
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 10 March 2023
Sec. General Cardiovascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1126093
This article is part of the Research Topic Cytokines, Novel Cell Death Models and Pathways in Cardiovascular Diseases View all 11 articles
Background: While insulin-like growth factor 1 (IGF-1) exerts a cardioprotective effect in the setting of atherosclerosis, insulin-like growth factor binding protein 2 (IGFBP-2) is involved in metabolic syndrome. Although IGF-1 and IGFBP-2 are known to be predictors for mortality in patients with heart failure, their use in clinic as prognostic biomarkers for acute coronary syndrome (ACS) requires investigation. We evaluated the relationship between IGF-1 and IGFBP-2 levels at admission and the risk of major adverse cardiovascular events (MACEs) in patients with ACS.
Methods: A total of 277 ACS patients and 42 healthy controls were included in this prospective cohort study. Plasma samples were obtained and analyzed at admission. Patients were followed for MACEs after hospitalization.
Results: Among patients who suffered acute myocardial infarction, plasma levels of IGF-1 and IGFBP-2 were lower and higher, respectively, as compared to healthy controls (both p < 0.05). The mean follow-up period was 5.22 (1.0–6.0) months and MACEs incidence was 22.4% (62 of 277 patients). Kaplan–Meier survival analysis revealed that patients with low IGFBP-2 levels had a greater event-free survival rate than patients with high IGFBP-2 levels (p < 0.001). Multivariate Cox proportional hazards analysis revealed IGFBP-2, but not IGF-1, to be a positive predictor of MACEs (hazard ratio 2.412, 95% CI 1.360–4.277; p = 0.003).
Conclusion: Our findings suggest that high IGFBP-2 levels are associated with the development of MACEs following ACS. Moreover, IGFBP-2 is likely an independent predictive marker of clinical outcomes in ACS.
Acute coronary syndrome (ACS) is characterized by a sudden decrease in blood flow to the heart. Worldwide, more than seven million people are annually diagnosed with ACS; approximately 5% of this patient population was reported to die prior to hospital discharge (1, 2). Although scientific advances have greatly facilitated implementation of effective secondary cardiovascular prevention strategies, previously unrecognized mediators of cardiovascular disease (CVD) continue to be discovered.
Insulin-like growth factors (IGFs) are conserved peptide hormones structurally similar to insulin that are expressed universally in multiple tissues (3). Interestingly, IGF-1 is not only found in the circulation but also in arteries, with studies having reported IGF-1 to exert cardioprotective effects in the setting of atherosclerosis (4, 5). Preclinical model studies reported that administration of IGF-1 suppresses cardiac fibrosis induced by angiotensin II (6), and treatment of sheep fetuses with IGF-1 was reported to stimulate growth of the coronary vasculature and myocardium (7). Furthermore, bone marrow mesenchymal stem cells overexpressing IGF-1 were reported to better resist apoptosis in myocardial infarction (8). In clinical practice, serum IGF-1 levels are decreased in heart failure (HF) patients (9) and serve as a predictor of cardiovascular mortality in this condition (10).
All six members of the IGF-binding protein (IGFBP) family regulate IGF bioavailability (11, 12). As the second most abundant protein of this family (13), IGFBP-2 plays critical roles in several pathological processes including carcinogenesis (14), pulmonary arterial hypertension (PAH) (15), obesity and insulin resistance (13). In addition to strongly predicting mortality in HF patients (16), IGFBP-2 has recently emerged as a novel candidate biomarker for cardiovascular risk assessment in patients with aortic stenosis who undergo transcatheter aortic valve implantation (17) and elderly men (18).
To date, relevant research has been limited to animal experiments, preclinical analyses or patients with HF, PAH or aortic stenosis. As such, the prognostic influences of circulating IGF-1 and IGFBP-2 in ACS patients remain unclear. Here, we evaluated the relationship of circulating IGF-1 and IGFBP-2 with major adverse cardiovascular events (MACEs) in ACS patients who underwent coronary angiography (CAG).
This study was a prospective cohort study conducted according to regulations set forth by the Declaration of Helsinki. This study was approved by the Ethics Committee of Liaocheng People's Hospital and all subjects enrolled provided informed consent. From June 1 2021 to October 1 2021, 304 ACS patients who underwent CAG and 42 site-matched controls free of clinical heart disease were initially enrolled in this study. All ACS patients enrolled met relevant diagnostic criteria for either ST-elevation myocardial infarction (STEMI) or non-ST-elevation ACS (19, 20). Patients with severe liver disease or renal failure, neoplasms of any kind or infectious or inflammatory conditions were excluded from this study. Patients lost to follow-up were also excluded from analyses. The treatment therapy, including intensive treatment with medicine, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) was decided by two experienced cardiologists according to the results of CAG and international standards and guidelines (21).
Venous blood samples were draw from study subjects for evaluation prior to administration of any medications. Blood samples were collected using tubes containing ethylenediaminetetraacetic acid and centrifuged at 3,000 rpm for 15 min immediately after collection. Samples were aliquoted and stored at −80°C until use. Plasma levels of IGF-1 and IGFBP-2 were assayed using the enzyme linked immunosorbent assays (ELISA) IGF-1 (DG100B) and IGFBP-2 (DGB 200) (both R&D Systems, USA), according to manufacturer's protocol. No significant cross-reactivity or interference of IGFBP/IGF-1 with IGFBP-2 was found in the immunoassay according to product description. ELISA IGFBP-2 kit did not measure IGFBP in complex with IGF-1.
Participants with one major coronary artery ≥50% stenosis were considered as single-vessel disease, whereas multi-vessel disease (MVD) was defined in cases of stenoses ≥50% in 2 or 3 major epicardial coronary arteries. Stenosis of the left main coronary artery ≥50% was regarded as the left main disease (22). Incomplete revascularization (IR) was defined as one or more vessels stenosis ≥50% being left untreated after revascularization (23).
The entire cohort was followed up for 6 months starting from date of hospitalization. Data were systematically obtained via phone interviews and review of medical records. A total of 27 ACS patients were lost of follow-up and excluded from this study. A total of 277 ACS patients who completed follow-up were finally evaluated and included 93 unstable angina (UA), 89 non-ST-elevation myocardial infarction (NSTEMI) and 95 STEMI patients. Cardiovascular death, angina, new-onset HF, recurrent myocardial infarction (MI) or any revascularization were all defined as MACEs.
The Shapiro–Wilk test was used to determine whether continuous data were normally distributed. Normally distributed continuous variables were expressed as mean ± standard deviation; data not normally distributed were expressed as median (interquartile range). Comparisons of continuous variables between two groups were performed using the Mann–Whitney U test or t-test. Comparisons of continuous variables among four groups were performed using one-way analysis of variance or the Kruskal–Wallis H test. The pairwise test for multiple comparisons was used to analyze intergroup differences for IGF-1 and IGFBP-2 levels after Kruskal–Wallis H analysis. Categorical variables were presented as frequency and percentage and compared using the chi-squared test. Spearman correlation analysis was performed to analyze the correlation of plasma IGF-1 and IGFBP-2 levels.
A receiver operator characteristics (ROC) curve was generated and the area under the curve (AUC) was calculated, and Z test were used to compare AUC values. Optimal IGF-1 and IGFBP-2 cutoff points for MACEs prediction were determined based on maximal Youden's index. Kaplan–Meier survival curves were constructed to analyze the short-term event-free survival (EFS) rate and comparisons were performed using the log-rank test. Cox proportional hazards regression analysis was performed to determine independent factors predictive for MACEs; confounders with unadjusted p-values <0.05 in univariate analysis were included in a multivariate regression model. A two-tailed p-value of <0.05 was considered as statistically significant. Statistical analyses were performed using SPSS 23.0 (IBM, USA).
Median values (interquartile ranges) of IGF-1 and IGFBP-2 concentrations in healthy controls (n = 42) were 170.39 (106.14) ng/ml and 199.1 (255.01) ng/ml, respectively. While IGF-1 levels in healthy controls were higher than in patients who suffered NSTEMI or STEMI (p < 0.05), IGFBP-2 levels showed the opposite pattern. Although IGFBP-2 levels in STEMI patients were higher than those in UA patients (p < 0.05), no significant difference between healthy controls and UA patients was found (p > 0.05; Table 1).
In a total of 277 ACS patients, 195 (70.4%) presented with MVD, 37 (13.4%) presented with left main vessel disease, and the number of patients with IR treatment was 32 (11.6%). 89 (32.1%) patients were treated with intensive medication, and 188 (67.9%) patients were treated with PCI or CABG. Patients were divided into two groups based on median IGF-1 concentrations: a high IGF-1 group (IGF-1 levels ≥126.92 ng/ml; n = 139) and a low IGF-1 group (IGF-1 levels <126.92 ng/ml; n = 138). High IGF-1 group patients were found to have a higher ejection fraction (EF), a lower rate of acute myocardial infarction (AMI) and a higher rate of multi-vessel lesion as compared to those in the low IGF-1 group. No other statistically significant differences in demographic characteristics or laboratory evaluations were noted between the two groups (Table 2).
Patients were divided into two groups based on median IGFBP-2 concentrations: a high IGFBP-2 group (IGFBP-2 ≥ 308.3 ng/ml; n = 139) and a low IGFBP-2 group (IGFBP-2 < 308.3 ng/ml; n = 138). As shown in Table 3, high IGFBP-2 group patients were older, had lower levels of hemoglobin, triglycerides and EF, higher D-dimer levels, and lower body mass indices (BMI). Moreover, high IGFBP-2 group patients were found to have a higher rate of AMI as compared to low IGFBP-2 group patients.
The correlation of plasma IGF-1 and IGFBP-2 levels was evaluated by Spearman correlation analysis. A significant negative correlation was found among IGF-1 and IGFBP-2 (r = −0.172, p = 0.002; Figure 1).
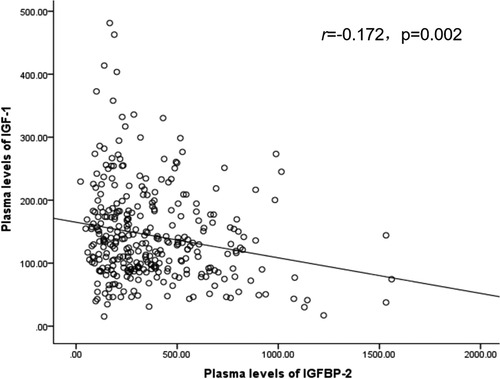
Figure 1. Correlation graph of IGF-1 and IGFBP-2. IGF-1: Insulin like growth factor 1; IGFBP-2: Insulin like growth factor binding protein 2.
The mean follow-up period was 5.22 (1.0–6.0) months. The incidence of MACEs was 22.4% (62 of 277 patients) and included instances of cardiovascular death (n = 8), angina (n = 21), HF (n = 13) and reinfarction or revascularization (n = 20). No patient died of non-cardiovascular causes. No statistical differences in MACEs rates between high and low IGF-1 group patients were found (p = 0.141). Total MACEs incidence was higher among high IGFBP-2 group patients as compared to low IGFBP-2 group patients (p < 0.001). Furthermore, incidences of angina (p = 0.003) and HF (p = 0.011) were higher among high IGFBP-2 group patients as compared to low IGFBP-2 group patients (Table 4).
Kaplan-Meier survival analysis revealed no statistically significant differences in event-free survival (EFS) between high and low IGF-1 group patients (p = 0.145; Figure 2A). Low IGFBP-2 group patients were found to have had a higher EFS as compared to high IGFBP-2 group patients (p < 0.001; Figure 2B). As such, patients with low levels of IGFBP-2 were found to have had a more favorable prognosis as compared to those with high levels of IGFBP-2.
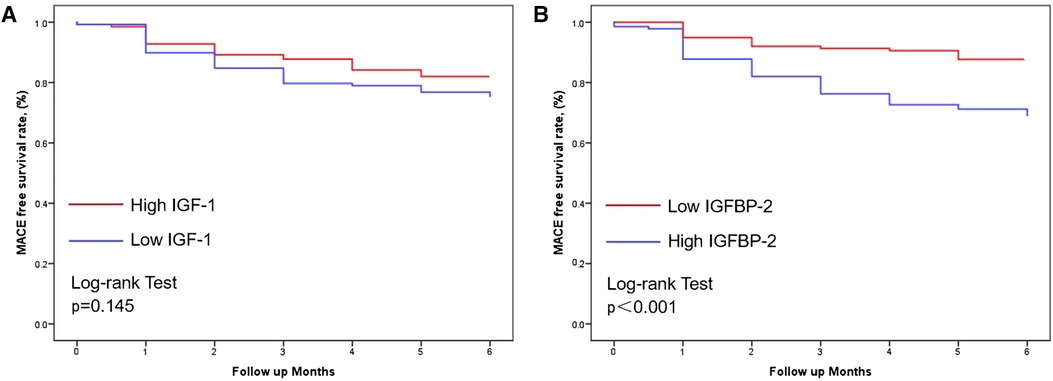
Figure 2. Kaplan-Meier curves in patients with ACS with individual levels of IGF-1 (A) and IGFBP-2 (B) during follow-up (p = 0.145 and <0.001, respectively). MACEs, Major adverse cardiovascular events; ACS, Acute coronary syndrome; IGF-1, Insulin like growth factor 1; IGFBP-2, Insulin like growth factor binding protein 2.
As shown in Table 5, we analyzed potential confounders for association with short-term MACEs using univariate analysis. Confounders with p-values of <0.05 in univariate analysis were included in multivariate Cox regression analysis. After correcting for age, diagnosis of AMI, creatinine level, EF, intensive medication therapy and left main vessel lesion, high IGFBP-2 level was confirmed to have positively predicted value for MACEs [adjusted hazard ratio: 2.412, 95% confidential interval (CI) 1.360–4.277; p = 0.003]. Furthermore, EF and left main vessel lesion were found to be independent predictors for MACEs, while IGF-1 level was not.
To evaluate the potential prognostic power of circulating IGFBP-2 and EF for MACEs prediction, ROC curves were generated. Analysis revealed that AUC values of plasma IGFBP-2 (Figure 3A) and EF (Figure 3B) for MACEs prediction in ACS patients were 0.722 (95% CI 0.640–0.804; p < 0.001) and 0.659 (95% CI 0.570–0.748; p < 0.001), respectively. No statistical differences in AUC values of IGFBP-2 and EF for MACEs prediction were found (Z = 1.02; p > 0.05).
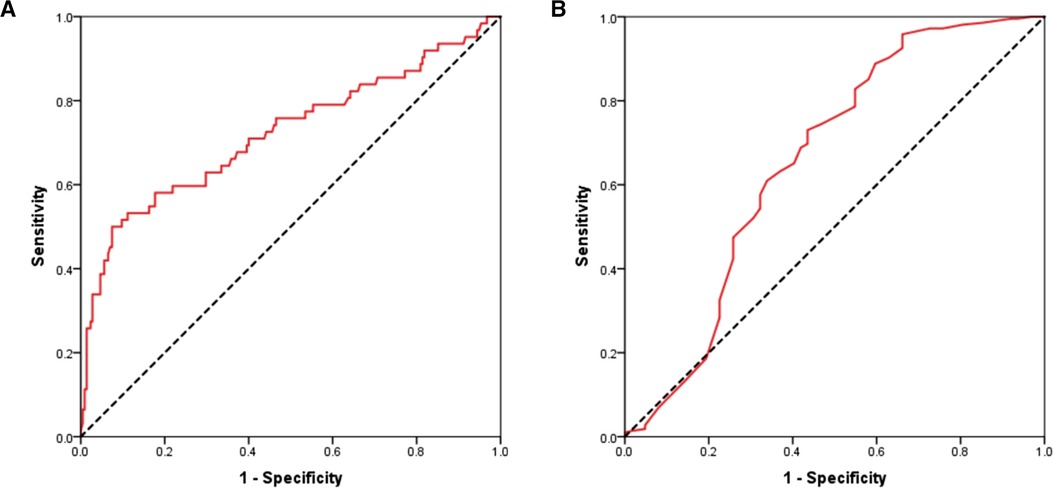
Figure 3. Receiver operating characteristic curves of circulating IGFBP-2 (A) and EF (B) for predicting MACEs in patients with ACS. IGFBP-2, Insulin like growth factor binding protein 2; EF, Ejection fraction; MACEs, Major adverse cardiovascular events; ACS, Acute coronary syndrome.
We evaluated a cohort of 277 ACS patients and 42 healthy controls to investigate the relationship between IGF-1 and IGFBP-2 levels and prognosis for short-term outcomes. Our findings revealed that (1) circulating IGF-1 levels in healthy controls were higher than in NSTEMI or STEMI patients, and IGFBP-2 levels showed an opposite pattern; (2) EFS was poor in patients with high levels of IGFBP-2; and (3) high IGFBP-2 levels, but not low IGF-1 levels, independently predicted for MACEs in ACS patients who underwent CAG.
IGF-1 levels were previously found to be lower in acute MI patients compared to healthy controls (24, 25). To date, however, studies evaluating the influence of IGFBP-2 levels on cardiovascular disease remain scarce. In this study, we not only confirmed IGF-1 levels in healthy controls to have been significantly higher than those in acute MI patients, but also found IGFBP-2 levels to have been higher in acute MI patients than those in healthy controls. Levels of IGF-1 and IGFBP-2 were previously reported to associate with EF and potentially serve as biomarkers for HF (9, 26–28). In agreement with previous studies, we found that IGF-1 levels positively associated with EF, whereas IGFBP-2 negatively associated with EF. Although HF incidence was greater in high IGFBP-2 group patients, no differences between groups were found for IGF-1 levels. We noted that high IGFBP-2 group patients had lower triglyceride levels and BMI. Our findings are in agreement with prior literature that reported IGFBP-2 to be a marker of metabolic syndrome and inversely correlate with BMI and triglyceride levels (29–31).
No differences in incidences of MACEs or death were found between low and high IGF-1 group patients in this study. Although no difference in mortality was noted between high and low IGFBP-2 group patients, the incidence of MACEs in high IGFBP-2 group patients was found significantly higher as compared to low IGFBP-2 group patients. Moreover, after adjusting for age and other variables, we found that IGFBP-2 was an independent predictive factor for MACEs, while IGF-1 was not. Iswandi et al. (32) reported that IGF-1 was not an independent predictor of cardiovascular mortality or morbidity in ACS patients over a 5-year follow-up period. Furthermore, Wallander et al. (33) found that IGF-1 levels at hospital admission were not related to cardiovascular death over a three-year follow-up period in patients with type 2 diabetes who suffered acute MI. Although our findings were in agreement with most previously reported, a study by Bourron and et al. (34) reported that low IGF-1 levels not only associate with increased mortality risk but also with risk of any MACEs in acute MI patients over 2 years of follow-up. As various populations may yield disparate findings, future studies should confirm whether IGF-1 is predictive for adverse outcomes in diverse groups.
Increasing evidence has highlighted the cardioprotective effects of IGF-1 in the setting of cardiovascular disease. Numerous in vivo and in vitro studies reported that IGF-1 facilitates resistance to apoptosis in hypoxic conditions (8), stimulates fetal cardiac growth (7), increases production of circulating angiogenic cytokines (35) and exerts positive inotropic and antioxidant effects (36). Translational research has further revealed that treatment with IGF-1 significantly reduces left ventricular volume, attenuates left ventricular mass and improves stroke volume in STEMI patients (37), and improves EF in HF patients (38). However, Conover et al. (39) found that transgenic overexpression of pregnancy-associated plasma protein-A increases IGF-1 activity and results in accelerated atherosclerotic lesion development. Hirai et al. (40) reported that IGF-1 promotes atherosclerosis by affecting endothelial function and increasing aging in rabbits fed a cholesterol-rich diet. As such, the roles of IGF-1 in cardiovascular disease remain unclear and warrant further study.
Previously, IGFBP-2 was reported to inversely correlate with BMI (41). Indeed, studies reported that low IGFBP-2 independently associates with an increased risk of metabolic syndrome as well as elevated fasting glucose levels (30). Higher circulating IGFBP-2 concentrations were also longitudinally associated with lower type 2 diabetes risk (42, 43). Although IGFBP-2 is considered to protect against cardiovascular risk factors, high levels of IGFBP-2 were reported to associate with poor prognoses in several diseases. Prior studies reported that IGFBP-2 independently predicts for adverse clinical outcomes in patients with HF (16, 44), severe aortic stenosis (17), dilated cardiomyopathy (45) and PAH (46). To date, studies about IGFBP-2 in CVD is seldom, and the present study first uncovered that IGFBP-2 is an independent predictor of MACEs in ACS patients. Additionally, although no significant differences in prognosis power among IGFBP-2 and EF were noted, the AUC value of circulating IGFBP-2 was found to be greater than that of EF. Studies enrolling more eligible patients may suggest the prognostic power of IGFBP-2 was superior to EF in ACS patients. The seemingly paradoxical influences of IGFBP-2 on cardiovascular risk factors and pathological processes warrant detailed study.
The potential mechanism of IGFBP-2 and MACE is thought to be multifactorial. IGFBP-2 plays a crucial role in regulating mitogen-activated protein kinase (MAPK) pathway, which is a driver of atherosclerosis and involved in inflammatory signaling and oxidative stress (47, 48). Moreover, IGFBP-2 regulates the phosphatidylinositol 3-kinase (PI3K)/alpha serine/threonine-protein kinase (Akt) signaling pathway, which has a fundamental role in the pathological processes of atherosclerosis (49, 50). In addition, IGFBP-2 enhances the migration and proliferation of vascular smooth muscle cells (VSMC), this process is associated with the development of atherosclerosis (51). Further studies about the underlying mechanisms of IGFBP-2 in CVD are certainly warranted.
Our study, although well-designed, was not without limitations. First, the participants in this study were predominantly male, our sample size was relatively small and the follow-up period was short. As such, sex differences were not investigated, and the low incidence of mortality limited the hard endpoint analysis of the study. In addition, a lack of significant findings concerning IGF-1 and MACEs prediction may have occurred due to a type II statistical error. Second, the mechanisms behind the association between circulating levels of IGF-1 and IGFBP-2 and the incidence of MACE were not elucidated. Third, healthy controls were not well-matched with ACS patients for gender or age. Finally, blood samples were collected at admission and lacked data concerning serial fluctuations in IGF-1 and IGFBP-2 levels during follow-up. Therefore, multi-center studies with a greater sample size and animal studies about potential biological mechanisms of IGF-1 and IGFBP-2 are needed to confirm these results.
Plasma IGFBP-2 levels were higher in patients who suffered acute MI compared to healthy controls. A high IGFBP-2 level, but not a low IGF-1 level, likely has clinical use as a prognostic biomarker for MACEs in patients with ACS. Although the underlying mechanisms for our findings remain unclear, we provide a foundation for further study of IGFBP-2 and improvements in clinical management of patients suffering ACS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Liaocheng People's Hospital. The patients/participants provided their written informed consent to participate in this study.
HY put forward conception and study design. KY, SZ and LH researched data, tested biomarkers. WW wrote the manuscript and contributed to statistical analysis. TL, DM and JL edited and contributed to the manuscript, data interpretation, and discussion. All authors contributed to the article and approved the submitted version.
This work was supported by the Horizontal research project of Shandong University (No. 13450012001901 and No. 23460012711702).
The authors thank the patients, nurses, study coordinators and all investigators involved in this study, without whom the study would not have been possible.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. (2022) 327(7):662–75. doi: 10.1001/jama.2022.0358
2. Collet JP, Thiele H. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation and coexistent atrial fibrillation—dual versus triple antithrombotic therapy. Eur Heart J. (2021) 42(20):2020–1. doi: 10.1093/eurheartj/ehaa909
3. Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. The insulin like growth factor and binding protein family: novel therapeutic targets in obesity & diabetes. Mol Metab. (2019) 19:86–96. doi: 10.1016/j.molmet.2018.10.008
4. Higashi Y, Gautam S, Delafontaine P, Sukhanov S. IGF-1 and cardiovascular disease. Growth Horm IGF Res. (2019) 45:6–16. doi: 10.1016/j.ghir.2019.01.002
5. Higashi Y, Sukhanov S, Shai SY, Danchuk S, Tang R, Snarski P, et al. Insulin-Like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation. (2016) 133(23):2263–78. doi: 10.1161/CIRCULATIONAHA.116.021805
6. Ock S, Ham W, Kang CW, Kang H, Lee WS, Kim J. IGF-1 protects against angiotensin II-induced cardiac fibrosis by targeting alphaSMA. Cell Death Dis. (2021) 12(7):688. doi: 10.1038/s41419-021-03965-5
7. Jonker SS, Giraud GD, Chang EI, Elman MR, Louey S. Coronary vascular growth matches IGF-1-stimulated cardiac growth in fetal sheep. FASEB J. (2020) 34(8):10041–55. doi: 10.1096/fj.202000215R
8. Lin M, Liu X, Zheng H, Huang X, Wu Y, Huang A, et al. IGF-1 enhances BMSC viability, migration, and anti-apoptosis in myocardial infarction via secreted frizzled-related protein 2 pathway. Stem Cell Res Ther. (2020) 11(1):22. doi: 10.1186/s13287-019-1544-y
9. Barroso MC, Kramer F, Greene SJ, Scheyer D, Kohler T, Karoff M, et al. Serum insulin-like growth factor-1 and its binding protein-7: potential novel biomarkers for heart failure with preserved ejection fraction. BMC Cardiovasc Disord. (2016) 16(1):199. doi: 10.1186/s12872-016-0376-2
10. De Giorgi A, Marra AM, Iacoviello M, Triggiani V, Rengo G, Cacciatore F, et al. Insulin-like growth factor-1 (IGF-1) as predictor of cardiovascular mortality in heart failure patients: data from the T.O.S.CA. Registry. Intern Emerg Med. (2022) 17(6):1651–60. doi: 10.1007/s11739-022-02980-4
11. Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol Metab. (2016) 27(6):375–91. doi: 10.1016/j.tem.2016.03.019
12. Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. (2002) 23(6):824–54. doi: 10.1210/er.2001-0033
13. Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. (2007) 56(2):285–94. doi: 10.2337/db06-0436
14. Sun L, Zhang X, Song Q, Liu L, Forbes E, Tian W, et al. IGFBP2 Promotes tumor progression by inducing alternative polarization of macrophages in pancreatic ductal adenocarcinoma through the STAT3 pathway. Cancer Lett. (2021) 500:132–46. doi: 10.1016/j.canlet.2020.12.008
15. Yang J, Griffiths M, Nies MK, Brandal S, Damico R, Vaidya D, et al. Insulin-like growth factor binding protein-2: a new circulating indicator of pulmonary arterial hypertension severity and survival. BMC Med. (2020) 18(1):268. doi: 10.1186/s12916-020-01734-3
16. Barutaut M, Fournier P, Peacock WF, Evaristi MF, Caubere C, Turkieh A, et al. Insulin-like growth factor binding protein 2 predicts mortality risk in heart failure. Int J Cardiol. (2020) 300:245–51. doi: 10.1016/j.ijcard.2019.09.032
17. Muessig JM, Lichtenauer M, Wernly B, Kelm M, Franz M, Baz L, et al. Insulin like growth factor binding protein 2 (IGFBP-2) for risk prediction in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI). Int J Cardiol. (2019) 277:54–9. doi: 10.1016/j.ijcard.2018.09.091
18. van den Beld AW, Blum WF, Brugts MP, Janssen JA, Grobbee DE, Lamberts SW. High IGFBP2 levels are not only associated with a better metabolic risk profile but also with increased mortality in elderly men. Eur J Endocrinol. (2012) 167(1):111–7. doi: 10.1530/EJE-12-0160
19. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. (2014) 64(24):e139–228. doi: 10.1016/j.jacc.2014.09.017
20. Jneid H, Addison D, Bhatt DL, Fonarow GC, Gokak S, Grady KL, et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on performance measures. J Am Coll Cardiol. (2017) 70(16):2048–90. doi: 10.1016/j.jacc.2017.06.032
21. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40(2):87–165. doi: 10.1093/eurheartj/ehy394
22. Su J, Li Z, Huang M, Wang Y, Yang T, Ma M, et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. (2022) 21(1):96. doi: 10.1186/s12933-022-01523-7
23. Gaba P, Gersh BJ, Ali ZA, Moses JW, Stone GW. Complete versus incomplete coronary revascularization: definitions, assessment and outcomes. Nat Rev Cardiol. (2021) 18(3):155–68. doi: 10.1038/s41569-020-00457-5
24. Conti E, Andreotti F, Sciahbasi A, Riccardi P, Marra G, Menini E, et al. Markedly reduced insulin-like growth factor-1 in the acute phase of myocardial infarction. J Am Coll Cardiol. (2001) 38(1):26–32. doi: 10.1016/S0735-1097(01)01367-5
25. Hajsadeghi S, Mohseni H, Moradi M, Rahmani E, Kordshakeri K, Manteghi MJ, et al. Evaluating the association between insulin-like growth factor-1 values and short-term survival rates following acute myocardial infarction. Clin Med Insights Cardiol. (2011) 5:7–11. doi: 10.4137/CMC.S6629
26. Bruno C, Silvestrini A, Calarco R, Favuzzi AMR, Vergani E, Nicolazzi MA, et al. Anabolic hormones deficiencies in heart failure with preserved ejection fraction: prevalence and impact on antioxidants levels and myocardial dysfunction. Front Endocrinol (Lausanne). (2020) 11:281. doi: 10.3389/fendo.2020.00281
27. D'Assante R, Arcopinto M, Rengo G, Salzano A, Walser M, Gambino G, et al. Myocardial expression of somatotropic axis, adrenergic signalling, and calcium handling genes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. ESC Heart Fail. (2021) 8(2):1681–6. doi: 10.1002/ehf2.13067
28. Prelevic V, Antunovic T, Radunovic D, Gligorovic Barhanovic N, Gledovic B, Bulatovic N, et al. Decreased insulin-like growth factor-1 (IGF-1) concentration correlates with reduced left-ventricle ejection fraction (LVEF) in hemodialysis patients. Int Urol Nephrol. (2020) 52(12):2385–91. doi: 10.1007/s11255-020-02595-8
29. Carter S, Li Z, Lemieux I, Almeras N, Tremblay A, Bergeron J, et al. Circulating IGFBP-2 levels are incrementally linked to correlates of the metabolic syndrome and independently associated with VLDL triglycerides. Atherosclerosis. (2014) 237(2):645–51. doi: 10.1016/j.atherosclerosis.2014.09.022
30. Heald AH, Kaushal K, Siddals KW, Rudenski AS, Anderson SG, Gibson JM. Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp Clin Endocrinol Diabetes. (2006) 114(7):371–6. doi: 10.1055/s-2006-924320
31. van den Beld AW, Carlson OD, Doyle ME, Rizopoulos D, Ferrucci L, van der Lely AJ, et al. IGFBP-2 and aging: a 20-year longitudinal study on IGFBP-2, IGF-I, BMI, insulin sensitivity and mortality in an aging population. Eur J Endocrinol. (2019) 180(2):109–16. doi: 10.1530/EJE-18-0422
32. Iswandi CP, van den Berg VJ, Simsek S, Velzen DV, Boekel ET, Cornel JH, et al. IGF-1 is not related to long-term outcome in hyperglycemic acute coronary syndrome patients. Diab Vasc Dis Res. (2021) 18(6):14791641211047436. doi: 10.1177/14791641211047436
33. Wallander M, Norhammar A, Malmberg K, Ohrvik J, Ryden L, Brismar K. IGF Binding protein 1 predicts cardiovascular morbidity and mortality in patients with acute myocardial infarction and type 2 diabetes. Diabetes Care. (2007) 30(9):2343–8. doi: 10.2337/dc07-0825
34. Bourron O, Le Bouc Y, Berard L, Kotti S, Brunel N, Ritz B, et al. Impact of age-adjusted insulin-like growth factor 1 on major cardiovascular events after acute myocardial infarction: results from the fast-MI registry. J Clin Endocrinol Metab. (2015) 100(5):1879–86. doi: 10.1210/jc.2014-3968
35. Haleagrahara N, Chakravarthi S, Mathews L. Insulin like growth factor-1 (IGF-1) causes overproduction of IL-8, an angiogenic cytokine and stimulates neovascularization in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci. (2011) 12(12):8562–74. doi: 10.3390/ijms12128562
36. Yeves AM, Burgos JI, Medina AJ, Villa-Abrille MC, Ennis IL. Cardioprotective role of IGF-1 in the hypertrophied myocardium of the spontaneously hypertensive rats: a key effect on NHE-1 activity. Acta Physiol (Oxf). (2018) 224(2):e13092. doi: 10.1111/apha.13092
37. Caplice NM, DeVoe MC, Choi J, Dahly D, Murphy T, Spitzer E, et al. Randomized placebo controlled trial evaluating the safety and efficacy of single low-dose intracoronary insulin-like growth factor following percutaneous coronary intervention in acute myocardial infarction (RESUS-AMI). Am Heart J. (2018) 200:110–7. doi: 10.1016/j.ahj.2018.03.018
38. Osterziel KJ, Blum WF, Strohm O, Dietz R. The severity of chronic heart failure due to coronary artery disease predicts the endocrine effects of short-term growth hormone administration. J Clin Endocrinol Metab. (2000) 85(4):1533–9. doi: 10.1210/jcem.85.4.6575
39. Conover CA, Bale LK, Harrington SC, Resch ZT, Overgaard MT, Oxvig C. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. Am J Physiol Cell Physiol. (2006) 290(1):C183–8. doi: 10.1152/ajpcell.00199.2005
40. Hirai H, Kanaya R, Maeda M, Qungfang D, Ina K, Hayashi T. The role of insulin growth factor on atherosclerosis and endothelial function: the effect on hyperlipidemia and aging. Life Sci. (2011) 88(9-10):425–31. doi: 10.1016/j.lfs.2010.12.021
41. Crowe FL, Key TJ, Allen NE, Appleby PN, Overvad K, Gronbaek H, et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European prospective investigation into cancer and nutrition (EPIC). Ann Hum Biol. (2011) 38(2):194–202. doi: 10.3109/03014460.2010.507221
42. Lappas M, Jinks D, Shub A, Willcox JC, Georgiou HM, Permezel M. Postpartum IGF-I and IGFBP-2 levels are prospectively associated with the development of type 2 diabetes in women with previous gestational diabetes mellitus. Diabetes Metab. (2016) 42(6):442–7. doi: 10.1016/j.diabet.2016.06.004
43. Wittenbecher C, Ouni M, Kuxhaus O, Jahnert M, Gottmann P, Teichmann A, et al. Insulin-like growth factor binding protein 2 (IGFBP-2) and the risk of developing type 2 diabetes. Diabetes. (2019) 68(1):188–97. doi: 10.2337/db18-0620
44. Brankovic M, Akkerhuis KM, Mouthaan H, Brugts JJ, Manintveld OC, van Ramshorst J, et al. Cardiometabolic biomarkers and their temporal patterns predict poor outcome in chronic heart failure (bio-SHiFT study). J Clin Endocrinol Metab. (2018) 103(11):3954–64. doi: 10.1210/jc.2018-01241
45. Hassfeld S, Eichhorn C, Stehr K, Naegele H, Geier C, Steeg M, et al. Insulin-like growth factor-binding proteins 2 and 3 are independent predictors of a poor prognosis in patients with dilated cardiomyopathy. Heart. (2007) 93(3):359–60. doi: 10.1136/hrt.2006.090092
46. Griffiths M, Yang J, Nies M, Vaidya D, Brandal S, Williams M, et al. Pediatric pulmonary hypertension: insulin-like growth factor-binding protein 2 is a novel marker associated with disease severity and survival. Pediatr Res. (2020) 88(6):850–6. doi: 10.1038/s41390-020-01113-x
47. Chakrabarty S, Kondratick L. Insulin-like growth factor binding protein-2 stimulates proliferation and activates multiple cascades of the mitogen-activated protein kinase pathways in NIH-OVCAR3 human epithelial ovarian cancer cells. Cancer Biol Ther. (2006) 5(2):189–97. doi: 10.4161/cbt.5.2.2333
48. Reustle A, Torzewski M. Role of p38 MAPK in atherosclerosis and aortic valve sclerosis. Int J Mol Sci. (2018) 19(12):3761. doi: 10.3390/ijms19123761
49. Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. (2007) 104(13):5563–8. doi: 10.1073/pnas.0609139104
50. Ghafouri-Fard S, Khanbabapour Sasi A, Hussen BM, Shoorei H, Siddiq A, Taheri M, et al. Interplay between PI3K/AKT pathway and heart disorders. Mol Biol Rep. (2022) 49(10):9767–81. doi: 10.1007/s11033-022-07468-0
51. Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase beta and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol. (2012) 32(20):4116–30. doi: 10.1128/MCB.01011-12
Keywords: acute coronary syndrome, insulin-like growth factor-1, insulin-like growth factor binding protein 2, cardiovascular prognosis, IGF-1, IGFBP-2
Citation: Wang W, Yu K, Zhao S, Mo D, Liu J, Han L, Li T and Yao H (2023) The impact of circulating IGF-1 and IGFBP-2 on cardiovascular prognosis in patients with acute coronary syndrome. Front. Cardiovasc. Med. 10:1126093. doi: 10.3389/fcvm.2023.1126093
Received: 17 December 2022; Accepted: 22 February 2023;
Published: 10 March 2023.
Edited by:
Yuli Huang, Southern Medical University, ChinaReviewed by:
Oriol Iborra Egea, Germans Trias i Pujol Health Science Research Institute (IGTP), Spain© 2023 Wang, Yu, Zhao, Mo, Liu, Han, Li and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heng-Chen Yao eWFvaGM2NkBob3RtYWlsLmNvbQ==
Specialty Section: This article was submitted to General Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.