- 1Department of Neurology, Shantou Central Hospital, Shantou, China
- 2School of Nursing, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 3Graduate School, Shantou University Medical College, Shantou, Guangdong, China
- 4Department of Cardiology, Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
- 5Graduate School, North China University of Science and Technology, Tangshan, China
- 6Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 7Department of Head and Neck, Cancer Hospital of Shantou University Medical College, Shantou, China
- 8Department of Cardiology, Kailuan General Hospital, Tangshan, China
Objective: We aimed to characterize the relationship of a combination of circulating non-high-density lipoprotein-cholesterol (non-HDL-C) concentration and brachial-ankle pulse wave velocity (baPWV) with cardiovascular disease (CVD).
Methods: We performed a prospective cohort study of the residents of the Kailuan community, with data from a total of 45,051 participants being included in the final analysis. The participants were allocated to four groups according to their non-HDL-C and baPWV status, each of which was categorized as high or normal. Cox proportional hazards models were used to explore the relationships of non-HDL-C and baPWV, individually and in combination, with the incidence of CVD.
Results: During the 5.04-year follow-up period, 830 participants developed CVD. Compared with the Normal non-HDL-C group independently, the multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for CVD in the High non-HDL-C was 1.25 (1.08–1.46). Compared with the Normal baPWV group independently, the HRs and 95% CIs for CVD in the High baPWV was 1.51 (1.29–1.76). In addition, compared with the Normal both non-HDL-C and baPWV group, the HRs and 95% CIs for CVD in the High non-HDL-C and normal baPWV, Normal non-HDL-C and high baPWV, and High both non-HDL-C and baPWV groups were 1.40 (1.07–1.82), 1.56 (1.30–1.88), and 1.89 (1.53–2.35), respectively.
Conclusion: High non-HDL-C concentration and high baPWV are independently associated with a higher risk of CVD, and individuals with high both non-HDL-C and baPWV are at a still higher risk of CVD.
1. Introduction
Arteriosclerosis is not only a sign of advanced vascular aging, but also a potent, independent risk factor for cardiovascular disease (CVD), renal failure, cognitive dysfunction, and all-cause mortality (1–4). There is a linear correlation between the severity of arteriosclerosis and CVD, and the Framingham study showed that each standard deviation (SD) increment in carotid-femoral artery pulse wave velocity (cfPWV) is associated with a 48% higher risk of cardiovascular disease (5, 6). Moreover, a meta-analysis of aortic pulse wave velocity (PWV) showed that an increase in aortic PWV of 1 m/s is associated with age-, sex-, and other risk factor-adjusted increases of 15, 14, and 15% in the risks of all-cause mortality, and total CVD morbidity and mortality, respectively (7).
In 2001, the National Cholesterol Education Program of the United States first proposed the utility of measuring the circulating concentration of non-high-density lipoprotein-cholesterol (non-HDL-C), which includes multiple types of lipoprotein and cholesterol that cause atherosclerosis, such as low-density lipoprotein-cholesterol (LDL-C) (8). However, no matter whether it is used to predict the morbidity or mortality associated with CVD or to evaluate the efficacy of lipid-lowering therapy, non-HDL-C has been shown to superior to LDL-C (9–11). Mora et al. (12) reported that female patients with LDL-C concentrations below the median value and non-HDL-C concentrations above the median have a threefold higher risk of CVD than those with non-HDL-C concentrations below the median value.
Atherosclerosis, as well as non-HDL-C specifically, is a risk factor for cardiovascular events. Several previous studies have shown a close correlation between non-HDL-C concentration and brachial-ankle pulse wave velocity (baPWV) (13–15). Many previous studies (16–18) have shown the correlation between non-HDL-C or baPWV with CVD separately, but few studies about the relationship of a combination of both non-HDL-C and baPWV with CVD. Therefore, to remedy this deficiency, we evaluated the relationship of non-HDL-C or baPWV with CVD separately, which was similar as previous studies. After that, we investigated the relationship of a combination of both non-HDL-C and baPWV with CVD. We used data from the Kailuan Study (Registration Number: CHICTR-TNRC-11001489) to explore the relationships of non-HDL-C and baPWV, individually and in combination, with CVD events.
2. Materials and methods
2.1. Study participants
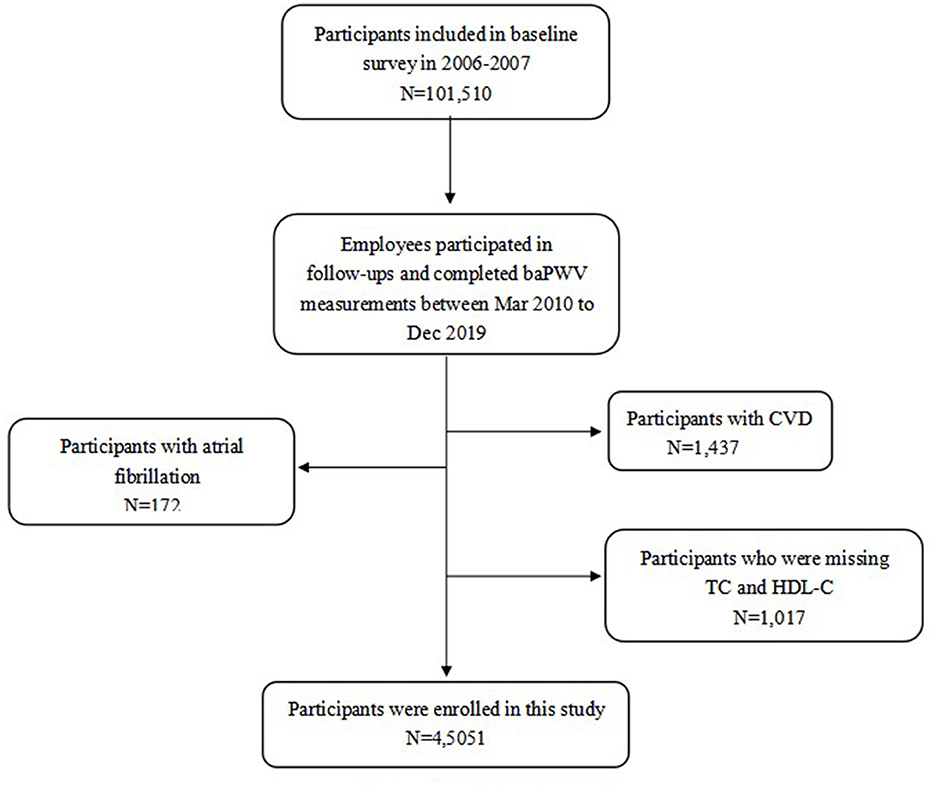
The Kailuan study (registration number: ChiCTR-TNRC-11001489) is an ongoing community-based study of the risk factors and interventions for CV and related diseases that commenced in 2006. According to standardized uniform design, face-to-face questionnaire interviews (demographic characteristics, disease history, lifestyles, etc.), physical examinations (body weight, height, waist circumference, blood pressure, etc.), and laboratory tests (fasting blood glucose, lipids profile, etc.) were conducted by trained physicians or nurses in every circle for 2 years, with a total of six being completed to date. In addition to the usual measurements performed on these occasions, including of blood lipid concentrations, baPWV measurement was commenced for some of the participants in 2010–2011. For the present study, individuals who participated in the follow-up examinations during 2010–2011, 2012–2013, 2014–2015, 2016–2017, and 2018–2019, and who underwent baPWV measurements, were studied. The study conformed with the Declaration of Helsinki and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006-05). All of the participants provided their written informed consent.
2.2. Inclusion and exclusion criteria
2.2.1. Inclusion criteria
① Individuals who participated in follow-up examinations during 2010/11, 2012/13, 2014/15, 2016/17, and 2017/18. ② Those who underwent baPWV measurements at each of these follow-up examinations. ③ Those who agreed to participate in the present study and provided their written informed consent.
2.2.2. Exclusion criteria
① Participants with a previous history of CVD. ② Those with electrocardiographic data suggestive of atrial fibrillation at the time of baPWV testing. ③ Those with missing data for circulating total cholesterol (TC), and HDL-C concentrations, as shown in Figure 1.
2.3. Data collection
2.3.1. Anthropometric parameters and related definitions
A standardized questionnaire was completed by members of the Kailuan study cohort at each follow-up visit to collect information regarding age, sex, lifestyle (smoking status, alcohol consumption status, and educational level), medical history, and the use of medication. Measurements of height, body mass, and blood pressure were performed by a professional according to a standard protocol and to 0.1 cm for height and 0.1 kg for body mass. A calibrated mercury column sphygmomanometer was used to measure the right brachial artery blood pressure, and three consecutive measurements were made at intervals of 1–2 min, and if the difference between the measurements exceeded 5 mmHg, a further three measurements were made. The mean of the three measurements was used in analyses. Body mass index (BMI) was calculated as body mass (kg)/height2 (m2). Physical activity was defined as aerobic exercise ≥3 times/week for ≥30 min on each occasion. A smoker was defined as someone who smoked a mean of at least one cigarette per day during the preceding year, and an alcohol consumer was defined as someone who drank a mean of 100 mL of liquor (50% alcohol or more) per day for at least 1 year. Hypertension was defined using a systolic blood pressure (SBP) ≥140 mmHg and/or a diastolic blood pressure (DBP) ≥90 mmHg, the use of anti-hypertensive drugs, or a self-reported history of hypertension (19). Diabetes mellitus was defined using a fasting blood glucose (FBG) ≥7 mmol/L, the use of hypoglycemic drugs, or a self-reported history of diabetes mellitus (20).
2.3.2. Biochemical index test
After fasting for at least 8 h, 5 mL of fasting elbow venous blood were drawn between 07:00 and 09:00 on the day of physical examination for the measurement of the TC, HDL-C, LDL-C, TG, Hs-CRP, and fasting blood glucose (FBG) concentrations. The serum concentrations of FBG, LDL-C, and HDL-C were measured by the hexokinase/glucose-6-phosphate dehydrogenase method, and direct method-surfactant clearance method, respectively, using a Hitachi 7600 autoanalyzer (Hitachi, Tokyo, Japan) at the central laboratory of Kailuan General Hospital.
Non-HDL-C concentration was calculated as the sum of the concentrations of lipoprotein-cholesterol other than HDL-C using non-HDL-C = TC – HDL-C.
2.3.3. baPWV testing
baPWV testing was performed on the day of follow-up examination between 07:00 and 09:00. The room temperature of the examination room was maintained at 22–25°C and measurements were made by trained professionals. A BP-203RPEIII networked atherosclerosis detection device [Omron Health Medical (China) Co., Ltd., Beijing, China] was used to measure baPWV, and the blood pressure values of the extremities were also recorded. The participants were asked not to smoke and to rest for more than 5 min prior to the measurements, and they remained supine during the test. The measurement was repeated twice for each participant and the second set of values obtained were used. The larger baPWV value for the left and right sides was used in the analyses.
2.4. Non-HDL-C and baPWV status
According to the 2016 Chinese Guidelines for the Management of Dyslipidemia in Adults (21), non-HDL status was classified as normal (<4.9 mmol/L, without the use of lipid-lowering drugs), or high (≥4.9 mmol/L or the use of lipid-lowering drugs). As in a previous study (22), we used age- and sex-specific cut-off values to categorize participants according to baPWV, because these two factors, age and sex, are key correlates of baPWV. Therefore, high baPWV was defined using a baPWV of less than the ≥5-year age- and sex-specific median value.
To determine the combined effects of high non-HDL-C and high baPWV, the participants were placed into four groups: Normal both non-HDL-C and baPWV, High non-HDL-C and normal baPWV, Normal non-HDL-C and high baPWV, and High both non-HDL-C and baPWV groups.
2.5. Outcomes
The start of the follow-up period was defined as the time point at which the participant underwent their first baPWV measurement and the end was defined as the time point when the individual participated in the 2018–2019 follow-up examination. The occurrence of a CVD was used as the endpoint.
The CVD outcomes were defined as myocardial infarction (MI) or ischemic stroke. We used ICD-10th revision codes to identify CVD cases I21 for MI, and I63 for ischemic stroke (23). The databases of CVD diagnoses were obtained from the Municipal Social Insurance Institution and the Hospital Discharge Register. These were updated annually during the follow-up period. An expert panel collected and reviewed the annual discharge records from the 11 participating hospitals to identify patients who were suspected of having CVD. A diagnosis of MI was made on the basis of the symptoms, electrocardiographic findings, and changes in myocardial enzyme activities, according to the World Health Organization's Multinational Monitoring of Trends and Determinants in Cardiovascular Disease criteria (24). Stroke was diagnosed on the basis of neurological signs, symptoms, and the findings of neuroimaging, including computed tomography and magnetic resonance imaging, according to the World Health Organization's criteria (25). For those who experienced two or more events, the time and nature of the first event was recorded as the endpoint, and those who did not experience an event underwent their last follow-up examination on December 31, 2019.
2.6. Statistical analysis
The baseline information used in this study was the data collected during the physical examination performed at the time of baPWV measurement. Normally distributed continuous data are expressed as mean ± standard deviation and one-way ANOVA was used for comparisons between multiple groups. Non-normally distributed continuous data were expressed as median (25th and 75th percentiles) and the Wilcoxon rank sum test was used for comparisons between groups. Categorical data were expressed as number of cases (%) and the chi-square test was used for comparisons between groups. Differences of basic characteristics between four groups were compared with Bonferoni correction.
The cumulative incidence of CVD for each group was calculated using the Kaplan-Meier method and they were compared using the log-rank test. First, multivariable Cox proportional hazards models were used to evaluate the individual relationships between non-HDL-C or baPWV and the incidence of CVD and to evaluate the relationships between the four groups and the incidence of CVD. We adjusted for age (continuous variable), sex, BMI (continuous variable), MAP (continuous variable), Hs-CRP concentration (continuous variable), LDL-C (continuous variable), smoking status (categorical variable, yes or no), alcohol consumption status (categorical variable, yes or no), physical activity (categorical variable, yes or no), educational level (categorical variable, yes or no), family history of diabetes (categorical variable, yes or no), and the use of anti-hypertensive agents (categorical variable, yes or no) and lipid-lowering drugs (categorical variable, yes or no) in the multivariable models. Covariates were selected on the basis of previous reports of their associations with CVD (26, 27).
The data were also analyzed after stratification according to age, sex, and whether or not to use lipid-lowering drugs. To test the robustness of the results, the following sensitivity analyses were performed: (1) the exclusion of individuals who developed CVD outcomes occurring within the first year of the study (n = 313); (2) the exclusion of individuals with ABI ≤ 0.9 (n = 967); (3) after the exclusion of participants who underwent treatment with anti-hypertensive, hypoglycemic, or lipid-lowering medications (n = 13,288). The data were analyzed using SAS v.9.4 (Cary, NC, USA) and differences were considered statistically significant when P < 0.05 (two-sided).
3. Results
3.1. Characteristics of the study participants
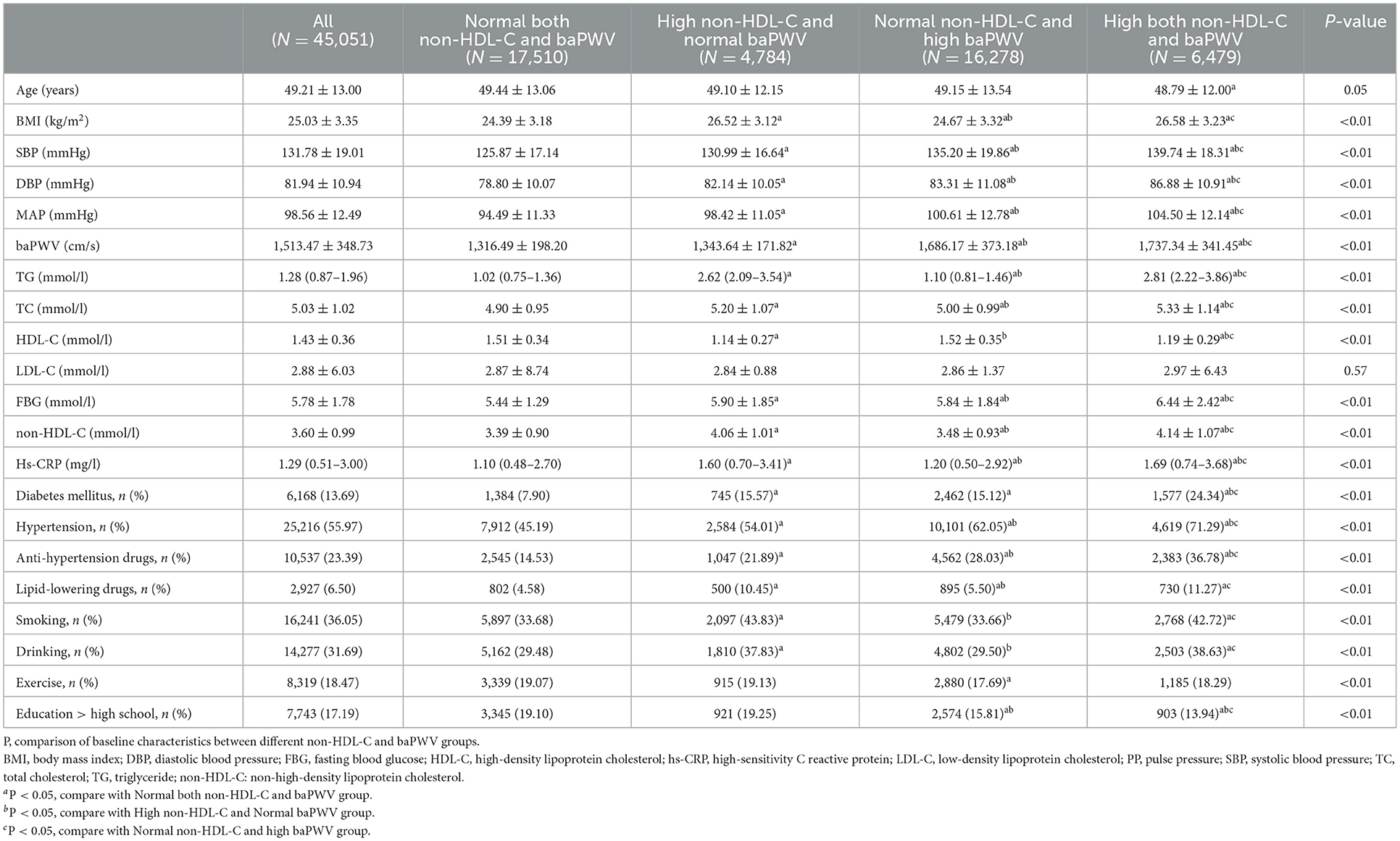
The mean age of the 45,051 participants was 49.21 ± 13.00 years. The mean serum non-HDL-C concentration was 3.60 ± 0.99 mmol/l and the mean baPWV was 1,513.5 ± 348.7 cm/s. The Normal both non-HDL-C and baPWV, High non-HDL-C and normal baPWV, Normal non-HDL-C and high baPWV, and High both non-HDL-C and baPWV groups contained 17,510, 4,784, 16,278, and 6,479 participants, respectively. Compared with Normal both non-HDL-C concentration and baPWV, those with High both non-HDL-C and baPWV were older; had higher SBP, DBP, BMI, and baPWV; had higher triglyceride (TG), TC, hs-CRP, and FBG concentrations; and had the highest prevalences of hypertension, diabetes, smoking, and the use of anti-hypertensive drugs (P < 0.01) (all P < 0.01; Table 1).
3.2. Cumulative incidence of CVD in the various groups
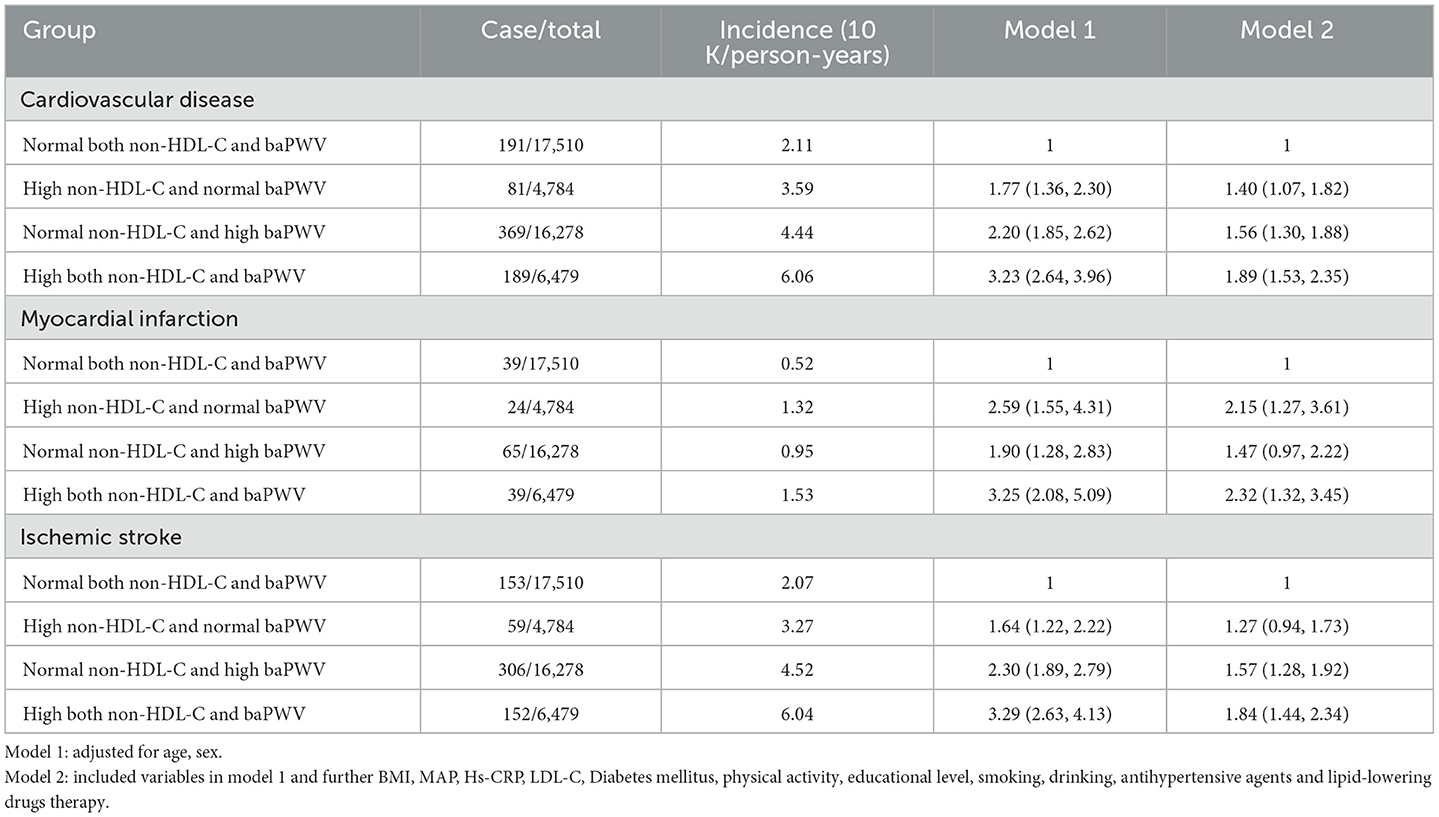
During a follow-up period of 5.04 ± 2.80 years, the cumulative incidences of CVD in the groups with High non-HDL-C and normal baPWV, Normal non-HDL-C and high baPWV, and High both non-HDL-C and baPWV showed a trend to increase vs. the group with normal non-HDL-C and normal baPWV (cumulative incidences of CVD for each group: 1.09, 2.96, 7.29, and 10.01; incidence densities for each group: 2.11/1,000, 3.59/1,000, 4.44/1,000, and 6.06/1,000 person-years; respectively); and the differences in the cumulative incidences of the endpoint events among the groups were statistically significant (log-rank test; all P < 0.05); as shown in Figure 2.

Figure 2. Kaplan–Meier incidence of CVD in groups with combination of normal or high non-HDL-C and baPWV. p < 0.05; log-rank test.
3.3. Relationships of high non-HDL-C concentration and high baPWV alone with the risk of CVD
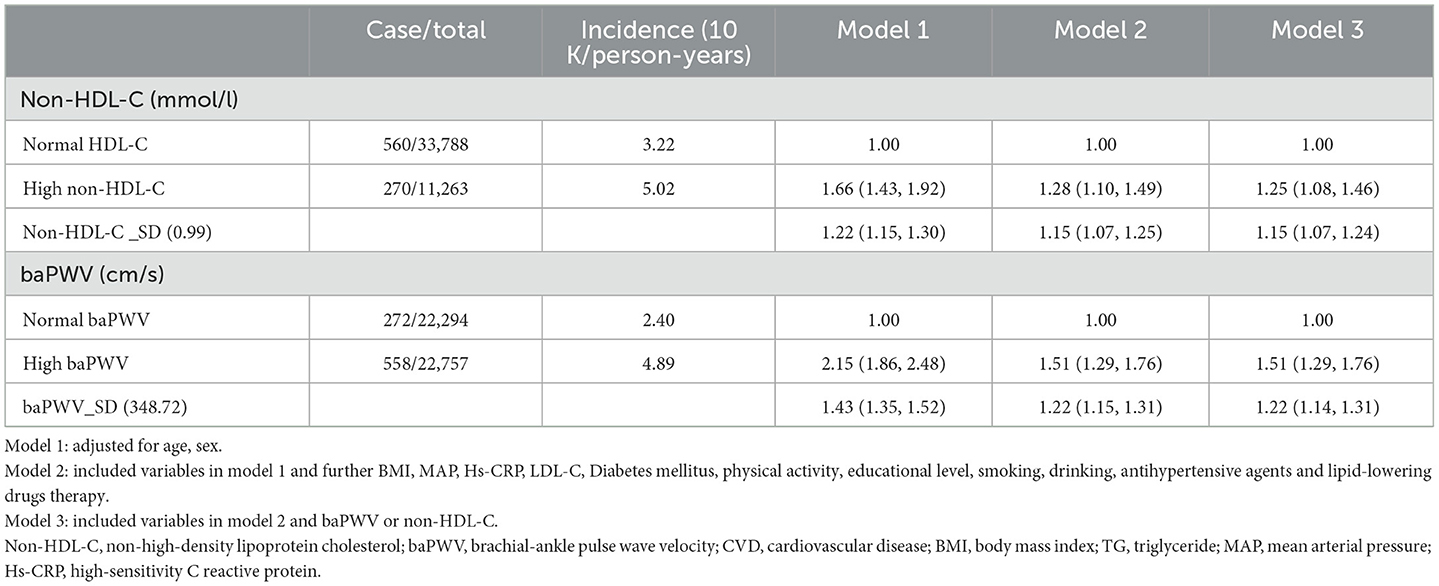
The results of multivariate Cox regression analysis, with the incidence of CVD as the dependent variable and the baPWV (Normal baPWV group as the control group) and non-HDL-C (Normal non-HDL-C group as the control group) subgroups as the independent variables, showed that compared with the Normal non-HDL-C group, and the HR for cardiovascular events was 1.15 (1.07–1.24) per standard deviation increase in non-HDL-C concentration. In addition, the HR for CVD was 1.51 (1.29–1.76) for the High baPWV group vs. the Normal baPWV group, and the HR for CVD was 1.22 (1.14–1.31) per standard deviation increase in baPWV, as shown in Table 2.
3.4. Relationships of non-HDL-C concentration and baPWV with the risk of CVD
Multivariate Cox regression analysis, with the occurrence of a CVD as the dependent variable and the various non-HDL-C and baPWV subgroups (Normal both non-HDL-C and baPWV group as the control group) as the independent variables, showed that compared with the Normal both non-HDL-C and baPWV group, the HRs for s in each group were 1.40 (1.07–1.82), 1.56 (1.30–1.88), and 1.89 (1.53–2.35), respectively (Table 3).
3.5. Results of the stratification and sensitivity analyses
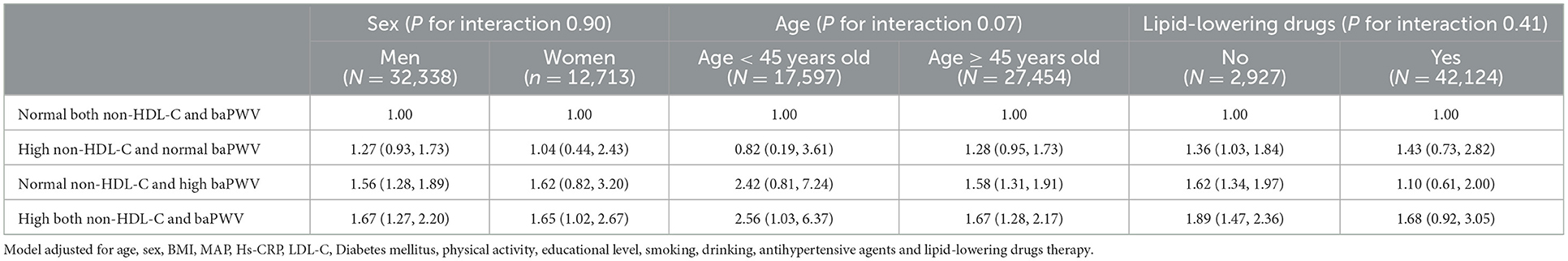
There were no significant interactions of age, sex for the association between non-HDL-C and baPWV and CVD. However, this result was invalidated in taking lipid-lowering medication populations, compared with the Normal both non-HDL-C and baPWV group, the HRs in each group were 1.43 (0.73,2.82), 1.10 (0.61,2.00), and 1.68 (0.92,3.05), respectively.
To exclude the effects of drugs and to verify their robustness, we repeated the multivariate Cox proportional risk regression analysis after the exclusion those participants who developed CVD the first year of the study, took anti-hypertensive, hypoglycemic, or lipid-lowering medications, and those with ABI ≤ 0.9. We found that the risk of CVD was higher in participants with both high non-HDL-C concentration and high baPWV, consistent with the results of the main outcome analysis, and as shown in Tables 4, 5.
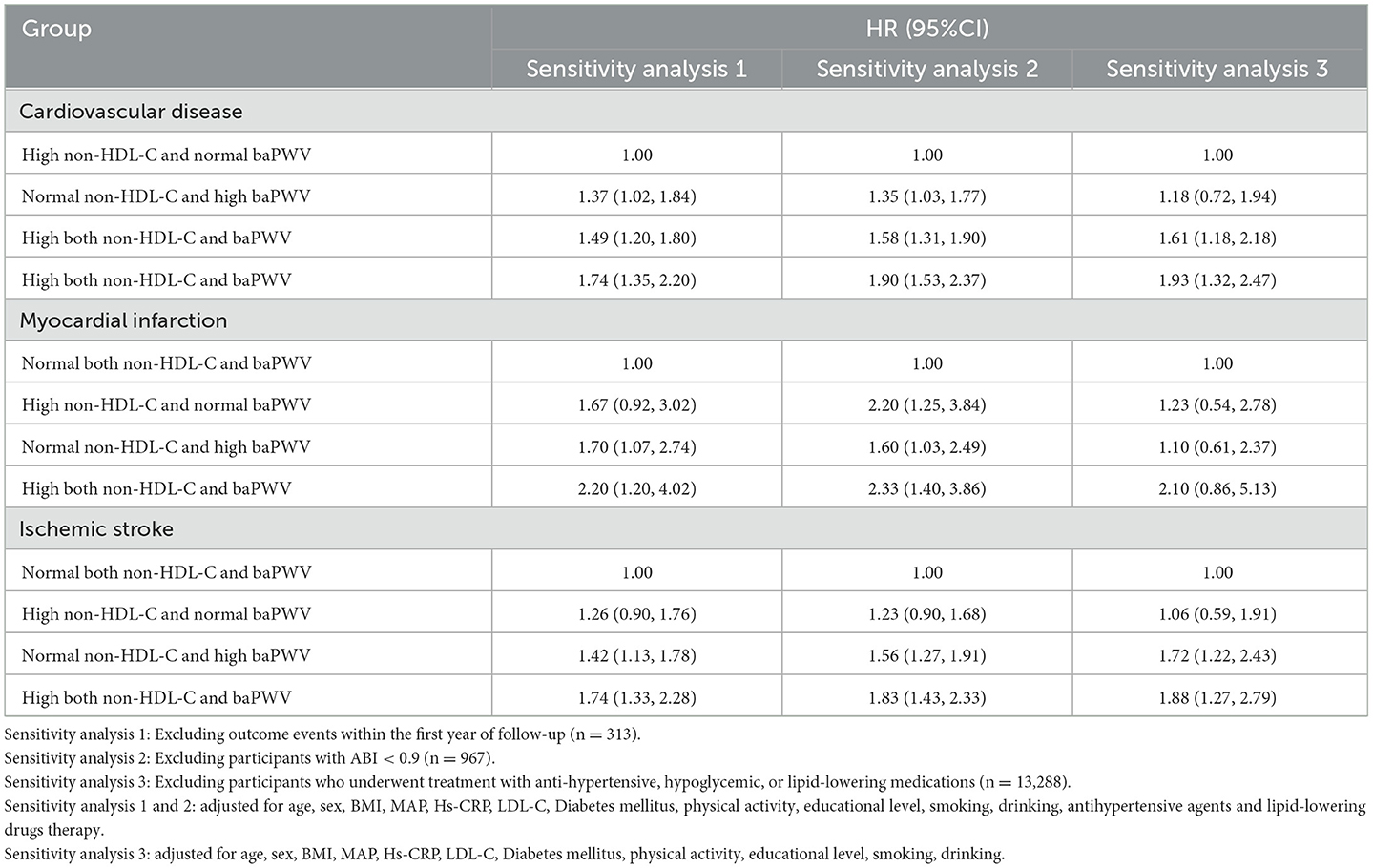
Table 5. Sensitivity analysis of the influence of the combined effects of non-HDL-C and baPWV on CVD.
4. Discussion
To the best of our knowledge, this is the largest longitudinal cohort study (45,051 participants, a 5.04-year follow-up period) conducted in China to explore the relationships of non-HDL-C concentration and baPWV with CVD. We found that both non-HDL-C and baPWV are positively associated with the risk of CVD. More importantly, a combination of high non-HDL-C concentration and high baPWV further increases the risk of CVD. In addition, taking lipid-lowering could remove those association.
In the present study, we found that relative to individuals with normal non-HDL-C or no arteriosclerosis, those with higher non-HDL-C concentration or arteriosclerosis tended to be at a higher risk of CVD. Moreover, participants with arteriosclerosis were at higher risk than those with a high non-HDL-C concentration. Previous studies have shown that the incidence and progression of arteriosclerosis are closely related to hypertension, smoking, obesity, and abnormal glucose and lipid metabolism. Not only abnormal lipid metabolism, but also a number of other factors, such as diabetes, hypertension, smoking, and obesity increase the risk of CVD (28–31). Therefore, compared to hypercholesterolemia, atherosclerosis has a larger impact on the incidence of CVD. The results of the present study are consistent with those of several previous large observational studies. Pencina et al. (18) showed that the risk of CVD in people with a high non-HDL-C concentration is three times higher than in people with a normal non-HDL-C concentration. In addition, in a meta-analysis of 14 Japanese cohort studies consisting of a total of 14,673 observations, Ohkuma et al. (17) found that the risk of CVD in the highest quintile group of baPWV is 3.50 times higher than that of the lowest quintile group. The results of these studies implied that both non-HDL-C and baPWV could be used as independent predictors of the risk of CVD.
In addition, we found that the highest risk of CVD was in individuals with both high non-HDL-C and high baPWV, and that this was 1.89 times higher than in individuals with both normal non-HDL-C concentration and baPWV. Although the present study is the first to demonstrate the combined effect of non-HDL-C and baPWV on the risk of CVD, similar analyses (32–34) of the combined effects of lipids and blood pressure on CVD risk have been performed previously. Yang et al. (34) showed that individuals with both hypertension and hypercholesterolemia are at a higher risk of CVD than those with hypertension or hypercholesterolemia alone in a study of 5,092 participants who were followed for 20.84 years.
Moreover, we repeated the analysis after the exclusion of outcome events within the first year of follow-up, participants with ABI < 0.9 and participants who underwent treatment with anti-hypertensive, hypoglycemic, or lipid-lowering medications. We found that the strength of association between non-HDL-C and IM or ischemic were weaker. The results are similar with those of several previous studies (35–38). Okamura et al. (35) showed that nor non-HDL-C cannot predict any subtype of stroke risk. Noda et al. (36) showed that higher concentrations of non-HDL-C were associated with an increased risk of mortality from coronary heart disease for men, but not for women. These inconsistent results may be caused by the differences in study design and method, participants' characteristics, and the covariates adjusted in the multivariate models (39).
The mechanism underpinning the relationship of a combination of arteriosclerosis and high non-HDL-C concentration with a higher risk of CVD remains poorly understood. On the basis of previous findings, we considered that it may involve the following. First, high non-HDL-C concentrations result in cholesterol accumulation in the arterial wall, which leads to atherosclerosis. Subsequently, the oxidative stress and chronic low-grade inflammation induced by the excessive lipid deposition stimulate leukocytes to release a variety of cytokines and adhesion molecules, causing them to adhere to the vascular endothelium and penetrate the intima, thereby increasing vascular resistance, leading to atherosclerosis and endothelial dysfunction, and ultimately increasing the risk of CVD (40–45).
Our study findings have important clinical implications. As we know, high baPWV and high non-HDL-C were positively associated with the risk of CVD independently. Our findings underscore that a higher risk of CVD in individuals with a combination of both high non-HDL-C and baPWV than in those with one of them alone. Furthermore, participants who underwent treatment with lipid-lowering medications could decrease the risk and remove those association between non-HDL-C and baPWV and CVD. Therefore, early identification of high non-HDL-C and high baPWV in the general population, should greatly assist the prevention of CVD, and lipid-lowering medications, such as statins are an effective treatment to reduce CVD for those individuals with high non-HDL-C or high baPWV.
There were several limitations to the present study. First, we assessed arterial stiffness using baPWV, rather than cfPWV, which is considered to be the gold standard method of evaluation. However, previous studies have shown a close correlation between baPWV and cfPWV (46). Second, the duration of follow-up was not very consistent among the participants, which may have influenced the findings. Third, most of the participants were men of a single ethnicity; therefore, the findings cannot be directly extrapolated to other ethnic groups. However, the Kailuan cohort comprises a large group with diverse occupations that has been closely followed; therefore, the present findings are likely to be representative and reliable. Four, we only gathered the all-cause mortality but not specific cause of death, which is the constraint on some outcomes to attain.
In summary, we have shown a higher risk of CVD in individuals with a combination of both high non-HDL-C and high baPWV than in those with one of them alone. Moreover, the closer relationship of high baPWV suggests a larger contribution to the risk. In addition, which taking lipid-lowering could remove those association between non-HDL-C and baPWV and CVD. Therefore, early identification of high non-HDL-C and high baPWV, and especially the measurement of baPWV in the general population, should greatly assist the prevention of CVD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Kailuan General Hospital (Approval Number: 2006-05). The patients/participants provided their written informed consent to participate in this study.
Author contributions
QZ, HW, XW, YL, WW, XY, and ZH designed the study idea. ZKC, ZFC, QL, HSZ, ML, and YZ analyzed and interpreted the data. QZ, YL, XW, QL, HSZ, YZ, KW, and HCZ drafted the manuscript. SW and YC reviewed the manuscript. All authors have read and approved the final manuscript.
Funding
This work has been supported by the National Natural Science Foundation of China (No. 15 81870312).
Acknowledgments
We appreciate all the participants of the Kailuan Study and the members of the Kailuan General Hospital and its affiliated hospitals.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
non-HDL-C: non-high-density lipoprotein cholesterol; baPWV, brachial-ankle pulse wave velocity; CVD, cardiovascular disease; TG, triglyceride; FPG, fasting plasma glucose; BMI, body mass index; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; TC, total cholesterol; Hs-CRP, high-sensitivity C-reactive protein; HDL-C, High-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HR, hazard ratio; CI, confidence interval.
References
1. Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. The metabolic syndrome: a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8
2. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. (2010) 12:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655
3. Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension. (2004) 43:163–8. doi: 10.1161/01.HYP.0000114571.75762.b0
4. Sharif S, Visseren FLJ, Spiering W, de Jong PA, Bots ML, Westerink J, et al. Arterial stiffness as a risk factor for cardiovascular events and all-cause mortality in people with Type 2 diabetes. Diabet Med. (2019) 36:1125–32. doi: 10.1111/dme.13954
5. Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. (2013) 81:984–91. doi: 10.1212/WNL.0b013e3182a43e1c
6. Vasan RS, Pan S, Xanthakis V, Beiser A, Larson MG, Seshadri S, et al. Arterial stiffness and long-term risk of health outcomes: the Framingham Heart Study. Hypertension. (2022) 79:1045–56. doi: 10.1161/HYPERTENSIONAHA.121.18776
7. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55:1318–27. doi: 10.1016/j.jacc.2009.10.061
8. Cleeman J. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
9. Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. (2005) 112:3375–83. doi: 10.1161/CIRCULATIONAHA.104.532499
10. HPS3/TIMI55–REVEAL Collaborative Group; Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. (2017) 377:1217–27. doi: 10.1056/NEJMoa1706444
11. Cholesterol Treatment Trialists' (CTT) Collaboration; Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. (2010) 376:1670–81. doi: 10.1016/S0140-6736(10)61350-5
12. Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. (2014) 129:553–61. doi: 10.1161/CIRCULATIONAHA.113.005873
13. Wen J, Huang Y, Lu Y, Yuan H. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertens Res. (2019) 42:1223–30. doi: 10.1038/s41440-019-0251-5
14. de Oliveira Alvim R, Mourao-Junior CA, Magalhães GL, de Oliveira CM, Krieger JE, Mill JG, et al. Non-HDL cholesterol is a good predictor of the risk of increased arterial stiffness in postmenopausal women in an urban Brazilian population. Clinics. (2017) 72:106–10. doi: 10.6061/clinics/2017(02)07
15. Wang Z, Li M, Xie J, Gong J, Liu N. Association between remnant cholesterol and arterial stiffness: a secondary analysis based on a cross-sectional study. J Clin Hypertens. (2022) 24:26–37. doi: 10.1111/jch.14384
16. Aggarwal DJ, Kathariya MG, Verma DPK. LDL-C, NON-HDL-C, and APO-B for cardiovascular risk assessment: looking for the ideal marker. Indian Heart J. (2021) 73:544–8. doi: 10.1016/j.ihj.2021.07.013
17. Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. (2017) 69:1045–52. doi: 10.1161/HYPERTENSIONAHA.117.09097
18. Pencina KM, Thanassoulis G, Wilkins JT, Vasan RS, Navar AM, Peterson ED, et al. Trajectories of non-HDL cholesterol across midlife: implications for cardiovascular prevention. J Am Coll Cardiol. (2019) 74:70–9. doi: 10.1016/j.jacc.2019.04.047
19. Williams B, Mancia G, Spiering W, Agabiti W, Rosei E, Azizi M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy439
20. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33(Suppl 1):S62–9. doi: 10.2337/dc10-S062
21. Joint Committee Issued Chinese Guideline for the Management of Dyslipidemia in Adults. 2016 Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. (2016) 44:833–53. doi: 10.3760/cma.j.issn.0253-3758.2016.10.005
22. Niiranen TJ, Kalesan B, Mitchell GF, Vasan RS. Relative contributions of pulse pressure and arterial stiffness to cardiovascular disease. Hypertension. (2019) 73:712–7. doi: 10.1161/HYPERTENSIONAHA.118.12289
23. Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. (2020) 75:2921–30. doi: 10.1016/j.jacc.2020.04.038
24. Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. (1994) 90:583–612. doi: 10.1161/01.CIR.90.1.583
25. WHO TF. Stroke-1989. Recommendations on stroke prevention, diagnosis, and therapy Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.STR.20.10.1407
26. Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. (2003) 290:891–7. doi: 10.1001/jama.290.7.891
27. Grundy SM, Pasternak R, Greenland P, Smith S, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. (1999) 100:1481–92. doi: 10.1161/01.CIR.100.13.1481
28. Tomiyama H, Shiina K. State of the art review: Brachial-Ankle PWV. J Atheroscler Thromb. (2020) 27:621–36. doi: 10.5551/jat.RV17041
29. Tomiyama H, Hashimoto H, Hirayama Y, Yambe M, Yamada J, Koji Y, et al. Synergistic acceleration of arterial stiffening in the presence of raised blood pressure and raised plasma glucose. Hypertension. (2006) 47:180–8. doi: 10.1161/01.HYP.0000198539.34501.1a
30. Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res. (2010) 33:398–410. doi: 10.1038/hr.2010.25
31. Tomiyama H, Shiina K, Vlachopoulos C, Iwasaki Y, Matsumoto C, Kimura K, et al. Involvement of arterial stiffness and inflammation in hyperuricemia-related development of hypertension. Hypertension. (2018) 72:739–45. doi: 10.1161/HYPERTENSIONAHA.118.11390
32. Wei L, Sun J, Xie H, Zhuang Q, Wei P, Zhao X, et al. Interaction analysis of abnormal lipid indices and hypertension for ischemic stroke: a 10-year prospective cohort study. Front Cardiovasc Med. (2022) 9:819274. doi: 10.3389/fcvm.2022.819274
33. Thomas F, Bean K, Guize L, Quentzel S, Argyriadis P, Benetos A. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (< 55 years) men and women. Eur Heart J. (2002) 23:528–35. doi: 10.1053/euhj.2001.2888
34. Yang Y, Li JX, Chen JC, Cao J, Lu XF, Chen SF, et al. Effect of elevated total cholesterol level and hypertension on the risk of fatal cardiovascular disease: a cohort study of Chinese steelworkers. Chin Med J. (2011) 124:3702–6. doi: 10.3760/cma.j.issn.0366-6999.2011.22.018
35. Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: the Suita study. Atherosclerosis. (2009) 203:587–92. doi: 10.1016/j.atherosclerosis.2008.07.020
36. Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H. Association between non-high-density lipoprotein cholesterol concentrations and mortality from coronary heart disease among Japanese men and women: the Ibaraki Prefectural Health Study. J Atheroscler Thromb. (2010) 17:30–6. doi: 10.5551/jat.1016
37. Imamura T, Doi Y, Ninomiya T, Hata J, Nagata M, Ikeda F, et al. Non-high-density lipoprotein cholesterol and the development of coronary heart disease and stroke subtypes in a general Japanese population: the Hisayama Study. Atherosclerosis. (2014) 233:343–8. doi: 10.1016/j.atherosclerosis.2014.01.005
38. Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, et al. Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events: the JALS-ECC. Circ J. (2010) 74:1346–56. doi: 10.1253/circj.CJ-09-0861
39. Tang M, Zhao Q, Yi K, Wu Y, Xiang Y, Cui S, et al. Association between four non-traditional lipids and ischemic stroke: a cohort study in Shanghai, China. Lipids Health Dis. (2022) 21:72. doi: 10.1186/s12944-022-01683-1
40. Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. (2018) 123:825–48. doi: 10.1161/CIRCRESAHA.118.312563
41. Chung TH, Shim JY, Kwon YJ, Lee YJ. High triglyceride to high-density lipoprotein cholesterol ratio and arterial stiffness in postmenopausal Korean women. J Clin Hypertens. (2019) 21:399–404. doi: 10.1111/jch.13484
42. Wang L, Zhi F, Gao B, Ni J, Liu Y, Mo X, et al. Association between lipid profiles and arterial stiffness: a secondary analysis based on a cross-sectional study. J Int Med Res. (2020) 48:300060520938188. doi: 10.1177/0300060520938188
43. Hansson GK. Inflammation and atherosclerosis: the end of a controversy. Circulation. (2017) 136:1875–7. doi: 10.1161/CIRCULATIONAHA.117.030484
44. Mozos I, Luca CT. Crosstalk between oxidative and nitrosative stress and arterial stiffness. Curr Vasc Pharmacol. (2017) 15:446–56. doi: 10.2174/1570161115666170201115428
45. Veiraiah A. Hyperglycemia, lipoprotein glycation, and vascular disease. Angiology. (2005) 56:431–8. doi: 10.1177/000331970505600411
Keywords: non-high-density lipoprotein cholesterol, brachial-ankle pulse wave velocity, cardiovascular diseases, Kailuan cohort, combined effect
Citation: Zheng Q, Wang H, Wang X, Lan Y, Wu W, Yu X, Huang Z, Chen Z, Cai Z, Lin Q, Zhou H, Zhu Y, Liu M, Wu K, Zheng H, Wu S and Chen Y (2023) Individual and combined contributions of non-high-density lipoprotein cholesterol and brachial-ankle pulse wave velocity to cardiovascular disease risk: Results of a prospective study using the Kailuan cohort. Front. Cardiovasc. Med. 10:1105464. doi: 10.3389/fcvm.2023.1105464
Received: 22 November 2022; Accepted: 18 January 2023;
Published: 09 February 2023.
Edited by:
Pasqualino Sirignano, Sapienza University of Rome, ItalyReviewed by:
Vittoria Cammisotto, Sapienza University of Rome, ItalyAntonio Gallo, Hôpitaux Universitaires Pitié Salpêtrière, France
Copyright © 2023 Zheng, Wang, Wang, Lan, Wu, Yu, Huang, Chen, Cai, Lin, Zhou, Zhu, Liu, Wu, Zheng, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shouling Wu,  ZHJ3dXNsQDE2My5jb20=; Youren Chen,
ZHJ3dXNsQDE2My5jb20=; Youren Chen,  eXJjaGVuM0BzdHUuZWR1LmNu
eXJjaGVuM0BzdHUuZWR1LmNu
†These authors have contributed equally to this work
 Qiongbing Zheng1†
Qiongbing Zheng1† Xianxuan Wang
Xianxuan Wang Zegui Huang
Zegui Huang Muyuan Liu
Muyuan Liu Shouling Wu
Shouling Wu Youren Chen
Youren Chen