- 1Department of Pharmacy, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
- 2Department of Pharmacy, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou, China
- 3School of Acupuncture and Tuina, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background: Danlou tablets exert auxiliary advantages in treating coronary heart disease (CHD), but a summary of evidence-based proof is lacking. This study aims to systematically evaluate Danlou tablets in treating CHD from two aspects, including efficacy and safety.
Methods: By a thorough retrieval of the four English databases, namely, PubMed, The Cochrane Library, Embase, and Web of Science, and the four Chinese databases, namely, CNKI, Wanfang, VIP database, and China Biomedical Literature Service System, we found all randomized controlled trials (RCTs) related to Danlou tablets in treating CHD. The retrieval time was from the construction of the database to April 2022. We engaged two researchers to screen the studies, extract the required data, and assess the risk of bias. We then used RevMan5.3 and STATA.14 software to conduct a meta-analysis. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to evaluate the quality of outcome indicators.
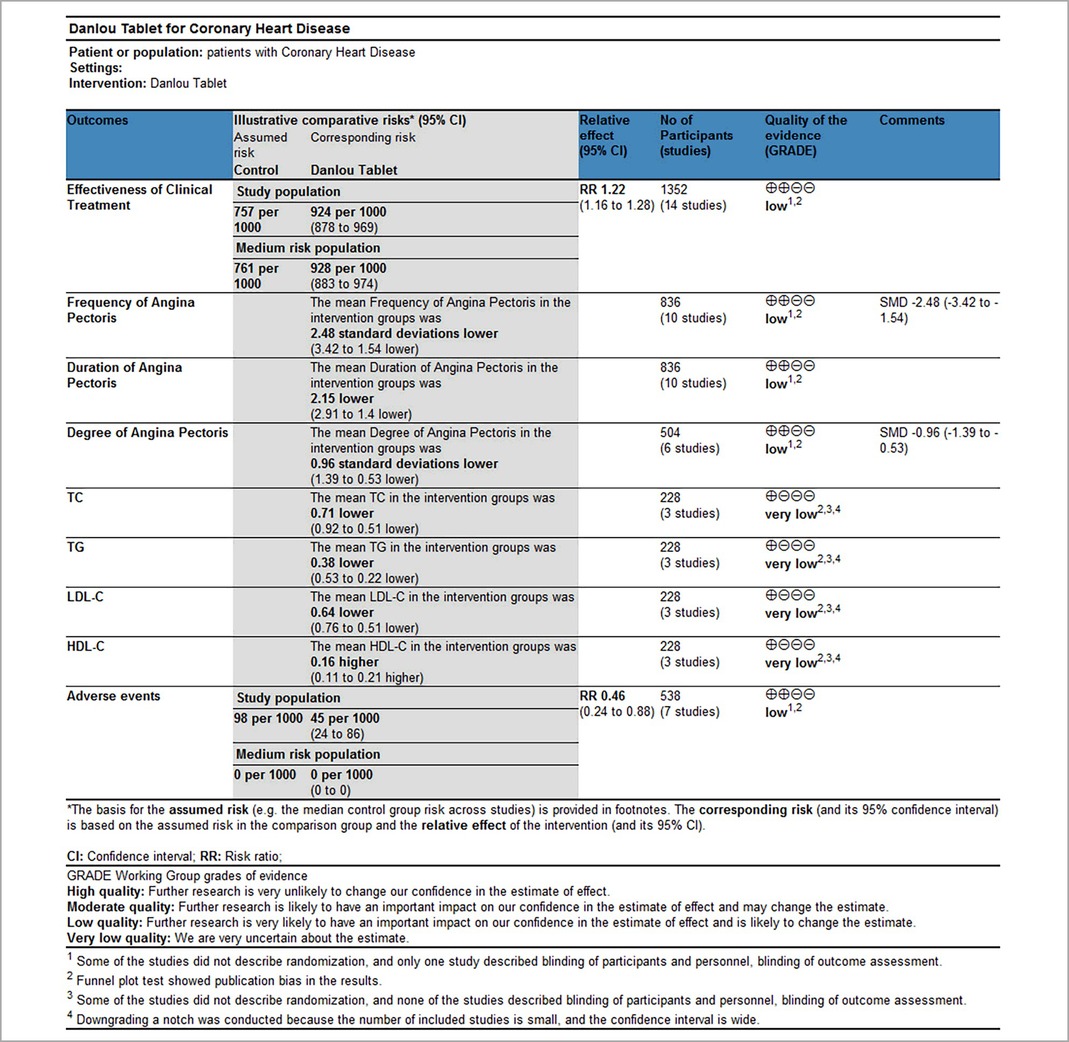
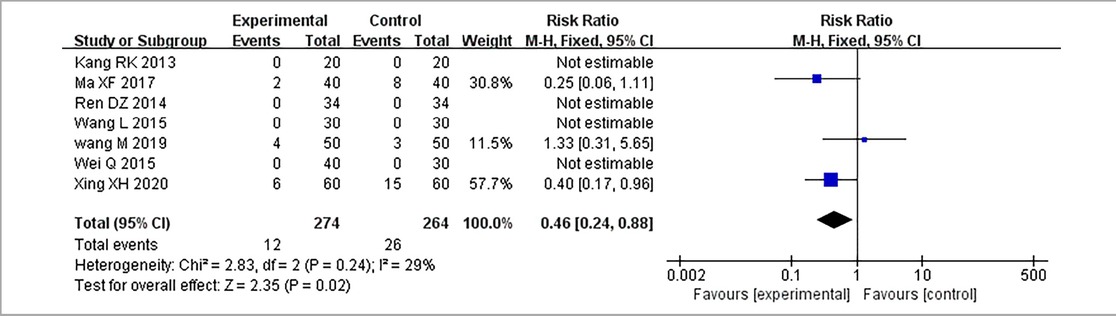
Results: Seventeen RCTs involving 1,588 patients were included. The meta-analysis results are displayed as follows: clinical treatment effect [risk ratio (RR) = 1.22, 95% confidence interval (CI): 1.16, 1.28, P < 0.00001], angina pectoris duration [MD = −0.2.15, 95% CI: −2.91, −1.04, P < 0.00001], angina pectoris frequency [standard mean difference (SMD) = −2.48, 95% CI: −3.42, −1.54, P < 0.00001], angina pectoris degree [SMD = −0.96, 95% CI: −1.39, −0.53, P < 0.0001], TC [MD = −0.71, 95% CI: −0.92, −0.51, P < 0.00001], TG [MD = −0.38, 95% CI: −0.53, −0.22, P < 0.00001], low-density lipoprotein cholesterol [MD = −0.64, 95% CI: −0.76, −0.51, P < 0.00001], high-density lipoprotein cholesterol [MD = 0.16, 95% CI: 0.11, 0.21, P < 0.00001], and adverse events [RR = 0.46, 95% CI: 0.24, 0.88, P = 0.02].
Conclusion: The current evidence suggests that the combination of Danlou tablets and Western medicine can enhance the efficacy of CHD and does not increase adverse events. However, because of the limited number and quality of the included studies, the results of our study should be treated with caution. Further large-scale RCTs are necessary to verify the benefits of this approach.
1. Introduction
Cardiovascular and cerebrovascular diseases remain the dominant causes of death (1). Cardiovascular disease killed 17.9 million people worldwide in 2016, according to the World Health Organization, of which about 34% were due to coronary heart disease (CHD). An estimated 23.3 million cardiovascular deaths will occur in 2030 (2, 3). CHD seriously harms human health and aggravates the public health burden with its extremely high mortality (1). CHD is a heart disease caused by myocardial ischemia and hypoxia resulting from coronary atherosclerosis that causes lumen hardening, stenosis, or obstruction (4). CHD patients mainly present chest tightness, chest pain, angina pectoris, and other symptoms, which may cause sudden death in severe cases, significantly affecting patients' daily lives and even threatening their lives (5).
CHD is caused by a combination of personal constitution, genetic background, and environmental factors, and its symptoms involve multiple organs and tissues, eventually spreading to the whole body (6). In the development of the disease, different groups present different clinical characteristics due to differences in personal fitness, environment, genetic background, and other factors. Individualized therapy has become a future development trend in medicine (7). Traditional Chinese medicine (TCM) emphasizes individualized treatment, of which the TCM syndrome is the core of diagnosis and the key to treatment (6). According to the characteristics of various symptoms, CHD is mainly divided into blood stasis, phlegm turbidity, cold coagulation, qi deficiency, yin deficiency, and yang deficiency syndromes (8). For example, as a representative of Chinese patent medicine for blood stasis syndrome, Shexiang Baoxin pills can improve the curative effect of Western medicine in treating CHD according to evidence-based medical research (9) and are strongly recommended for treating CHD and angina pectoris in the prevention and treatment guidelines for CHD in China (10). Danlou tablets, a representative medicine of TCM in treating CHD with blood stasis and phlegm turbidity syndrome, have the effects of clearing qi, comforting the chest, relieving phlegm, dispersing the knot, invigorating blood circulation, and eliminating stasis (11). They can also significantly reduce atherosclerosis, ischemia, and reperfusion injury and improve myocardial dysfunction (12). Experimental studies have indicated that these tablets regulate oxidative stress, prevent inflammation, and reduce lipid deposition (13).
The results of a large-scale survey involving 5,284 patients with coronary disease displayed the top three TCM syndromes: blood stasis (79.3%), qi deficiency (56.5%), and phlegm turbidity (41.1%) (7). Compared with Shexiang Baoxin pills, Danlou tablets seem suitable for more CHD patients. However, in the Clinical Application Guidelines for the Treatment of CHD of Chinese Patent Medicine (2020), the recommended intensity of Danlou tablets in treating CHD is weak (14). Therefore, more high-quality clinical studies and standardized systematic reviews on Danlou tablets in treating coronary heart disease are needed. Some clinical studies on Danlou tablets in treating coronary heart disease have been published. However, the design methods, efficacy evaluation criteria, and treatment courses of different studies are different. Therefore, our systematic evaluation aims to provide evidence-based support for the guidance of clinical and scientific medical work by evaluating the effectiveness and safety of Danlou tablets in treating CHD.
2. Methods
2.1. Study registration
This study was conducted per the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (the checklist is shown in Supplementary Table S1). In addition, the protocol and registration information can be found at https://www.crd.york.ac.uk/prospero/#searchadvanced (registration number: CRD42021274916).
2.2. Inclusion criteria
(1) Research type: Randomized controlled trials (RCTs) were included.
(2) Diagnostic criteria: Patients diagnosed with CHD, which belongs to phlegm turbidity, blood stasis, or phlegm turbidity combined with blood stasis in TCM, were included. CHD diagnostic criteria refer to the nomenclature and criteria for the diagnosis of ischemic heart disease formulated by the WHO (15). The TCM syndrome diagnostic criteria refer to the Guiding Principles for Clinical Research of TCM New Drugs. There is no limit on nationality, race, age, gender, or disease duration.
(3) Interventions: The treatment group was treated orally with Danlou tablets combined with Western medicine, and the control group was given the same Western medicine alone or combined with Danlou tablets as a placebo.
(4) Outcome indicators: The outcomes are as follows:
(1) Primary outcomes: These involved clinical treatment effects. According to WHO criteria, the efficacy of CHD is divided into the following categories (5): Significantly effective: angina pectoris symptoms disappeared obviously and electrocardiogram (ECG) was normal; effective: angina pectoris symptoms improved to a certain extent and the ECG was improved; ineffective: angina symptoms were not relieved and the ECG was not changed. The clinical treatment effect represents the proportion of significantly and effectively effective patients to total patients.
(2) Secondary outcomes: These involved improvement of angina pectoris (including the frequency, duration, and pain degree of angina pectoris); it was defined as an improvement of angina pectoris only when the frequency, duration, and pain degree of angina pectoris were all improved. Angina frequency was defined as the number of angina attacks per day or week after treatment. The duration of angina pectoris was defined as the duration of each episode of angina pectoris after the end of treatment, measured in minutes. The degree of pain from angina should be evaluated as the angina score at the end of treatment. Blood lipid improvement [including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)] and the incidence of adverse events were also recorded.
2.3. Exclusion criteria
(1) Studies without diagnostic criteria or with diagnostic criteria errors;
(2) Studies without outcome indicators or with incorrect or incomplete data;
(3) Studies including patients after percutaneous coronary intervention (PCI); and
(4) Repetitive studies or studies with duplicate study data.
2.4. Search methods for identifying studies
The RCTs of Danlou tablets in treating CHD were comprehensively searched in Chinese and English databases from the database establishment to April 2021. Retrieval databases included PubMed, the Cochrane Library, CNKI, EMBASE, Web of Science, Wanfang, and VIP database. The search terms were “Danlou tablet,” “coronary diseases,” “coronary heart diseases,” “angina pectoris,” etc. Supplementary Table S2 shows the search strategy in PubMed.
2.5. Data collection and analysis
2.5.1. Data extraction and management
Two researchers (PL and RW) screened the literature back to back, referring to the criteria above, and then cross-checked. A third researcher (WM) discussed the results after cross checking for any disagreement. After confirming the final included studies, we carefully read the complete study and extracted the required data.
The extracted data included the following: (1) general information (first author, publication year, samples in each group, baseline information, course of disease); (2) treatment protocol (name, medication frequency, and treatment course of oral drugs in each group); (3) risk bias assessment factors in RCTs; and (4) outcome indicators.
2.5.2. Assessment of risk of bias
We assessed the risk bias according to the RCT risk assessment tool recommended by the Cochrane Collaboration Handbook. Two researchers (PL and RW) completed risk bias assessments for each study. After completion, they cross-checked the data, and discussion was required in case of differences. If no agreement could be reached, negotiation with a third researcher (WM) was performed, and a final consensus would be reached.
2.5.3. Data synthesis
RevMan5.3 software was used for data synthesis. The dichotomous variables were expressed as a relative risk ratio (RR); for continuous outcomes, the weighted mean difference (WMD) and standard mean difference (SMD) were used as effect sizes for consistency and inconsistency between measuring tools and measuring units, respectively. All were presented as 95% confidence intervals (CIs). We used χ2 and I2 values to determine the heterogeneity. P ≥ 0.1, I2 ≤ 50% indicated low heterogeneity, and we chose a fixed-effects model. P < 0.1, I2 > 50% indicated significant heterogeneity, and then the heterogeneity was analyzed. We used subgroup or sensitivity analysis to explore the origin of heterogeneity and then used a random-effects model for merging after excluding apparent clinical and methodological heterogeneity. As recommended in the Cochrane manual, one-by-one elimination would be used in the sensitivity analysis to test the stability of meta-analysis results of indicators. For the primary outcome indicators, the publication bias was qualitatively detected by a funnel plot, and the potential publication bias was quantitatively evaluated by Egger's and Begg's tests.
2.5.4. Evidence quality evaluation
Two researchers (PL and RW) graded the outcome indicators by the Grading of Recommendation, Development, and Evaluation (GRADE) (16). Similarly, after completing the assessment, the two researchers cross-checked with each other, discussed with the third researcher (WM) for any disagreement, and finally reached a consensus.
3. Results
3.1. Studies' characteristics
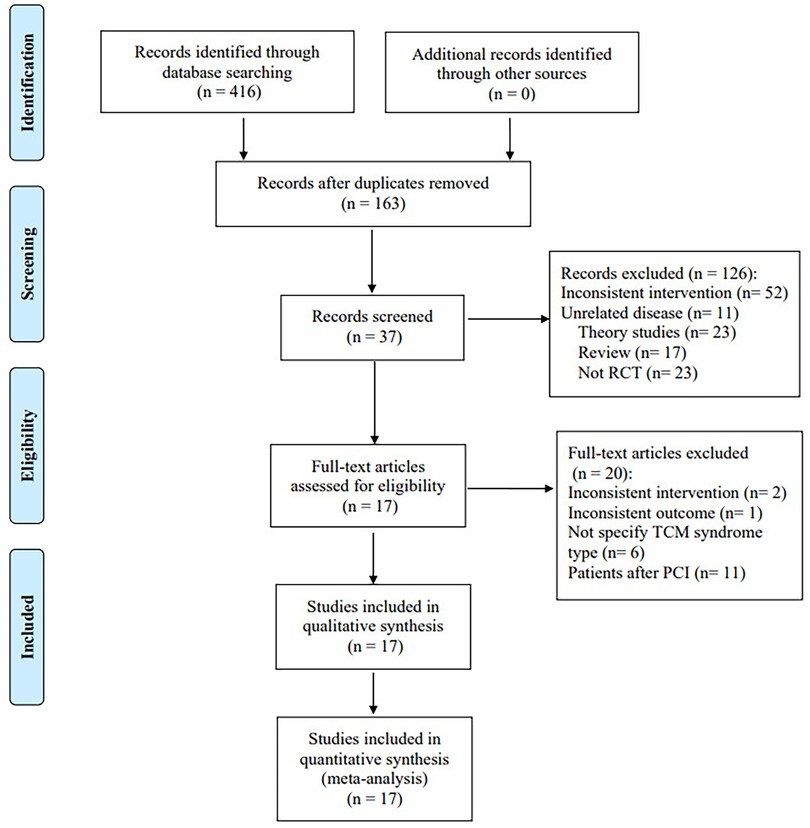
A total of 416 pieces of related literature were obtained after preliminary examination, and 163 were obtained after eliminating duplication, of which 126 were excluded after reading titles and abstracts, and 37 remained. The full text of 37 studies was read, and 20 articles were excluded. Finally, 17 studies were included. Among the 20 excluded articles, 11 included patients after PCI as intervention objects, 6 did not specify the TCM syndrome type, 2 did not conform to the intervention plan, and 1 did not have the required outcome indicators. Figure 1 depicts a flow diagram of the literature screening process.
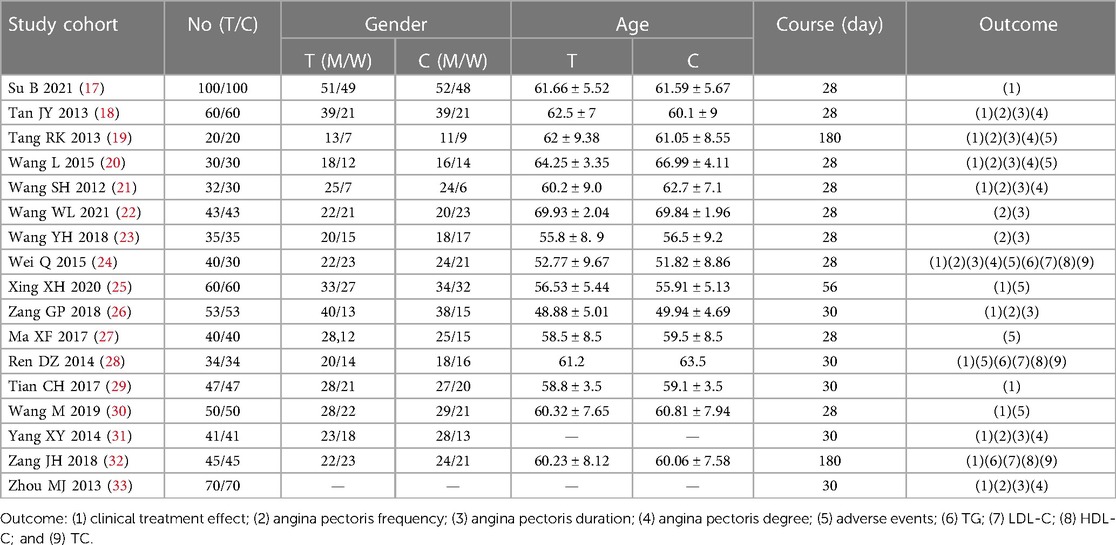
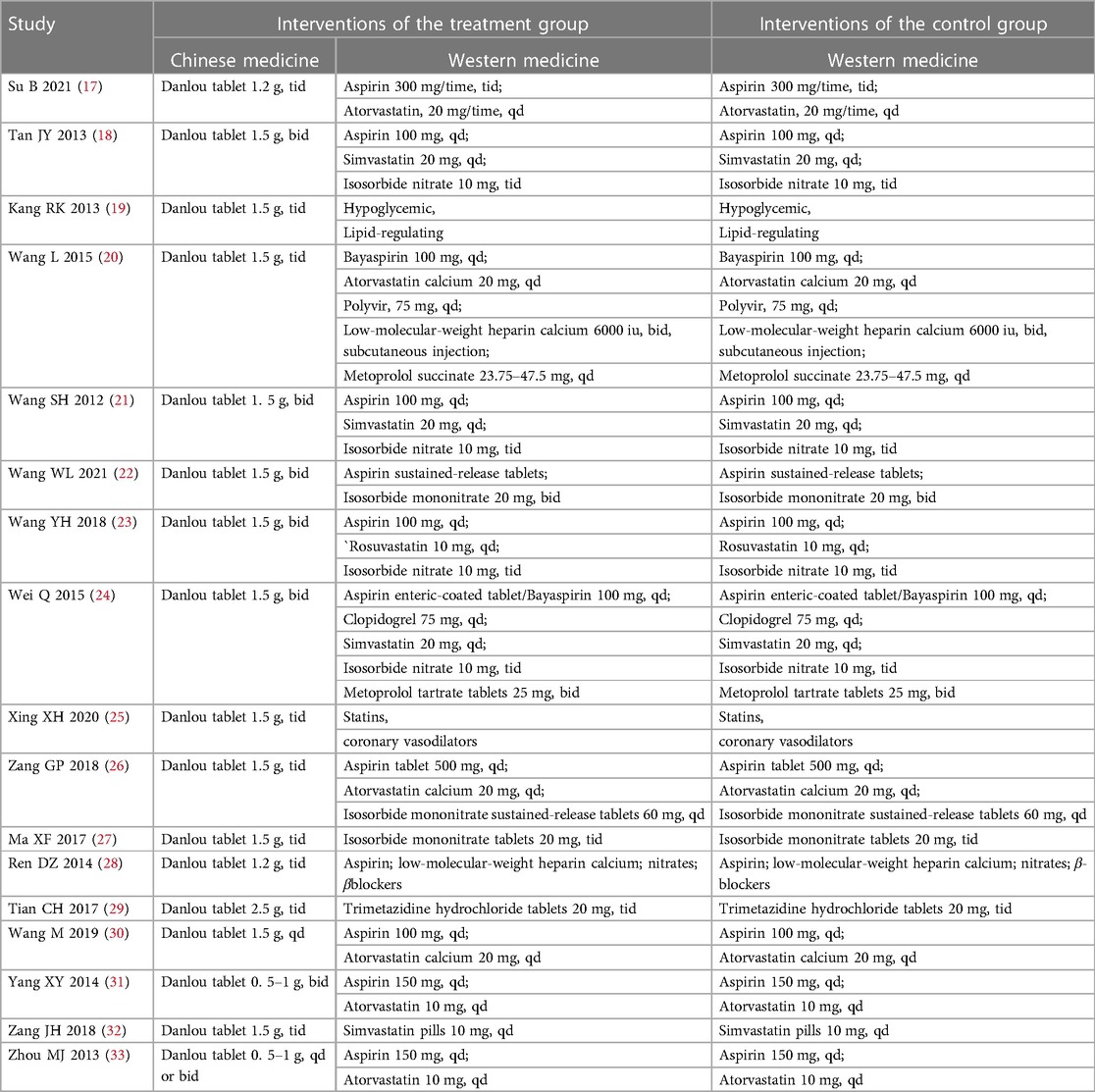
The essential characteristics of the included studies are presented in Table 1. Patients in the treatment group took Danlou tablets combined with Western medicine, and patients in the control group took only Western medicine. The characteristics of the intervention measures are presented in Table 2.
3.2. Risk of bias assessment
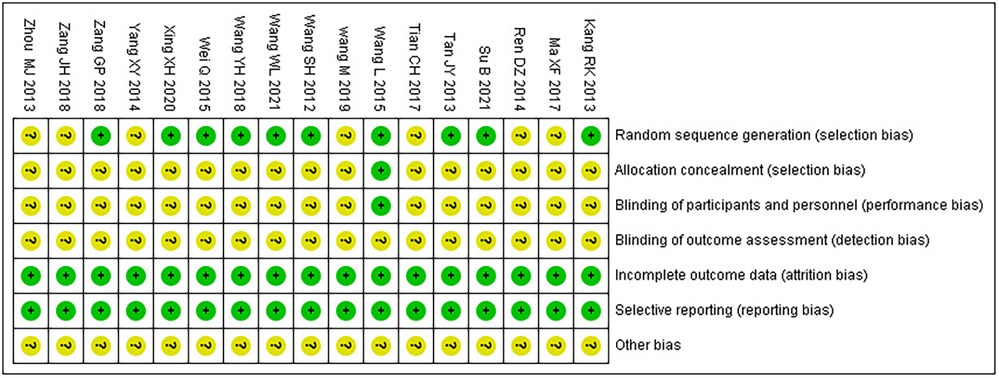
Among the 17 RCTs, 10 (17–26) were randomly assigned by the random number table method and rated as low risk, while the rest (27–33) did not describe the method of random sequence generation and were rated as unclear; only one (20) used double-blind and placebo controls, and the use of blindness and allocation hiding was rated as low risk, while the rest (17–19, 21–33) did not mention the use of blindness and allocation hiding and were rated as unclear; none of the studies (17–33) reported outcome indicators with incomplete information, and none of them had a selective reporting; thus, their risk rating was evaluated as low. For other biases, all (17–33) were evaluated as unclear. The bias risk assessment results are presented in Table 3.
3.3. Outcome indicators
3.3.1. Clinical treatment effect
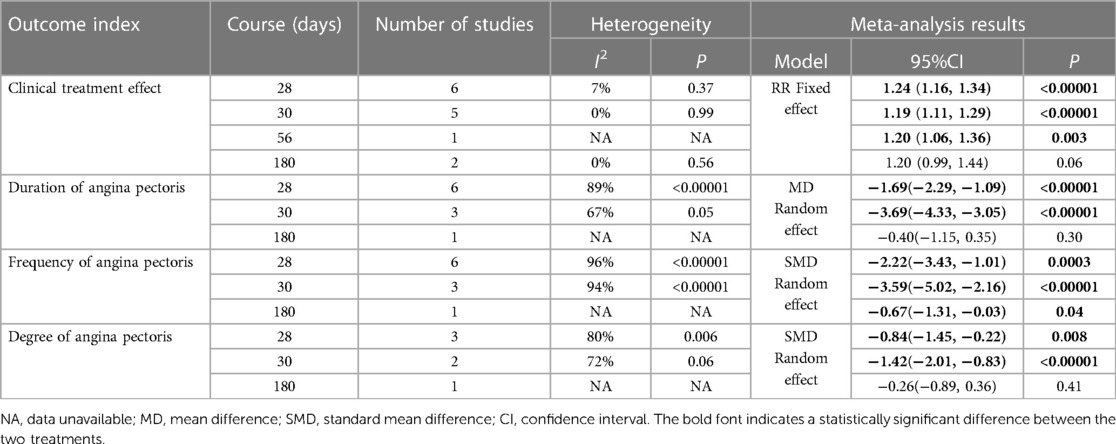
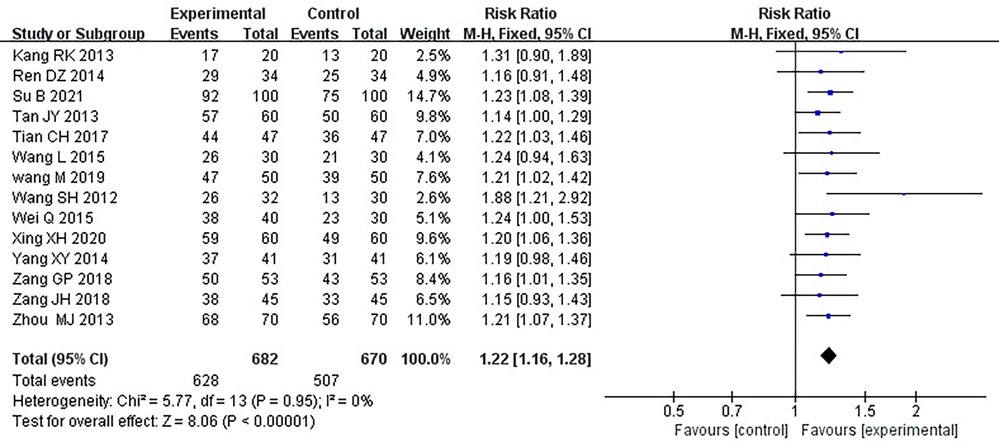
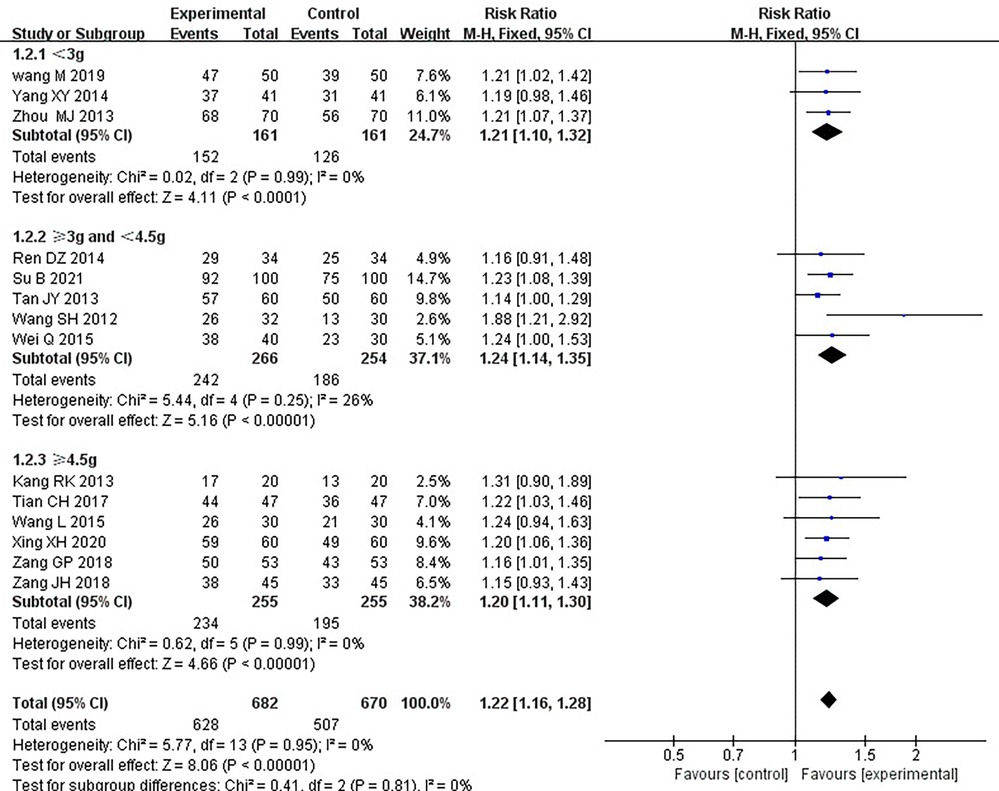
Fourteen RCTs (17–21, 24–26, 28–33), including 1,352 patients, reported the effectiveness of clinical treatment, and heterogeneity test results indicated no heterogeneity (P = 0.95, I2 = 0%). By using a fixed-effects model, meta-analysis results showed a better effect in the treatment group than in the control group [(RR = 1.22, 95% CI: 1.16, 1.28, P < 0.00001)] (P < 0.05, Table 4). Due to the difference in the daily dose of Danlou tablets among the patients, we divided them into three subgroups, <3, ≥3, and <4.5, ≥4.5 g, according to the daily dose. Heterogeneity test results indicated no significant heterogeneity of the three subgroups: <3 g (P = 0.99, I2 = 0%), ≥3 and <4.5 g (P = 0.25, I2 = 26%), and ≥4.5 g (P = 0.99, I2 = 0%). By using a fixed-effects model, the results are as follows: <3 g [(RR = 1.21, 95% CI: 1.10, 1.32, P < 0.0001)], ≥3 and <4.5 g [(RR = 1.24, 95% CI: 1.14, 1.35, P < 0.00001)], and ≥4.5 g [(RR = 1.20, 95% CI: 1.11, 1.30, P < 0.0001)], indicating a better effect in the treatment group than in the control group in three subgroups (Table 5).
3.3.2. Duration of angina pectoris
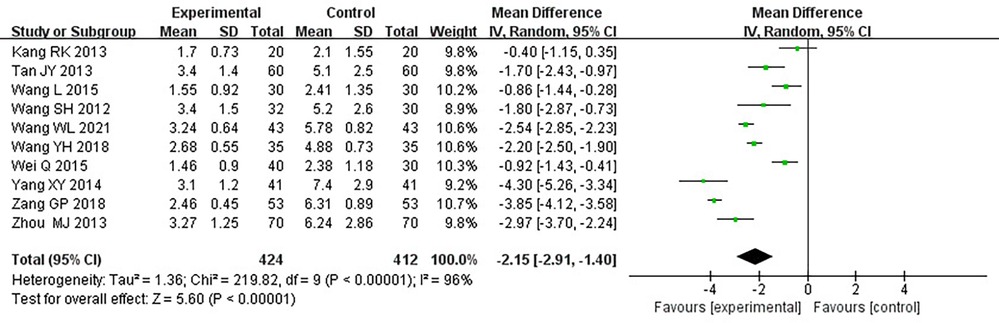
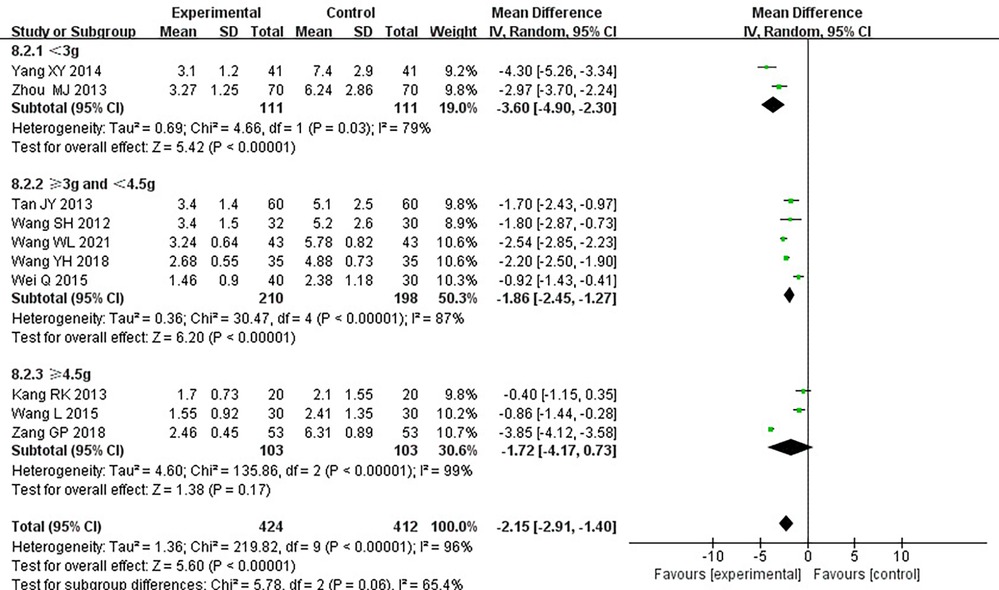
Ten RCTs (18–24, 26, 31, 33), including 836 patients, reported the angina pectoris duration, and the results of the heterogeneity test suggested significant heterogeneity (P < 0.00001, I2 = 96%). We explored the source of heterogeneity through sensitivity analysis. The exclusion of any study had no significant effect on the heterogeneity results, indicating that interstudy heterogeneity did not affect the results, so we used a random-effects model to combine them. Results displayed lower angina pectoris duration in the treatment group than that in the control group [(MD = −0.2.15, 95% CI: −2.91, −1.04, P < 0.00001), Table 6]. In terms of the treatment course, among all the included studies, the treatment course in Tang (19) was 180 days, while it was 28 or 30 days in other studies. After excluding Tang (19), I2 was found to be 96%, and the heterogeneity did not change significantly. We divided them into three subgroups, <3, ≥3 and <4.5, and ≥4.5 g (Table 7), according to the daily dose of the Danshen tablet. Results showed significant intergroup heterogeneity of the three subgroups: <3 g (P = 0.03, I2 = 79%), ≥3 and <4.5 g (P < 0.00001, I2 = 87%), and ≥4.5 g (P < 0.00001, I2 = 87%). Results from a random-effect model are as follows: <3 g [(MD = −3.60, 95% CI: −4.90, −2.30, P < 0.0001)], ≥3 and <4.5 g [(MD = −1.86, 95% CI: −2.45, −1.27, P < 0.00001)], and ≥4.5 g [(MD = −1.72, 95% CI: −4.17, 0.73, P = 0.17)], indicating lower angina pectoris duration in the treatment group than that in the control group when the daily dose of Danlou tablets was <4.5 g (P < 0.05). There was no difference when the daily dose of Danlou tablets was more significant than or equal to 4.5 g (P > 0.05).
3.3.3. Frequency of angina pectoris
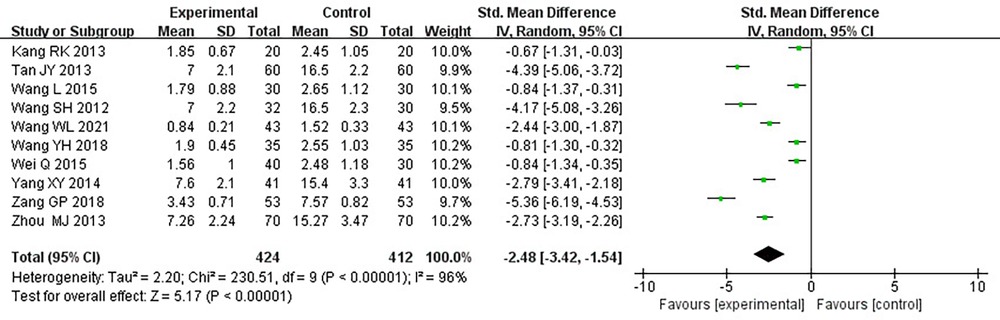
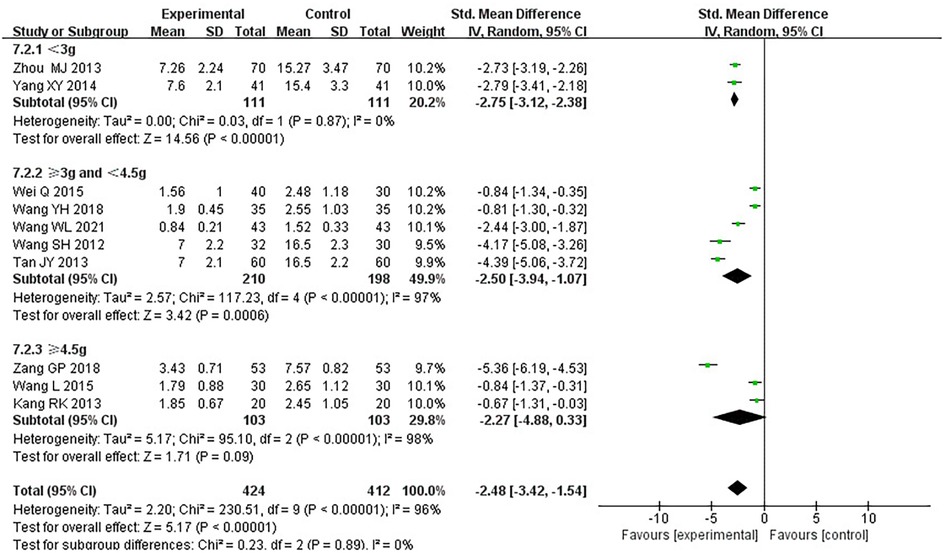
Ten RCTs (18–24, 26, 31, 33), including 836 patients, reported the frequency of angina pectoris, and the results of the heterogeneity test suggested significant heterogeneity (P < 0.00001, I2 = 96%). We explored the source of heterogeneity through sensitivity analysis. The exclusion of any study had no prominent effect on the heterogeneity results, indicating no effect of interstudy heterogeneity on the results, so we combined them through a random-effects model. Since the measurement units of angina pectoris frequency differed in different research centers, SMD was used as a valid indicator for meta-analysis. Results indicated lower angina pectoris frequency in the treatment group than in the control group [(SMD = −2.48, 95% CI: −3.42, −1.54, P < 0.00001), Table 8]. In terms of the treatment course, among all the included studies, the treatment course in Tang (19) was 180 days, while in other studies, it was 28 or 30 days. After excluding Tang (19), I2 was found to be 96%, and the heterogeneity did not change significantly. We divided them into three subgroups: <3, ≥3 and <4.5, and ≥4.5 g subgroups (Table 9) according to the daily dose of the Danshen tablet. Heterogeneity test results showed a loss of heterogeneity in the <3 g subgroup (P = 0.87, I2 = 0%) and significant heterogeneity in the ≥3 and <4.5 g subgroup (P < 0.00001, I2 = 97%) and in the ≥4.5 g subgroup (P < 0.00001, I2 = 96%). Meta-analysis results, through a random-effects model, revealed that: <3 g [(SMD = −2.75, 95% CI: −3.12, −2.38, P < 0.0001)], ≥3 and <4.5 g [(SMD = −2.50, 95% CI: −3.94, −1.07, P = 0.0006)], and ≥4.5 g [(SMD = −2.27, 95% CI: −4.88, 0.33, P = 0.09)], indicating lower angina pectoris frequency in the treatment group than the control group when the daily dose of Danlou tablets was <4.5 g (P < 0.05), and there was no difference when the daily dose of Danlou tablets was ≥4.5 g (P > 0.05).
3.3.4. Degree of angina pectoris
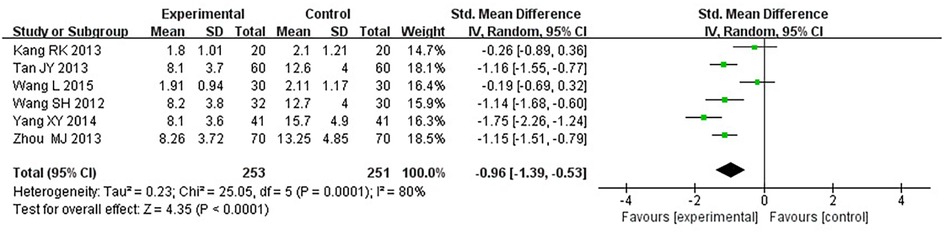
Six RCTs (18–21, 25, 33), including 504 patients, reported the angina pectoris degree (P = 0.0001, I2 = 80%). We explored the source of heterogeneity through sensitivity analysis. The exclusion of any study had no significant effect on the heterogeneity results, indicating that interstudy heterogeneity did not affect the result; thus, we combined them through a random-effect model. Since the measurement units of the degree of angina pectoris differed in different research centers, SMD could be used as a valid indicator in meta-analysis. Results displayed a lower angina pectoris degree in the treatment group vs. the control group [(SMD = −0.96, 95% CI: −1.39, −0.53, P < 0.0001), Table 10].
3.3.5. Total cholesterol
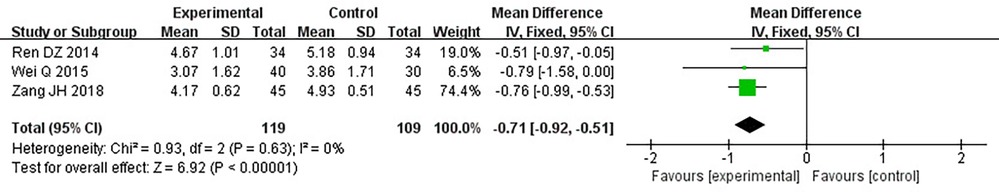
Three RCTs (24, 28, 32) including 228 patients reported TC. No heterogeneity could be seen from the heterogeneity test results (P = 0.63, I2 = 0%). Through a fixed-effect model, the results displayed a lower TC for the treatment group than that for the control group [(MD = −0.71, 95% CI: −0.92, −0.51, P < 0.00001), Table 11].
3.3.6. Triglyceride
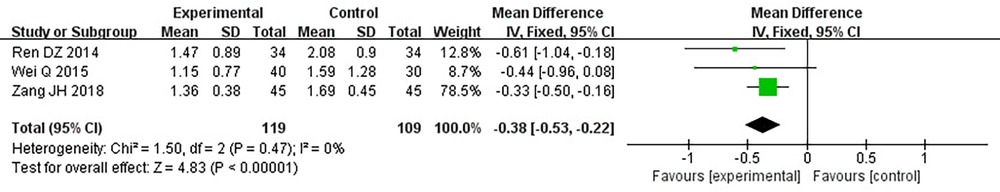
Three RCTs (24, 28, 32), including 228 patients, reported TG. No heterogeneity could be seen from the heterogeneity test results (P = 0.47, I2 = 0%). Through a fixed-effect model, the results displayed a lower TG for the treatment group than that for the control group [(MD = −0.38, 95% CI: −0.53, −0.22, P < 0.00001), Table 12].
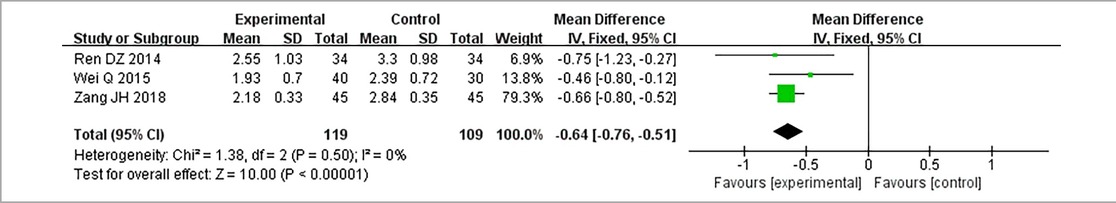
3.3.7. Low-density lipoprotein cholesterol
Three RCTs (24, 28, 32), including 228 patients, reported LDL-C. No heterogeneity could be seen from the heterogeneity test results (P = 0.50, I2 = 0%). Through a fixed-effect model, the results displayed lower LDL-C for the treatment group than that for the control group [(MD = −0.64, 95% CI: −0.76, −0.51, P < 0.00001), Table 13].
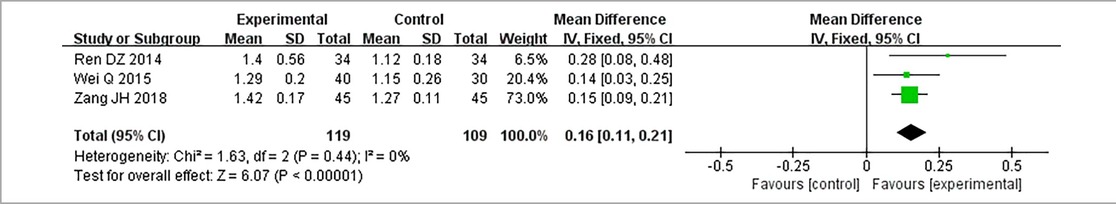
3.3.8. High-density lipoprotein cholesterol
Three RCTs (24, 28, 32), including 228 patients, reported HDL-C. No heterogeneity could be seen from the heterogeneity test results (P = 0.44, I2 = 0%). Through a fixed-effect model, results displayed higher HDL-C for the treatment group than that for the control group [(MD = 0.16, 95% CI: 0.11, 0.21, P < 0.00001), Table 14].
3.3.9. Adverse events
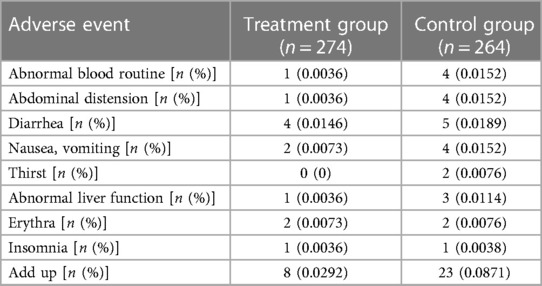
Seven RCTs (19, 20, 24, 25, 27, 28, 30), including 538 patients, reported an adverse event rate, of which four RCTs (19, 20, 24, 28) showed no adverse events in any of the groups. Meta-analysis results of adverse events reported in the remaining three RCTs (25, 27, 30) are presented in Table 15, indicating fewer adverse events in the treatment group than those in the control group [(RR = 0.46, 95% CI: 0.24, 0.88, P = 0.02), Table 15]. The proportion of adverse events is shown in Table 16.
3.4. Subgroup analysis
We conducted a subgroup analysis based on the course of treatment (Table 17). In the case of the clinical treatment effect, we used a fixed-effect model, which showed greater efficacy than the control group in the subgroups of 28, 30, and 56 days. However, the 180-day subgroup showed no difference in efficacy between the treatment and control groups [28 days: (RR = 1.24, 95% CI: 1.16, 1.34, P < 0.00001); 30 days: (RR = 1.19, 95% CI: 1.11, 1.29, P < 0.00001); 56 days: (RR = 1.20, 95% CI: 1.06, 1.36, P = 0.0003); 180 days: (RR = 1.20, 95% CI: 0.99, 1.44, P = 0.06)]. In the case of the duration of angina pectoris, we used a random-effects model, which showed greater efficacy than that of the control group in the subgroups of 28 and 30 days, but the 180-day subgroup showed no difference in efficacy between the treatment and control groups [28 days: (MD = −1.69, 95% CI: −2.29, −1.09, P < 0.00001); 30 days: (MD = −3.69, 95% CI: −4.33, −3.05, P < 0.00001); 180 days: (MD = −0.40, 95% CI: −1.15, 0.35, P = 0.30)]. In the case of the frequency of angina pectoris, we used a random-effects model, and the results indicated that each subgroup showed greater efficacy than the control group [28 days: (SMD = −2.22, 95% CI: −3.43, −1.01, P = 0.0003); 30 days: (SMD = −3.59, 95% CI: −5.02, −2.16, P < 0.0001); 180 days: (SMD = −0.67, 95% CI: −1.31, −0.03, P = 0.04%)]. In the case of the degree of angina pectoris, we used a random-effect model. The results showed that the subgroups with 28 and 30 days of treatment showed greater efficacy than the control group. However, the 180-day subgroup showed no difference in efficacy between the treatment and control groups [28 days: (SMD = −0.84, 95% CI: −1.45, −0.22, P = 0.008); 30 days: (SMD = −1.42, 95% CI: −2.01, −0.83, P < 0.00001); 180 days: (SMD = −0.26, 95% CI: −0.89, 0.36, P = 0.41)].
3.5. Sensitivity analysis
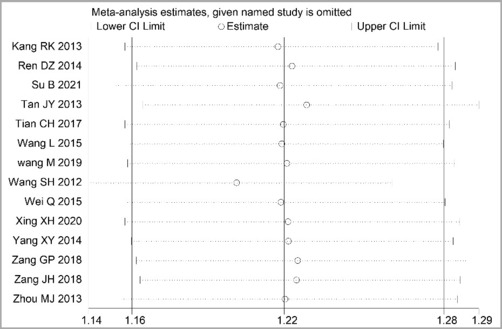
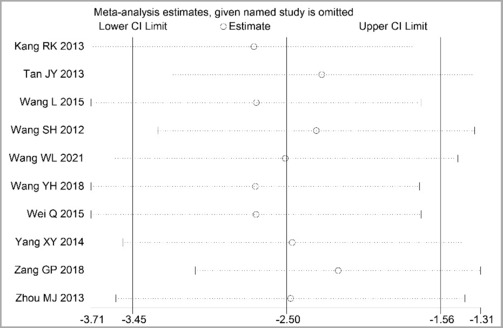
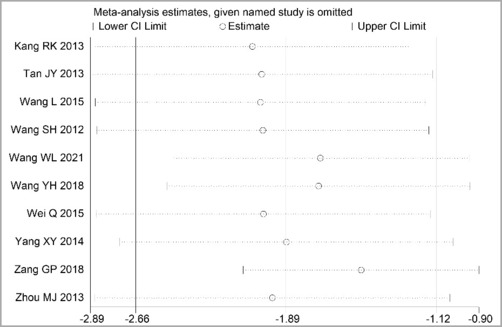
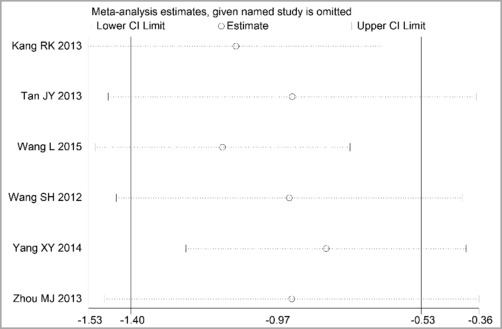
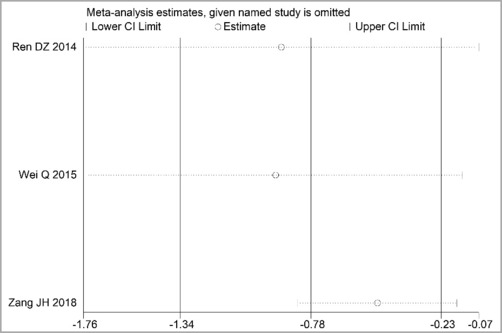
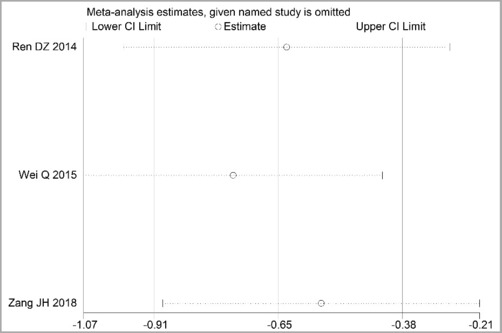
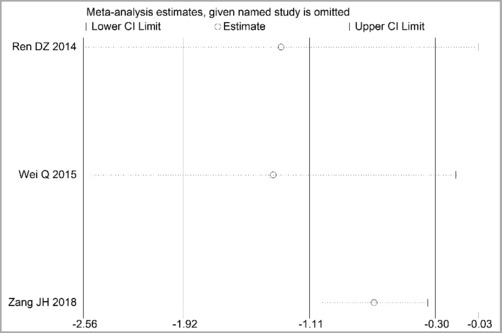
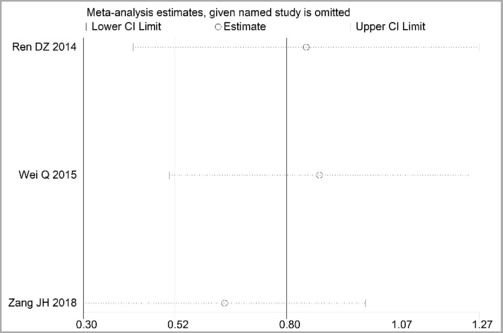
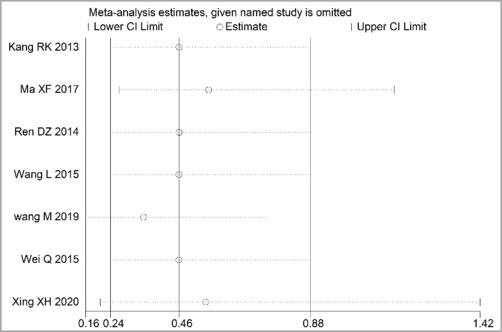
Through STATA.14 software, sensitivity analysis was conducted for all outcome indicators, including clinical treatment effect, improvement of angina pectoris (pain frequency, duration, and degree), blood lipid status (TC, TG, LDL-C, and HDL-C), and adverse events. Results showed no significant change in the size of the effect of the outcome indicators after excluding any study, indicating reliable and stable meta-analysis results (Figures 2–10).
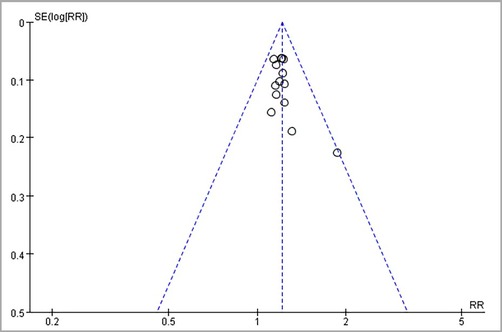
3.6. Publication bias
A funnel plot detected publication bias in the primary outcome indicators. As shown in the figure, the asymmetric funnel plot indicated that publication bias might exist (Figure 11). Subsequently, Egger's and Begg's tests were used. Egger's test (P = 0.0726 > 0.05) suggested no publication bias, and Begg's test (P = 0.0487 < 0.05) suggested publication bias. To sum up, publication bias exists.
3.7. Overall quality of evidence by GRADE
The available evidence was evaluated using the GRADE method. Clinical treatment effect, improvement of angina pectoris (duration, frequency, and degree), and incidence of adverse events were rated as “low.” The downgraded contents included the following: (1) some studies did not describe randomization, and only one study described participants, personnel, and outcome assessments; and (2) publication bias from a funnel plot test was shown. Improvement of blood lipids (TC, TG, LDL-C, and HDL-C) was rated as “very low,” and the downgraded contents included the following: (1) some studies did not describe randomization, and only one study described participants, personnel, and outcome assessments; (2) due to the small number of studies included and the wide confidence interval, the downgrade was carried out; and (3) publication bias from a funnel plot test was shown (Table 18).
4. Discussion
The deposition of coronary artery lipids, the formation of atherosclerotic plaque, and disorders of lipid metabolism are involved in the pathogenesis of CHD. Therefore, while improving the myocardial blood supply, it is essential to regulate the concentration of blood lipids, enhance the cardiomyocyte's tolerance to ischemia, and improve the state of blood hypercoagulation (34). Guideline-recommended drugs such as aspirin, statins, angiotension converting enzyme inhibitors (ACEI)/angiotonin receptor blockers (ARBs), and β-blockers are widely used to prevent and treat CHD (35). However, the actual use situation and clinical efficacy are not optimistic (36, 37), related to individual patient differences, compliance, and adverse drug reactions (7, 38). Therefore, guideline-based standardized therapy should also consider individual patient differences, and seeking complementary or alternative therapies for CHD is necessary.
In the concept of TCM, CHD belongs to “Xiong bi” (chest obstruction) and “zhen xin tong” (absolute heart pain) and is the disease of intermingled deficiency and excess, with asthenia in origin and superficiality. The deficiency is dominated by qi deficiency, and the excess is dominated by blood stasis and phlegm turbidity (39, 40). Danlou tablets are composed of Danshen (Radix Salviae Miltiorrhiae), Gualou (Fructrs Trichosanthis), Gegen (Radix Puerariae), Chishao (Radix Paeoniae Rubra), Xiebai (Bulbus Allii Macrostemi), Chuanxiong (Rhizoma Chuanxiong), Yujin (Radix Curcumae), Zexie (Rhizoma Alismatis), Huangqi (Radix Astragali), and Gusuibu (Rhizoma Drynariae), which has the effect of relieving chest, dispelling phlegm, dispersing knot, and activating blood to remove stasis. Therefore, it plays a good role in treating CHD of blood stasis and phlegm turbidimetry syndromes (41). Experimental studies have fully exemplified that Danlou tablets can reduce myocardial ischemia and reperfusion injury (42), regulate procholesterol efflux, and perform anti-inflammation by activating the PPARα/ABCA1 signaling pathway; concurrently, the NF-κB signaling pathway is prevented, thereby playing its role in alleviating atherosclerosis (13). The main chemical components of Danlou tablets include flavonoids, tanshinones, protostane triterpenoids, and paeoniflorin (43), which have antioxidant, antiplatelet aggregation, and antithrombosis effects and are the main bioactive compounds used to treat cardiovascular diseases (6).
Our results indicated a better effect of Danlou tablets combined with Western medicine than Western medicine alone in treating CHD, mainly in improving the clinical treatment effect, reducing the angina pectoris attack frequency, shortening the angina pectoris attack duration, reducing the angina pectoris degree, reducing TC, TG, and LDL-C levels, and improving HDL-C levels. We evaluated the quality of evidence for outcome indicators using the GRADE method. The quality of evidence for clinical treatment effect, improvement of angina pectoris (duration, frequency, and degree), and incidence of adverse events were “Low.” The quality of evidence regarding the improvement of blood lipids (TC, TG, LDL-C, and HDL-C) was “very low.” Clinical treatment efficacy is a common index for evaluating the TCM curative effect, which can reflect the overall therapeutic effect. The results showed a better clinical treatment effect of Danlou tablets combined with Western medicine than in treating CHD alone. Subgroup analysis results showed that a high dose (≥4.5 g per day) or a low dose (<4.5 g per day) of Danlou tablets could improve the therapeutic effect. Combined with the effect size, the efficacy of a low dose was better than that of a high dose. It can be seen from the results of the meta-analysis that adding Danlou tablets to conventional Western medicine treatment could improve the frequency, duration, and pain degree of angina pectoris. Subgroup analysis results displayed no difference in the angina pectoris frequency and the duration between the two groups when the dose of Danlou tablets was ≥4.5 g per day (P > 0.05). Since the observation time of one study (19) was 6 months, much longer than that of the other studies, we suspected that the treatment course affected the difference between the two groups. However, when we excluded this study, the results did not change, and the sensitivity analysis showed that excluding any of the studies would not change the robustness of the results. We conducted a subgroup analysis based on the course of treatment to explore the effects of different treatments on the results. The results showed the worst outcome for 180 days of treatment, and even no difference in efficacy from the control group, which may be associated with two studies of 180 days in the high-dose group (≥4.5 g per day) (19, 32). Therefore, low-dose Danlou tablets may have a better effect on angina pectoris. Dyslipidemia is a significant critical risk factor for CHD, and prevention and reasonable control of dyslipidemia can significantly change the morbidity and mortality of cardiovascular diseases (44, 45). Meta-analysis results showed that Danlou tablets had positive efficacy in reducing TC, TG, and LDL-C levels and improving HDL-C levels. Despite the high homogeneity of the study results and robust results by sensitivity analysis, only three studies reported changes in blood lipids with a small sample size; therefore, more large clinical studies are required to confirm this conclusion. Adverse events are crucial indicators to evaluate the feasibility of treatment. Meta-analysis results showed no increase in the incidence of adverse events from Danlou tablets, but it was not clear whether Danlou tablets could reduce the adverse reactions caused by Western drugs because most of the studies reporting adverse reactions were conducted for a short period (6 months in one study, 8 weeks in one study, and 4 weeks in the others). Adverse events were observed in only three studies, so more long-term follow-up studies are needed to evaluate the impact of Danlou tablets on adverse events.
Heterogeneity analysis indicated that the results of the heterogeneity test in regard to the frequency, duration, and degree of angina pectoris showed significant heterogeneity; although subgroup analysis based on the dose of the Danshen tablet was performed, the heterogeneity was not eliminated. We also analyzed heterogeneity through the course of treatment. Since one of the included studies (19) had a course of 6 months and the remaining studies had a course of 28 or 30 days, heterogeneity remained the same when we excluded the study with a long course. Therefore, the dose and course of treatment of Danlou tablets may not be the primary sources of heterogeneity. Through a detailed comparison of the characteristics of the included studies, it was found that differences in Western medicine treatment options may have brought about more pronounced heterogeneity since the types and doses of Western oral medicine were not wholly the same among patients in all studies, and some studies did not report the name and dose of western medicine. In addition, there was also a specific difference in the patients' ages, which ranged from 48.88 ± 5.01 to 69.93 ± 2.04, with a large span. Despite the heterogeneity of some outcome indicators, sensitivity analysis showed that all meta-analysis results were robust.
Our meta-analysis had the following limitations: (1) The Western medicine treatment regimens in all the studies were not identical, and the age span of the patients in the study was large, which may increase clinical heterogeneity. (2) The blind method and concealment of distribution concealment were not reported in most studies, which may lead to a bias in the efficacy of Danlou tablets. (3) A small sample size was included in most studies conducted in just one clinical trial center.
5. Conclusion
The current evidence suggests that the combination of Danlou tablets and Western medicine can enhance the efficacy of CHD and does not increase adverse events. However, because of the limited number and quality of the included studies, the results of our study should be treated with caution. Further large-scale RCTs are necessary to verify the benefits of this approach.
Author contributions
Conception and design: WM, PL, and RW; design of data synthesis analysis scheme: WM, KM, PL, RW, YL, and JH; manuscript writing: WM, PL, and ZF; and final approval of manuscript: JW, WM, PL, and RW. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the Zhejiang Provincial Natural Science Foundation of China under Grant no. LYQ20H300001.
Acknowledgments
The authors would like to thank Freescience (www.home-for-researchers.com) for the help with the English language.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer Lin Li declared a shared affiliation with the authors RW and YL to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fcvm.2023.1100006/full#supplementary-material.
Abbreviations
CHD, coronary heart disease; RCTs, randomized controlled trials; WHO, World Health Organization; TCM, traditional Chinese medicine; GRADE, Grading of Recommendations Assessment, Development and Evaluation; ECG, electrocardiogram; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; RR, risk ratio; WMD, weighted mean difference; SMD, standard mean difference; CIs, confidence intervals.
References
1. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, et al. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J. (2018) 39(7):508–79. doi: 10.1093/eurheartj/ehx628
2. Yu YF, Zhou ML, Luo XX, Zhao YC, Zhou XH, Zhang YF, et al. Meta analysis and trial sequential analysis of colchicine in the treatment of coronary heart disease. Chin Circ J. (2021) 36(7):659–66. CNKI:SUN:ZGXH.0.2021-07-006
3. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3(11):e442. doi: 10.1371/journal.pmed.0030442
4. Liu GP, Li GZ, Wang YL, Wang YQ. Modelling of inquiry diagnosis for coronary heart disease in traditional Chinese medicine by using multi-label learning. BMC Complement Altern Med. (2010) 10(13):37. doi: 10.1186/1472-6882-10-37
5. Liu Q, Dong T, Xi M, Gou L, Bai Y, Hou L, et al. Tongxinluo capsule combined with atorvastatin for coronary heart disease: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2021) 2021:9413704. doi: 10.1155/2021/9413704
6. Zhou W, Wang Y. A network-based analysis of the types of coronary artery disease from traditional Chinese medicine perspective: potential for therapeutics and drug discovery. J Ethnopharmacol. (2014) 151(1):66–77. doi: 10.1016/j.jep.2013.11.007
7. Gao ZY, Xu H, Shi DZ, Wen C, Liu BY. Analysis on outcome of 5284 patients with coronary artery disease: the role of integrative medicine. J Ethnopharmacol. (2012) 141(2):578–83. doi: 10.1016/j.jep.2011.08.071
8. Ren Y, Zhang M, Chen K, You S, Li J, Guo L, et al. Clinical and epidemiological investigation of TCM syndromes of patients with coronary heart disease in China. Evid Based Complement Alternat Med. (2012) 2012:714517. doi: 10.1155/2012/714517
9. Dong T, Wang J, Ma X, Ma R, Wen J, Chen N, et al. Shexiang Baoxin pills as an adjuvant treatment for chronic heart failure: a system review and meta-analysis. Evid Based Complement Alternat Med. (2018) 2018:6949348. doi: 10.1155/2018/6949348
10. Fan WH, Wu ZG, Shi HM. Consensus of Chinese experts on treating angina pectoris of coronary heart disease with Shexiang Baoxin pill. Chin J Integr Tradit West Med. (2018) 38(2):145–53. doi: 10.7661/j.cjim.20200321.010
11. Liu Y, Liu CF. Research progress on pharmacological action and clinical application of Danlou tablet. Hebei Med J. (2019) 41(18):2861–5. CNKI:SUN:HBYZ.0.2019-18-036
12. Li Z, Yang L, Liu Y, Xu H, Wang S, Liu Y, et al. Anti-inflammatory and antioxidative effects of Dan-Lou tablets in the treatment of coronary heart disease revealed by metabolomics integrated with molecular mechanism studies. J Ethnopharmacol. (2019) 240:111911. doi: 10.1016/j.jep.2019.111911
13. Hao D, Danbin W, Maojuan G, Chun S, Bin L, Lin Y, et al. Ethanol extracts of Danlou tablet attenuate atherosclerosis via inhibiting inflammation and promoting lipid effluent. Pharmacol Res. (2019) 146:104306. doi: 10.1016/j.phrs.2019.104306
14. Standardization project group of Clinical Application Guide for Dominant Diseases of Chinese Patent Medicine Treatment. Clinical application guide of Chinese patent medicine in treating coronary heart disease (2020). Chin J Integr Tradit West Med. (2021) 41(4):391–417. CNKI:SUN:ZZXJ.0.2021-04-003
15. Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. (1979) 59(3):607–9. doi: 10.1161/01.cir.59.3.607
16. Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. Br Med J. (2014) 349:5630. doi: 10.1136/bmj.g5630
17. Su B. Effect of Danlou tablet on electrocardiogram and serum sPLA2, LP-PLA2 and ox-LDL levels in patients with coronary heart disease with mutual obstruction of phlegm and stasis syndrome. Guangming J Chin Med. (2021) 36(11):1748–50. CNKI:SUN:GMZY.0.2021-11-006
18. Tan JY. A randomized parallel control study of Danlou Tablet combined with Western medicine in the treatment of Angina pectoris of coronary heart disease with mutual obstruction of phlegm and stasis syndrome. J Pract Tradit Chin Intern Med. (2013) 27(13):20–1. CNKI:SUN: SYZY.0.2013-13-011
19. Tang RK. Clinical observation of Danlou tablet in treating stable angina pectoris of coronary heart disease (Phlegm turbidiasis and blood stasis syndrome). Master. J Heilongjiang Univ Tradit Chin Med. (2013):35–40.
20. Wang L. Clinical study of Danlou tablet in treating unstable angina pectoris with phlegm and blood stasis blocking collaterals syndrome. Master. J Changchun Univ Chin Med. (2015):24–32.
21. Wang SH, Wang J, Li J, Xiong XJ, Ye Y, Zhu MJ. Clinical evaluation of Danlou tablet in treating angina pectoris of coronary heart disease with mutual obstruction of phlegm and blood stasis syndrome. Chin J Integr Tradit West Med. (2012) 32(8):1051–5. CNKI:SUN:ZZXJ.0.2012-08-014
22. Wang WL. Effect of Danlou tablet combined with isosorbide mononitrate on angina attack and hemodynamic indicators in elderly patients with coronary heart disease. Chin J Drug Abuse Prev Treat. (2021) 27(4):596–8. doi: 10.15900/j.cnki.zylf1995.2021.04.041
23. Wang YH, Tong SH, Ding Y, Lu C, Zhuang SW. Clinical effect of Danlou tablet on angina pectoris of coronary heart disease and its effect on vascular endothelial function. Guangxi Med J. (2018) 40(21):2524–6. CNKI:SUN:GYYX.0. 2018-21-003
24. Wei Q, Wei M. Clinical study on angina pectoris of coronary heart disease treated by integrated traditional Chinese and Western medicine. J Basic Chin Med. 2015;21(12):1530–2. CNKI:SUN:ZYJC.0.2015-12-023
25. Xing XH, Jiang A, Li B Clinical application of Danlou tablet combined with Omaha case management model in patients with chronic stable coronary heart disease. Chin J Mod Nurs. 2020;26(25):3499–503. doi: 10.3760/cma.j.cn115682-20200311-01626
26. Zhang GP. Curative effect of Danlou tablet on angina pectoris of coronary heart disease with mutual obstruction of phlegm and blood stasis syndrome. Curr Med Res Pract. (2018) 3(24):94–5. doi: 10.19347/j.cnki.2096-1413.201824044
27. Ma XF. Analysis of electrocardiogram and TCM syndrome of Danlou tablet in treatment of stable angina pectoris of coronary heart disease with phlegm and stasis interaction syndrome. Guangming J Chin Med. (2017) 32(24):3558–60. CNKI:SUN:GMZY.0.2017-24-024
28. Ren DZ, Zhang JR, Shen XL. Clinical observation of Danlou tablet in treating unstable angina pectoris of coronary heart disease with phlegm and blood stasis syndrome. Chin J Integr Med Cardio-Cerebrovasc Dis. (2014) 12(8):1022–3. CNKI:SUN:ZYYY.0.2014-08-063
29. Tian CH, Zhang LC, Jia XD. Effect of Danlou tablet combined with trimetazidine on unstable angina pectoris. Cardiovasc Dis Electron J Integr Tradit Chin West Med. (2017) 5(30):82. doi: 10.16282/j.cnki.cn11-9336/r.2017.30.064
30. Wang M. Danlou tablet for treating 50 cases of coronary heart disease with phlegm and blood stasis syndrome. J China Prescription Drug. (2019) 17(8):116–7. CNKI:SUN:ZGCF.0.2019-08-067
31. Yang XY. Clinical effect of Danlou tablet on angina pectoris of senile coronary heart disease with mutual obstruction of phlegm and blood stasis syndrome. Contemp Med Symp. (2014) 12(15):33. CNKI:SUN:QYWA.0.2014-15-029
32. Zhang JH, Zhao ZH, Li HX, Yan SY. Effect of Danlou tablet on blood lipid and hemorheology in patients with coronary heart disease complicated with hyperlipidemia. Chin J Integr Med Cardio-Cerebrovasc Dis. (2018) 16(10):1319–23. doi: 10.12102/j.issn.1672-1349.2018.10.003
33. Zhou MJ. Clinical observation of Danlou tablet in treating 70 cases of angina pectoris of coronary heart disease with mutual obstruction of phlegm and blood stasis syndrome. Chin J Ethnomed Ethnopharm. (2013) 22(18):98. CNKI:SUN:MZMJ.0.2013-18-079
34. Kalyuzhin VV, Teplyakov AT, Pushnikova EY, Bespalova LD, Kalyuzhina EV, Kolesnikov RN. Comparative evaluation of the impact of four-week therapy with amlodipine and atenolol on quality of life and blood lipid composition in patients with coronary heart disease associated with metabolic syndrome. Ter Arkh. (2013) 85(5):68–72. doi: 10.2147/PPA.S46990
35. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41(3):407–77. doi: 10.1093/eurheartj/ehz425
36. Tian CY, Huang Y, Sun X, Guo R, Guan YY, Wu XX, et al. The efficacy and safety of clopidogrel and aspirin in coronary heart disease with angina pectoris: a systematic review and meta-analysis. Asian Toxicol Res. (2020) 2(3):97–108. doi: 10.12032/ATR20200602
37. Hobbs FD, Erhardt L. Acceptance of guideline recommendations and perceived implementation of coronary heart disease prevention among primary care physicians in five European countries: the Reassessing European Attitudes about Cardiovascular Treatment (REACT) survey. Fam Pract. (2002) 19(6):596–604. doi: 10.1093/fampra/19.6.596
38. Puri R, Nissen SE, Libby P, Shao M, Ballantyne CM, Barter PJ, et al. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation. (2013) 128(22):2395–403. doi: 10.1161/CIRCULATIONAHA.113.004243
39. Wang ZY, Fan JR. Research progress of coronary heart disease angina pectoris in modern Chinese medicine. Chin J Integr Med Cardio-Cerebrovasc Dis. (2020) 18(24):4161–4. doi: 10.12102/j.issn.1672-1349.2020.24.013
40. Xing DM, Zhu MJ, Liu CX, Wang H. Outcome measures in clinical trials of traditional Chinese medicine for stable angina pectoris. Acupunct Herb Med. (2021) 1(2):99–106. doi: 10.1097/HM9.0000000000000014
41. Yang G, He H, Li H, Shen Z, Zhou S, Lu B, et al. Effects of Danlou tablet for the treatment of stable angina pectoris: a study protocol of a randomized, double-blind, and placebo-controlled clinical trial. Medicine (Baltimore). (2020) 99(49):23416. doi: 10.1097/MD.0000000000023416
42. Qi JY, Wang L, Gu DS, Guo LH, Zhu W, Zhang MZ. Protective effects of Danlou tablet against murine myocardial ischemia and reperfusion injury in vivo. Chin J Integr Med. (2018) 24(8):613–20. doi: 10.1007/s11655-016-2448-7
43. Dong J, Zhu Y, Gao X, Chang Y, Wang M, Zhang P. Qualitative and quantitative analysis of the major constituents in Chinese medicinal preparation Dan-Lou tablet by ultra high performance liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. (2013) 80:50–62. doi: 10.1016/j.jpba.2013.02.011
44. Yang K. Clinical observation of Danlou tablet combined with aspirin in treating angina pectoris of senile coronary heart disease. Chin Arch Tradit Chin Med. (2019) 37(2):369–71. doi: 10.13193/j.issn.1673-7717.2019.02.027
Keywords: Danlou tablet, coronary heart disease, randomized controlled trial, meta-analysis, complementary alternative therapy
Citation: Mao W, Lu P, Wan R, Mao K, Lv Y, Hu J, Fu Z and Wang J (2023) Efficacy and safety of Danlou tablets in traditional Chinese medicine for coronary heart disease: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1100006. doi: 10.3389/fcvm.2023.1100006
Received: 16 November 2022; Accepted: 9 May 2023;
Published: 7 June 2023.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Bao Li, Beijing University of Technology, ChinaLin Li, Tianjin University of Traditional Chinese Medicine, China
Li Liang, Xuzhou Medical University, China
© 2023 Mao, Lu, Wan, Mao, Lv, Hu, Fu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wang d2FuZ2p1bjE5ODNoeUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 WeiLi Mao
WeiLi Mao Peng Lu
Peng Lu Renhong Wan
Renhong Wan Kaili Mao1
Kaili Mao1 Jie Hu
Jie Hu