral annular disjunction; MV, mitral valve; PVC, premature ventricular contraction; VA, ventricular arrhythmia; VF, ventricular fibrillation; VT, ventricular tachycardia.
ICD therapy
Specific data on ICD therapy for VAs in patients with MVP are lacking. Accordingly, ICD therapy is recommended following general principles of current recommendations for non-ischemic cardiomyopathy (37, 38). Placement of an ICD for secondary prevention of SCD is indicated for patients with MVP and a documented history of sudden cardiac arrest with ventricular fibrillation or sustained VT without reversible causes. For primary prevention, an ICD is recommended in symptomatic heart failure with a LVEF ≤ 35% on optimal medical therapy. Following the recent expert European Heart Rhythm Association (EHRA) consensus statement on arrhythmic MVP and MAD complex, an ICD should be considered for MVP patients with unexplained syncope and high risk VA, defined as non-sustained VT runs ≥180 beats/min detected by ECG or Holter monitoring (17). The option of an ICD may be reasonable for asymptomatic MVP with non-sustained VT and two or more phenotypic risk features (T-wave inversion in inferior leads, repetitive polymorphic PVCs, MAD, redundant MV leaflets, enlarged LA or a LVEF ≤ 50%, and fibrosis within the mitral apparatus by cardiac MRI).
Pharmacological treatment
There are no specific data on the efficacy of beta-blockers for the prevention of SCD in the setting of organic MVP, but they are widely used as first-line medical therapy for suppressing frequent symptomatic or complex PVCs and VT. Other anti-arrhythmic agents may also be effective in the management of MVP-associated VAs but must be used with caution, given their potential to cause adverse events. Reduction of VA burden using flecainide combined with beta-blockers after excluding structural heart disease has been recently reported in patients with MVP (39).Class I C sodium-channel blockers should be avoided in cases of MVP with prior myocardial infarction (MI) or hemodynamically significant MR (35).
Catheter ablation
In the event of non-responsiveness or contraindication to antiarrhythmic agents, catheter ablation (CA) of symptomatic VAs originating from the PPM in the presence MVP has been reported to be effective (40–44). Ablation for VAs may be considered for patients with appropriate ICD therapies and in cases of PVC-induced cardiomyopathy (37, 38). Catheter stability during the mapping and ablation of MVP-associated VA originating from the papillary muscle may be challenging. Significant progress has been recently made in CA techniques, such as the development of catheters with contact force sensors, which improve safety and allow the creation of larger and deeper lesions. The use of new tools, such as intracardiac echocardiography, which improves catheter positioning on the papillary muscles, or cryoablation catheters that ensure better catheter stability during freezing, has led to higher acute and long-term success rates (43).
A recent study demonstrated the efficacy of CA to reduce the VA burden for patients with MVP and MAD (45). In the presence of MAD, the arrhythmogenic substrate, defined by abnormal local electrograms (low voltage, long-duration, and fractionated, isolated mid-diastolic potentials) on electro-anatomical mapping, was identified in the anterolateral mitral annulus or MAD area (45). An exhaustive list of studies reporting the results of CA for VAs for patients with MVP or MAD is presented in Table 2.
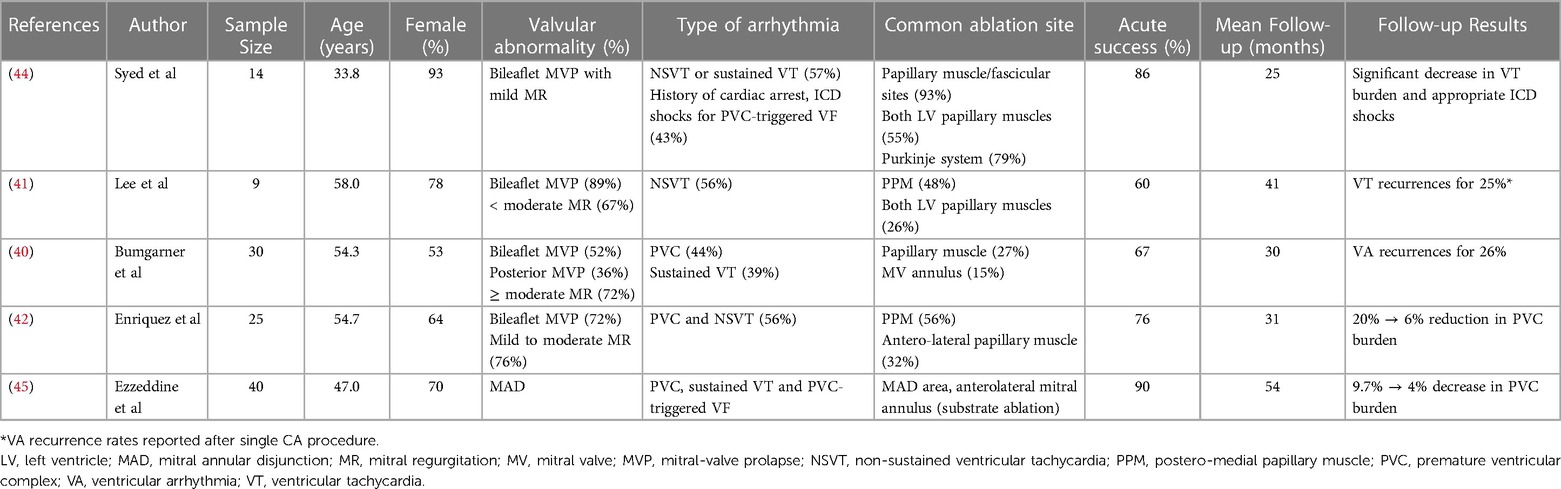
Table 2. List of major studies reporting results of catheter ablation of ventricular arrhythmias in patients with MVP.
Current ESC and AHA/ACC/HRS guidelines for the management of patients with VAs and the prevention of SCD indicate CA for PVCs that trigger recurrent VF as a class I indication (37, 38). CA should be considered after failure of one or more antiarrhythmic agents or according to the patient's preference as a class I (AHA/ACC/HRS) or IIa (ESC) recommendation for symptomatic patients with papillary muscle tachycardia. Regardless of MR severity, frequent PVC and non-sustained VT in the presence of extended phenotypic characteristics of arrhythmic MVP, such as MAD, leaflet redundancy, and T-wave inversion, should lead cardiologists to intensify beta-blocker therapy and discuss CA (35).
Knowledge gaps and perspectives
Seminal data suggest that VA are common in patients with MVP but rarely severe and that severe VA is linked to excess mortality. While literature remains sparse and contrasted, recent prospective data provided a definition of the arrhythmic MVP complex, ascertained in an expert consensus to guide future research and clinical trials in order to define the appropriate diagnosis and therapeutic approach.
Holter monitoring is not systematically recommended and the diagnosis of VAs in MVP is still at the discretion of the cardiologist. Arrhythmic MVP is a particular phenotype of MVP that should be part of all MVP comprehensive assessment and trigger episodic or frequent Holter monitoring during follow-up. Little is known about the prevalence and patterns of VAs, in particular transient and subclinical VAs, in mild MVP. Thus, the results and indications of cardiac monitoring by 12-lead Holter monitoring and more recent diagnostic tools, such as sensitive loop recorders, should be prospectively determined in this setting. Establishing the burden, type, sites of origin, and severity of VAs using these methods and exploiting large registries is a starting point for further appropriate prospective research that warrants careful planning. Our understanding of LV remodeling in the setting of MVP at early stages is still insufficient and additional research is needed to elucidate the potential link between VA burden and LV dysfunction.
The severity and prognostic implications of VAs for patients with MVP will require large registries providing sufficient power to define the clinical determinants of outcome. The role of electrophysiological testing and imaging techniques, including cardiac MRI for the detection of ventricular fibrosis and the definition of patterns of the arrhythmogenic substrate, needs to be determined. Further quantification of the identified abnormalities will serve as the basis for establishing data-driven thresholds of clinical severity of arrhythmic MVP.
Pilot data suggest that targeted therapies for MVP-related VAs may be effective, but larger clinical trials will be required to define the indications and optimal timing of CA and its impact on the quality of life and survival. While the risk of life-threatening VA appears to be attenuated after mitral valve surgery, the benefits and indications for ICDs for primary prevention are still poorly defined. More data is needed to identify the burden and character of VAs following valvular surgery using loop recorders and 12-lead Holter monitors.
Despite current knowledge and undeniable recent progress in our understanding of the underlying mechanisms, the association of MVP with VAs is still underestimated. Recent contributions to the arrhythmic MVP complex will set the basis for large and well-defined trials to gather more evidence on genetic, phenotypic, and therapeutic aspects.
Author contributions
MK: data collection, wrighting. BE: wrighting. HIM: evaluation. ME-S: evaluation. CT: global evaluation. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Savage DD, Levy D, Garrison RJ, Castelli WP, Kligfield P, Devereux RB, et al. Mitral valve prolapse in the general population. 3. Dysrhythmias: the framingham study. Am Heart J. (1983) 106(3):582–6. doi: 10.1016/0002-8703(83)90706-8
2. Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. (2013) 62(3):222–30. doi: 10.1016/j.jacc.2013.02.060
3. Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. (2015) 132(7):556–66. doi: 10.1161/CIRCULATIONAHA.115.016291
4. Delling FN, Aung S, Vittinghoff E, Dave S, Lim LJ, Olgin JE, et al. Antemortem and post-mortem characteristics of lethal mitral valve prolapse among all countywide sudden deaths. JACC Clin Electrophysiol. (2021) 7(8):1025–34. doi: 10.1016/j.jacep.2021.01.007
5. Duren DR, Becker AE, Dunning AJ. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. (1988) 11(1):42–7. doi: 10.1016/0735-1097(88)90164-7
6. Grigioni F, Enriquez-Sarano M, Ling LH, Bailey KR, Seward JB, Tajik AJ, et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. (1999) 34(7):2078–85. doi: 10.1016/s0735-1097(99)00474-x
7. Nishimura RA, McGoon MD, Shub C, Miller FA Jr, Ilstrup DM, Tajik AJ. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N Engl J Med. (1985) 313(21):1305–9. doi: 10.1056/NEJM198511213132101
8. Hourdain J, Clavel MA, Deharo JC, Asirvatham S, Avierinos JF, Habib G, et al. Common phenotype in patients with mitral valve prolapse who experienced sudden cardiac death. Circulation. (2018) 138(10):1067–9. doi: 10.1161/CIRCULATIONAHA.118.033488
9. Essayagh B, Sabbag A, Antoine C, Benfari G, Yang LT, Maalouf J, et al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol. (2020) 76(6):637–49. doi: 10.1016/j.jacc.2020.06.029
10. Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. (2018) 72(8):823–34. doi: 10.1016/j.jacc.2018.06.048
11. Essayagh B, Sabbag A, Antoine C, Benfari G, Batista R, Yang L-T, et al. The mitral annular disjunction of mitral valve prolapse. JACC: Cardiovasc Imaging. (2021) 14(11):2073–87. doi: 10.1016/j.jcmg.2021.04.029
12. Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A, Senage T, Filippetti L, Piriou N, et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation. (2021) 143(18):1763–74. doi: 10.1161/CIRCULATIONAHA.120.050214
13. Han HC, Parsons SA, Curl CL, Teh AW, Raaijmakers AJA, Koshy AN, et al. Systematic quantification of histologic ventricular fibrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm. (2021) 18(4):570–6. doi: 10.1016/j.hrthm.2020.12.021
14. Bains S, Tester DJ, Asirvatham SJ, Noseworthy PA, Ackerman MJ, Giudicessi JR. A novel truncating variant in FLNC-encoded filamin C may serve as a proarrhythmic genetic substrate for arrhythmogenic bileaflet mitral valve prolapse syndrome. Mayo Clin Proc. (2019) 94(5):906–13. doi: 10.1016/j.mayocp.2018.11.028
15. Guenancia C, Pace N, Hossu G, Selton-Suty C, Mandry D, Beaumont M, et al. Prevalence and determinants of PVCs originating from the mitral apparatus in patients with MVP. JACC Clin Electrophysiol. (2022) 8(4):526–8. doi: 10.1016/j.jacep.2021.12.005
16. Kubala M, de Chillou C, Bohbot Y, Lancellotti P, Enriquez-Sarano M, Tribouilloy C. Arrhythmias in patients with valvular heart disease: gaps in knowledge and the way forward. Front Cardiovasc Med. (2022) 9:792559. doi: 10.3389/fcvm.2022.792559
17. Sabbag A, Essayagh B, Barrera JDR, Basso C, Berni A, Cosyns B, et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC council on valvular heart disease and the European association of cardiovascular imaging endorsed cby the heart rhythm society, by the Asia pacific heart rhythm society, and by the latin American heart rhythm society. Europace. (2022) 24(12):1981–2003. doi: 10.1093/europace/euac125
18. Essayagh B, Iacuzio L, Civaia F, Avierinos JF, Tribouilloy C, Levy F. Usefulness of 3-tesla cardiac magnetic resonance to detect mitral annular disjunction in patients with mitral valve prolapse. Am J Cardiol. (2019) 124(11):1725–30. doi: 10.1016/j.amjcard.2019.08.047
19. Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. (2008) 1(3):294–303. doi: 10.1016/j.jcmg.2008.01.013
20. Bui AH, Roujol S, Foppa M, Kissinger KV, Goddu B, Hauser TH, et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart. (2017) 103(3):204–9. doi: 10.1136/heartjnl-2016-309303
21. Perazzolo Marra M, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi B, et al. Morphofunctional abnormalities of mitral Annulus and arrhythmic mitral valve prolapse. Circulation Cardiovasc Imaging. (2016) 9(8):e005030. doi: 10.1161/CIRCIMAGING.116.005030
22. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38(36):2739–91. doi: 10.1093/eurheartj/ehx391
23. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143(5):e72–e227. doi: 10.1161/CIR.0000000000000923
24. Essayagh B, Mantovani F, Benfari G, Maalouf JF, Mankad S, Thapa P, et al. Mitral annular disjunction of degenerative mitral regurgitation: three-dimensional evaluation and implications for mitral repair. J Am Soc Echocardiogr. (2022) 35(2):165–75. doi: 10.1016/j.echo.2021.09.004
25. Yang LT, Ahn SW, Li Z, Benfari G, Mankad R, Takeuchi M, et al. Mitral valve prolapse patients with less than moderate mitral regurgitation exhibit early cardiac chamber remodeling. J Am Soc Echocardiogr. (2020) 33(7):815–825.e2. doi: 10.1016/j.echo.2020.01.016
26. Han HC, Ha FJ, Teh AW, Calafiore P, Jones EF, Johns J, et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. (2018) 7(23):e010584. doi: 10.1161/JAHA.118.010584
27. Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, Vohra JK, et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. (2019) 105(2):144–51. doi: 10.1136/heartjnl-2017-312932
28. Zugwitz D, Fung K, Aung N, Rauseo E, McCracken C, Cooper J, et al. Mitral annular disjunction assessed using CMR imaging: insights from the UK biobank population study. JACC Cardiovasc Imaging. (2022) 15(11):1856–66. doi: 10.1016/j.jcmg.2022.07.015
29. Chivulescu M, Krohg-Sorensen K, Scheirlynck E, Lindberg BR, Dejgaard LA, Lie OH, et al. Mitral annulus disjunction is associated with adverse outcome in Marfan and Loeys-Dietz syndromes. Eur Heart J Cardiovasc Imaging. (2021) 22(9):1035–44. doi: 10.1093/ehjci/jeaa324
30. Asher SB, Chen R, Kallish S. Mitral valve prolapse and aortic root dilation in adults with hypermobile Ehlers-Danlos syndrome and related disorders. Am J Med Genet A. (2018) 176(9):1838–44. doi: 10.1002/ajmg.a.40364
31. Faletra FF, Leo LA, Paiocchi VL, Schlossbauer SA, Pavon AG, Ho SY, et al. Morphology of mitral annular disjunction in mitral valve prolapse. J Am Soc Echocardiogr. (2022) 35(2):176–86. doi: 10.1016/j.echo.2021.09.002
32. El-Eshmawi A, Pandis D, Miller MA, Boateng P, Dukkipati SR, Reddy VY, et al. Surgical cryoablation of papillary muscle PVCs during mitral valve surgery: therapeutic consideration for malignant MVP. J Am Coll Cardiol. (2020) 76(25):3061–2. doi: 10.1016/j.jacc.2020.10.037
33. Van Dessel PF, Van Hemel NM, Van Swieten HA, De Bakker JM, Jessurun ER. Successful surgical ablation of sustained ventricular tachycardia associated with mitral valve prolapse guided by a multielectrode basket catheter. Pacing Clin Electrophysiol: PACE. (2001) 24(6):1029–31. doi: 10.1046/j.1460-9592.2001.01029.x
34. Olafiranye O, Hochreiter CA, Borer JS, Supino PG, Herrold EM, Budzikowski AS, et al. Nonischemic mitral regurgitation: prognostic value of nonsustained ventricular tachycardia after mitral valve surgery. Cardiology. (2013) 124(2):108–15. doi: 10.1159/000347085
35. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur Heart J. (2015) 36(41):2793–867. doi: 10.1093/eurheartj/ehv316
36. Martinez-Rubio A, Schwammenthal Y, Schwammenthal E, Block M, Reinhardt L, Garcia-Alberola A, et al. Patients with valvular heart disease presenting with sustained ventricular tachyarrhythmias or syncope: results of programmed ventricular stimulation and long-term follow-up. Circulation. (1997) 96(2):500–8. doi: 10.1161/01.cir.96.2.500
37. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. (2022) 43(40):3997–4126. doi: 10.1093/eurheartj/ehac262
38. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2018) 72(14):1677–749. doi: 10.1016/j.jacc.2017.10.053
39. Aabel EW, Dejgaard LA, Chivulescu M, Helle-Valle TM, Edvardsen T, Hasselberg NE, et al. Flecainide in patients with arrhythmic mitral valve syndrome: a case series. Heart Rhythm. (2022) 20(4):635–36. doi: 10.1016/j.hrthm.2022.12.024
40. Bumgarner JM, Patel D, Kumar A, Clevenger JR, Trulock KM, Popovic Z, et al. Management and outcomes in mitral valve prolapse with ventricular arrhythmias undergoing ablation and/or implantation of ICDs. Pacing Clin Electrophysiol: PACE. (2019) 42(4):447–52. doi: 10.1111/pace.13613
41. Lee A, Hamilton-Craig C, Denman R, Haqqani HM. Catheter ablation of papillary muscle arrhythmias: implications of mitral valve prolapse and systolic dysfunction. Pacing Clin Electrophysiol: PACE. (2018) 41(7):750–8. doi: 10.1111/pace.13363
42. Enriquez A, Shirai Y, Huang J, Liang J, Briceno D, Hayashi T, et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: electrophysiologic substrate and catheter ablation outcomes. J Cardiovasc Electrophysiol. (2019) 30(6):827–35. doi: 10.1111/jce.13900
43. Gordon JP, Liang JJ, Pathak RK, Zado ES, Garcia FC, Hutchinson MD, et al. Percutaneous cryoablation for papillary muscle ventricular arrhythmias after failed radiofrequency catheter ablation. J Cardiovasc Electrophysiol. (2018) 29(12):1654–63. doi: 10.1111/jce.13716
44. Syed FF, Ackerman MJ, McLeod CJ, Kapa S, Mulpuru SK, Sriram CS, et al. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. (2016) 9(5):e004005. doi: 10.1161/CIRCEP.116.004005
45. Ezzeddine FM, Siontis KC, Giudicessi J, Ackerman MJ, Killu AM, Deshmukh AJ, et al. Substrate characterization and outcomes of catheter ablation of ventricular arrhythmias in patients with mitral annular disjunction. Circ Arrhythm Electrophysiol. (2022) 15(9):e011088. doi: 10.1161/CIRCEP.122.011088
Keywords: mitral valve prolapse (MPV), premature ventricular contractions, ventricular tachycardia (VT), sudden cardiac death (SCD), risk stratification
Citation: Kubala M, Essayagh B, Michelena HI, Enriquez-Sarano M and Tribouilloy C (2023) Arrhythmic mitral valve prolapse in 2023: Evidence-based update. Front. Cardiovasc. Med. 10:1130174. doi: 10.3389/fcvm.2023.1130174
Received: 22 December 2022; Accepted: 3 April 2023;
Published: 18 April 2023.
Edited by:
Pompilio Faggiano, Fondazione Poliambulanza Istituto Ospedaliero, ItalyReviewed by:
Elena Galli, Centre Hospitalier Universitaire (CHU) de Rennes, FranceGloria Santangelo, Santi Paolo e Carlo Hospital, Italy
Emanuele Bertaglia, Clinica Cardiologica - Azienda Ospedale Università, Italy
© 2023 Kubala, Essayagh, Michelena, Enriquez-Sarano and Tribouilloy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christophe Tribouilloy dHJpYm91aWxsb3kuY2hyaXN0b3BoZUBjaHUtYW1pZW5zLmZy
Specialty Section: This article was submitted to Heart Valve Disease, a section of the journal Frontiers in Cardiovascular Medicine
 Maciej Kubala
Maciej Kubala Benjamin Essayagh
Benjamin Essayagh Hector I. Michelena
Hector I. Michelena Maurice Enriquez-Sarano
Maurice Enriquez-Sarano Christophe Tribouilloy
Christophe Tribouilloy