
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 25 April 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1124276
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2022 View all 39 articles
Background: Recently, the survival rate of patients with cancer has improved annually due to advancements in cancer diagnosis and treatment technologies. Meanwhile, late-onset complications associated with cancer treatment significantly affect survival and quality of life. However, different from pediatric cancer survivors, there is no unified view on the follow-up of late complications in elderly cancer survivors. We reported a case of congestive heart failure as a late-onset complication of doxorubicin (DXR) in an elderly cancer survivor.
Case report: The patient is an 80-year-old woman with hypertension and chronic renal failure. She received six cycles of chemotherapy for Hodgkin's lymphoma that started in January 201X-2. The total dose of DXR was 300 mg/m2, and a transthoracic echocardiogram (TTE) performed in October 201X-2, showed good left ventricular wall motion (LVWM). In April 201X, she suddenly developed dyspnea. Upon arrival at the hospital, a physical examination revealed orthopnea, tachycardia, and leg edema. A chest radiograph showed cardiac enlargement and pleural effusion. A TTE showed diffusely reduced LVWM and a left ventricular ejection fraction in the 20% range. After close examination, the patient was diagnosed with congestive heart failure due to late-onset DXR-induced cardiomyopathy.
Conclusion: Late-onset DXR-induced cardiotoxicity is considered high-risk from 250 mg/m2 or higher. Elderly cancer survivors are at higher risk of cardiotoxicity than non-elderly cancer survivors and may require closer follow-up.
Recently, advancements in cancer diagnosis and treatment techniques have improved the survival rate of patients with cancer, including those with Hodgkin's lymphoma, annually (1, 2). On the other hand, late-onset complications associated with cancer treatment significantly affect survival and quality of life (3). In particular, doxorubicin (DXR) cardiotoxicity, which affects survival, is speculated to impair DXR-induced mitochondrial function, ultimately leading to cardiomyocyte death, and correlates with cumulative DXR dosage (4). The incidence of DXR-induced congestive heart failure is as high as 16% when the cumulative dose of DXR exceeds 500 mg/m2 and is a critical problem for patients receiving DXR (5). Therefore, the Japanese DXR's package insert includes a dose limit of up to 500 mg/m2 of cumulative dosage. However, since a history of radiation therapy to the heart and long-term survival after DXR administration are risks for the development of DXR cardiotoxicity, the European Society of Cardiology guidelines recommend risk-based monitoring such as echocardiography and troponin I (TnI) for pediatric cancer survivors with risk factors, even if the cumulative dose of DXR is lower than 500 mg/m2 (6–8). Furthermore, the efficacy of dexrazoxane as prophylaxis for DXR cardiotoxicity has been reported, but effective treatment for DXR cardiotoxicity remains unclear (9, 10). Thus, late-onset complications are considered a problem in oncology for children, adolescents and young adults, and adults, and guidelines have been developed for cancer survivors, including methods for long-term follow-up (6, 11–14). However, there is no unified view on late complications in elderly cancer survivors, who are at risk of cardiac complications, and most of the survivors remain unrecognized. In this study, we reported a case of congestive heart failure as a late-onset complication of DXR in an elderly Hodgkin's lymphoma survivor.
The patient was an 80-year-old woman with coexisting hypertension, dyslipidemia, and chronic renal failure (CRF). She routinely took enalapril 5 mg once per day and atorvastatin 10 mg once per day. She had no smoking history and no family history of cardiac disease. A transthoracic echocardiogram (TTE) performed in December 201X-3, showed a left ventricular (LV) ejection fraction (LVEF) of 63.4%. She received six cycles of DXR, bleomycin, vinblastine, and dacarbazine (ABVD) for Hodgkin's lymphoma (Lugano classification, stage III) that started in January 201X-2. The total dose of DXR was 300 mg/m2, and a TTE performed on October, 201X-2, showed good LV wall motion (LVEF, 65.4%) and no evidence of heart failure. Subsequently, the patient did not experience any recurrence of Hodgkin's lymphoma. In April 201X, the patient suddenly developed dyspnea and was rushed to the emergency room. Upon arrival at the hospital, a physical examination revealed the following: body temperature, 36.9°C; heart rate, 144 beats/min; blood pressure, 160/114 mmHg; respiratory rate, 25 breaths/minute; and oxygen saturation, 98% (under 5 L of oxygen administration). The patient's consciousness was also impaired [Glasgow Coma Scale score, 13 (E3V4M6); Japan Coma Scale score, 10], and her eyelid and conjunctiva were pale. The patient had no heart murmur and no chest pain, but she had mild wheezing, orthopnea, and bilateral leg pitting edema. Blood test results (Table 1) revealed markedly elevated B-type natriuretic peptide (BNP) (1,155 pg/ml) level and slightly increased TnI level (0.08 ng/ml). The patient's hemoglobin (Hb) A1c, free thyroxine, and D-dimer levels were within the normal ranges. The patient also showed decreased Hb level (8.7 g/dl) and increased serum creatinine level (1.48 mg/dl), but these two values had not changed over time for more than a year. An electrocardiogram (ECG) showed sinus tachycardia (Supplementary Figure S1). A chest radiograph showed an enlarged heart and pleural effusion. A TTE showed a diffusely reduced LV wall motion and an LVEF of 21.6% (Figure 1). The patient was diagnosed with clinical scenario 1 acute heart failure (15) and underwent close examination and treatment in the intensive care unit. Thallium-201 myocardial perfusion scintigraphy showed transient ischemic dilatation and old myocardial infarction (OMI) of the left circumflex artery. LV perfusion, LV wall motion, and LV end-diastolic volume results were consistent with tachycardia-induced cardiomyopathy (Figure 2, Supplementary Figure S2). No chest symptoms or significant ECG changes appeared at the time of loading. Since the patient had no evidence of progressive anemia, exacerbation of chronic renal failure, arrhythmia, or acute coronary syndrome and the cumulative dose of DXR was 300 mg/m2, the patient was diagnosed with congestive heart failure due to late-onset DXR-induced cardiomyopathy.
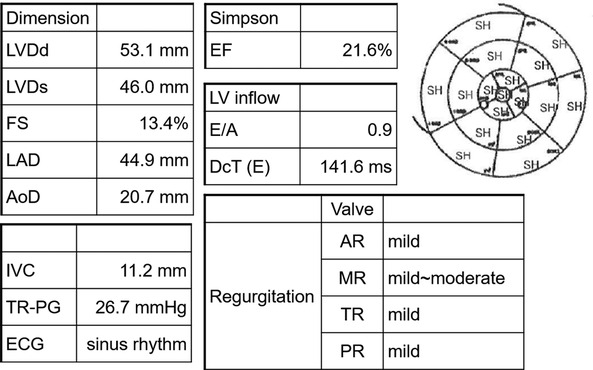
Figure 1. Transthoracic echocardiogram findings at the emergency department visit. LVDd, left ventricular internal dimension in diastole; LVDs, left ventricular internal dimensions in systole; FS, fractional shortening; LAD, left atrial dimension; AoD, aortic root diameter; IVC, inferior vena cava; TR-PG, tricuspid regurgitation peak gradient; ECG, electrocardiogram; EF, ejection fraction; LV, left ventricle; E/A, early-to-late transmitral velocity; DcT, deceleration time; AR, aortic regurgitation; MR, mitral regurgitation; TR, tricuspid regurgitation; PR, pulmonary regurgitation; SH, severe hypokinesis.
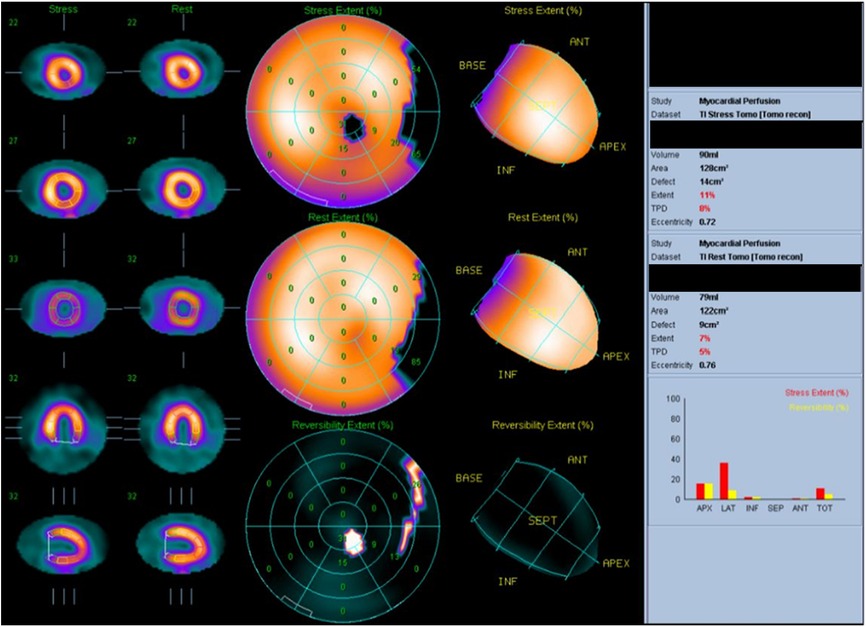
Figure 2. Thallium-201 myocardial perfusion: transient ischemic dilatation and old myocardial infarction of the left circumflex artery.
We administered furosemide (1 mg/h) and nitroglycerin (2 mg/h) in the hyperacute phase and added bisoprolol (2.5 mg/day) in the early phase. After the patient's congestive heart failure improved, we continued bisoprolol and added spironolactone (50 mg/day) and azosemide (60 mg/day). After treatment, symptoms, such as leg edema, tachycardia, and orthopnea, disappeared, BNP level decreased to 75.7 pg/ml, and the patient's LVEF improved to 51.2% on TTE in September 201X (Figure 3).
We presented a case of an elderly cancer survivor with late-onset DXR-induced heart failure, whose cardiac function improved with therapeutic intervention.
DXR is a key drug in Hodgkin's and non-Hodgkin's lymphomas and breast and endometrial cancers (16–19). A previous study demonstrated that the 6-year overall survival rate for previously untreated patients with stage III or IV classic Hodgkin lymphoma was 89.4% in the ABVD group (16). Another study reported a 5-year survival rate of 86%–90% for adjuvant chemotherapy containing DXR for recurrent high-risk breast cancer (18).
Pediatric cancer survivors who receive anthracyclines, including DXR, have a persistent risk of developing cardiovascular disease over time. However, the risk of cancer recurrence decreases over time (20, 21). This trend is also evident in adult patients with Hodgkin's lymphoma and breast cancer (22). Patients with breast cancer, even the elderly, reported more deaths from cardiovascular disease than breast cancer 10 years after diagnosis (23).
One potential mechanism for this persistent DXR cardiotoxicity is that DXR accumulates in the mitochondria of cardiomyocytes (24). Accumulated DXR increases the concentration of iron in the mitochondria and produces reactive oxygen species (ROS). ROS may cause myocardial damage, which is a possible reason why the iron chelator dexrazoxane is effective in protecting against DXR-induced myocardial damage (9, 25). Furthermore, topoisomerase IIβ expressed in the myocardium is a key molecular mediator of DXR-induced cardiotoxicity and is thought to impair myocardial repair and irreversibly reduce cardiac function (26–28). Other mechanisms associated with DXR cardiotoxicity include calcium hemostasis imbalance inducing DNA damage and disruption of the neuroglial/ErbB signaling pathway resulting in apoptosis and mitochondrial dysfunction (4). There were recent reports of an association between cardiac syndrome X and DXR-induced endothelial cell damage (29). DXR-induced endothelial cell damage triggers the onset and progression of cardiomyopathy by reducing the release and activity of critical endothelial factors and inducing endothelial cell death. That may be an important mechanism for microvascular angina development in young patients without risk factors or comorbid conditions. The present case was out of the characteristics of DXR-induced cardiac syndrome X due to the presence of OMI, hypertension, and CRF, as well as the patient's advanced age.
DXR-induced cardiotoxicity is dose-dependent, with LV dysfunction occurring in 3%–5% of patients at a cumulative dose of 400 mg/m2 compared to 7%–26% at a dose of 550 mg/m2 (28, 30). Therefore, the Japanese package insert states that the cumulative dose of DXR should be up to 500 mg/m2, but for late cardiotoxicity, DXR has a higher risk starting at 250 mg/m2 or higher (6, 31). The risk of cardiovascular disease would be higher in DXR-treated patients aged over 65 years compared to those aged under 65 years (5, 6). In addition, the average life expectancies for Japanese women are 15.6 years at age 75 years and 11.4 years at age 80 years, and follow-up for late complications is necessary even in elderly patients (32). Therefore, elderly patients receiving DXR should be followed up more closely than non-elderly patients, paying attention to late complications. The patient in this case report also received ABVD regimen for Hodgkin's lymphoma at age 78 years, with a cumulative dose of 300 mg/m2. Therefore, the risk of late-onset DXR-induced heart failure is high, and close follow-up is necessary.
Although DXR-induced cardiotoxicity is generally considered irreversible, there have been reports of improved cardiac function with early therapeutic interventions with β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics (28, 33–35). In the present case, early diuretic and β-blockers administration improved cardiac function with the improvement of sinus tachycardia and reduction of cardiothoracic ratio associated with congestive heart failure (Figure 3, Supplementary Figure S1). Thus, the early diagnosis and treatment of late-onset DXR-induced heart failure may have effectively improved patients' outcomes.
Recently, there have been reports on the prediction of the risk of developing DXR-induced cardiovascular disease based on genes and the value of global longitudinal strain and TnI for the early detection of DXR-induced cardiotoxicity (36, 37).
In conclusion, elderly cancer survivors with an estimated life expectancy of 5–10 years or more are at higher risk of cardiovascular disease and other late complications than non-elderly cancer survivors. Therefore, elderly cancer survivors with a history of DXR treatment may benefit from closer follow-up with BNP and TnI testing and early detection and treatment of DXR-induced heart failure.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: HS, MS, and AO. Methodology: HS and MS. Investigation: HS, MS, YI, and AO. Data curation: HS, MS, YI, and AO. Writing—original draft preparation: HS. Writing—review and editing: HS, MS, YI, and AO. Supervision: MS and AO. All authors contributed to the article and approved the submitted version.
The authors would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1124276/full#supplementary-material.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Welch HG, Kramer BS, Black WC. Epidemiologic signatures in cancer. N Engl J Med. (2019) 381:1378–86. doi: 10.1056/NEJMsr1905447
3. Kadowaki H, Akazawa H, Ishida J, Komuro I. Cancer therapeutics-related cardiac dysfunction—insights from bench and bedside of onco-cardiology. Circ J. (2020) 84:1446–53. doi: 10.1253/circj.CJ-20-0467
4. Sheibani M, Azizi Y, Shayan M, Nezamoleslami S, Eslami F, Farjoo MH, et al. Doxorubicin-induced cardiotoxicity: an overview on pre-clinical therapeutic approaches. Cardiovasc Toxicol. (2022) 22:292–310. doi: 10.1007/s12012-022-09721-1
5. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. (2003) 97:2869–79. doi: 10.1002/cncr.11407
6. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 Esc guidelines on cardio-oncology developed in collaboration with the European hematology association (eha), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
7. Bates JE, Howell RM, Liu Q, Yasui Y, Mulrooney DA, Dhakal S, et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the childhood cancer survivor study. J Clin Oncol. (2019) 37:1090–101. doi: 10.1200/JCO.18.01764
8. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. (2005) 23:2629–36. doi: 10.1200/JCO.2005.12.121
9. Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. (2004) 351:145–53. doi: 10.1056/NEJMoa035153
10. Chow EJ, Aggarwal S, Doody DR, Aplenc R, Armenian SH, Baker KS, et al. Dexrazoxane and long-term heart function in survivors of childhood cancer. J Clin Oncol. (2023):Jco2202423. doi: 10.1200/JCO.22.02423
11. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2014) 27:911–39. doi: 10.1016/j.echo.2014.07.012
12. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. (2017) 35:893–911. doi: 10.1200/JCO.2016.70.5400
13. Armenian SH, Armstrong GT, Aune G, Chow EJ, Ehrhardt MJ, Ky B, et al. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. (2018) 36:2135–44. doi: 10.1200/JCO.2017.76.3920
14. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: esmo consensus recommendations. Ann Oncol. (2020) 31:171–90. doi: 10.1016/j.annonc.2019.10.023
15. Mebazaa A, Gheorghiade M, Piña IL, Harjola VP, Hollenberg SM, Follath F, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med. (2008) 36(1):S129–39. doi: 10.1097/01.CCM.0000296274.51933.4C
16. Ansell SM, Radford J, Connors JM, Długosz-Danecka M, Kim WS, Gallamini A, et al. Overall survival with brentuximab vedotin in stage III or IV hodgkin’s lymphoma. N Engl J Med. (2022) 387:310–20. doi: 10.1056/NEJMoa2206125
17. Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. (2022) 386:351–63. doi: 10.1056/NEJMoa2115304
18. Miller KD, O’Neill A, Gradishar W, Hobday TJ, Goldstein LJ, Mayer IA, et al. Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer (E5103). J Clin Oncol. (2018) 36:2621–9. doi: 10.1200/JCO.2018.79.2028
19. Nomura H, Aoki D, Michimae H, Mizuno M, Nakai H, Arai M, et al. Effect of taxane plus platinum regimens vs doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at a high risk of progression: a randomized clinical trial. JAMA Oncol. (2019) 5:833–40. doi: 10.1001/jamaoncol.2019.0001
20. van der Pal HJ, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. (2012) 30:1429–37. doi: 10.1200/JCO.2010.33.4730
21. Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. (2014) 32:1218–27. doi: 10.1200/JCO.2013.51.1055
22. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. (2019) 40:3889–97. doi: 10.1093/eurheartj/ehz766
23. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. (2011) 13:R64. doi: 10.1186/bcr2901
24. Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. (2014) 124:617–30. doi: 10.1172/JCI72931
25. Jones RL, Wagner AJ, Kawai A, Tamura K, Shahir A, Van Tine BA, et al. Prospective evaluation of doxorubicin cardiotoxicity in patients with advanced soft-tissue sarcoma treated in the announce phase III randomized trial. Clin Cancer Res. (2021) 27:3861–6. doi: 10.1158/1078-0432.CCR-20-4592
26. Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. (2012) 18:1639–42. doi: 10.1038/nm.2919
27. Vejpongsa P, Yeh ET. Topoisomerase 2β: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther. (2014) 95:45–52. doi: 10.1038/clpt.2013.201
28. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. (2015) 12:547–58. doi: 10.1038/nrcardio.2015.65
29. Avagimyan A, Mkrtchyan L, Abrahomovich O, Sheibani M, Guevorkyan A, Sarrafzadegan N, et al. Ac-mode of chemotherapy as a trigger of cardiac syndrome x: a case study. Curr Probl Cardiol. (2022) 47:100994. doi: 10.1016/j.cpcardiol.2021.100994
30. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 Esc position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the esc committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European society of cardiology (esc). Eur J Heart Fail. (2017) 19:9–42. doi: 10.1002/ejhf.654
31. Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the international late effects of childhood cancer guideline harmonization group. Lancet Oncol. (2015) 16:e123–36. doi: 10.1016/S1470-2045(14)70409-7
32. Iwamoto M, Nakamura F, Higashi T. Estimated life expectancy and risk of death from cancer by quartiles in the older Japanese population: 2010 vital statistics. Cancer Epidemiol. (2014) 38:511–4. doi: 10.1016/j.canep.2014.07.005
33. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. (2010) 55:213–20. doi: 10.1016/j.jacc.2009.03.095
34. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. (2015) 131:1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777
35. Ohtani K, Fujino T, Ide T, Funakoshi K, Sakamoto I, Hiasa KI, et al. Recovery from left ventricular dysfunction was associated with the early introduction of heart failure medical treatment in cancer patients with anthracycline-induced cardiotoxicity. Clin Res Cardiol. (2019) 108:600–11. doi: 10.1007/s00392-018-1386-0
36. Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dubé MP, Al-Saloos H, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. (2012) 30:1422–8. doi: 10.1200/JCO.2010.34.3467
Keywords: doxorubicin, cardiotoxicity, onco-cardiology, cancer survivor, elderly, troponin I, global longitudinal strain
Citation: Suto H, Suto M, Inui Y and Okamura A (2023) Late-onset doxorubicin-induced congestive heart failure in an elderly cancer survivor: A case report. Front. Cardiovasc. Med. 10:1124276. doi: 10.3389/fcvm.2023.1124276
Received: 15 December 2022; Accepted: 11 April 2023;
Published: 25 April 2023.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Mohammad Sheibani, Iran University of Medical Sciences, Iran© 2023 Suto, Suto, Inui and Okamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hirotaka Suto aGlyb3Rha2Euc3V0b0BqZmNyLm9yLmpw Makiko Suto bWFraTExMjlAaG90bWFpbC5jby5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.