- 1Department of Cardiology and Vascular Medicine, Saiful Anwar General Hospital - Faculty of Medicine Universitas Brawijaya, Malang, Indonesia
- 2Center for Cardiovascular Studies, Universitas Brawijaya, Malang, Indonesia
- 3Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Indonesia, National Cardiovascular Center Harapan Kita (Indonesia), Jakarta, Indonesia
- 4Department Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Background: Genetic factors contribute to the AF pathophysiology by altering the structural and functional properties of proteins involved in different cellular activities. MicroRNAs (miRNAs), which take part in structural and electrical remodeling during the AF evolution, are important genetic elements that must be considered. The aim of study is to determine correlation between the expression of miRNAs and the development of AF, as well as to explain any potential importance of genetic factors in the AF diagnosis.
Methods and Results: Online scientific databases, including Cochrane, ProQuest, PubMed, and Web of Science were used to conduct the literature search. The keywords were associated with or characterized the relationship between miRNAs and AF. The pooled sensitivity and specificity statistical parameters were analyzed using a random-effects model. The miRNAs had a combined sensitivity and specificity of 0.80 (95% CI = 0.70–0.87) and 0.75 (95% CI = 0.64–0.83) for the diagnosis of AF, respectively. The area under the SROC was 0.84 (95% CI = 0.81–0.87). The DOR was 11.80 (95% CI = 6.79–20.50). This study also revealed that miRNAs had a pooled PLR of 3.16 (95% CI = 2.24–4.45) and NLR of 0.27 (95% CI = 0.18–0.39) for the diagnosis of AF. The miR-425-5p demonstrated the highest sensitivity (0.96, 95% CI, 0.89–0.99).
Conclusion: The meta-analysis revealed substantial connection between miRNA expression dysregulation and AF, supporting the potential diagnostic role of miRNAs. The miR-425-5p has potential role as a biomarker for AF.
Introduction
The most frequent supraventricular arrhythmia, atrial fibrillation (AF), is associated with high rates of morbidity and death and significantly raises the risk of stroke, systemic thromboembolism, and heart failure (1, 2). The estimated prevalence of AF in adults is currently between 2% and 4%. Due to increased lifespan among the general population and a more intense screening for undiagnosed AF, a 2.3-fold increase is anticipated (2). Several well-known risk factors, such as male sex, older age, hypertension, diabetes mellitus, obstructive sleep apnea, heart failure, valvular heart disease, left atrial enlargement, and chronic obstructive pulmonary disease, play an essential role in the development of AF (3). In addition to those risk factors, family history is also thought to play a crucial role in the development of AF. Family history is associated with genetic factors, such as autosomal dominant inheritance, where epigenetic mechanisms can contribute to the AF pathophysiology by altering the structural and functional properties of proteins involved in different cellular activities (4, 5). MicroRNAs (miRNAs), which take part in structural and electrical remodeling during the AF evolution, are important genetic elements that must be considered (6).
Small non-coding RNAs known as miRNAs are encoded by nuclear DNA and transcribed by RNA polymerase II. Their main role is to control post-transcriptional gene expression by binding to complementary target sequences in messenger RNA (mRNA), which prevents the transcribed target from being translated or degraded (6). In other words, miRNAs play a role in the post-transcriptional regulation of protein expression and take part in the pathogenesis of the disease (7). The dysregulated miRNAs may be used as a new disease-specific diagnostic biomarker and treatment target (8, 9). The performance of miRNAs in detecting several cardiovascular diseases, including acute myocardial infarction, heart failure, and stroke, has been well-recognized (10–13). Several studies have investigated the connection between miRNAs and AF or AF recurrence following catheter ablation procedures in recent years (14–16). In total, 51 consistently dysregulated miRNAs linked to AF were identified (17). Beside the role of miRNA as diagnostic tolls of AF, several studies also conclude that miRNA can be used as Major Adverse Cardiovascular Events (MACE) in patient with AF (18). However, the performance of miRNA as the potential biomarker for AF diagnosis has not been widely explored. The goal was to determine whether there is a correlation between the expression of miRNAs and the development of AF, as well as to explain any potential importance of genetic factors in the AF diagnosis.
Methods
Study design
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement was followed in this systematic review and meta-analysis study (19). Because this study was a systematic review and meta-analysis, patient-informed consent and ethical approval were not required.
Literature search strategy
A conscientious literature search was conducted to find eligible original studies for this systematic review and meta-analysis. Online scientific databases, including Cochrane, ProQuest, PubMed, and Web of Science were used to conduct the literature search. The keywords were associated with or characterized the relationship between miRNAs and AF. The keywords were described or associated with the relationship between miRNAs and AF. We searched the potential articles with the following keywords: “micro-RNA” OR “micro RNA” OR “miRNA” AND “atrial fibrillation” OR “AF” OR “Afib” AND “diagnostic” OR “diagnosis” AND “sensitivity” OR “specificity.” The literature search was completed in November 2022.
Eligibility criteria and quality of study assessment
The inclusion criteria of this systematic review and meta-analysis study were; (1) observational study; (2) exploring the diagnostic performance of miRNAs concentration for AF; (3) providing the sensitivity and specificity data of miRNAs in detecting AF. Articles were excluded according to the following criteria: (1) full-text unavailability; (2) not written in English; (3) non-human studies; (4) editorials; (5) letters to the editor, (6) article reviews; and (7) studies with inadequate information regarding diagnostic performance, sensitivity or specificity. QUADAS-2 tools for diagnostic studies were used to evaluate the quality of the included studies. It consists of four domains as follows: (1) patient selection, (2) the index test, (3) the reference standard, and (4) flow and timing (20).
Data extraction
The data extraction included the following information: (1) first author's name, (2) publication year, (3) design, (4) enrolment period; (5) number of participants in the AF and sinus rhythm groups: (6) specimen; (7) miRNAs studied; (8) miRNAs quantification method; (9) miRNAs expression in AF; and (10) AF detection method. The number of participants with true-positive (TP), true-negative (TN), false-positive (FP), and false-negative (FN) results was also extracted. The authors calculated these values for investigations in which such information was not directly provided according to the associated sensitivity and specificity.
Statistical analysis
For the statistical analysis, STATA 17.0 was utilized. Statistically significant was defined as p-value <0.05. The pooled sensitivity and specificity statistical parameters were analyzed using a random-effects model. The summary receiver operating characteristic (SROC) curve was used to assess the overall diagnostic efficacy (21). The hierarchical summary receive operating characteristic (HSROC) curve was used to validate bivariate model data The diagnostic odds ratio (DOR), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were also assessed (10). Using the heterogeneity index (I2) and the Cochran-Q test, the heterogeneity was evaluated (22). The significant heterogeneity was identified when the Cochran-Q test's p-value was less than 0.01 and the I2 value was greater than 50%. The potential publication bias of diagnostic research was evaluated using Deeks' funnel plot (23).
Results
Study selection
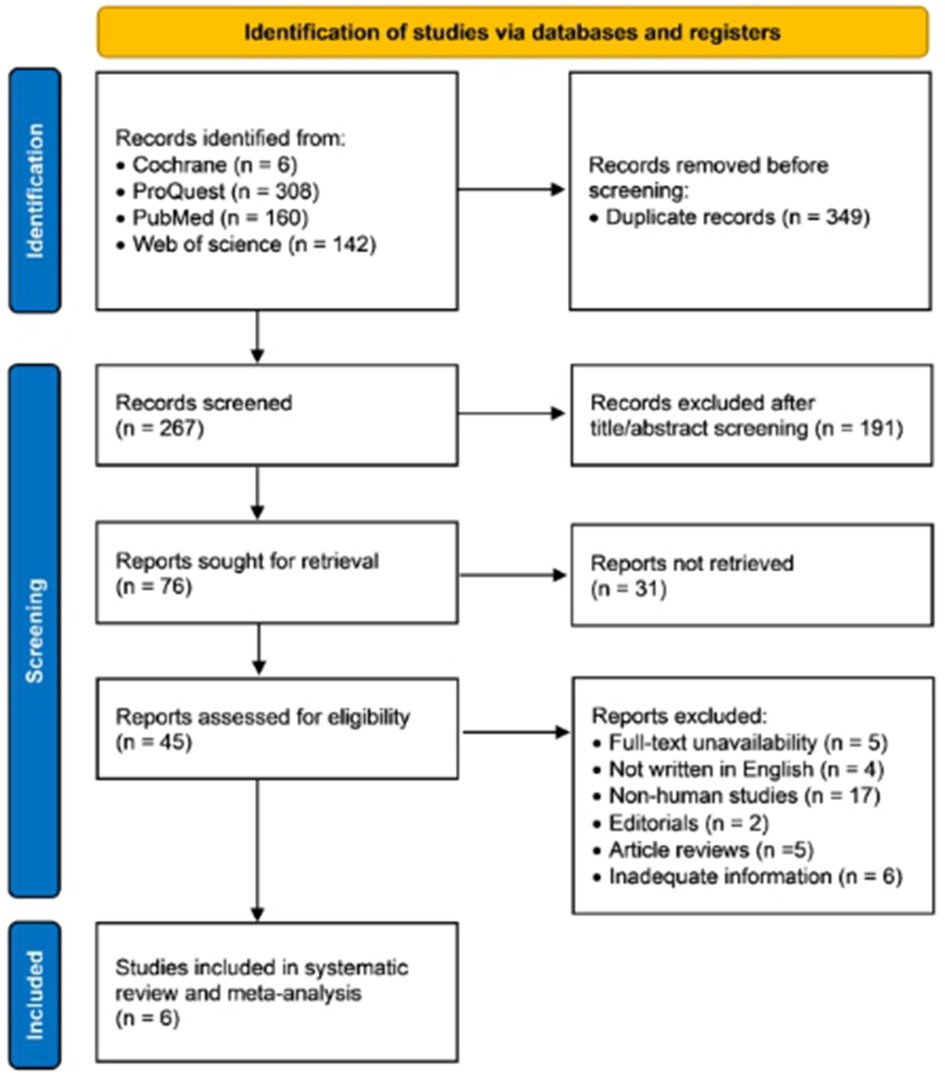
A total of 616 articles were identified from Cochrane (n = 6), ProQuest (n = 308), PubMed (n = 160), and Web of Science (n = 142) databases, among which 349 records were excluded due to duplications. During the title/abstract screening, we excluded 191 records due to mismatch with our purposes. In the next step, 31 reports were not retrieved. The remaining 45 articles were included in the further selection, and 39 articles were excluded due to: (1) full-text unavailability (n = 5); (2) not written in English (n = 4); (3) non-human studies (n = 17); (4) editorials (n = 2); article reviews (n = 5), and inadequate information (n = 6). Finally, 6 studies evaluating the diagnostic performance of miRNAs expression in AF were included in the meta-analysis. Figure 1 provides a schematic representation of the literature review and the selection criteria for the study.
Baseline characteristics
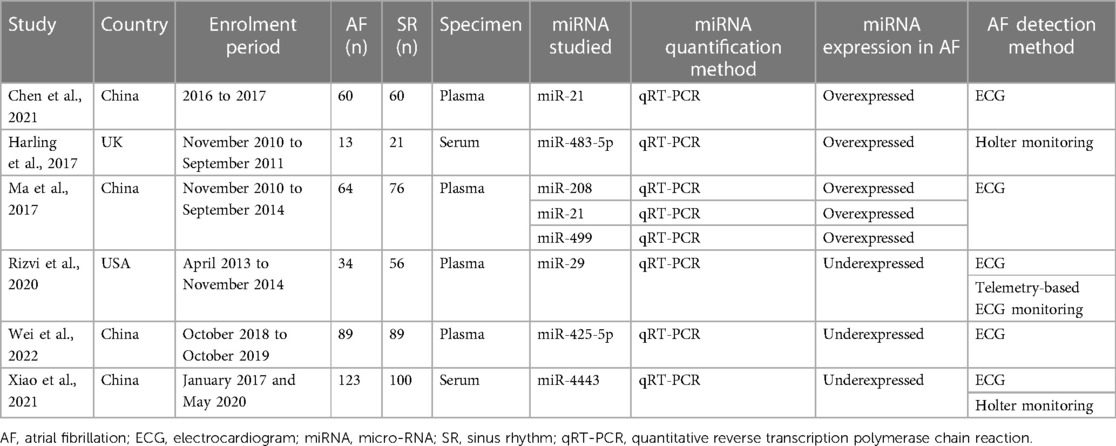
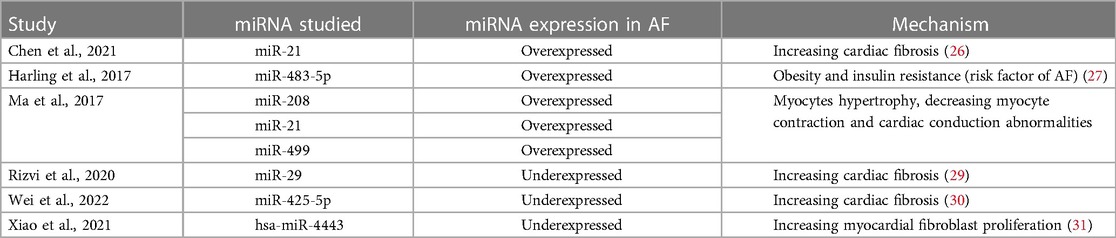
A total of 1,065 participants, including 547 and 518 participants in the AF and SR groups from 6 studies, were included in the data analysis. Four studies used plasma samples, whereas two studies used serum for miRNA quantification. MiRNAs were measured using quantitative reverse transcription polymerase chain reaction (qRT-PCR). The miRNA studied were miR-21, miR-483-5p, miR-208, miR-499, miR-29, miR-425-5p, and hsa-miR-4443. The baseline characteristics of the included studies are summarized in Table 1. According to the QUADAS-2 assessment, studies with a high risk of bias were not found (Figure 2).
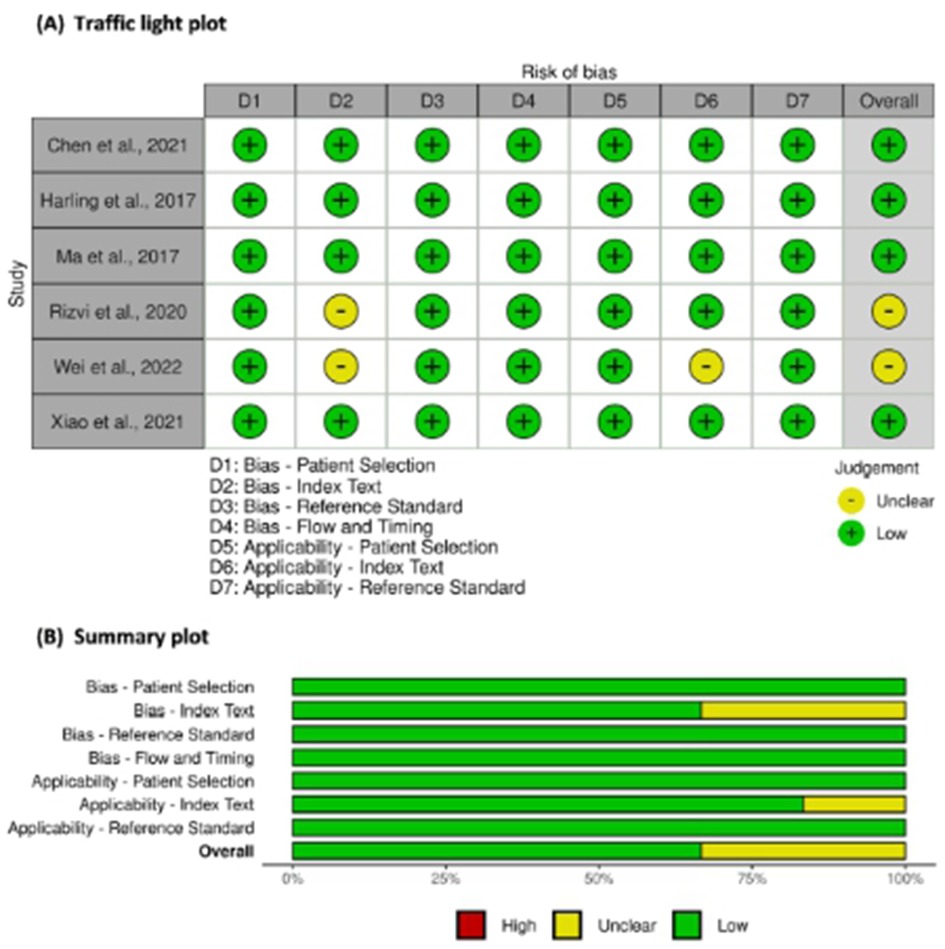
Figure 2. Risk of bias assessment of studies using QUADAS-2. (A) Traffic light plot and (B) Summary plot.
Diagnostic performance of MiRNAs for AF
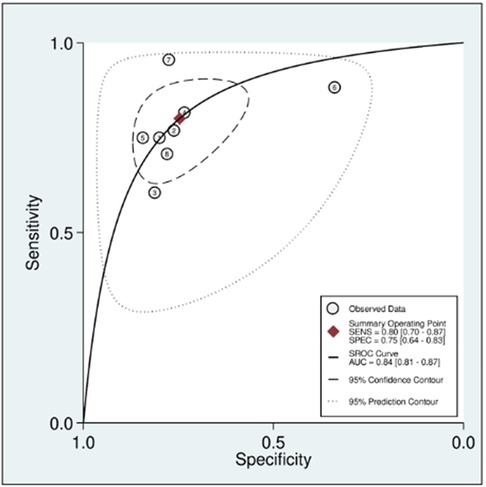
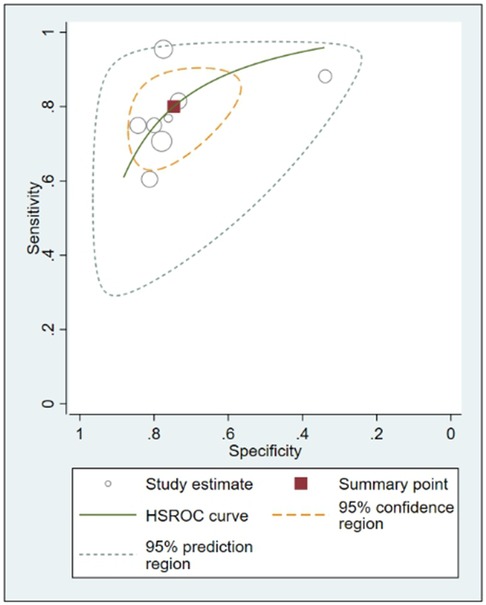
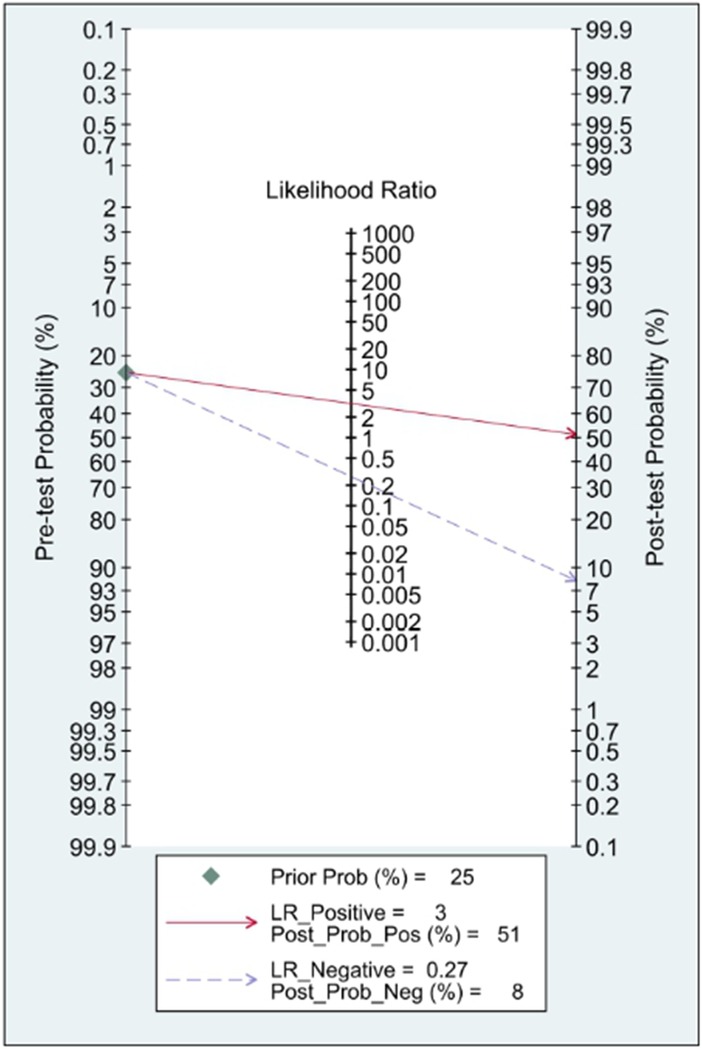
In this systematic review and meta-analysis study we explored the diagnostic performance or miRNAs for AF that included several parameters such as sensitivity, specificity, area under curve of SROC, PLR, and NLR. MiRNAs had a combined sensitivity and specificity of 0.80 (95% CI = 0.70–0.87) and 0.75 (95% CI = 0.64–0.83) for the diagnosis of AF, respectively (Figure 3). Using SROC curve analysis, the combined diagnostic performance of miRNAs was evaluated (Figure 4). The area under the SROC was 0.84 (95% CI = 0.81–0.87). In the results given by the HSROC model (Figure 5 and Supplementary Figure S1), the β (beta) estimation and the 95% CI were −0.02 (95% CI = −0.82–0.79), and z = −0.04, p = 0.97 (p > 0.5). The λ (lambda) estimation and the 95% CI were 2.47 (95% CI, 1.91–3.02). Those results are consistent. Additionally, the DOR was assessed (Figure 6). The DOR was 11.80 (95% CI = 6.79–20.50). This study also revealed that miRNAs had a pooled PLR of 3.16 (95% CI = 2.24–4.45) and NLR of 0.27 (95% CI = 0.18–0.39) for the diagnosis of AF (Figure 7). Figure 8 demonstrates the likelihood of correctly diagnosing a patient with AF is 51% if the miRNA test yields a positive result. The likelihood of misdiagnosing a patient without AF as having AF is 8% if the miRNA test results are negative.
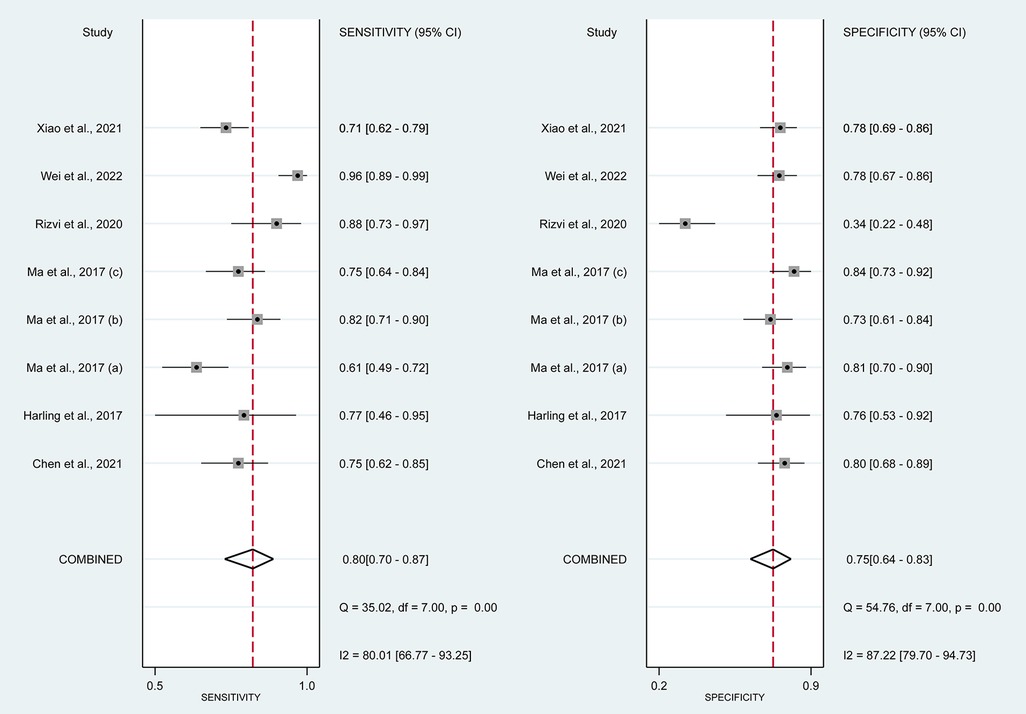
Figure 3. Forrest plot of estimates of sensitivity and specificity. MiRNAs had combined sensitivity 0.80 (95% CI = 0.70–0.87, p < 0.01) and specificity 0.75 (95% CI, 0.64–0.83; p < 0.01).
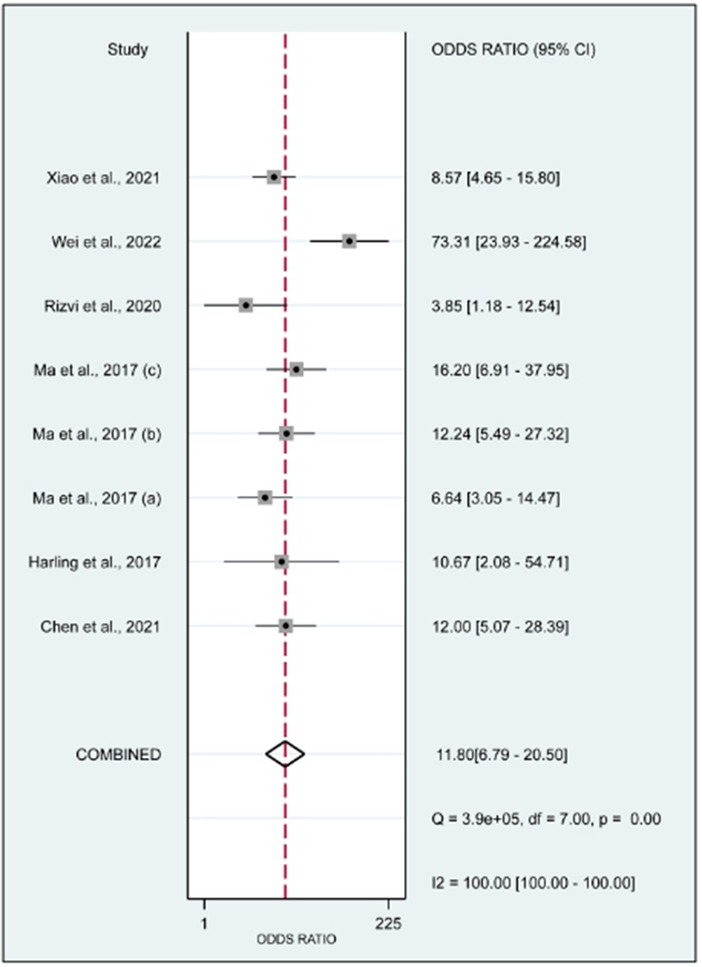
Figure 6. Forrest plot of the diagnostic odds ratio. The DOR was 11.80 (95% CI, 6.79–20.50; p < 0.01).
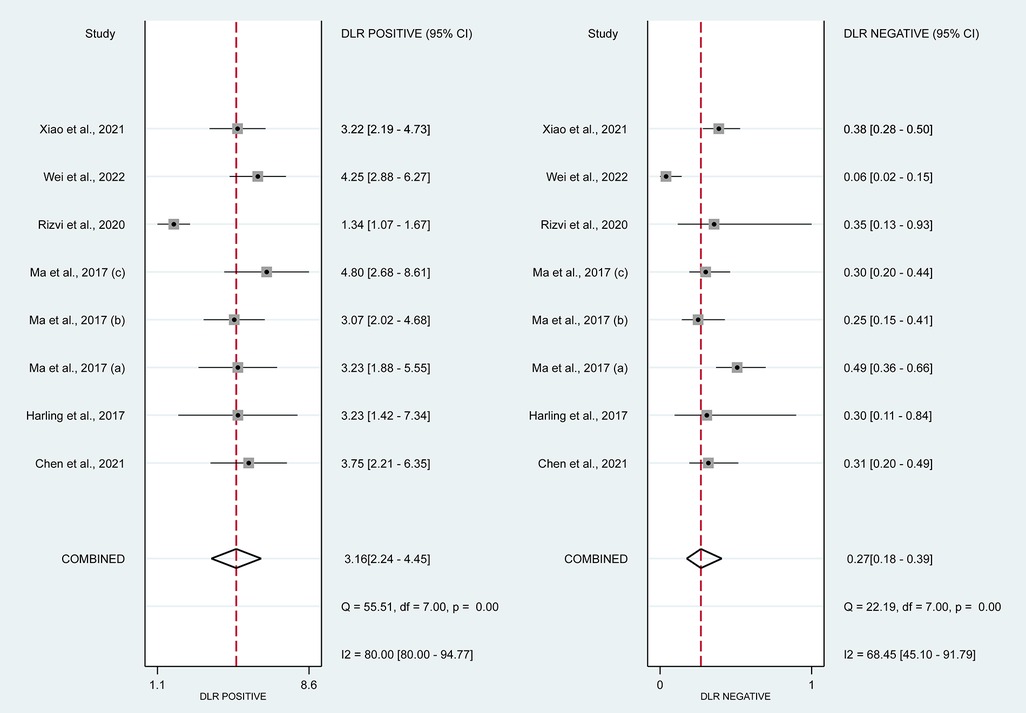
Figure 7. Forrest plot of estimates of positive likelihood ratio and negative likelihood ratio. The PLR was 3.16 (95% CI, 2.24–4.45; p < 0.01) and The DLR was 0.27 (95% CI, 0.18–0.39; p < 0.01).
Publication bias
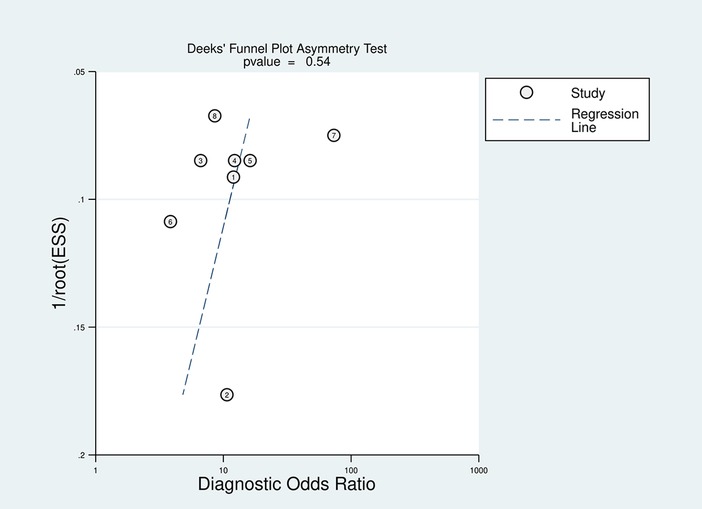
Using STATA 17.0, Deeks' funnel plot was created to assess the underlying publishing bias. That plot revealed a p-value of 0.54, indicating a low publication bias probability (Figure 9).
Discussion
MiRNAs regulate essential target genes and perform crucial roles in the development of AF. Multiple experimental studies have demonstrated that miRNAs have a role in atrial electric remodeling in AF by targeting essential genes. Multiple strategic miRNA-related pathways, including Ca2+-dependent signaling pathways, inflammatory and immunological pathways, apoptotic and cycle pathways, have been identified, indicating that miRNAs are likely to be the therapeutic target for AF (24). Multiple studies have examined the potential use of miRNAs as biomarkers for AF. In this study, the relationship between dysregulation of miRNAs and the occurrence of AF was originally established using a pooled analysis of previously published data. Quantitative approaches were then utilized to corroborate this association. In addition, we compared the diagnostic value of miRNAs in various studies and discovered that miR-425-5p reported by Wei et al. (2022) (25) demonstrated the highest sensitivity (0.96, 95% CI, 0.89–0.99, Figure 3) and diagnostic value (73.31, 95% CI, 23.93–224.58, Figure 5), suggesting the potential role of miR-425–5p as a biomarker for AF. This meta-analysis revealed a substantial connection between miRNA expression dysregulation and AF, supporting the potential diagnostic role of miRNAs.
AF known to be initiated and perpetuated by electrical and structural remodeling of the atrium. In this meta-analysis, 2 studies found that over expression of miR-21 had diagnostic role of AF. Previous study found that miR-21 was found to be higher in atrial tissue patient with AF compared to those in sinus rhythm. Its over expression decreasing L-Type Ca2+ current expression, which is the process of electrical remodeling (26). Another study found that higher miR-21 concentration had correlation with atrial tissue fibrosis, the key process of atrial tissue structural remodeling (27). Harling et al. found used miR-483-5p in their study. This type of miRNA had a role of manipulating cytokine signaling by targeting SOCS3 (suppressor of cytokine signaling-3). SOCS3 previously found to be important in the development of metabolic syndrome by increasing the risk of obesity and insulin resistance (28).
Other mechanisms of how different miRNA act on initiation and perpetuation are summarized in Table 2.
Based on the first systematic review of its kind to investigate the miRNA expression markers of AF (17), the previous study need for a meta-analysis to produce specific and useful miRNA data stems from the wide variety of technology platforms and inconsistencies in sample sources. A total of 51 miRNAs showed systematic abnormal expression across all studies. A total of five microRNAs (miRNA-328, miRNA-223-3p, miRNA-21, miRNA-29b, and miRNA-1-5p) were found through additional investigation as possible biomarkers of atrial fibrillation. MiRNA-21 was identified as being overexpressed in two studies, both of which were included in the meta-analysis (32–34). We recommend further study of miRNA-21 to identify more specific miRNA that can be utilized as a more accurate diagnostic tool for atrial fibrillation.
A single biomarker, miRNA-328-3p, was found to be strongly related with an elevated risk of AF in another meta-analysis investigation. This finding suggests that miR-328-3p was crucial in the diagnosing process and could be a significant and effective biomarker for detecting AF (35). However, miR-328-3p has also been linked to a wide variety of other diseases and disorders. Numerous studies have shown that miR-328-3p can inhibit tumor growth (36). Several limitations were discovered in our meta-analysis. Some miRNAs may be differentially expressed between paroxysmal and chronic AF. However, this was not investigated in the current study. Moreover, the examined group showed heterogeneities in comorbidities such valve disease and rheumatic heart disease, which may have served as confounding factors, adding to the heterogeneity of miRNA expression.
Recently, a meta-analysis published in early 2023 revealed strong correlation between circulating miRNA and Atrial fibrillation. Unlike our study, this study did not evaluate diagnostic performance including pooled sensitivity and specificity. They analyzed each type of the OR of each miRNA and then measured the overall OR. The overall OR was 2.51 (95% CI, 1.99-3.16). The miRNA—150 had highest OR (3.77; 95% CI, 1.50–9.46) (37).
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
AR: Idea formulation, study method construction, manuscript writing. YW: Idea formulation, study method construction, conducting meta-analysis process, manuscript writing. AW: Idea formulation, study method construction, manuscript writing. YY: Concept, editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1128708/full#supplementary-material.
References
1. Waranugraha Y, Rizal A, Syaban MFR, Faratisha IFD, Erwan NE, Yunita KC. Direct comparison of non-vitamin K antagonist oral anticoagulant versus warfarin for stroke prevention in non-valvular atrial fibrillation: a systematic review and meta-analysis of real-world evidences. Egypt Hear J. (2021) 73(1):1–17. doi: 10.1186/s43044-021-00194-1
2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
3. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. (2017) 136(6):583–96. doi: 10.1161/CIRCULATIONAHA.116.023163
4. Liu L, Yu Y, Hu LL, Dong QB, Hu F, Zhu LJ, et al. Potential target genes in the development of atrial fibrillation: a comprehensive bioinformatics analysis. Med Sci Monit. (2021) 27:e928366. doi: 10.12659/MSM.928366
5. Rizal A, Yuniadi Y. Epigenetic implication in atrial fibrillation: a potential biomarker? J Lab Precis Med. (2019) 4:33–33. doi: 10.21037/jlpm.2019.09.02
6. Lozano-Velasco E, Franco D, Aranega A, Daimi H. Genetics and epigenetics of atrial fibrillation. Int J Mol Sci. (2020) 21(16):5717. doi: 10.3390/ijms21165717
7. Chavda V, Madhwani K. Coding and non-coding nucleotides’: the future of stroke gene therapeutics. Genomics. (2021) 113(3):1291–307. doi: 10.1016/j.ygeno.2021.03.003
8. Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. (2014) 13(8):622–38. doi: 10.1038/nrd4359
9. Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. (2016) 6(3):235–46. doi: 10.1158/2159-8290.CD-15-0893
10. Wang Q, Ma J, Jiang Z, Wu F, Ping J, Ming L. Identification of microRNAs as diagnostic biomarkers for acute myocardial infarction in Asian populations: a systematic review and meta-analysis. Medicine (Baltimore). (2017) 96(24):e7173. doi: 10.1097/MD.0000000000007173
11. Zhao J, Yu H, Yan P, Zhou X, Wang Y, Yao Y. Circulating microRNA-499 as a diagnostic biomarker for acute myocardial infarction: a meta-analysis. Dis Markers. (2019) 2019:6121696. doi: 10.1155/2019/6121696
12. Yan H, Ma F, Zhang Y, Wang C, Qiu D, Zhou K, et al. miRNAs as biomarkers for diagnosis of heart failure: a systematic review and meta-analysis. Medicine (Baltimore). (2017) 96(22):e6825. doi: 10.1097/MD.0000000000006825
13. Deng Y, Huang P, Zhang F, Chen T. Association of microRNAs with risk of stroke: a meta-analysis. Front Neurol. (2022) 13:865265. doi: 10.3389/fneur.2022.865265
14. Galenko O, Jacobs V, Knight S, Taylor M, Cutler MJ, Muhlestein JB, Carlquist JL, Knowlton KU, Jared Bunch T. The role of microRNAs in the development, regulation, and treatment of atrial fibrillation. J Interv Card Electrophysiol. (2019) 55(3):297–305. doi: 10.1007/s10840-018-0495-z
15. McManus DD, Tanriverdi K, Lin H, Esa N, Kinno M, Mandapati D, et al. Plasma microRNAs are associated with atrial fibrillation and change after catheter ablation (the miRhythm study). Hear Rhythm. (2015) 12(1):3–10. doi: 10.1016/j.hrthm.2014.09.050
16. Liu T, Zhong S, Rao F, Xue Y, Qi Z, Wu S. Catheter ablation restores decreased plasma miR-409-3p and miR-432 in atrial fibrillation patients. Europace. (2016) 18(1):92–9. doi: 10.1093/europace/euu366
17. Shen NN, Zhang C, Li Z, Kong LC, Wang XH, Gu ZC, et al. MicroRNA expression signatures of atrial fibrillation: the critical systematic review and bioinformatics analysis. Exp Biol Med (Maywood). (2020) 245(1):42–53. doi: 10.1177/1535370219890303
18. de los Reyes-García AM, Zapata-Martínez L, Águila S, Lozano ML, Martínez C, González-Conejero R. microRNAs as biomarkers of risk of major adverse cardiovascular events in atrial fibrillation. Front Cardiovasc Med. (2023) 10:1–8. doi: 10.3389/fcvm.2023.1135127
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
20. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
21. Zhang H, Ding R, Chen D. Value of miR-21 levels as potential biomarkers in the early diagnosis of hepatocellular carcinoma: a meta-analysis. Biomarkers. (2021) 26(7):586–97. doi: 10.1080/1354750X.2021.1955976
22. Waranugraha Y, Rizal A, Yuniadi Y. A systematic review and meta-analysis of the direct comparison of second-generation cryoballoon ablation and contact force-sensing radiofrequency ablation in patients with paroxysmal atrial fibrillation. J Pers Med. (2022) 12(2):298. doi: 10.3390/jpm12020298
23. Lin L, Chu H, Murad MH, Hong C, Qu Z, Cole SR, et al. Empirical comparison of publication bias tests in meta-analysis. J Gen Intern Med. (2018) 33(8):1260–7. doi: 10.1007/s11606-018-4425-7
24. Shen NN, Zhang ZL, Li Z, Zhang C, Li H, Wang JL, et al. Identification of microRNA biomarkers in atrial fibrillation. Medicine (Baltimore). (2019) 98(30):e16538. doi: 10.1097/md.0000000000016538
25. Wei F, Ren W, Zhang X, Wu P, Fan J. miR-425-5p is negatively associated with atrial fibrosis and promotes atrial remodeling by targeting CREB1 in atrial fibrillation. J Cardiol. (2022) 79(2):202–10. doi: 10.1016/j.jjcc.2021.09.012
26. Barana A, Matamoros M, Dolz-Gaiton P, Perez-Hernandez M, Amoros I, Núñez M, et al. Chronic atrial fibrillation increases microRNA-21 in human atrial myocytes decreasing L-type calcium current. Circ Arrhythmia Electrophysiol. (2014) 7(5):861–8. doi: 10.1161/CIRCEP.114.001709
27. Adam O, Löhfelm B, Thum T, Gupta SK, Puhl SL, Schäfers HJ, et al. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. (2012) 107(5):278. doi: 10.1007/s00395-012-0278-0
28. Gallo W, Esguerra JLS, Eliasson L, Melander O. miR-483-5p associates with obesity and insulin resistance and independently associates with new onset diabetes mellitus and cardiovascular disease. PLoS One. (2018) 13(11):e0206974. doi: 10.1371/journal.pone.0206974
29. Smyth A, Callaghan B, Willoughby CE, O’Brien C. The role of miR-29 family in TGF-β driven fibrosis in glaucomatous optic neuropathy. Int J Mol Sci. (2022) 23(18):10216. doi: 10.3390/ijms231810216
30. de la Rocha AM, González-Huarriz M, Guruceaga E, Mihelson N, Tejada-Solís S, Díez-Valle R, et al. miR-425-5p, a SOX2 target, regulates the expression of FOXJ3 and RAB31 and promotes the survival of GSCs. Arch Clin Biomed Res. (2020) 4(3):221–38. doi: 10.26502/acbr.50170100
31. Xiao J, Zhang Y, Tang Y, Dai H, OuYang Y, Li C, et al. hsa-miR-4443 inhibits myocardial fibroblast proliferation by targeting THBS1 to regulate TGF-β1/α-SMA/collagen signaling in atrial fibrillation. Braz J Med Biol Res. (2021) 54(4):e10692. doi: 10.1590/1414-431X202010692
32. Chen H, Zhang F, Zhang YL, Yang XC. Relationship between circulating miRNA-21, atrial fibrosis, and atrial fibrillation in patients with atrial enlargement. Ann Palliat Med. (2021) 10(12):12742–9. doi: 10.21037/apm-21-3518
33. Harling L, Lambert J, Ashrafian H, Darzi A, Gooderham NJ, Athanasiou T. Elevated serum microRNA 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur J Cardio-thorac Surg. (2017) 51(1):73–8. doi: 10.1093/ejcts/ezw245
34. Paul A, Pai PG, Ariyannur PS, Joy RA. MiR-499 is a diagnostic biomarker of paroxysmal atrial fibrillation involved in the development of atrial fibrillation. Int J Clin Exp Pathol. (2017) 10(4):4221–31. doi: 10.1016/j.ihj.2021.06.018
35. Huang H, Chen H, Liang X, Chen X, Chen X, Chen C. Upregulated miR-328-3p and its high risk in atrial fibrillation: a systematic review and meta-analysis with meta-regression. Medicine (Baltimore). (2022) 101(9):e28980. doi: 10.1097/MD.0000000000028980
36. Wang X, Xia Y. microRNA-328 inhibits cervical cancer cell proliferation and tumorigenesis by targeting TCF7L2. Biochem Biophys Res Commun. (2016) 475(2):169–75. doi: 10.1016/j.bbrc.2016.05.066
Keywords: atrial fibrillation, genetic, micro-RNA (miRNA), biomarker, diagnostic
Citation: Rizal A, Waranugraha Y, Wikananda AP and Yuniadi Y (2023) Identification of microRNAs as diagnostic biomarkers for atrial fibrillation: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1128708. doi: 10.3389/fcvm.2023.1128708
Received: 21 December 2022; Accepted: 7 April 2023;
Published: 28 April 2023.
Edited by:
Tania Martins-Marques, University of Coimbra, PortugalReviewed by:
Xavier Vidal-Gomez, INSERM U1046 Physiologie et Médecine Expérimentale du Coeur et des Muscles, FranceJie Du, Shanxi Medical University, China
© 2023 Rizal, Waranugraha, Wikananda and Yuniadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoga Yuniadi eW9nYXkxMzZAZ21haWwuY29t
 Ardian Rizal1,2
Ardian Rizal1,2 Yoga Waranugraha
Yoga Waranugraha Yoga Yuniadi
Yoga Yuniadi