- 1Center for Reproductive Medicine, Department of Pediatrics, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
- 2Department of Clinical Laboratory Medicine, Sir Run Run Shaw Hospital Xiasha Campus, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Neurosurgery, People’s Hospital of Pan’an County, Jinhua, China
- 4Center for General Practice Medicine, Department of General Practice Medicine, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
Background: In recent years, red cell distribution width (RDW) has been found to be associated with the prognosis of patients with heart failure (HF) in Western countries. However, evidence from Asia is limited. We aimed to investigate the relationship between RDW and the risk of 3-month readmission in hospitalized Chinese HF patients.
Methods: We retrospectively analyzed HF data from the Fourth Hospital of Zigong, Sichuan, China, involving 1,978 patients admitted for HF between December 2016 and June 2019. The independent variable in our study was RDW, and the endpoint was the risk of readmission within 3 months. This study mainly used a multivariable Cox proportional hazards regression analysis. Smoothed curve fitting was then used to assess the dose-response relationship between RDW and the risk of 3-month readmission.
Results: In the original cohort of 1,978 patients with HF (42% male and 73.1% aged ≥70 years), 495 patients (25.0%) were readmitted within 3 months after discharge. Smoothed curve fitting showed a linear correlation between RDW and the risk of readmission within 3 months. In the multivariable-adjusted model, every 1% increase in RDW was associated with a 9% increased risk of readmission within 3 months (hazard ratio = 1.09, 95% confidence interval: 1.00–1.15; P < 0.005).
Conclusions: A higher RDW value was significantly associated with a greater risk of 3-months readmission in hospitalized patients with HF.
1. Introduction
Heart failure (HF) is one of the major global public health issues and a common cause of hospitalization in middle-aged and older adults (1). Traditional and innovative therapies have been used to treat and manage HF, leading to a significant reduction in HF mortality (2, 3). However, the high rate of readmission of HF patients within 3 months of discharge is an emerging problem that can cause a clinical and economic burden. Readmission of HF patients within 3 months after discharge may be seen as a warning sign of a worse subsequent prognosis for the patient. A study from Japan concluded that patients with HF who were readmitted within 3 months had a higher risk of death at long-term follow-up (4); therefore, early identification of this group of patients with HF could optimize treatment more quickly, improve symptoms, increase patient survival, reduce readmission rates, and reduce the economic burden on families and society.
Several variables have been associated with readmission in patients with HF. Apostolos et al. conducted a propensity score-matched observational study that included HF patients with ejection fraction use from the U.S. Medicare-related OPTIMIZE-HF registry from March 2003 to December 2004 and found that systolic blood pressure (SBP) <120 mmHg at discharge was associated with an increased risk of readmission in these patients (5). However, this study included only patients with HF with an ejection fraction of ≥50%; therefore, it is not possible to infer whether an SBP of <120 mmHg at discharge is associated with an increased risk of readmission in all HF patients. Ioanna et al. also studied 671 HF patients from the OPTIMIZE-HF registry (6). They found that psychosocial factors were associated with 1-year readmission risk in HF patients. Still, their study sample size was small, and the questionnaire for assessing psychosocial factors was too cumbersome and complex for clinical replication. Therefore, we need to find an earlier and easier indicator to detect the risk of readmission and better manage this group of inpatients with HF.
Red cell distribution width (RDW), an indicator of changes in red blood cell (RBC) size and shape, is easily obtained from a complete blood count and is a simple, inexpensive measure (7). The RDW typically ranges from 11% to 15%, and a higher RDW value indicates greater variability in size (8). Traditionally, RDW has been used to differentiate the cause of anemia (9). Recent clinical evidence suggests that changes in RBC size are associated with the development and adverse outcomes of non-hematologic diseases, such as stroke, osteoporosis, dementia, and cardiovascular disease (10–13). Especially in patients with HF, RDW has been shown to be associated with first hospitalization and poor prognosis in HF (14, 15). However, most of the available evidence is focused on the United States and Europe, and there are few studies related to Asia, especially China.
Thus, this study aimed to investigate the relationship between RDW and the risk of 3-month readmission in hospitalized Chinese HF patients.
2. Materials and methods
2.1. Study population
This study used a single-center database included in PhysioNet (16). This dataset collected information on a total of 2,008 adult HF patients from December 2016 to June 2019 at the Fourth People's Hospital in Zigong, Sichuan, China, to understand the characteristics of the Chinese HF population (17). The data collected in this dataset included demographic data, baseline clinical characteristics, co-morbidities, laboratory test results, medications, and outcomes. This study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement and was designed to investigate whether elevated RDW is correlated with an increased risk of rehospitalization. The Ethics Committee of the Fourth People's Hospital of Zigong approved this study (approval number:2020-010). The requirement for informed consent was waived owing to the retrospective design of this study. This study was conducted in strict accordance with the Declaration of Helsinki. HF was defined according to the criteria of the European Society of Cardiology (18).
2.2. Study variables
In this study, the exposure variable was RDW, and the primary outcome variable was the risk of readmission within 3 months. We also collected additional data from the database, including age, sex, Charlson Comorbidity Index (CCI) score, New York Heart Association (NYHA) cardiac function classification, type of HF (right, left, both), systolic blood pressure, diastolic blood pressure, body mass index, brain natriuretic peptide, white blood cell count, RBC count, hematocrit, hemoglobin, mean cell volume, mean cell hemoglbin content, mean cell hemoglobin concentration, platelet, total protein, albumin, alanine aminotransferase, aspartate transaminase, lactate dehydrogenase, alkaline phosphatase, γ-glutamyltranspeptidase, and estimated glomerular filtration rate (eGFR, ml/min per 1.73 m2)).
2.3. Statistical analysis
Categorical variables were analyzed using percentages, and normally continuous variables were expressed as mean ± standard deviation. Creatinine levels were included in the Chronic Kidney Disease Epidemiology Collaborative Study formula to estimate the glomerular filtration rate. The patients were divided into three groups according to the tertiles of RDW. First, this study used linear regression models and χ2 tests to compare the baseline characteristics of the patients in different groups. Second, univariate and multivariate Cox proportional hazards regression analyses were applied to estimate the correlation between RDW and the probability of readmission within 3 months. Three models were simultaneously calculated as follows: a non-adjusted model, not adjusted for potential confounders; a minimally adjusted model, adjusted for age and sex; a multivariable-adjusted model, adjusted for confounders in the minimally adjusted model + body mass index, CCI score, NYHA cardiac function classification, type of HF, brain natriuretic peptide, RBC, HB, total protein, albumin, alanine aminotransferase, lactate dehydrogenase, and eGFR. The covariates selected for adjustment were based on the fact that their addition to the model changed the regression coefficient by at least 10%. 95% confidence intervals (CIs) and hazard ratios (HRs) were estimated for all models. Third, sensitivity analyses were conducted in this study to test the robustness of the results. The RDW was converted into a categorical tertile variable. The P for the trend was calculated. We examined whether the results were consistent with those of RDW as a continuous variable. Stratified analyses and interactions were implemented according to age, sex, CCI score, NYHA cardiac function classification, HF type, eGFR, and absolute iron deficiency. Absolute iron deficiency referred to an RDW > 15% with: either a mean cell volume <80 fl or a mean cell hemoglobin content <27 pg or a mean cell hemoglobin concentration <32 g/dl (19).
We used smooth curve fittings (penalized spline method) to evaluate the dose-response relationship between RDW and the risk of 3-month readmission. A cumulative 3-month readmission-free probability analysis was performed using Kaplan–Meier curves with log-rank statistics according to the different groups.
All tests were two-sided, and P < 0.05 was considered statistically significant. Data were analyzed using the R statistical package (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org; version 3.6.3) and Free Statistics software version 1.7.
3. Results
3.1. Patient selection
The database included 2008 patients with HF, 30 of whom were excluded because of missing RDW values on admission. Therefore, 1,978 patients were included in the analysis.
3.2. Baseline characteristics
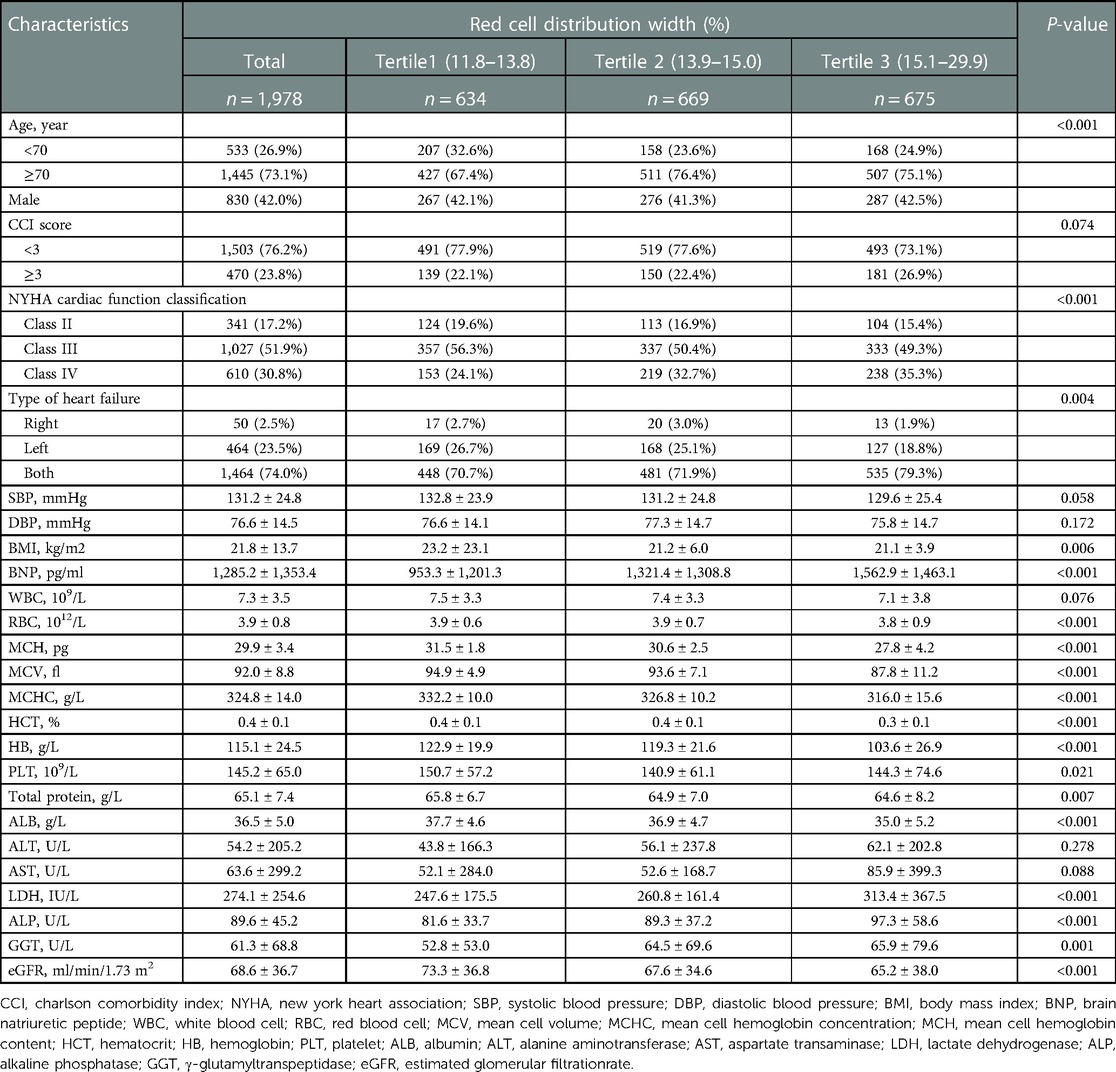
The selected patient characteristics are shown in Table 1. Of these patients, 73.1% were older than 70 years, and 42% were male. Compared with the patients in the lower RDW groups (tertile 1 and tertile 2), patients with a higher RDW (tertile 3) were more likely to be male, have total HF, have higher CCI scores, NYHA class, brain natriuretic peptide, lactate dehydrogenase, alkaline phosphatase, γ-glutamyltranspeptidase, and have lower body mass index, RBC count, mean cell volume, mean cell hemoglobin content, mean cell hemoglobin concentration, hematocrit, HB, total protein, albumin, and eGFR (P < 0.01).
3.3. Association between RDW and the risk of readmission within 3 months
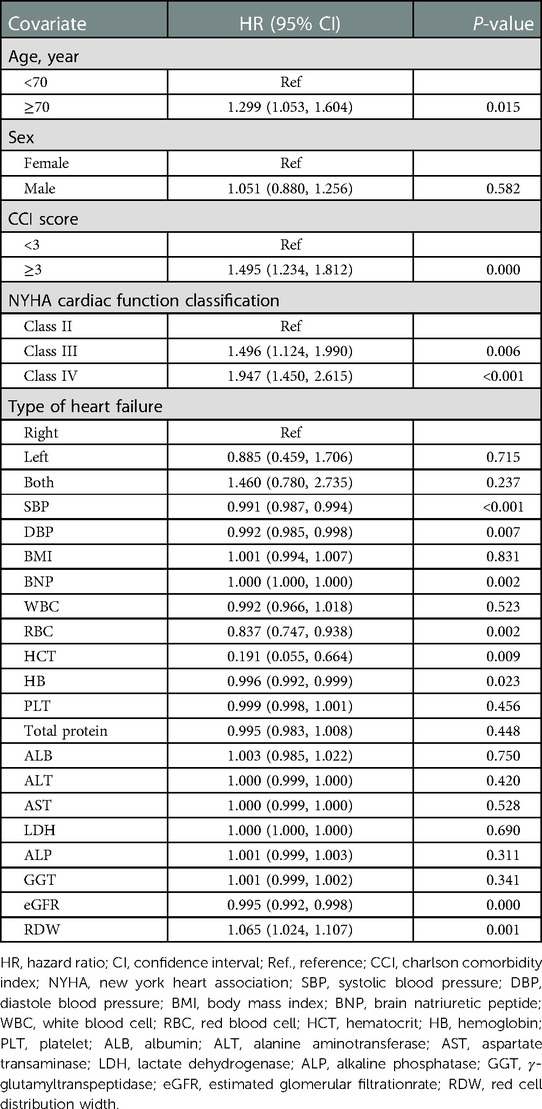
Univariate analysis suggested that age, CCI score, cardiac function class, NYHA cardiac function classification, systolic blood pressure, diastolic blood pressure, RBC, hematocrit, HB, eGFR, and RDW were associated with readmission risk within 3 months (P < 0.05) (Table 2).
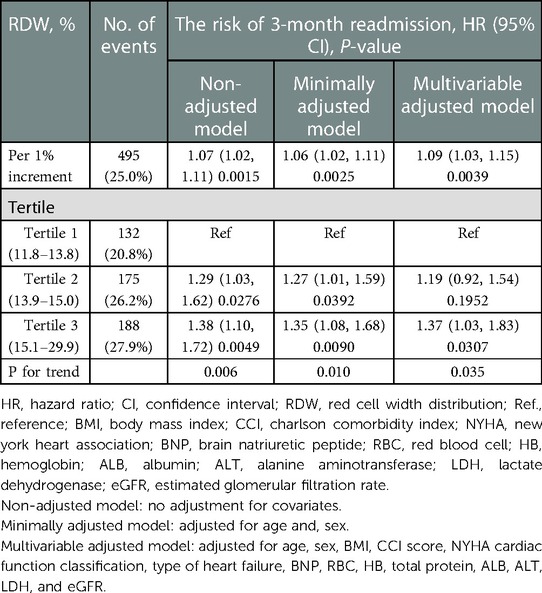
Table 3 shows that 495 (25.0%) participants were readmitted within 3 months. In the RDW tertile 1–3 groups, 132 (20.8%), 175 (26.2%), and 188 (27.9%) patients with HF, respectively, were readmitted within 3 months. The results of the multivariable Cox proportional hazards regression analysis suggested that an elevated RDW was associated with an increased risk of readmission within 3 months. In the multivariable-adjusted model, every 1% increase in RDW was associated with a 9% increased risk of readmission within 3 months (HR = 1.09, 95% CI: 1.00–1.15, P = 0.003). We also analyzed RDW as a categorical variable one more time. Compared with participants with a RDW lower than 13.8% (tertile 1), the probability of readmission within 3 months increased by 37% in those with RDW levels of 15.1%–29.9% (tertile 3) in the multivariable-adjusted model. P-values for the trend tests were all less than 0.05, indicating that our findings were robust.
3.4. Dose-response relationship between RDW and the risk of readmission within 3 months
The correlation between RDW and the risk of readmission within 3 months was evaluated on a continuous scale using smoothed curve fitting (restricted cubic spline method) based on the Cox proportional hazards models. The fully adjusted smoothed curve fitting showed a linear association between RDW and the risk of readmission within 3 months, with a P-value for non-linearity of 0.993 (Figure 1). When the RDW was greater than 14.4%, the HR of readmission within 3 months for patients with HF was greater than 1.
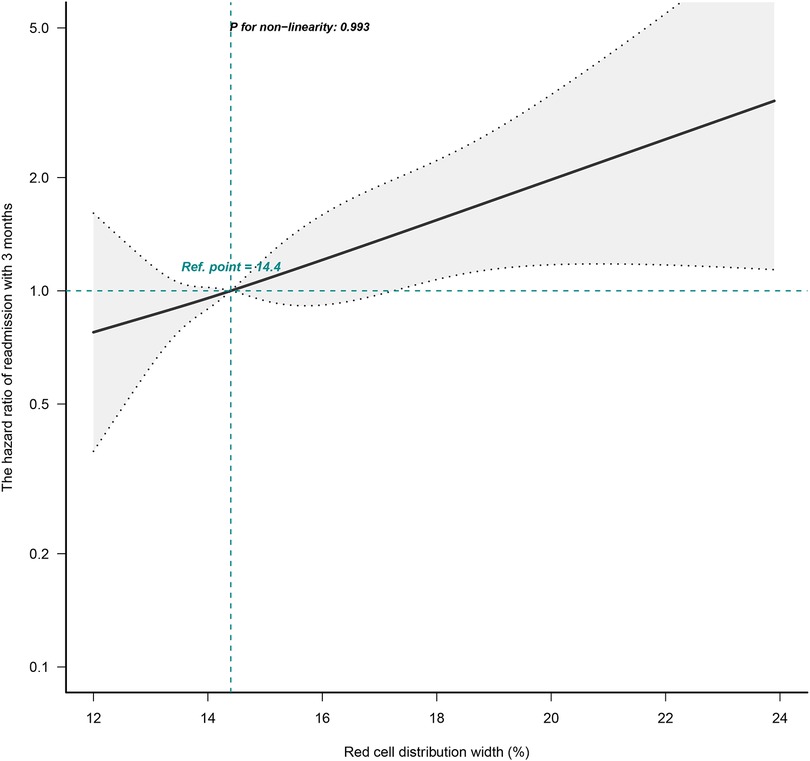
Figure 1. Dose-response relationships between RDW and the hazard ratio of readmission within 3 months. The black solid line represents the estimated risk of readmission within 3 months, with dashed lines showing 95% confidence intervals. The blue histogram at the bottom represents the distribution of RDW. Analyses were adjusted for age, sex, BMI, CCI score, NYHA cardiac function classification, type of heart failure, BNP, RBC, HB, total protein, ALB, ALT, LDH, and eGFR. RDW, red cell distribution width; BMI, body mass index; CCI, charlson comorbidity index; NYHA, new york heart association; BNP, brain natriuretic peptide; RBC, red blood cell, HB, hemoglobin; ALB, albumin; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate.
3.5. Subgroup analysis
The stratification and interaction analyses of the correlation between RDW and the risk of readmission within 3 months for patients with HF are shown in Figure 2. The results of the subgroup analysis were highly consistent with those of the multivariate Cox regression analysis. Interaction analysis results showed no interactive roles in the subgroup.
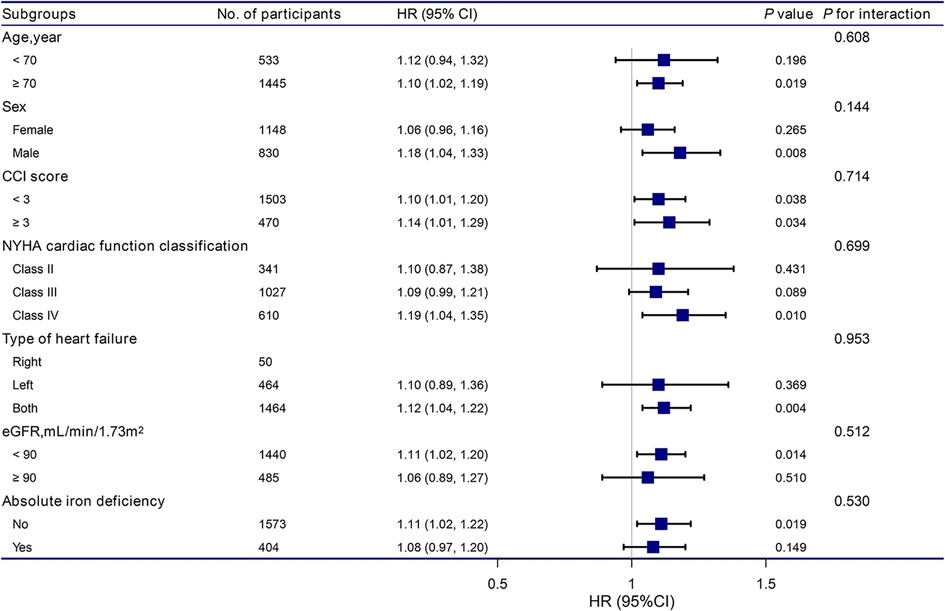
Figure 2. Association between RDW and the hazard ratio of readmission within 3 months according to subgroup. Analyses were adjusted for age, sex, BMI, CCI score, NYHA cardiac function classification, type of heart failure, BNP, RBC, HB, total protein, ALB, ALT, LDH, eGFR, and absolute ID, except for the stratification variable. RDW, red cell distribution width; BMI, body mass index; CCI, charlson comorbidity index; NYHA, new york heart association; BNP, brain natriuretic peptide; RBC, red blood cell, HB, hemoglobin; ALB, albumin; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate.
3.6. Kaplan–Meier survival curve
Patients with a lower RDW (tertile 1) had a significantly higher 3-month readmission-free probability than those with a higher RDW (tertiles 2 and, 3) (P = 0.014), as shown in Figure 3.
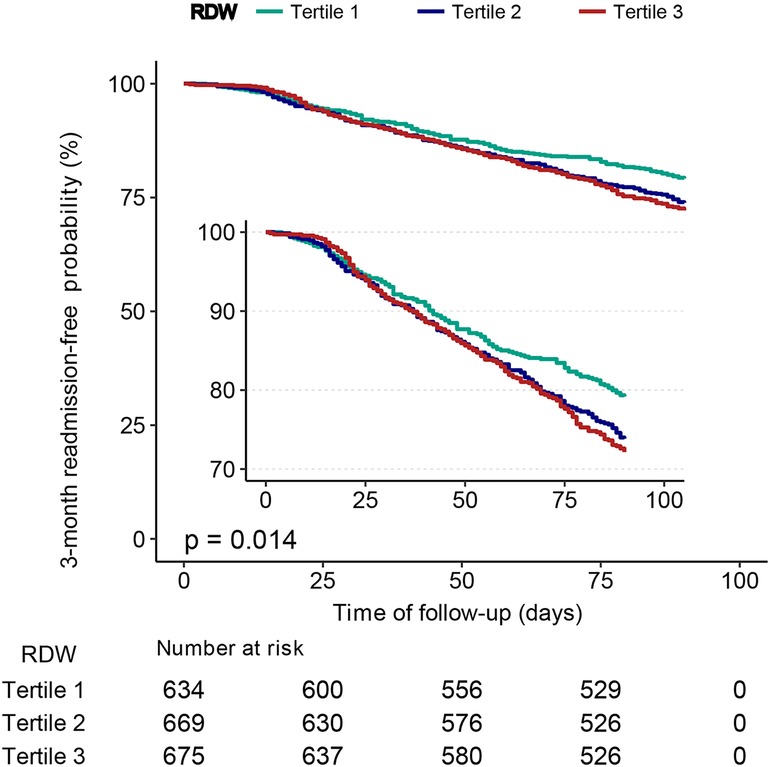
Figure 3. Kaplan–Meier curves for 3-month readmission-free probability in the patients with heart failure for categories of RDW tertiles. RDW, red cell distribution width.
4. Discussion
This retrospective cohort study focused on determining the association between RDW and the risk of readmission within 3 months in patients with HF. It suggested that RDW was linearly and positively associated with the risk of readmission within 3 months in middle-aged and elderly patients with HF in China after adjusting for several major confounding factors. In the multivariable-adjusted model, we found that every 1% increase in RDW was correlated with a 9% increased risk of readmission within 3 months (HR = 1.09, 95% CI: 1.03–1.15, P < 0.005) and the highest RDW group (tertile3) had a higher risk of 3-month readmission compared with the lowest group (tertile1). These results were consistent for each subgroup.
RDW is strongly associated with the prognosis of patients with HF. Consistent with our results, previous studies have shown that the RDW at admission is associated with a multifactorial adjusted risk of rehospitalization. Remo et al. showed that patients with high RDW had a risk ratio of 1.55 (95% CI: 1.08–2.22) for death or reoccurrence of HF from any cause compared with patients with a low RDW (20). Kyoung et al. found that readmission rates for older patients were higher in the highest group of the RDW category (7.4% in the lowest group and 15.8% in the highest group, P-trend < 0.001) (21). However, some studies have reported inconsistent results. Tan et al. included patients with HF in China by using multivariate logistic regression models to predict the risk of 3-month readmission and concluded that simple observation of RDW from clinical blood test reports could not be used to visually determine the risk of readmission (22); the area under the red cell volume distribution width curve also confirmed its weaker discriminatory ability (C statistic = 0.61); however, their study included was only 350 patients, and the study population was limited to the HF population in central China. Our study had a larger sample size, and the patients were not limited to central China to further investigate the relationship between RDW and the risk of readmission within 3 months.
RDW is a common clinical measure of heterogeneity in RBC size (23). A higher RDW indicates a defect in the maturation or degradation of blood cells (24). An elevated RDW has been shown to be associated with anemia, inflammatory diseases (e.g., infections and tumors), chronic kidney disease, diabetes mellitus, and cardiovascular diseases (e.g., HF, hypertension, stroke, and atrial fibrillation) (25–30). However, the specific mechanisms underlying the association between RDW and poor prognosis in chronic diseases, including HF, are not fully understood. Multiple interrelated pathological mechanisms, including nutritional deficiency, ineffective erythropoiesis, reduced iron mobilization, oxidative stress, chronic inflammation, adrenergic stimulation, and enhanced immune system activation, are thought to be associated with an increase in RDW and poor prognosis in HF patients (31, 32). The main role of erythrocytes is to transport oxygen and carbon dioxide from the lungs to the tissues and to maintain the acid-base balance of the body (33). Recent studies suggest that erythrocytes are involved in nitric oxide metabolism and have secretory functions (release of nitric oxide, nitric oxide metabolites, and adenosine triphosphate) (33). The mediators released by erythrocytes play a key role in cardiovascular regulation and have a direct impact on the heart. Nutritional deficiencies lead to inadequate erythropoiesis and increased RDW (34). Nutritional factors are critical for improving the medium-to-long-term prognosis of patients with HF. Chronic inflammation and oxidative stress can lead to increased RDW by suppressing effective bone marrow erythropoiesis and increasing RBC variability (35). On the one hand, inflammatory markers (interleukin-6 and tumor necrosis factor-α) disrupt erythropoiesis by directly inhibiting erythroid precursors, affect iron metabolism, and reduce erythropoiesis; it also decreases erythropoietin secretion from the kidney (36, 37). On the other hand, oxidative stress decreases erythrocyte survival and leads to increased circulating premature erythrocytes, resulting in anisocytosis and increased erythrocyte lysis, which leads to increased free radicals and adverse effects on the heart (38, 39). Thus, higher oxidative stress is another potential mechanism linking RDW to a poor prognosis in patients with HF.
Based on the subgroup analysis, we found that the association between RDW and the risk of readmission within 3 months was stronger in men than in women (HR: 1.18 vs. 1.06). Sex differences exist in almost all aspects of HF. First, there are differences in cardiac anatomy between men and women, with women without heart disease having a higher left ventricular ejection fraction than men (40). Women with HF with preserved ejection fraction have smaller, stiffer hearts and more frequent concentric remodeling than men with HF with preserved ejection fraction (41). Second, from a pathophysiological point of view, women with HF with reduced left ventricular ejection fraction have better adaptation of the myocardium to stress and a lower risk of ventricular tachycardia, atrial fibrillation, and sudden cardiac death than men (42, 43). Third, several neurohormonal modulators have been shown to have better therapeutic effects in women with HF, but even before the recent introduction of neurohormonal modulators into HF treatment, clinical outcomes for women with HF with preserved or reduced ejection fraction were consistently better than for men (44). Mechanistic studies to explain these sex-related differences in HF treatment are lacking, and further research is required to verify these phenomena. An analysis of PARADIGM-HF suggested that mortality and hospitalization rates are lower in women for reasons that are not yet clear (45). Moreover, future consideration of gender-based differences in treatment is needed to reduce the risk of readmission.
In the subgroup analysis, we found that the association between RDW and the risk of readmission within 3 months was stronger among patients with more co-morbidities (CCI score ≥3) and poor cardiac function (NYHA cardiac function class IV). Previous studies have demonstrated that co-morbidities are common in hospitalized elderly patients and are associated with long-term prognosis (46). Moreover, other studies have documented co-morbidities and cardiac function class as independent adverse prognostic factors in elderly patients with acute HF (47), consistent with our results.
Our study has the following strengths. First, this study had a larger sample size than previous studies. Second, the effect modification factor analysis allowed better use of the data to draw stable conclusions across different subgroups. However, this study has some limitations. First, because this was an observational study, we could not confirm a causal relationship between RDW and the risk of readmission within 3 months. Second, this study only assessed RDW values at the first admission. Therefore, it is impossible to evaluate the influence of dynamic changes in RDW on the risk of readmission within 3 months. Third, the subjects of this study were all patients with HF in China; therefore, there are some deficiencies in the extrapolation and generalizability of the study.
In conclusion, we found that RDW was positively and linearly related to the risk of readmission within 3 months in patients with HF. Patients with HF with higher RDW values also had a higher risk of readmission within 3 months. Further studies on the ability of RDW to predict the risk of readmission are needed.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.13026/8a9e-w734.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zigong Fourth People's Hospital (approval number:2020-010). The patients/participants provided their written informed consent to participate in this study.
Author contributions
FG and HW: writing—original draft preparation. XJ: validation. CK and WZ: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Zhejiang Province Health Science and Technology Project (grant no. 2021KY467, 2021KY529) and Zhejiang Province Traditional Chinese Medicine Science and Technology Program (grant no. 2022ZB039).
Acknowledgment
The authors thank Professor Zhongheng Zhang and his team at the Fourth Hospital in Zigong, Sichuan, China, for creating and sharing this database. The authors also thank Jie Liu of Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rethy L, Petito LC, Vu THT, Kershaw K, Mehta R, Shah NS, et al. Trends in the prevalence of self-reported heart failure by race/ethnicity and age from 2001 to 2016. JAMA Cardiol. (2020) 5(12):1425–9. doi: 10.1001/jamacardio.2020.3654
2. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383(15):1413–24. doi: 10.1056/NEJMoa2022190
3. Nassif M, Kosiborod M. Effect of glucose-lowering therapies on heart failure. Nat Rev Cardiol. (2018) 15(5):282–91. doi: 10.1038/nrcardio.2017.211
4. Kitakata H, Kohno T, Kohsaka S, Shiraishi Y, Parizo JT, Niimi N, et al. Prognostic implications of early and midrange readmissions after acute heart failure hospitalizations: a report from a Japanese multicenter registry. J Am Heart Assoc. (2020) 9(10):e014949. doi: 10.1161/JAHA.119.014949
5. Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, et al. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol. (2018) 3(4):288–97. doi: 10.1001/jamacardio.2017.5365
6. Sokoreli I, Pauws SC, Steyerberg EW, de Vries G-J, Riistama JM, Tesanovic A, et al. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA-HF study. Eur J Heart Fail. (2018) 20(4):689–96. doi: 10.1002/ejhf.1112
7. Diez-Silva M, Dao M, Han J, Lim C-T, Suresh S. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull. (2010) 35(5):382–8. doi: 10.1557/mrs2010.571
8. Buttarello M, Plebani M. Automated blood cell counts: state of the art. Am J Clin Pathol. (2008) 130(1):104–16. doi: 10.1309/EK3C7CTDKNVPXVTN
9. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52(2):86–105. doi: 10.3109/10408363.2014.992064
10. Hong L, Fang K, Ling Y, Yang L, Cao W, Liu F, et al. Red blood cell distribution width is associated with collateral flow and final infarct volume in acute stroke with large artery atherosclerosis. Semin Thromb Hemost. (2020) 46(4):502–6. doi: 10.1055/s-0039-3400257
11. Sakai Y, Wakao N, Matsui H, Watanabe T, Iida H, Katsumi A. Elevated red blood cell distribution width is associated with poor outcome in osteoporotic vertebral fracture. J Bone Miner Metab. (2021) 39(6):1048–57. doi: 10.1007/s00774-021-01242-1
12. Jiang Z, Han X, Wang Y, Hou T, Cong L, Tang S, et al. Red cell distribution width and dementia among rural-dwelling older adults: the MIND-China study. J Alzheimer’s Dis. (2021) 83(3):1187–98. doi: 10.3233/JAD-210517
13. Amar D, Sinnott-Armstrong N, Ashley EA, Rivas MA. Graphical analysis for phenome-wide causal discovery in genotyped population-scale biobanks. Nat Commun. (2021) 12(1):350. doi: 10.1038/s41467-020-20516-2
14. Al-Najjar Y, Goode KM, Zhang J, Cleland JGF, Clark AL. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail. (2009) 11(12):1155–62. doi: 10.1093/eurjhf/hfp147
15. Salvatori M, Formiga F, Moreno-Gónzalez R, Chivite D, De Amicis MM, Cappellini MD, et al. Red blood cell distribution width as a prognostic factor of mortality in elderly patients firstly hospitalized due to heart failure. Kardiol Pol. (2019) 77(6):632–8. doi: 10.33963/KP.14818
16. Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. Physiobank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. (2000) 101(23):E215–20. doi: 10.1161/01.CIR.101.23.e215
17. Zhang Z, Cao L, Chen R, Zhao Y, Lv L, Xu Z, et al. Electronic healthcare records and external outcome data for hospitalized patients with heart failure. Sci Data. (2021) 8(1):46. doi: 10.1038/s41597-021-00835-9
18. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18(8):891–975. doi: 10.1002/ejhf.592
19. Aung N, Ling HZ, Cheng AS, Aggarwal S, Flint J, Mendonca M, et al. Expansion of the red cell distribution width and evolving iron deficiency as predictors of poor outcome in chronic heart failure. Int J Cardiol. (2013) 168(3):1997–2002. doi: 10.1016/j.ijcard.2012.12.091
20. Melchio R, Rinaldi G, Testa E, Giraudo A, Serraino C, Bracco C, et al. Red cell distribution width predicts mid-term prognosis in patients hospitalized with acute heart failure: the RDW in acute heart failure (RE-AHF) study. Intern Emerg Med. (2019) 14(2):239–47. doi: 10.1007/s11739-018-1958-z
21. Kim KM, Nerlekar R, Tranah GJ, Browner WS, Cummings SR. Higher red cell distribution width and poorer hospitalization-related outcomes in elderly patients. J Am Geriatr Soc. (2022) 70(8):2354–62. doi: 10.1111/jgs.17819
22. Tan B-Y, Gu J-Y, Wei H-Y, Chen L, Yan S-L, Deng N. Electronic medical record-based model to predict the risk of 90-day readmission for patients with heart failure. BMC Med Inform Decis Mak. (2019) 19(1):193. doi: 10.1186/s12911-019-0915-8
23. Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. (2008) 112(10):4284–91. doi: 10.1182/blood-2008-04-154112
24. Higgins JM, Mahadevan L. Physiological and pathological population dynamics of circulating human red blood cells. Proc Natl Acad Sci U S A. (2010) 107(47):20587–92. doi: 10.1073/pnas.1012747107
25. Yeh H-C, Lin Y-T, Ting IW, Huang H-C, Chiang H-Y, Chung C-W, et al. Variability of red blood cell size predicts all-cause mortality, but not progression to dialysis, in patients with chronic kidney disease: a 13-year pre-ESRD registry-based cohort. Clin Chim Acta. (2019) 497:163–71. doi: 10.1016/j.cca.2019.07.035
26. Petrella F, Casiraghi M, Radice D, Prisciandaro E, Rizzo S, Spaggiari L. Prognostic value of red blood cell distribution width in resected pN1 lung adenocarcinoma. Cancers. (2020) 12(12):3677. doi: 10.3390/cancers12123677
27. Wang Z-H, Fu B-Q, Lin Y-W, Wei X-B, Geng H, Guo W-X, et al. Red blood cell distribution width: a severity indicator in patients with COVID-19. J Med Virol. (2022) 94(5):2133–8. doi: 10.1002/jmv.27602
28. Jiang Y, Jiang F-Q, Kong F, An M-M, Jin B-B, Cao D, et al. Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: a preliminary observational study. Ann Intensive Care. (2019) 9(1):67. doi: 10.1186/s13613-019-0542-7
29. Hong J, Hu X, Liu W, Qian X, Jiang F, Xu Z, et al. Impact of red cell distribution width and red cell distribution width/albumin ratio on all-cause mortality in patients with type 2 diabetes and foot ulcers: a retrospective cohort study. Cardiovasc Diabetol. (2022) 21(1):91. doi: 10.1186/s12933-022-01534-4
30. Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, Ghandehari M, Shafiee M, Rahmani F, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors. (2019) 45(4):507–16. doi: 10.1002/biof.1518
31. Tang Y-D, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. (2006) 113(20):2454–61. doi: 10.1161/CIRCULATIONAHA.105.583666
32. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. (2011) 17(11):899–906. doi: 10.1016/j.cardfail.2011.08.003
33. Kuhn V, Diederich L, Keller TCS, Kramer CM, Lückstädt W, Panknin C, et al. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, Anemia. Antioxid Redox Signal. (2017) 26(13):718–42. doi: 10.1089/ars.2016.6954
34. Pernow J, Mahdi A, Yang J, Zhou Z. Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc Res. (2019) 115(11):1596–605. doi: 10.1093/cvr/cvz156
35. Feng G-H, Li H-P, Li Q-L, Fu Y, Huang R-B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol. (2017) 2(3):172–5. doi: 10.1136/svn-2017-000071
36. Valletta S, Thomas A, Meng Y, Ren X, Drissen R, Sengül H, et al. Micro-environmental sensing by bone marrow stroma identifies IL-6 and TGFβ1 as regulators of hematopoietic ageing. Nat Commun. (2020) 11(1):4075. doi: 10.1038/s41467-020-17942-7
37. Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, et al. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. (2006) 397(1):61–7. doi: 10.1042/BJ20060215
38. Minetti M, Agati L, Malorni W. The microenvironment can shift erythrocytes from a friendly to a harmful behavior: pathogenetic implications for vascular diseases. Cardiovasc Res. (2007) 75(1):21–8. doi: 10.1016/j.cardiores.2007.03.007
39. Pagan LU, Gomes MJ, Martinez PF, Okoshi MP. Oxidative stress and heart failure: mechanisms, signalling pathways, and therapeutics. Oxid Med Cell Longevity. (2022) 2022:9829505. doi: 10.1155/2022/9829505
40. Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas heart study. Circulation. (2006) 113(12):1597–604. doi: 10.1161/CIRCULATIONAHA.105.574400
41. Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis. (2007) 49(4):241–51. doi: 10.1016/j.pcad.2006.08.011
42. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J. (2019) 40(47):3859–3868c. doi: 10.1093/eurheartj/ehz835
43. Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. (2019) 25(11):1657–66. doi: 10.1038/s41591-019-0643-8
44. Martínez-Sellés M, Doughty RN, Poppe K, Whalley GA, Earle N, Tribouilloy C, et al. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur J Heart Fail. (2012) 14(5):473–9. doi: 10.1093/eurjhf/hfs026
45. McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation. (2020) 141(5):338–51. doi: 10.1161/CIRCULATIONAHA.119.044491
46. Buurman BM, Frenkel WJ, Abu-Hanna A, Parlevliet JL, de Rooij SE. Acute and chronic diseases as part of multimorbidity in acutely hospitalized older patients. Eur J Intern Med. (2016) 27:68–75. doi: 10.1016/j.ejim.2015.09.021
Keywords: red cell distribution width (RDW), prognosis, readmission, heart failure, China
Citation: Gu F, Wu H, Jin X, Kong C and Zhao W (2023) Association of red cell distribution width with the risk of 3-month readmission in patients with heart failure: A retrospective cohort study. Front. Cardiovasc. Med. 10:1123905. doi: 10.3389/fcvm.2023.1123905
Received: 14 December 2022; Accepted: 16 February 2023;
Published: 7 March 2023.
Edited by:
Elisabetta Salvioni, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Ermes Carulli, University of Milan, ItalyDarlington Okonko, King’s College London, United Kingdom
© 2023 Gu, Wu, Jin, Kong and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenyan Zhao emhhb3dlbnlhbkBobWMuZWR1LmNu Cheng Kong d3ptY2tjQDEyNi5jb20=
†These authors have contributed equally to this work
‡These authors contributed equally to this work and share correspondence
Specialty Section: This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
Abbreviations CCI, charlson comorbidity index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HB, hemoglobin; HF, heart failure; HR, hazard ratio; NYHA, new york heart association; RBC, red blood cell; RDW, red cell distribution width.
 Fang Gu
Fang Gu Han Wu2,†
Han Wu2,† Wenyan Zhao
Wenyan Zhao