- 1Department of Cardiology, Capital Institute of Pediatrics-Peking University Teaching Hospital, Beijing, China
- 2Department of Cardiology, Children’s Hospital Capital Institute of Pediatrics, Beijing, China
- 3Graduate School of Peking Union Medical College, Capital Institute of Pediatrics, Beijing, China
Backgrounds: Vasovagal syncope (VVS) is a common form of syncope. In children with VVS, recurrent syncope or presyncope can affect the physical and mental health of both children and parents, which markedly impairs quality of life.
Objectives: We aimed to identify factors at baseline that can predict the recurrence of syncope or presyncope over a 5-year follow-up period, and further to develop a prognostic nomogram model.
Methods: This cohort is bidirectional in design. From July 2017 to August 2022, children with VVS were included and followed up every 3 to 6 months. Head-up Tilt Test (HUTT) was performed for diagnosing VVS. Data were analyzed using STATA software, and risk estimates are presented as hazard ratio (HR) and 95% confidence interval (CI).
Results: Total 352 children with VVS who had complete information were included in this study. Median follow-up time was 22 months. Overall, supine mean arterial pressure (MAP-supine) in HUTT and baseline urine specific gravity (USG) were associated with the significant risk of syncope or presyncope recurrence (HR: 0.70 and 3.00, respectively; both P < 0.05). Calibration and discrimination analyses revealed that the addition of MAP-supine and USG can result in a better fit. A prognostic nomogram model based on significant factors annexed with five traditional promising factors was finally constructed, with strong discriminative and predictive abilities (C-index approaching 0.700, P < 0.05).
Conclusions: Our findings indicated that MAP-supine and USG can independently predict the significant risk of syncope recurrence in children with VVS, and the prediction was more obvious in a nomogram model.
1. Introduction
Syncope is a major public health concern with approximately 17% of children 2 to 18 years of age experiencing at least one episode of syncope (1). As a common form of syncope, vasovagal syncope (VVS) accounts for about 60 to 80% of all pediatric cases (2). VVS tends to have a good prognosis, yet its recurrent episodes can affect the physical and mental health of children, as well as their parents, which markedly impairs the quality of life (3). As the development of syncope is a complex process, it is of clinical and healthcare importance to identify potential factors attributable to the recurrence of syncope in children affected with VVS.
Currently, research on the risk profiles of recurrent syncope or presyncope in children with VVS has been less conducted in medical literature. For example, some investigators have reported that the number of previous episodes of syncope (4, 5), onset age of syncope, and family history (6) were identified as promising predictors for recurrent syncope in VVS. In addition, others revealed that venous blood routine hemoglobin concentration and mean corpuscular hemoglobin were candidate biomarkers in predisposition to syncope recurrence (7). Further, heart rate variability (HRV), body mass index (BMI), and therapeutic regimens can also affect the prognosis of VVS (8–10). Thus far, no consensus has been attained which and how many factors actually precipitate the development of recurrent syncope in children with VVS, likely due to differences in genetic underpinnings, statistical power or participant characteristics. Given that VVS etiology is complex and usually involve a multistep, multifactorial process, the impact of any single factors is believed to be small, but may be more evident when assessed in combination. However, a literature search did not trace any hints concerning the joint contribution of various factors to recurrent syncope in VVS.
To fill this gap in knowledge and yield more references for future studies, we, in a large cohort of children with VVS, aimed to identify factors at baseline that can predict the recurrence of syncope over a 5-year follow-up period, and meanwhile for clinical application, to develop a prognostic nomogram model based on promising significant attributes.
2. Methods
2.1. Ethical approval and informed consent
The conduct of this study was approved by the Ethics Committee of Children's Hospital Capital Institute of Pediatrics (SHERLLM2021025), and written informed consent was obtained from parents or supervisors of participating children.
2.2. Study children
The present cohort is bidirectional in design. From July 2017 to August 2022, children diagnosed with VVS were hospitalized for the first time at the Department of Cardiology, Children's Hospital Capital Institute of Pediatrics, and after discharge they were followed up at the Out-Patient Department every 3–6 months. The latest follow-up was competed on October 7, 2022. In case of no-show at the scheduled time, we reached out to their parents or supervisors through phone. The minimal follow-up time was set at 1 month.
2.3. Inclusion and exclusion criteria
Children were included if all of the following criteria were satisfied simultaneously: (i) <18 years of age; (ii) hospitalized children; (iii) confirmed diagnosis of VVS. The clinical diagnostic criteria of VVS refer to the expert consensus on the diagnosis of syncope in children (11).
Children were excluded if they complicated any of the following criteria: (i) cardiovascular diseases, such as hypertension, arrhythmias, and congenital heart disease; (ii) neuropsychological diseases, such as anxiety and depression; (iii) metabolic diseases, such as hyperthyroidism and metabolic syndrome; (iv) convulsant syncope confirmed as seizure based on their medical histories as well as electroencephalograph and brain magnetic resonance imaging examination.
2.4. Follow-up assessment
The details of treatment regimens for children with VVS can be found in the Supplementary Materials (Appendix A.1). During each follow-up, autonomic exercises, oral rehydration salts, and drug regimens were recorded. Disease-specific survival was calculated from the date of diagnosis to the date of syncope or presyncope recurrence or the date of the last follow-up visit, whichever came first. Presyncope is defined as symptoms before syncope, and it includes extreme lightheadedness, visual sensations (such as tunnel vision and graying out), and variable degrees of altered consciousness without complete loss of consciousness. If the child had a transient loss of consciousness associated with inability to maintain postural tone with rapid and spontaneous recovery, syncope was confirmed. Recurrence information was obtained from either parents or supervisors. Children with VVS were divided into two groups: children with syncope or presyncope recurrence and children free of syncope or presyncope recurrence.
2.5. Head-up tilt test
Participating children were requested to lie on the electric tilting bed (SHUT -100A, STANDARD, Beijing, China) in the supine position for 10 min. A baseline head-up tilt test (HUTT) was conducted with the child tilted for 45 min without medication. If syncope did not develop, the child was given a sublingual nitroglycerin (4–6 μg/kg, maximum ≤0.3 mg) to provoke syncope within 20 min. Automatic cuff blood pressure, heart rate, and electrocardiograph were recorded continuously by electric tilting instrument. If syncope occurred during the test, the tilt table would be quickly lowered to the supine position. Positive responses to HUTT involve syncope or presyncope in addition to four key factors, including (i) drops in blood pressure (systolic blood pressure [SBP] ≤80 mmHg diastolic blood pressure [DBP] ≤50 mmHg; (ii) a sinus arrest with junctional escape rhythm; (iii) an atrioventricular (AV) conduction block no less than II° or a cardiac arrest for three seconds; (iv) a decrease in heart rate of <75 beats per minute(bpm) in children aged 4–6 years, <65 bpm in those aged 7–8 years, and <60 bpm in those aged 8 years (11–13). In addition, VVS is divided into three types according to the changes in blood pressure and heart rate. The cases with lower blood pressure were defined as the vaso inhibitive type, the cases with significantly lower heart rate were defined as the VVS cardiac inhibitory type, and the cases with both lower heart rate and lower blood pressure as the VVS mixed type.
2.6. Data collection
For children admitted for the first time to our hospital, a standardized questionnaire was adopted to glean demographic information at baseline, including age, sex, BMI, medical history, number of syncope, and family history of syncope. Routine blood tests, cardiac enzymes, urine routine, 24-hour urine output and electrolytes, cardiac function, and 24-hour Holter during hospitalization were also recorded. All above tests were completed prior to the HUTT test. The first morning urine specimens were measured using an automatic urinary sediment analyzer (AX-4030, Arkray, Kyoto, Japan). Heart rate and arterial pressure were measured twice after affected children had stayed in a supine position for 10 min and again at 60-degree tilting when stable within the first 1 min.
In addition, the following indicators were also collected. Mean arterial pressure in the supine position for 10 min (MAP-supine), and mean arterial pressure in the first 1 min at 60 degrees of inclination (MAP-tilt) were calculated SBP and DBP in tilt position. MAP was calculated as DBP plus 0.333 × (SBP - DBP). Change-SBP was calculated as SBP in tilt immediately minus SBP in supine, and Change-DBP as DBP in tilt immediately minus DBP in supine. Change-HR was calculated as heart rate in tilt immediately minus heart rate in supine.
2.7. Statistical analysis
Continuous parameters are presented as mean ± standard deviation (SD) or as median (interquartile range) depending on whether the parameter was deviated from the Gaussian distribution. Where appropriate, the t-test or Mann-Whitney test was used to compare differences of continuous parameters between groups. Categorical parameters are presented as percentage (count) and compared using the χ2 test or Fisher's exact test. Potential factors related to recurrence were initially identified by the univariate Cox proportional hazards regression analyses. Then, the multivariate Cox proportional hazards regression analyses were undertaken to control for covariates, including age, sex, BMI, medical history, number of syncope, family history of syncope, and therapeutic regimens.
A series of discrimination and calibration statistics were used to evaluate prediction accuracy. To justify the improvement in prediction accuracy, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) (14, 15) were calculated. If the outcome is less than 0.05, adding significant risk factors might greatly increase the model's ability in prediction. The area under the Harrell C-statistic is to examine whether the traditional model can distinguish between children who had presyncope or syncope recurrence when significant factors are added. To determine how closely the prediction probability for the addition of significant factors reflected actual observed risk and the overall fit of the improved risk model, the Akaike information criterion (AIC), Bayesian information criterion (BIC), and likelihood ratio test were utilized (16). For the AIC and BIC indices, smaller values indicate better models.
By using decision curve analysis (DCA), the net benefit of the addition of significant factors over the conventional model was clearly demonstrated (17). The DCA graph's X-axis represents the recurrence thresholds, while the Y-axis represents the net benefits for various thresholds. If the “model” curve is further from the solid curve line under all recurrence assumptions and the dotted horizontal line with none recurrence assumptions, a bigger net benefit is reported.
A predicted nomogram for the 1-year, 2-year, and 3-year recurrence rates from VVS was constructed among all research patients based on baseline demographic and clinical data with statistical significance, improving clinical interpretation. The predictive precision and discriminative power of this prognostic nomogram were assessed using the concordance index (C-index) and calibration curve. The area under the receiver operating characteristic curve and the C-index are equivalent. According to the calibration curve, the 45° line illustrates how far the prognostic nomogram's predicted probabilities differ from actual outcomes.
The regression modeling strategies (RMS) package (https://cran.r-project.org/web/packages/rms/index.html) in the R- language (version 4.2.1) was used to create the prognostic nomogram model. A nomogram is a graphic calculator that has been scaled and is used to determine an approximation of a function. In this study, a simple 10-point scale was used as the basis for the nomogram.
All of the aforementioned statistical analyses were conducted using STATA/SE software (version 14.0, Stata Corp, TX, USA). Probability less than 5% was considered statistically significant.
3. Results
3.1. Sample size
In total, 409 children were diagnosed with VVS during the study period, and 37 of them were excluded because of anxiety and depression (n = 23), hyperthyroidism (n = 7), arrhythmias (n = 3), hypertension (n = 3), and abnormal origin coronary artery (n = 1). Twenty children were lost during follow-up, leaving 352 patients with complete follow-up data in the final analysis. The median time to an endpoint (syncope or presyncope recurrence) event occurred was 22 months (from 1 day to 63 months).
3.2. Baseline characteristics
The baseline characteristics of study patients are showed in Table 1. Urine specific gravity (USG) before diagnosis and the time used oral rehydration salts (ORS) during follow-ups differed significantly between children with or without recurrence (P < 0.05). No significance was noted for the rest characteristics.

Table 1. Baseline characteristics of participating children by the presence and absence of recurrence of vasovagal syncope.
Comparison of baseline characteristics between children analyzed and lost to follow-ups is provided in Supplementary Table S1.
3.3. Identification of significant factors
The two factors, MAP-supine and USG, were associated with the significant risk of syncope or presyncope recurrence in VVS patients before adjustment at a 5% level of significance (Table 2). After adjusting for age, sex, BMI, medical history, number of syncope, family history of syncope, and therapeutic regimens, three factors were consistently and independently associated with the significant risk of recurrence in VVS, including MAP-supine (HR, 95% CI, P: 0.700, 0.567 to 0.869, 0.001), MAP-tilt (0.796, 0.654 to 0.969, 0.035), and USG (3.000, 1.03 to 8.741, 0.044).

Table 2. Identification of factors independently and significantly associated with the recurrence of vasovagal syncope.
3.4. Prediction accuracy assessment
Because MAP-supine had collinearity with MAP- tilt, MAP- tilt was removed from the following analyses. To evaluate the predictive power of the major factors identified, two models—the basic model and the full model—were built. The basic model includes general characteristics (age, sex, BMI, medical history, family history of syncope), and the full model includes general characteristics and the two significant factors (MAP-supine and USG).
The prediction accuracy gained by adding the two major factors to the basic model was assessed using both calibration and discrimination statistics (Table 3). Compared with the basic model, the prediction accuracy of the full model was significantly improved. The AIC and BIC in full model were smaller, and as revealed by the likelihood ratio test, both models differed significantly in prediction performance (P = 0.001). NRI and IDI statistics showed significant improvement after adding MAP-supine and USG to the basic model from the aspect of reclassification performance. Harrell's C-statistic indicated that the addition of MAP-supine and USG can improve the predictive ability of the basic model.
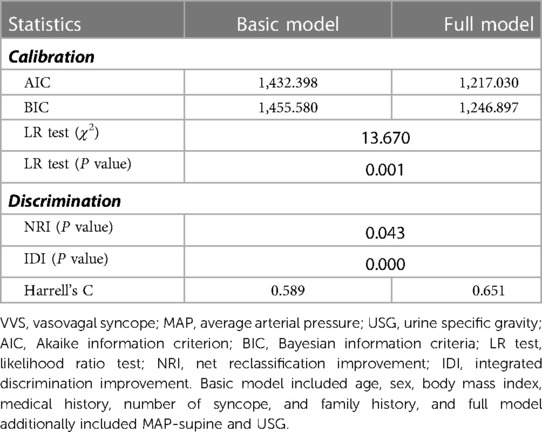
Table 3. Predictive accuracy of risk model with and without MAP-supine or USG for the prediction of VVS recurrence.
Furthermore, DCA graph indicated the obvious net benefits gained by adding the two significant factors to the basic model (Figure 1).
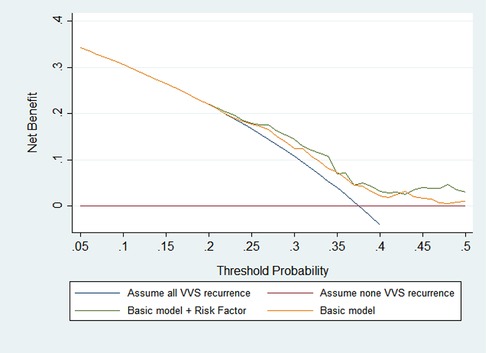
Figure 1. Prognostic decision curves for the basic model and the addition of MAP-supine and urine specific gravity in vasovagal syncope patients. The basic model included age, sex, body mass index, medical history, and family history.
3.5. Risk prognostic nomogram model
To facilitate clinical interpretation, a prognostic nomogram model was constructed on the basis of the two significant factors aforementioned and some acknowledged conventional factors (Figure 2). For an example of clinical usefulness of this nomogram, assuming a girl (1.5 points) aged ten years (3.5 points), with a family history of syncope (1.8 points), BMI of 16 kg/m2 (1 points), MAP-supine 90 mmHg (2.9 points), and USG 1.025 (2.8 points), she would have an estimated 71% chance to experience no-recurrence of syncope or presyncope at 1st year and 65% chance at 2nd year, and 58% chance at 3rd year. Thus, the recurrence rate in 1st year, 2nd year, and 3rd year were estimated to be 29%, 35%, and 42%, respectively.
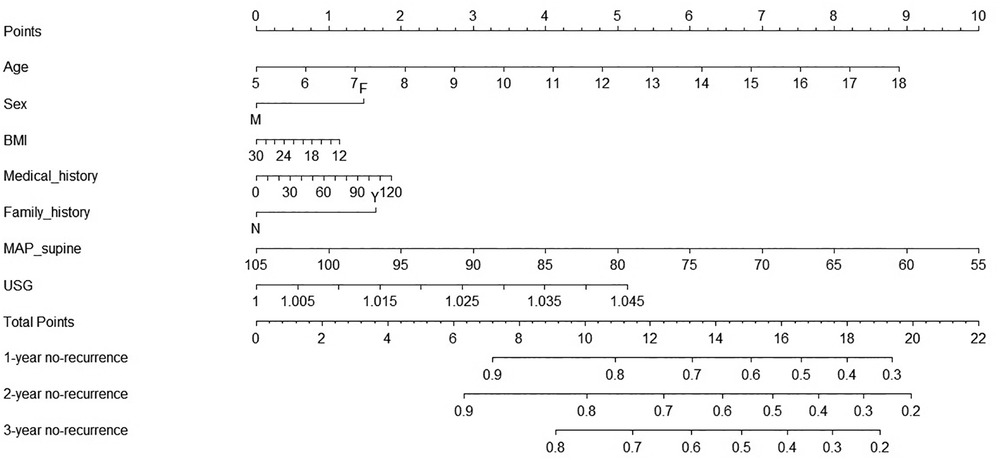
Figure 2. Prognostic nomogram of the prediction of significant parameters for 1-year, 2-year, and 3-year no-recurrence of vasovagal syncope among all study children. Abbreviations: F, female; M, male; Y, with family history of syncope; N, without family history of syncope; BMI, body mass index; MAP, average arterial pressure; USG, urine specific gravity.
This nomogram had a good prediction accuracy, with the C-index approaching 70%. In addition, the calibration curves for the probabilities of no-recurrence at 1-year, 2-year, and 3-year diagnosed showed the best possible agreement between this prognostic nomogram's forecast and the actual observations (Figure 3).
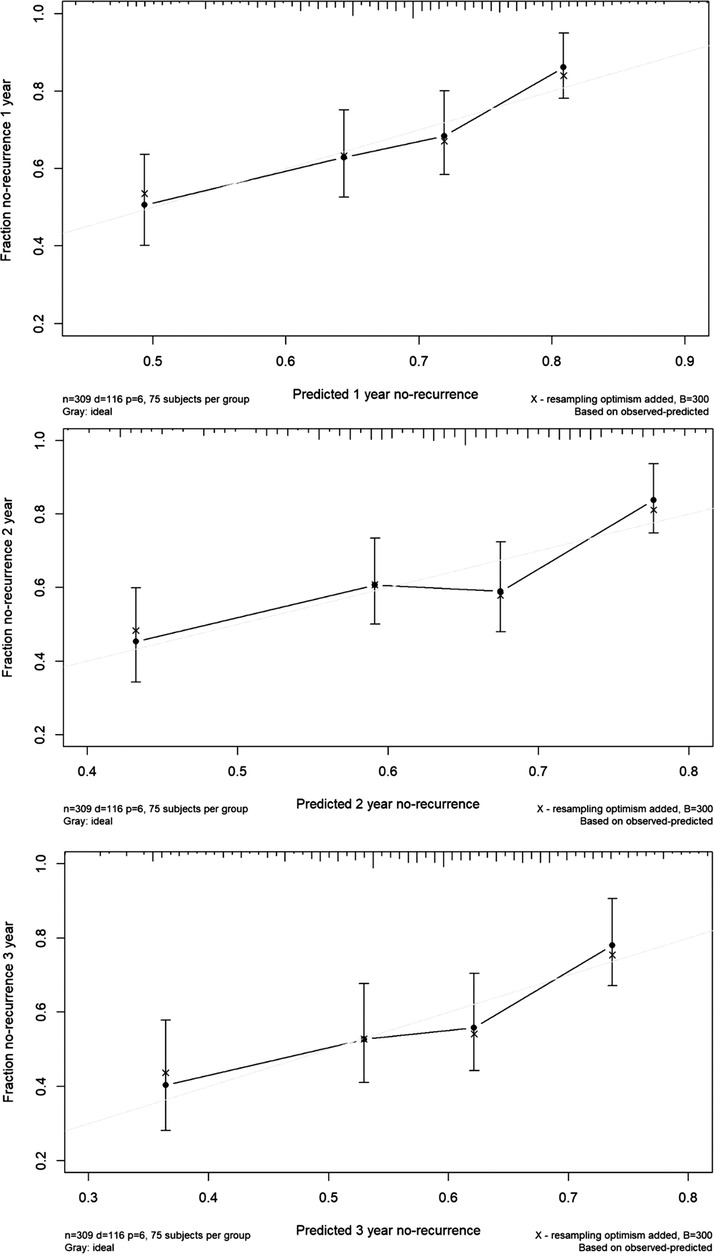
Figure 3. The calibration curves for predicting the risk of no-recurrence of vasovagal syncope at 1-year (the upper panel), 2-year (the middle panel) and 3-year (the lower panel) among all study children. Nomogram-predicted probability of no-recurrence is plotted on the X-axis, and actual no-recurrence is plotted on the Y-axis.
4. Discussion
The aim of this cohort study was to identify factors at baseline that can predict the recurrence of syncope or presyncope in children with VVS and develop a prognostic nomogram model based on promising significant attributes. It is worth noting that MAP-supine and USG can independently predict the significant risk of syncope or presyncope recurrence in VVS, especially in a nomogram model annexed with 5 other established factors. As far as we know, this is thus far the largest study that has examined factors in a joint manner associated with syncope or presyncope recurrence in children with VVS in the literature.
It is widely recognized that age is a promising factor for syncope recurrence among adults with VVS, and the recurrence rates increase with aging (18, 19). Similarly in this study, our findings supported the contribution of age to syncope recurrence in children with VVS. A possible explanation is that the epinephrine (Epi)/norepinephrine (NE) ratio increases to a greater extent in younger fainters (20). Other factors, such as number of previous syncope and family history, also played a contributory role in the recurrence of syncope or presyncope in VVS. For example, in a Canadian study of 51 children with VVS the higher number of previous syncope was linked to the greater likelihood of syncope or presyncope recurrence (4). Another study from Turkey showed that age, family history, and several prior syncope onsets were factors attributable to syncope recurrence (6). Yet, in the present study, we failed to detect statistical significance for above demographic factors, and instead we focused on laboratory indicators by taking these factors as confounders. After a careful analysis among 352 children with VVS, our findings indicated that MAP-supine and USG were independently and significantly associated with the risk of syncope or presyncope recurrence in children with VVS, differing from the results of a previous study (21) of 63 children with VVS, which identified hemoglobin and MCH as two recurrence-predisposition factors. Given the complex nature of syncope recurrence, we agree that identification of more promising factors that can accurately predict VVS prognosis is still in the process of exploration, perfection and renewal.
In fact, USG, a non-invasive biomarker and objective substitutability for water homeostasis, are widely used in clinical practice (22). The USG fluctuates depending on the amount of fluids consumed, and it reflects the total amount of water consumed and lost (23, 24). An increase in USG indicates low water intake or excessive water loss. Prior studies have shown that USG had a specific predictive value in diagnosing VVS in children and adolescents (25). However, there is a paucity of data on its role in predicting syncope of presyncope recurrence. Our study showed that elevated USG was a statistically significant risk factor for syncope or presyncope recurrence in VVS, and for practical reasons, USG can be routinely monitored for the early identification of possible recurrence. Moreover, MAP is the average of arterial blood pressure during a cardiac cycle. The median or mean MAP was regularly higher than MAP of 65 mmHg (range 70–114 mmHg) (26, 27). Considering the fact that children are in a period of growth and development, blood pressure is determined based on age, gender, BMI, and so there is currently no uniform value for MAP. In the present study, lower the MAP-supine and MAP-tilt, were found to be associated with the greater likelihood of syncope or presyncope recurrence in VVS. Previous studies showed that children with VVS usually had high levels of catecholamine, which can cause excessive contraction of the heart and abnormal Bezold-Jarish reflex (10). Then, the imbalance of sympathetic impulses and vagal impulses may reduce MAP, which is commonly seen in our recurrence group. Lower MAP in the recurrence group in this study indicated that BP can be easily decreased in the presence of triggering factors, causing the symptoms of pre-syncope. Further decrease in BP annexed with sudden falls in cerebral blood flow can lead to syncope eventually (28, 29). Both USG and MAP were for the first time identified as predisposing factors for syncope or presyncope recurrence in children with VVS, and more large-scale, well-designed studies are warranted to confirm or refute our findings.
Besides the obvious advantages, including large sample size, extended follow-up, and effective calibration/discrimination assessment, some possible limitations should be acknowledged. Firstly, because this study was conducted at a single site, our conclusions would be broadly applied pending consistently validated in other independent cohorts. Secondly, all assessable children with VVS were enrolled between July 2017 and August 2022, and during the 5-year period remarkable advances in techniques and deepened knowledge about this disease can introduce a potential bias that understates the influence of factors on the recurrence of syncope or presyncope. Thirdly, the findings presented here were based merely on children with VVS free of comorbidities and thereby cannot be extrapolated to all VVS populations. Last but not the least, only baseline biomarkers were assayed in this cohort, and frequently monitoring of these biomarkers and analyzing their dynamic variations may be of added interest. We agree that more investigations are warranted to confirm or refute the factors associated with VVS recurrence in this study.
Conclusions
Taken together, our findings indicated that MAP-supine and USG can independently predict the significant risk of syncope or presyncope recurrence in VVS, and the prediction was more obvious in a nomogram model. For practical reasons, we hope that this study will not only serve as an endpoint but also a new beginning for future large, well-designed studies to explore the risk profiles of syncope or presyncope recurrence in children with VVS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Children's Hospital Capital Institute of Pediatrics Beijing, China. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
XL and RS designed the study. RS, YK collected and analyzed the data. RS drafted the manuscript and MZ, HW revised the paper. LS, XL contributed to the interpretation of the results and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the “Peak Climbing” Talents Development Program of Beijing Hospital Authority (DFL20181301).
Acknowledgments
We thank the parents, guardians, and children who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1099115/full#supplementary-material.
References
1. Hu E, Liu X, Chen Q, Wang C. Investigation on the incidence of syncope in children and adolescents aged 2–18 years in Changsha. Front Pediatr. (2021) 9:638394. doi: 10.3389/fped.2021.638394
2. Jin H, Du J. Pediatric syncope: where are we now? Chin Med J Publishing House. (2014) 127(21):3681–3. doi: 10.3760/cma.j.issn.0366-6999.20132944
3. Kenny RA, Bhangu J, King-Kallimanis BL. Epidemiology of syncope/collapse in younger and older western patient populations. Prog Cardiovasc Dis. (2013) 55(4):357–63. doi: 10.1016/j.pcad.2012.11.006
4. Díaz JF, Tercedor L, Moreno E, García R, Álvarez M, Sánchez J, et al. Vasovagal syncope in pediatric patients: a medium-term follow-up analysis. Rev Esp Cardiol. (2002) 55(5):487–92. doi: 10.1016/S0300-8932(02)76640-2
5. Kouakam C, Vaksmann G, Pachy E, Lacroix D, Rey C, Kacet S. Long-term follow-up of children and adolescents with syncope; predictor of syncope recurrence. Eur Heart J. (2001) 22(17):1618–25. doi: 10.1053/euhj.2000.2577
6. Yılmaz ST, Binnetoğlu K, Babaoğlu K, Altun G. Predictors of vasovagal syncope recurrence in children and adolescents and value of head-up tilt table test. Anadolu Kardiyol Derg. (2013) 13(7):688–94. doi: 10.5152/akd.2013.250
7. Yan H, Wang S, Cai H, Zhang J, Liu P, Wang Y, et al. Prognostic value of biomarkers in children and adolescents with orthostatic intolerance. Front Pediatr. (2021) 9:752123. doi: 10.3389/fped.2021.752123
8. Tao C-Y, Chen S, Li X-Y, Tang C-S, Du J-B, Jin H-F. Body mass index is a promising predictor of response to oral rehydration saline in children with vasovagal syncope. Chin Med J. (2021) 134(04):463–8. doi: 10.1097/CM9.0000000000001168
9. Zhao T, Wang S, Wang M, Cai H, Wang Y, Xu Y, et al. Research progress on the predictive value of electrocardiographic indicators in the diagnosis and prognosis of children with vasovagal syncope. Front Cardiovasc Med. (2022) 9:916770. doi: 10.3389/fcvm.2022.916770
10. Li H-X, Gao L, Yuan Y. Advance in the understanding of vasovagal syncope in children and adolescents. World J Pediatr. (2021) 17(1):58–62. doi: 10.1007/s12519-020-00367-z
11. Pediatric Cardiovascular Group CMA. Guideline for diagnosis of syncope in children (updated in 2016). Chin J Pediatr. (2016) 54(4):246–50. doi: 10.3760/cma.j.issn.0578-1310.2016.04.003
12. Brignole M, Moya A, de Lange FJ, Deharo J-C, Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. (2018) 39(21):1883–948. doi: 10.1093/eurheartj/ehy037
13. Shen W-K, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2017) 70(5):e39–e110. doi: 10.1016/j.jacc.2017.03.003
14. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. (2008) 27(2):157–72. doi: 10.1002/sim.2929
15. Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. (2010) 48(12):1703–11. doi: 10.1515/CCLM.2010.340
16. Aho K, Derryberry D, Peterson T. Model selection for ecologists: the worldviews of AIC and BIC. Ecology. (2014) 95(3):631–6. doi: 10.1890/13-1452.1
17. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. (2006) 26(6):565–74. doi: 10.1177/0272989X06295361
18. Guo Y, Chen X, Zeng T, Wang L, Cen L. Age is a predictor for the syncope recurrence in elderly vasovagal syncope patients with a positive head-up tilt test. Front Cardiovasc Med. (2021) 8:609. doi: 10.3389/fcvm.2021.667171
19. Pournazari P, Sahota I, Sheldon R. High remission rates in vasovagal syncope: systematic review and meta-analysis of observational and randomized studies. JACC: Clin Electrophysiol. (2017) 3(4):384–92. doi: 10.1016/j.jacep.2016.10.012
20. Benditt DG, Detloff BL, Adkisson WO, Lu F, Sakaguchi S, Schussler S, et al. Age-dependence of relative change in circulating epinephrine and norepinephrine concentrations during tilt-induced vasovagal syncope. Heart Rhythm. (2012) 9(11):1847–52. doi: 10.1016/j.hrthm.2012.07.028
21. Song J, Li H, Li X, Wang Y, Jin H, Du J. Relationship between blood routine test parameters and syncopal recurrence of vasovagal syncope in children. Chin J Pediatr. (2017) 55(1):59–63. doi: 10.3760/cma.j.issn.0578-1310.2017.01.012
22. Vanhaecke T, Dolci A, Fulgoni VL, Lieberman HR. Associations between urinary hydration markers and metabolic dysfunction: a cross-sectional analysis of NHANES data, 2008–2010. Eur J Nutr. (2021) 60(8):4229–41. doi: 10.1007/s00394-021-02575-3
23. Khan NA, Westfall DR, Jones AR, Sinn MA, Bottin JH, Perrier ET, et al. A 4-d water intake intervention increases hydration and cognitive flexibility among preadolescent children. J Nutr. (2019) 149(12):2255–64. doi: 10.1093/jn/nxz206
24. Zhang J, Ma G, Du S, Zhang N. The relationships between water intake and hydration biomarkers and the applications for assessing adequate total water intake among young adults in Hebei, China. Nutrients. (2021) 13(11):3805. doi: 10.3390/nu13113805
25. Liu P, Zeng X, Mei W, Wang Y, Zou R, Wang C. The predictive value of urine specific gravity in the diagnosis of vasovagal syncope in children and adolescents. Ital J Pediatr. (2021) 47(1):1–7. doi: 10.1186/s13052-020-00935-z
26. Sekhon MS, Gooderham P, Menon DK, Brasher PM, Foster D, Cardim D, et al. The burden of brain hypoxia and optimal mean arterial pressure in patients with hypoxic ischemic brain injury after cardiac arrest. Crit Care Med. (2019) 47(7):960–9. doi: 10.1097/CCM.0000000000003745
27. Rikhraj KJ, Wood MD, Hoiland RL, Thiara S, Griesdale DE, Sekhon MS. Determining optimal mean arterial pressure after cardiac arrest: a systematic review. Neurocrit Care. (2021) 34(2):621–34. doi: 10.1007/s12028-020-01027-w
28. Liao Y, Du J. Pathophysiology and individualized management of vasovagal syncope and postural tachycardia syndrome in children and adolescents: an update. Neurosci Bull. (2020) 36(6):667–81. doi: 10.1007/s12264-020-00497-4
Keywords: vasovagal syncope, children, recurrence of syncope, prognosis, nomogram model
Citation: Sun R, Kang Y, Zhang M, Wang H, Shi L and Li X (2023) Development of prognostic nomogram model to predict syncope recurrence in children with vasovagal syncope. Front. Cardiovasc. Med. 10:1099115. doi: 10.3389/fcvm.2023.1099115
Received: 15 November 2022; Accepted: 17 February 2023;
Published: 8 March 2023.
Edited by:
Zhen-Yu Zhang, KU Leuven, BelgiumReviewed by:
Régis Guieu, Aix Marseille Université, FranceJulian Mark Stewart, New York Medical College, United States
© 2023 Sun, Kang, Zhang, Wang, Shi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Li bHhobWFnZ2llQDEyNi5jb20=
†These authors have contributed equally to this work and shared first authorship
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Cardiovascular Medicine
 Rui Sun
Rui Sun Yingying Kang2,3,†
Yingying Kang2,3,† Lin Shi
Lin Shi Xiaohui Li
Xiaohui Li