- 1Department of Urology, Changhai Hospital, Naval Medical University, Shanghai, China
- 2Department of Critical Care Medicine, Huashan Hospital, Fudan University, Shanghai, China
- 3Department of Plastic and Reconstructive Surgery, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
- 4Luodian Clinical Drug Research Center, Shanghai Baoshan Luodian Hospital, Shanghai University, Shanghai, China
Background: Erection dysfunction has been associated with hypertension in several epidemiological and observational studies. But the causal association between hypertension and erectile dysfunction requires further investigation.
Methods: A two-sample Mendelian randomization (MR) was conducted to analyze the causal effect of hypertension on risk of erection dysfunction. Large-scale publicly available genome-wide association study data were used to estimate the putative causality between hypertension and risk of erectile dysfunction. A total of 67 independent single nucleotide polymorphisms were selected as instrumental variables. Inverse-variant weighted, maximum likelihood, weighted median, penalized weighted median, and MR-PRESSO approaches were utilized in MR analyses. Heterogeneity test, horizontal pleiotropy test, and leave-one-out method were used to prove the stability of the results.
Results: In total, all P values were less than 0.05, demonstrating a positive causal link between hypertension and risk of erectile dysfunction in multiple MR methods, such as inverse-variant weighted (random and fixed effect) (OR 3.8315, 95% CI 2.3004–6.3817, P = 0.0085), maximum likelihood (OR 3.8877, 95% CI 2.3224–6.5081, P = 0.0085), weighted median (OR 4.9720, 95% CI 2.3645–10.4550, P = 0.0309), penalized weighted median (OR 4.9760, 95% CI 2.3201–10.6721, P = 0.0355), and MR-PRESSO (OR 3.6185, 95% CI 2.2387–5.8488, P = 0.0092). Sensitivity analysis detected no evidence of heterogeneity, pleiotropy, or outlier single nucleotide polymorphisms.
Conclusion: The study revealed a positive causal link between the presence of hypertension and the risk of erectile dysfunction. More attention should be paid during the management of hypertension with the purpose of preventing erectile dysfunction or improving erectile function.
Introduction
Erectile dysfunction (ED) is defined as the inability of attaining or maintaining sufficient and satisfying erection for sexual intercourse (1). Further diagnosis and precise grading are mainly based on the International Index for Erectile Function (IIEF) (2, 3) or Brief Male Sexual Function Inventory for Urology (BMSFI) (4). Sexual function is one of the most fundamental biological characteristics of Homo sapiens, and erectile function holds great importance for a majority of males. ED is regarded as a major health problem that affects up to millions of men in the world and keeps causing a negative impact on the quality of life (QoL) of the patients and their partners. In the Massachusetts Male Aging Study (MMAS), a population-based prospected randomized study on aging men, it was found that over 52% men between 40 and 70 years old reported ED. The incidence rate of ED was about 26 cases per 1,000 man-years (5). Among men under 49 years old, the prevalence of ED was approximately 6%. By the age of 50–59, 16% of men were estimated to suffer from ED, and this number grew to 32% for those between 60 and 69, and even higher to 44% for those aged 70–79 (6). The epidemiology data of ED mainly depend on query or self-reporting. Hence, real-life incidence may be even higher than the estimated number. According to predictions, by 2025, the estimated cases of ED would reach 322 million worldwide (7). The continuous rising prevalence of ED brings a heavy burden on healthcare, finance, and society. Hence, the prevention of, and early intervention for, the risk factors of ED hold considerable significance and a sense of urgency. A variety of risk factors of ED has been investigated in previous studies, such as hypertension (8), cardiovascular diseases (9), diabetes mellitus (10), obesity (11), unhealthy lifestyle (12), and so on.
Hypertension is one of the risk factors for ED and affects the blood supply to the penis, with a strong association due to shared intrinsic mechanisms such as endothelial dysfunction (13). The mechanisms linking hypertension and ED involve intertwined procontractile signaling pathways, resulting in reduced vascular flexibility (14, 15). Hypertension is estimated to affect up to 1.39 billion people globally (16, 17). The number of people with both hypertension and ED is significantly high, and the health management and quality of life of these patients is extremely important. A lot of clinical studies, including large-scale cross-national cohorts, have explored the correlation between hypertension and ED (18–23). However, some studies have reached contradictory conclusions (24–26). Most of these studies mentioned above mainly focused on the potential of ED as a predictor for cardiovascular diseases, especially the coronary artery disease, instead of further investigating the relationship between hypertension and ED. Some studies proposed that not hypertension but antihypertensive drugs trigger ED (27, 28). Only if the causal relationship and the direction of this relationship are definitely established will answers be found to the above questions.
To provide high-level clinical evidence and validate causality, Mendelian randomization (MR) was adopted to investigate the causality between hypertension and ED. MR could compensate for the deficiency of observational studies and obtain unbiased estimates without conducting a randomized controlled trial (RCT) (29, 30). Although RCTs are considered to have the highest level of evidence, with the increase in the study scale, comes the complexity of organizational and financial support, as well as the challenges of passing various legal and ethical reviews. These factors can all contribute to a higher implementation cost for RCTs. Therefore, despite RCTs providing a higher level of evidence, they also bring more challenges. In contrast, MR studies tend to be less costly and easier to conduct compared with RCTs (31). In addition, MR has the advantage of being able to establish causality between exposure and outcome, while RCTs can establish only association. This is because MR studies use genetic variation as an instrument for exposure, which eliminates the effects of reverse causality and confounding, making the causal inference more robust (32).
The Mendelian randomization method utilizes genetic variants to determine the causality between exposure and outcome (33). The genetic variants are significantly linked with the exposure, independent of confounding factors associated with exposure and outcome, and affect the outcome only via exposure. MR typically uses single nucleotide polymorphism (SNP) as a proxy for the exposure factor. SNP is a variation at a single nucleotide site in the genome and is randomly allocated among individuals, so that they are not affected by environmental or lifestyle factors. Moreover, SNPs do not change over a lifetime, making them useful as long-term biological markers (34). To gain access to genetic data, publicly available genome-wide association studies (GWAS) were explored. The statistics was then analyzed by MR. By excluding confounding bias factors, confounding and reverse causality could be avoided in order to gain an unaffected causal link between exposure and outcome (35). In the present study, a two-sample MR based on the large-scale publicly available GWAS data was applied to estimate the putative causality between hypertension and risk of ED.
Materials and methods
The study was conducted to obtain a comprehensive and reliable conclusion of the casual link between hypertension and risk of ED.
Data sets
We used the GWAS statistics obtained from large-scale genetic consortia of ED patients. Bovijn et al. identified the risk locus for ED by performing a genome-wide study among 6,175 European ED patients (36). Hypertension GWAS summary data were obtained from the Medical Research Council Integrative Epidemiology Unit at the University of Bristol (MRC IEU). As many as 54,358 European patients diagnosed with hypertension and 40,8652 controls were included in the database. All the above-mentioned data sets are available on https://gwas.mrcieu.ac.uk/ and used for MR analysis.
SNPs with a strong association with ED of genome-wide significance (P < 5 × 10−8) were selected and SNPs were pruned in linkage disequilibrium (R2 > 0.001 within a 10,000 kb window) with the clump data function in the TwoSampleMR software package in R. The filtered SNPs were finally qualified for the following MR analysis. The strength of the genetic instruments was indicated by the F-statistic.
Mendelian randomization
Mendelian randomization analysis aims to apply genetic variants as instrumental variables to estimate the causal effect between exposure and outcome, namely hypertension and ED in this study (Figure 1).
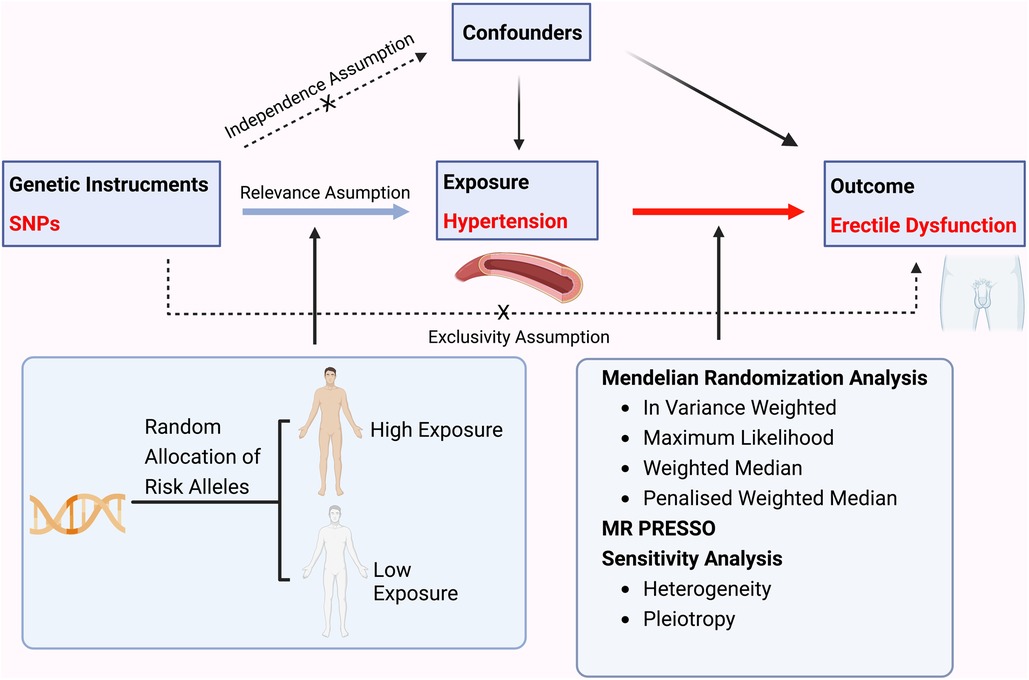
Figure 1. A flow chart illustrating the causality of the relationship between hypertension and erectile dysfunction.
An inversed-variance weighted (IVW) (random and fixed effect) meta-analysis of each Wald ratio was performed to obtain MR estimate since there is no evidence of directional pleiotropy (37). The outlier variables were detected by the MR-PRESSO method in IVW analysis by comparing the actual distance from the regression, assuming the absence of horizontal pleiotropy, and evaluating the causal estimates after removing outliers (38). In addition, several statistical analyses were performed to assess the stability of the MR results, including maximum likelihood, weighted median, and penalized weighted median approaches.
Pleiotropy and sensitivity analysis
Heterogeneity was detected by inverse-variance weighted and MR-Egger regression and quantified by using Cochran's Q test. A P-value <0.05 would be considered as significant heterogeneity. The potential horizontal pleiotropic effects of the instrumental variables were assessed by the intercept term in MR-Egger regression. If the intercept term converged to 0 (<0.1) as well as P > 0.05, it was indicated that no evidence of horizontal pleiotropy was detected in the analysis and the results of the MR analyses were reliable.
Moreover, a “leave-one-out” sensitivity analysis was repeated to test the potentially influential SNPs. In this analysis, each SNP would be left out in turn and the MR would be repeated over again.
Mendelian randomization analyses were performed by using the “TWO-SampleMR” package and “MR-PRESSO” in R software 4.0.5. All P values were two-sided.
Results
Selection of variables
In total, 67 qualified SNPs related to ED and hypertension were collected in this study. All the instrumental variables met the generally accepted genome-wide significance threshold (P < 5 × 10−8, r2 < 0.001, kb = 10,000) for exposure. The F-statistic indicated no weak instrument bias (all F-statistic > 10).
Mendelian randomization estimates
Mendelian randomization analysis demonstrated a significant association of hypertension with ED outcomes. The results were consistent with IVW (random and fixed effect) (OR 3.8315, 95% CI 2.3004–6.3817, P = 0.0085), maximum likelihood (OR 3.8877, 95% CI 2.3224–6.5081, P = 0.0085), weighted median (OR 4.9720, 95% CI 2.3645–10.4550, P = 0.0309), penalized weighted median (OR 4.9760, 95% CI 2.3201–10.6721, P = 0.0355), and MR-PRESSO (OR 3.6185, 95% CI 2.2387–5.8488, P = 0.0092) (Figures 2, 3A).
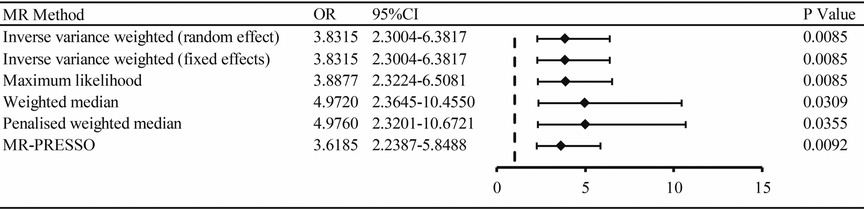
Figure 2. The association of hypertension with ED outcomes by MR analysis through different methods (random effect and fixed effects inverse variance weighted method, maximum likelihood, weighted median, penalized weighted median, MR-PRESSO). OR: odds ratio; CI: confidence interval.
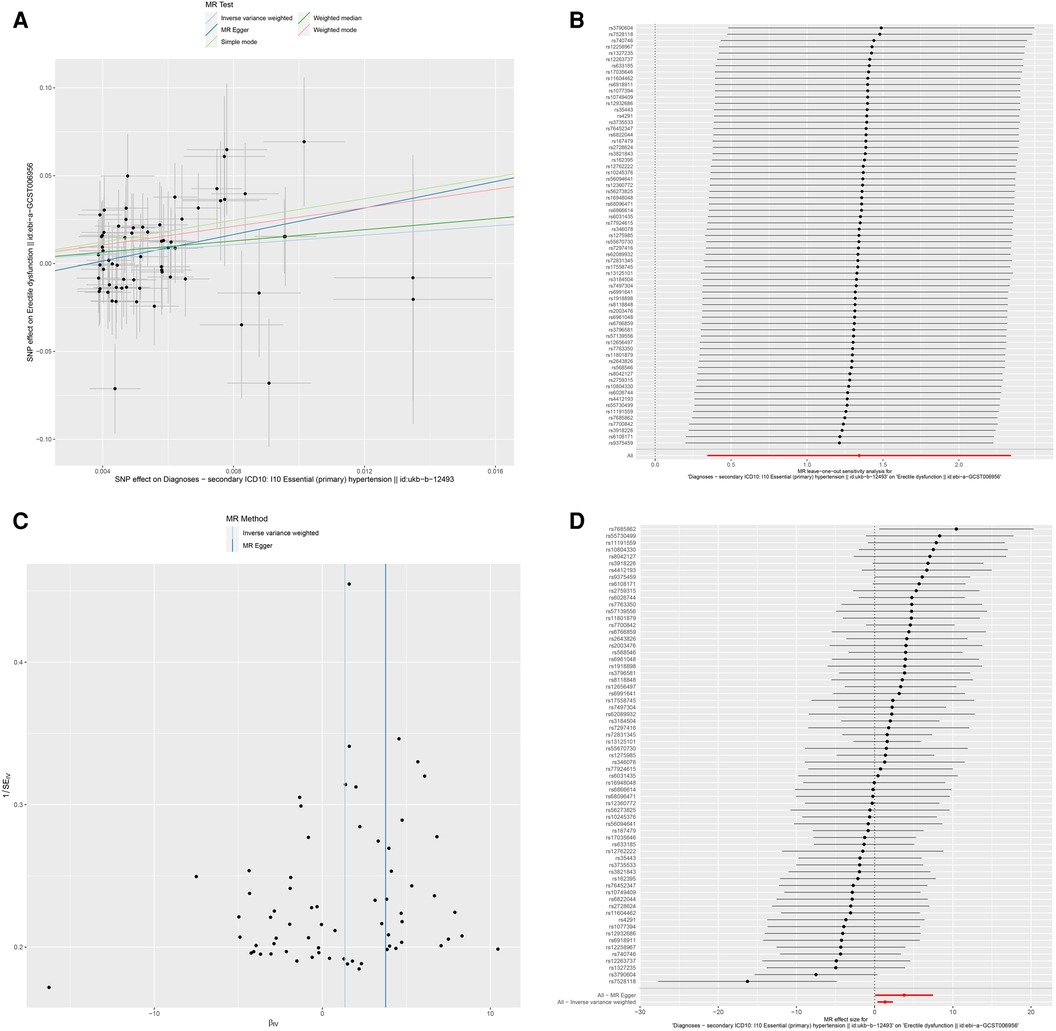
Figure 3. Association between hypertension and risk of erectile dysfunction. (A) multiple MR tests showed the SNP effects; (B) leave-one-out sensitivity analysis; (C) funnel plot for hypertension risk of ED; (D) effect size of each SNP. MR Mendelian Randomization, SNP single nucleotide polymorphism, ED erectile dysfunction.
Pleiotropy and sensitivity analysis
To explore the sensitivity of the analysis, we conducted the Cochren's Q test, which indicated no evidence of heterogeneity. The horizontal pleiotropy between SNPs and outcome was assessed by MR-Egger regression. No evidence of horizontal pleiotropy was found. The results of the leave-one-out analysis validated that no potentially influential SNP biased the casual link, and our conclusion was stable (Figure 3B). The funnel demonstrated the displayed symmetric pattern of each SNP on ED and indicated no apparent horizontal pleiotropy (Figure 3C). The effect size of each SNP is shown in Figure 3D.
Discussion
In this study, a two-sample MR analysis was performed to investigate the potential causal effect of hypertension on ED outcomes and revealed the suggested association of hypertension with an increased risk of ED. By employing several Mendelian tools, the results were proved reliable for achieving stability in the sensitivity analysis. In previous MR studies, the casual effects of insomnia (39), snoring (40), educational attainment (41), and COVID-19 (42) on risk of ED have been sufficiently investigated.
Based on the etiology, ED could be categorized as organic, psychogenic, or mixed ED. Noteworthily, the vast majority of patients are actually affected by mixed causes. In other words, organic lesion could be found in most patients. The pathological classification of ED includes vasculogenic, endocrinologic, neurogenic, anatomical, drug-related, psychogenic, or mixed causes (43). The arterial insufficiency is the primary cause of ED. The blood supply for penis mainly comes from the iliac and the pudendal artery and flows to the penile arterial system. An impairment in any segment of this arterial system may lead to ED. Hypertension as a major detrimental factor for vascular impairment could largely damage the blood flow to the penis (44).
During the past several years, a few large-scale observational studies on the incidence of ED among hypertensive patients have revealed that hypertension is closely correlated to an elevated risk of ED (18–20). A multicenter, prospective, open, observational study in Spain found a high incidence of ED in male patients with hypertension (975/2,130, 45.8%) compared with that in a normotensive male population (45). Furthermore, ED was found to be more prevalent in patients with long-duration or severe hypertension, which further illustrates the link between hypertension and risk of ED (46). The results of these mentioned studies were consistent with our findings.
Actually, ED shares not only various common risk factors (unhealthy lifestyle, obesity, aging, alcohol and tobacco use, etc.) (47), but also multiple intrinsic mechanisms such as endothelial and vascular smooth muscle dysfunction. A variety of vasoconstrictors (angiotensin II, endothelin 1, aldosterone, etc.) and vasodilators (nitric oxide, hydrogen sulfide, Nrf2, etc.) are strongly associated with the pathophysiologic pathways of hypertension and ED.
As a modifiable risk factor, the management of hypertension also significantly interferes with the treatment of ED. According to the recommendations given by the Princeton Consensus Conferences on optimizing sexual dysfunction and preserving cardiovascular health, ED patients with asymptomatic-controlled hypertension can receive treatment for ED in the first place and continue sexual activity without the fear of significant cardiac risk. Otherwise, ED patients with uncontrolled hypertension are stratified as a high-risk group. For these patients, treatment for ED should always be secondary to the management of hypertension or other cardiovascular diseases. More importantly, any form of sexual activity is strictly forbidden (48).
Noteworthily, although satisfying blood pressure control levels are closely related to erectile function benefits, antihypertensive therapy may independently trigger or worsen ED. According to the results of the MMAS study, one of the most valuable epidemiologic studies on ED, receiving treatment for hypertension strongly elevates the risk of ED (5). Different antihypertensive drugs have distinct effects on ED (49). Some β-blockers could negatively influence erectile function by blocking β-2 receptors, thus resulting in the constriction of the penile arteries (50), while nebivolol as a new-generation selective β-BLOCKER has a positive effect on erectile function (51). Diuretics is also considered to exert detrimental effects on ED as a common side effect (52). Angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) could be beneficial, or at least neutral, to erectile function and sexual activity (53).
In our study, a causal link between hypertension and risk of ED has been established with all confounding factors being excluded. Arterial hypertension brings more burden than any other diseases globally, which affects more than 1 billion people worldwide (17, 54). Considering the higher prevalence of ED in hypertensive patients, antihypertensive therapy regardless of side effects on ED no doubt would impair the QoL, cause a heavy mental burden, affect medication compliance, and eventually aggravate the vicious circle of hypertension and ED in a large number of patients and their partners. Hence, antihypertensive therapy, which is beneficial for erectile function, should be attempted on a large scale on untreated patients. For those patients who receive antihypertensive drugs with detrimental effects on erectile function, a switch in the therapeutic regimen with caution may be a wise decision. The coadministration of selective phosphodiesterase type 5 inhibitors (PDE5I) could be an alternative for avoiding the increased risk brought by the change to antihypertensive therapy. Currently, four potent PDE5I drugs are approved for ED treatment, namely, sildenafil, tadalafil, vardenafil, and avanafil. PDE5Is were effective in approximately 60%–70% of hypertensive patients, and some RCTs validated the safety of PD5EI in these patients (27, 55, 56). However, since hypertension is a modifiable risk factor of ED, priority should always be to prevent ED from initiating.
The main strength of this study is the merit of the MR design, which could evaluate the independent causal effects of hypertension on ED. To the best of our knowledge, this is the very first MR study on hypertension and ED based on large-scale consortium data. The application of MR avoids several limitations in retrospective studies. In addition, 67 qualified SNPs in the European population were used as instrumental variables, which constructed a well-powered MR analysis. Hence, the results were unlikely impacted by population stratification.
Inevitably, certain limitations exist in our study. First, due to the lack of original data in the GWAS data set, we could not fully evaluate the severity of ED and hypertension. Second, the most adaptable inquiry for the epidemiological data of ED is self-reporting and questionnaire survey. Owing to patients' reluctance to disclose their sexual dysfunction problems, the prevalence of ED in hypertensive patients may be underestimated. Third, our study was mainly based on the genetic data of European ancestry, and the results may be inconsistent in other ethnic populations. Also, each MR method has its own strength and weakness, and we cannot completely rule out potential bias.
Our results suggested that hypertension would increase the risk of ED outcomes. But a definite causal relationship requires conducting more RCTs with high quality and more in-depth studies in the future.
Our results confirmed a positive causal link between the presence of hypertension and the risk of ED in the general population. This MR study could serve as high-level clinical evidence of the impact of hypertension on erectile function, revealing causality and providing reference for clinical diagnosis and treatment, with the aim of improving the treatment effect of ED in the hypertension population, as well as providing certain guidance for the medication regimen of antihypertensive drugs to protect erectile function.
Data availability statement
Publicly available data sets were analyzed in this study. These data sets can be found here: https://gwas.mrcieu.ac.uk/. Please refer to the supplementary materials for the original code of this study.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The current analyses are based on publicly available summary data and therefore do not require ethical approval. Original studies have been approved by ethic committees and written informed consent was obtained from study participants or caregivers. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Author contributions
LW, DX, and ZL designed and supported the study. ZW, YW, and JX collected and analyzed the data. ZH, YY, and ZW wrote the manuscript. XG, YZ, and YB contributed to manuscript preparation and revision. WS, YW, and AJ contributed to data visualization and validation. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Kaiwen Wu for the assistance in data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YWe declared a shared parent affiliation with the author YWa to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1121340/full#supplementary-material.
References
1. NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. (1993) 270(1):83–90. doi: 10.1001/jama.1993.03510010089036
2. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. (1997) 49(6):822–30. doi: 10.1016/s0090-4295(97)00238-0
3. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. (1999) 11(6):319–26. doi: 10.1038/sj.ijir.3900472
4. O'Leary MP, Fowler FJ, Lenderking WR, Barber B, Sagnier PP, Guess HA, et al. A brief male sexual function inventory for urology. Urology. (1995) 46(5):697–706. doi: 10.1016/S0090-4295(99)80304-5
5. Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. (2000) 163(2):460–3. doi: 10.1016/S0022-5347(05)67900-1
6. Eardley I. The incidence, prevalence, and natural history of erectile dysfunction. Sex Med Rev. (2013) 1(1):3–16. doi: 10.1002/smrj.2
7. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. (1999) 84(1):50–6. doi: 10.1046/j.1464-410x.1999.00142.x
8. Wang XY, Huang W, Zhang Y. Relation between hypertension and erectile dysfunction: a meta-analysis of cross-section studies. Int J Impot Res. (2018) 30(3):141–6. doi: 10.1038/s41443-018-0020-z
9. Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. (2014) 65(5):968–78. doi: 10.1016/j.eururo.2013.08.023
10. Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. (2011) 25(2):129–36. doi: 10.1016/j.jdiacomp.2010.03.003
11. Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. (2004) 291(24):2978–84. doi: 10.1001/jama.291.24.2978
12. Sivaratnam L, Selimin DS, Abd Ghani SR, Nawi HM, Nawi AM. Behavior-related erectile dysfunction: a systematic review and meta-analysis. J Sex Med. (2021) 18(1):121–43. doi: 10.1016/j.jsxm.2020.09.009
13. de Oliveira AA, Nunes KP. Hypertension and erectile dysfunction: breaking down the challenges. Am J Hypertens. (2021) 34(2):134–42. doi: 10.1093/ajh/hpaa143
14. Nunes KP, de Oliveira AA, Mowry FE, Biancardi VC. Targeting toll-like receptor 4 signalling pathways: can therapeutics pay the toll for hypertension? Br J Pharmacol. (2019) 176(12):1864–79. doi: 10.1111/bph.14438
15. Calmasini FB, Klee N, Webb RC, Priviero F. Impact of immune system activation and vascular impairment on male and female sexual dysfunction. Sex Med Rev. (2019) 7(4):604–13. doi: 10.1016/j.sxmr.2019.05.005
16. Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1923–94. doi: 10.1016/S0140-6736(18)32225-6
17. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. (2016) 134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912
18. Manolis A, Doumas M. Sexual dysfunction: the ‘prima ballerina’ of hypertension-related quality-of-life complications. J Hypertens. (2008) 26(11):2074–84. doi: 10.1097/HJH.0b013e32830dd0c6
19. Nicolosi A, Moreira ED Jr., Shirai M, Bin Mohd Tambi MI, Glasser DB. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. (2003) 61(1):201–6. doi: 10.1016/s0090-4295(02)02102-7
20. Ponholzer A, Temml C, Mock K, Marszalek M, Obermayr R, Madersbacher S. Prevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaire. Eur Urol. (2005) 47(1):80–5, discussion 85–6. doi: 10.1016/j.eururo.2004.08.017
21. Gazzaruso C, Solerte SB, Pujia A, Coppola A, Vezzoli M, Salvucci F, et al. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: a potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol. (2008) 51(21):2040–4. doi: 10.1016/j.jacc.2007.10.069
22. Bohm M, Baumhakel M, Teo K, Sleight P, Probstfield J, Gao P, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: the ongoing telmisartan alone and in combination with ramipril global endpoint trial/telmisartan randomized assessment study in ace intolerant subjects with cardiovascular disease (ONTARGET/TRANSCEND) trials. Circulation. (2010) 121(12):1439–46. doi: 10.1161/CIRCULATIONAHA.109.864199
23. Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, Lieber MM, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. (2009) 84(2):108–13. doi: 10.4065/84.2.108
24. Hotaling JM, Walsh TJ, Macleod LC, Heckbert S, Pocobelli G, Wessells H, et al. Erectile dysfunction is not independently associated with cardiovascular death: data from the vitamins and lifestyle (vital) study. J Sex Med. (2012) 9(8):2104–10. doi: 10.1111/j.1743-6109.2012.02826.x
25. Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet. (1985) 1(8422):181–4. doi: 10.1016/s0140-6736(85)92023-9
26. Jaffe A, Chen Y, Kisch ES, Fischel B, Alon M, Stern N. Erectile dysfunction in hypertensive subjects. Assessment of potential determinants. Hypertension. (1996) 28(5):859–62. doi: 10.1161/01.hyp.28.5.859
27. Doumas M, Boutari C, Viigimaa M. Arterial hypertension and erectile dysfunction: an under-recognized duo. J Clin Hypertension. (2016) 14(4):1–7.
28. Doumas M, Douma S. The effect of antihypertensive drugs on erectile function: a proposed management algorithm. J Clin Hypertens. (2006) 8(5):359–64. doi: 10.1111/j.1524-6175.2005.05285.x
29. Wang RZ, Yang YX, Li HQ, Shen XN, Chen SD, Cui M, et al. Genetically determined low income modifies Alzheimer's disease risk. Ann Transl Med. (2021) 9(15):1222. doi: 10.21037/atm-21-344
30. Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): explanation and ELABORATION. BMJ (2021) 375:n2233. doi: 10.1136/bmj.n2233
31. Thanassoulis G, O'Donnell CJ. Mendelian randomization: nature's randomized trial in the post-genome era. JAMA. (2009) 301(22):2386–8. doi: 10.1001/jama.2009.812
32. Davey Smith G, Paternoster L, Relton C. When will Mendelian randomization become relevant for clinical practice and public health? JAMA. (2017) 317(6):589–91. doi: 10.1001/jama.2016.21189
33. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
34. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (2018) 362:k601. doi: 10.1136/bmj.k601
35. Yarmolinsky J, Relton CL, Lophatananon A, Muir K, Menon U, Gentry-Maharaj A, et al. Appraising the role of previously reported risk factors in epithelial ovarian cancer risk: a Mendelian randomization analysis. PLoS Med. (2019) 16(8):e1002893. doi: 10.1371/journal.pmed.1002893
36. Bovijn J, Jackson L, Censin J, Chen CY, Laisk T, Laber S, et al. GWAS identifies risk locus for erectile dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am J Hum Genet. (2019) 104(1):157–63. doi: 10.1016/j.ajhg.2018.11.004
37. Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. (2017) 14(10):577–90. doi: 10.1038/nrcardio.2017.78
38. Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiatry. (2019) 76(4):399–408. doi: 10.1001/jamapsychiatry.2018.4175
39. Xiong Y, Zhang FX, Zhang YC, Wu CJ, Qin F, Yuan JH. Genetically predicted insomnia causally increases the risk of erectile dysfunction. Asian J Androl. (2022). [Epub ahead of print]. doi: 10.4103/aja202261
40. Xiong Y, Zhong X, Zhang F, Wang W, Zhang Y, Wu C, et al. Genetic evidence supporting a causal role of snoring in erectile dysfunction. Front Endocrinol. (2022) 13:896369. doi: 10.3389/fendo.2022.896369
41. Wang M, Jian Z, Gao X, Yuan C, Jin X, Li H, et al. Causal associations between educational attainment and 14 urological and reproductive health outcomes: a Mendelian randomization study. Front Public Health. (2021) 9:742952. doi: 10.3389/fpubh.2021.742952
42. Zhang K, Gao H, Chen M. Genetic susceptibility to COVID-19 may increase the risk of erectile dysfunction: a two-sample Mendelian randomization study. Andrologia. (2022) 54(10):e14527. doi: 10.1111/and.14527
43. Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. (2010) 7(1 Pt 2):445–75. doi: 10.1111/j.1743-6109.2009.01624.x
44. Jiang R, Chen JH, Jin J, Shen W, Li QM. Ultrastructural comparison of penile cavernous tissue between hypertensive and normotensive rats. Int J Impot Res. (2005) 17(5):417–23. doi: 10.1038/sj.ijir.3901329
45. Aranda P, Ruilope LM, Calvo C, Luque M, Coca A, Gil de Miguel A. Erectile dysfunction in essential arterial hypertension and effects of sildenafil: results of a Spanish national study. Am J Hypertens. (2004) 17(2):139–45. doi: 10.1016/j.amjhyper.2003.09.006
46. Doumas M, Tsakiris A, Douma S, Grigorakis A, Papadopoulos A, Hounta A, et al. Factors affecting the increased prevalence of erectile dysfunction in Greek hypertensive compared with normotensive subjects. J Androl. (2006) 27(3):469–77. doi: 10.2164/jandrol.04191
47. Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. (2005) 294(23):2996–3002. doi: 10.1001/jama.294.23.2996
48. Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, Buvat J, et al. The Princeton III consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. (2012) 87(8):766–78. doi: 10.1016/j.mayocp.2012.06.015
49. Manolis A, Doumas M. Antihypertensive treatment and sexual dysfunction. Curr Hypertens Rep. (2012) 14(4):285–92. doi: 10.1007/s11906-012-0276-5
50. Chrysant SG. Antihypertensive therapy causes erectile dysfunction. Curr Opin Cardiol. (2015) 30(4):383–90. doi: 10.1097/HCO.0000000000000189
51. Doumas M, Tsakiris A, Douma S, Grigorakis A, Papadopoulos A, Hounta A, et al. Beneficial effects of switching from beta-blockers to nebivolol on the erectile function of hypertensive patients. Asian J Androl. (2006) 8(2):177–82. doi: 10.1111/j.1745-7262.2006.00076.x
52. Papatsoris AG, Korantzopoulos PG. Hypertension, antihypertensive therapy, and erectile dysfunction. Angiology. (2006) 57(1):47–52. doi: 10.1177/000331970605700107
53. Baumhakel M, Schlimmer N, Bohm M, Investigators D-I. Effect of irbesartan on erectile function in patients with hypertension and metabolic syndrome. Int J Impot Res. (2008) 20(5):493–500. doi: 10.1038/ijir.2008.28
54. Brouwers S, Sudano I, Kokubo Y, Sulaica EM. Arterial hypertension. Lancet. (2021) 398(10296):249–61. doi: 10.1016/s0140-6736(21)00221-x
55. Albuquerque DC, Miziara LJ, Saraiva JF, Rodrigues US, Ribeiro AB, Wajngarten M. Efficacy, safety and tolerability of sildenafil in Brazilian hypertensive patients on multiple antihypertensive drugs. Int Braz J Urol. (2005) 31(4):342–53, discussion 354–5. doi: 10.1590/s1677-55382005000400008
56. van Ahlen H, Wahle K, Kupper W, Yassin A, Reblin T, Neureither M. Safety and efficacy of vardenafil, a selective phosphodiesterase 5 inhibitor, in patients with erectile dysfunction and arterial hypertension treated with multiple antihypertensives. J Sex Med. (2005) 2(6):856–64. doi: 10.1111/j.1743-6109.2005.00150.x
Keywords: antihypertensive drug, hypertension, erectile dysfunction, Mendelian randomization, PDE5 inhibitor
Citation: Wang Z, Wang Y, Xiong J, Gan X, Bao Y, Jiang A, Zhou Y, Huangfu Z, Yang Y, Liu Z, Xia D and Wang L (2023) Causal effects of hypertension on risk of erectile dysfunction: A two-sample Mendelian randomization study. Front. Cardiovasc. Med. 10:1121340. doi: 10.3389/fcvm.2023.1121340
Received: 13 December 2022; Accepted: 17 February 2023;
Published: 21 March 2023.
Edited by:
Ouyang Chen, Duke University, United StatesReviewed by:
Haiyang Wu, Tianjin Medical University, ChinaYu Wei, Fudan University, China
Nengwang Yu, Shandong University, China
Ran Guo, Duke University, United States
© 2023 Wang, Wang, Xiong, Gan, Bao, Jiang, Zhou, Huangfu, Yang, Liu, Xia and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linhui Wang wanglinhui@smmu.edu.cn Demeng Xia demengxia@163.com
†These authors have contributed equally to this work
Specialty Section: This article was submitted to General Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
 Zheng Wang
Zheng Wang