Abstract
Background:
We aimed to test the differences in peak VO2 between males and females in patients diagnosed with heart failure (HF), using combined stress echocardiography (SE) and cardiopulmonary exercise testing (CPET).
Methods:
Patients who underwent CPET and SE for evaluation of dyspnea or exertional intolerance at our institution, between January 2013 and December 2017, were included and retrospectively assessed. Patients were divided into three groups: HF with preserved ejection fraction (HFpEF), HF with mildly reduced or reduced ejection fraction (HFmrEF/HFrEF), and patients without HF (control). These groups were further stratified by sex.
Results:
One hundred seventy-eight patients underwent CPET-SE testing, of which 40% were females. Females diagnosed with HFpEF showed attenuated increases in end diastolic volume index (P = 0.040 for sex × time interaction), significantly elevated E/e' (P < 0.001), significantly decreased left ventricle (LV) end diastolic volume:E/e ratio (P = 0.040 for sex × time interaction), and lesser increases in A-VO2 difference (P = 0.003 for sex × time interaction), comparing to males with HFpEF. Females diagnosed with HFmrEF/HFrEF showed diminished increases in end diastolic volume index (P = 0.050 for sex × time interaction), mostly after anaerobic threshold was met, comparing to males with HFmrEF/HFrEF. This resulted in reduced increases in peak stroke volume index (P = 0.010 for sex × time interaction) and cardiac output (P = 0.050 for sex × time interaction).
Conclusions:
Combined CPET-SE testing allows for individualized non-invasive evaluation of exercise physiology stratified by sex. Female patients with HF have lower exercise capacity compared to men with HF. For females diagnosed with HFpEF, this was due to poorer LV compliance and attenuated peripheral oxygen extraction, while for females diagnosed with HFmrEF/HFrEF, this was due to attenuated increase in peak stroke volume and cardiac output. As past studies have shown differences in clinical outcomes between females and males, this study provides an essential understanding of the differences in exercise physiology in HF patients, which may improve patient selection for targeted therapeutics.
1. Introduction
In patients with heart failure (HF), the assessment of functional capacity by cardiopulmonary exercise testing (CPET) is used in evaluating physical function and patient prognosis and identifying patients suitable for cardiac transplantation. However, the current cutoffs for transplantation are more representative of male populations and extrapolated to females (1). It is known that peak VO2 is lower in healthy females compared to males and potentially much lower for females in the setting of HF and poor ventricular function (2). Due to the under-representation of females in HF trials and in the literature surrounding functional capacity, our understanding of potential sex differences that may exist and that could affect therapy, functional assessment, and recommendations for females with HF, are limited (3).
Any factor that limits peak oxygen consumption (VO2) by reducing the rate of O2 delivery or its utilization by peripheral tissue can lead to limitations in overall exercise capacity. The physiology of O2 delivery and its utilization is determined by a series of steps that ultimately result in aerobic mitochondrial respiration in the peripheral musculature. This cascade includes alveolar ventilation, O2 diffusion into plasma and red blood cells, transport of oxygen through the cardiovascular (CV) system by the heart [cardiac output (CO)] and peripheral vessels to the skeletal muscles, and ultimately followed by entry into the mitochondria. Each of these steps in the O2 pathway can be quantified by using protocols combining cardiopulmonary stress tests (CPET) with invasive hemodynamic assessment through cardiac catheterization (4). However, this pathway of testing is limited due to its invasive nature and leads to limitations through selection bias and its limited anatomical data.
Previous studies (5, 6) have shown the benefit of combined CPET and stress echo (SE) protocol, allowing non-invasive comprehension of the mechanism of exercise intolerance in patients with HF, and its potential for clinical management. However, there is limited literature evaluating the differences in the mechanism between males and females diagnosed with HF, by using this novel CEPT-SE protocol (5, 6).
Therefore, we aimed to test the mechanisms for differences in peak VO2 between males and females diagnosed with HF through the non-invasive CEPT-SE protocol, assessing multiple hemodynamic responses to exercise, in predefined activity levels.
2. Methods
2.1. Study population
Between January 2013 and December 2017, 248 combined CPET and SE exams were performed using our novel protocol. All patients were clinically stable and ambulatory and referred for the evaluation of effort intolerance or dyspnea. We excluded patients who presented with primary valvular disease (aortic valve replacement n = 5, aortic stenosis n = 5, rheumatic mitral stenosis n = 12, organic mitral regurgitation n = 3), hypertrophic cardiomyopathy (n = 20), sinoatrial block (n = 1), active ischemia (n = 2), atrial septal defect (n = 1), primary pulmonary hypertension (n = 2), and mitochondrial myopathy (n = 1), as those disorders might influence the results of the test. Furthermore, none of the patients had congenital heart disease. We also excluded patients who were unable to complete exercise on a semi-recumbent bicycle (respiratory exchange ratio < 1.0; n = 15) or were with inadequate acoustic windows (n = 3). The remaining 178 patients were divided into three groups (control group, HFpEF, HFmrEF/HFrEF). The rationale for combining patients diagnosed with HFmrEF and HFrEF into one group was according to the ESC 2021 HF guidelines suggesting those two groups will benefit from similar therapies (7). Each group was divided based on sex [control group; M = 23 (61%), F = 15 (39%), HFpEF; M = 43 (55%), F = 35 (45%), and HFmrEF/HFrEF; M = 40 (65%), F = 22 (35%)]. The control group of patients all had normal exercise capacity and normal baseline echocardiography. Diagnosis of HFpEF and HFmrEF/HFrEF was made prior to testing, and was based on clinical signs and symptoms of HF as defined by the criteria of Rich et al. (8). Diagnosis of HFpEF was defined as patients with resting echocardiography ejection fraction EF ≥50%, while HFmrEF/HFrEF was defined as resting echocardiography with EF < 50%. The retrospective collection and analysis of data was approved by the institutional review board (IRB Committee project approval number 0346-13-TLV).
2.2. Sub-group analysis
We have previously shown that effort induced LV and RV, hemodynamic, and peripheral changes differ significantly between patients with normal cardiovascular effort capacity, HFpEF, and HFmrEF/HFrEF (5). Thus, in this study we performed subgroup analyses comparing CV and peripheral responses to exercise in males and females stratified within three groups: control group, patients with HFpEF, and patients with HFmrEF/HFrEF.
2.3. Exercise protocol
A symptom-limited graded ramp bicycle exercise test was performed in the semi-supine position on a tilting dedicated microprocessor-controlled eddy current brake stress echo cycle ergo meter (Ergoselect 1000 L, CareFusion, USA). We estimated the expected peak VO2 max based on the patient's age, height, and weight, while also including the patient's history. We then calculated the work rate increment necessary to reach each individual patient's estimated peak VO2 in 8–12 min. The protocol included 3 min of unloaded pedaling, a symptom limited ramp graded exercise, and 2 min of recovery. Breath-by-breath minute ventilation (VE), carbon dioxide production (VCO2), and VO2 were measured using a Medical Graphics metabolic cart (ZAN, nSpire Health Inc, Germany). Peak VO2 was the highest averaged 30-s VO2 during exercise (9). Anaerobic threshold was determined manually using the modified V-slope method. VE/VCO2 was defined as the lowest immediately after anaerobic threshold, and was expressed as absolute nadir VE/VCO2 (9, 10). A 12-lead electrocardiogram (ECG) and non-invasive arterial saturation were monitored continuously, heart rate and blood pressure were measured at rest and every minute during exercise.
2.4. Exercise echocardiography testing
Echocardiography images were obtained concurrently with breath-by-breath gas exchange measurements at rest, immediately upon reaching anaerobic threshold, and at maximal exercise capacity. Data collected at each time period included left ventricle (LV) end diastolic volume (LVEDV), end systolic volume (LVESV), EF, stroke volume (SV), Peak E- and A-wave velocities, E wave deceleration time (DT), and e′ in the septal mitral annulus. LVEDV, LVESD, and EF were calculated based on the single plane ellipsoid apical 4 chamber area-length method (11). SV was calculated by multiplying the LV outflow tract area at rest by the LV outflow tract velocity–time integral measured by pulsed-wave Doppler during each activity levels. E/e' ratio was calculated at all effort stages. During sinus tachycardia whenever merging of mitral E and A velocities occurred, peak E wave velocity, DT, A wave velocity, e' and E/e' ratio were measured by the methods used by Nagueh et al. (12). A-VO2 difference was calculated by using the Fick equation as: VO2/echo calculated cardiac output at each activity level (5, 10).
2.5. Statistical analysis
Descriptive results were expressed as mean ± SD for continuous variables and as percentages for categorical variables. For the analysis of differences in echocardiography and exercise variables between the male and female patients we used ANOVA for continuous, normally distributed, Wilcoxon test for other continuous, and Fisher's exact test or Chi-square test, for categorical variables. We used the repeated measures linear model analysis to define the within-group effect for each parameter over time, the between group differences over time, and the group by time interactions. Using an analysis “sex × time” interaction we referred to the difference between the groups overtime. While “group” tested the difference between the results between the two sexes, and “time” tested the in-group increment differences, “sex × time” compared the temporal change between the groups. This allows comparison of the sex-specific response to exercise for each tested parameter. All computations were performed using JMP statistical software for Windows (Version 13.0; SAS Institute Inc).
3. Results
3.1. Complete cohort results
Clinical and baseline echocardiography characteristics of our cohort in its entirety and stratified by sex are presented in Table 1. Most baseline LV parameters were larger in male patients compared to females, even following adjustment for body surface area (BSA). Of note, although functional parameters for LV (Cardiac index, EF) and RV (fractional area change) were similar in both sexes, E/e' was significantly higher in female patients compared to males (21.4 ± 15.4 vs. 14.5 ± 10.1, P = 0.004). Echocardiographic and combined CPET-SE parameters of the entire cohort, stratifies by sex groups in each of the exercise phases are presented in Table 2. Male patients showed a gradual increase in LVEDV when approaching the anaerobic threshold stage, which, combined with the attenuated change in LVESV, resulted in an early SV increase by ≈25% (mean 88.2 vs. 71.6 ml; P < 0.0001). LVEDV and LVESV volume decreased between the anaerobic threshold and maximal exercise phases resulting in a maintained average SV in the final part of the effort. However, in female patients, the gradual increase in LV volumes up to the anaerobic threshold was attenuated (P < 0.05 for sex × time interaction) resulting in a trend toward reduction in increases in SV during effort (P = 0.09 for sex × time interaction). Most importantly, female patients showed attenuated increases in A-VO2 difference (P = 0.04 for sex × time interaction), suggesting that the decrease in exercise capacity in females compared to male patients is related in part to peripheral factors.
Table 1
| Males | Females | P-value | |
|---|---|---|---|
| Age, years | 59.3 (±17.1) | 61.2 (±13.0) | 0.007 |
| Body surface area, m2 | 1.96 (±0.17) | 1.75 (±0.19) | <0.001 |
| Heart rate, beats per minute | 79.2 (±13.4) | 82.3 (±17.1) | 0.136 |
| Systolic blood pressure, mmHg | 133.3 (±38.5) | 140.1 (±26.6) | 0.047 |
| Diastolic blood pressure, mmHg | 75.1 (±23.1) | 80.8 (±12.5) | 0.04 |
| Left ventricle end diastolic diameter, mm | 51.6 (±8.6) | 47.6 (±6.5) | <0.001 |
| Left ventricle end systolic diameter, mm | 35.5 (±10.7) | 30.3 (±8.7) | <0.001 |
| Left ventricle ejection fraction, % | 51.3 (±16.7) | 54.1 (±15.1) | 0.239 |
| Stroke volume index, ml/m2 | 36.8 (±9.5) | 38.6 (±11.0) | 0.267 |
| Cardiac index, ml/min/m2 | 2.8 (±0.8) | 3.0 (±0.8) | 0.100 |
| Left ventricle end diastolic volume index, ml | 79.4 (±30.8) | 66.6 (±20.4) | 0.002 |
| Left ventricle end systolic volume index, ml | 40.9 (±26.1) | 31.2 (±16.7) | 0.005 |
| Left ventricle mass index, gr/m2 | 123 (±39) | 103 (±29) | 0.002 |
| Left atrial diameter, mm | 41.5 (±11.7) | 42.8 (±8.9) | 0.397 |
| Left atrial volume index, ml/m2 | 38.1 (±18.1) | 44.8 (±23.1) | 0.060 |
| E wave cm/s base | 78.5 (±30.1) | 107.6 (±55.7) | <0.001 |
| A wave cm/s base | 60.5 (±27.7) | 73.1 (±40.6) | 0.060 |
| E/A ratio | 1.4 (±0.6) | 1.4 (±0.6) | 0.168 |
| Deceleration time, ms | 216 (±89) | 286 (±177) | 0.006 |
| E' cm/s | 6.2 (±2.5) | 5.9 (±2.3) | 0.509 |
| E/e' | 14.5 (±10.1) | 21.4 (±15.4) | 0.004 |
| Systolic pulmonary artery pressure, mmHg | 33.3 (±10.8) | 36.0 (±12.7) | 0.397 |
| S wave, cm/s | 5.6 (±2.1) | 4.9 (±1.4) | 0.003 |
| Right atrial area, cm2 | 17.8 (±6.1) | 15.6 (±5.1) | 0.01 |
| Right ventricle end diastolic area, cm2 | 24.5 (±6.9) | 20.3 (±5.1) | <0.001 |
| Right ventricle end systolic area, cm2 | 14.7 (±5.1) | 12.1 (±4.1) | 0.001 |
| Right ventricle fractional area change, % | 0.39 (±0.1) | 0.41 (±0.13) | 0.789 |
| FEV1, % predicted | 87.4 (±17.7) | 85.3 (±19.1) | 0.062 |
| FVC, % predicted | 82.6 (±17.5) | 82.1 (±18.6) | 0.689 |
| FEV1-FVC, % predicted | 109.2 (±16.6) | 106.9 (±11.4) | 0.020 |
| Peak VO2 | 1.6 (±0.7) | 1.0 (±0.4) | <0.001 |
| Peak VO2/kg | 19.3 (±9.1) | 13.9 (±5.6) | <0.001 |
Baseline characteristics according to sex.
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; VO2, rate of oxygen consumption.
Table 2
| Measurement | Baseline |
Anaerobic
threshold |
Maximal
effort |
Group | Time |
Time*group
interaction |
|
|---|---|---|---|---|---|---|---|
| End diastolic volume, ml | Male | 155.3 (±64.5) | 172.1 (±71.6) | 159.2 (±60.2) | <0.001 | 0.010 | 0.050 |
| Female | 114.0 (±34.0) | 125.0 (±44.4) | 115.7 (±40.3) | ||||
| End diastolic volume index | Male | 79.4 (±30.8) | 87.7 (±34.3) | 81.6 (±29.7) | 0.010 | 0.020 | 0.030 |
| Female | 40.9 (±26.1) | 71.8 (±25.3) | 66.7 (±24.1) | ||||
| End systolic volume, ml | Male | 80.3 (±52.1) | 84.6 (±48.4) | 78.0 (±53.7) | 0.005 | 0.050 | 0.237 |
| Female | 53.8 (±30.6) | 52.0 (±31.1) | 53.2 (±32.2) | ||||
| Ejection fraction, % | Male | 51.3 (±16.7) | 53.9 (±19.8) | 53.4 (±18.4) | 0.974 | 0.070 | 0.974 |
| Female | 54.1 (±15.1) | 58.8 (±15.4) | 54.5 (±18.9) | ||||
| Tissue Doppler RV S', cm/s |
Male | 5.6 (±2.1) | 7.3 (±2.8) | 7.5 (±3.1) | 0.542 | <0.001 | 0.542 |
| Female | 4.9 (±1.4) | 6.7 (±2.4) | 6.6 (±2.7) | ||||
| Right ventricle end diastolic area, cm2 | Male | 24.5 (±6.9) | 26.6 (±5.8) | 25.1 (±6.1) | <0.001 | 0.020 | 0.081 |
| Female | 20.3 (±5.1) | 21.5 (±4.0) | 22.3 (±4.4) | ||||
| Right ventricle end systolic area, cm2 | Male | 14.7 (±5.1) | 15.6 (±5.0) | 14.9 (±5.4) | 0.004 | 0.164 | 0.545 |
| Female | 12.1 (±4.1) | 12.9 (±3.9) | 12.8 (±4.5) | ||||
| Right ventricle fractional area change, % | Male | 0.39 (±0.1) | 0.41 (±0.12) | 0.40 (±0.15) | 0.138 | 0.572 | 0.138 |
| Female | 0.41 (±0.13) | 0.40 (±0.15) | 0.43 (±0.15) | ||||
| Tissue Doppler e', cm/s |
Male | 6.2 (±2.5) | 10.2 (±4.8) | 11.4 (±6.4) | 0.818 | <0.001 | 0.818 |
| Female | 5.9 (±2.3) | 8.8 (±4.7) | 9.2 (±4.9) | ||||
| E/e' | Male | 14.5 (±10.1) | 13.8 (±9.4) | 15.4 (±10.4) | 0.001 | 0.714 | 0.295 |
| Female | 21.4 (±15.4) | 22.3 (±17.1) | 22.3 (±16.2) | ||||
| Stroke volume, ml | Male | 71.6 (±18.9) | 88.2 (±27.4) | 84.5 (±29.0) | 0.030 | <0.001 | 0.090 |
| Female | 65.9 (±16.4) | 80.9 (±21.4) | 68.9 (±26.7) | ||||
| Stroke volume index, ml/m | Male | 36.8 (±9.5) | 45.2 (±14.4) | 43.9 (±14.8) | 0.038 | <0.001 | 0.040 |
| Female | 38.6 (±11.0) | 46.6 (±12.9) | 39.5 (±14.6) | ||||
| Heart rate, BPM | Male | 79.2 (±13) | 107 (±21) | 127 (±15) | 0.963 | <0.001 | 0.963 |
| Female | 82.3 (±17) | 108 (±23) | 123 (31) | ||||
| Cardiac output, L/min | Male | 5.5 (±1.6) | 9.3 (±3.4) | 10.8 (±4.7) | 0.100 | <0.001 | 0.362 |
| Female | 5.3 (±1.4) | 9.0 (±2.9) | 8.8 (±4.0) | ||||
| VO2, L/min | Male | 0.4 (±0.1) | 1.1 (±0.4) | 1.6 (±0.7) | <0.001 | <0.001 | <0.001 |
| Female | 0.3 (±0.1) | 0.8 (±0.3) | 1.0 (±0.4) | ||||
| A-VO2 diff, L/L | Male | 0.08 (±0.03) | 0.11 (±0.03) | 0.15 (±0.05) | 0.002 | <0.001 | 0.040 |
| Female | 0.07 (±0.02) | 0.09 (±0.03) | 0.13 (±0.06) | ||||
Echocardiographic and combined cardiopulmonary exercise- stress echocardiography parameters of the entire cohort according to sex and stratified to the exercise phase.
BPM, beat per minute; VO2, rate of oxygen consumption.
3.2. Cardiovascular and peripheral responses in the control group
Clinical and baseline echocardiographic characteristics of the control group stratified by sex are shown in Supplementary Table 1. Echocardiographic and combined CPET-SE parameters for each sex during each of the exercise phases in the control group are presented in Table 3 and Figure 1. Female patients showed diminished increases in LV volumes when approaching anaerobic threshold (P = 0.033 for sex × time interaction), and in VO2 and A-VO2 difference at peak exercise (P < 0.001 and P = 0.010, respectively for sex × time interaction), when compared to males in the control group.
Table 3
| Measurement control | Baseline | Anaerobic threshold | Maximal effort | Group | Time |
Time*group
interaction |
|
|---|---|---|---|---|---|---|---|
| End diastolic volume, ml | Males | 161.7 (±72.7) | 187.0 (±69.3) | 158.8 (±58.2) | 0.033 | 0.020 | 0.033 |
| Females | 106.7 (±17.9) | 123.0 (±24.8) | 107.4 (±29.1) | ||||
| End diastolic volume index | Males | 72.3 (±17.9) | 90.3 (±25.4) | 73.8 (±20.0) | 0.010 | <0.001 | 0.030 |
| Females | 66.5 (±14.2) | 71.9 (±17.8) | 64.0 (±21.7) | ||||
| End systolic volume, ml | Males | 78.3 (±54) | 70.5 (±52.8) | 68.1 (±49.3) | 0.944 | 0.050 | 0.944 |
| Females | 42.5 (±18.4) | 40.6 (±12.5) | 34.2 (±20.2) | ||||
| Ejection fraction, % | Males | 64.0 (±16.1) | 64.8 (±14.1) | 66.7 (±14.8) | 0.865 | 0.010 | 0.865 |
| Females | 61.0 (±13.4) | 65.4 (±13.6) | 67.3 (±19.0) | ||||
| Tissue Doppler RV S', cm/s | Males | 7.1 (±1.5) | 10.2 (±2.6) | 10.6 (±3.2) | 0.913 | <0.001 | 0.913 |
| Females | 6.1 (±1.2) | 9.0 (±2.5) | 8.8 (±2.4) | ||||
| Right ventricle end diastolic area, cm2 | Males | 26.5 (±7.3) | 28.4 (±5.9) | 23.0 (±4.8) | 0.020 | 0.786 | 0.291 |
| Females | 21.3 (±5.5) | 21.2 (±2.9) | 22.7 (±4.1) | ||||
| Right ventricle end systolic area, cm2 | Males | 15.2 (±3.8) | 14.3 (±4.1) | 13.6 (±3.9) | 0.030 | 0.070 | 0.547 |
| Females | 12.8 (±5.2) | 10.8 (±2.8) | 10.9 (±4.5) | ||||
| Right ventricle fractional area change, % | Males | 0.4 (±0.09) | 0.49 (±0.1) | 0.41 (±0.1) | 0.191 | 0.001 | 0.191 |
| Females | 0.4 (±0.1) | 0.49 (±0.1) | 0.51 (±0.2) | ||||
| Tissue Doppler e', cm/s | Males | 8.2 (±2.7) | 14.9 (±4.8) | 16.5 (±7.2) | 0.281 | <0.001 | 0.281 |
| Females | 8.0 (±2.8) | 10.9 (±4.7) | 14.8 (±3.9) | ||||
| E/e' | Males | 9.1 (±4.2) | 8.6 (±3.9) | 7.4 (±3.3) | 0.884 | 0.123 | 0.884 |
| Females | 9.3 (±4.0) | 8.5 (±4.3) | 7.7 (±3.0) | ||||
| LVEDVi:E/e' | Males | 9.5 (±3.0) | 11.2 (±3.4) | 10.2 (±2.7) | 0.751 | 0.459 | 0.751 |
| Females | 7.8 (±5.0) | 8.5 (±2.9) | 8.3 (±2.5) | ||||
| Stroke volume, ml | Males | 76.2 (±20.4) | 108.7 (±28.3) | 102.7 (±26.8) | 0.100 | <0.001 | 0.296 |
| Females | 71.4 (±12.0) | 94.6 (±15.2) | 88.9 (±26.4) | ||||
| Stroke volume index, ml/m | Males | 41.3 (±11.0) | 58.5 (±14.0) | 53.6 (±14.4) | 0.446 | <0.001 | 0.446 |
| Females | 42.6 (±9.2) | 54.9 (±11.3) | 46.0 (±17.0) | ||||
| Heart rate, BPM | Males | 76.9 (±19.7) | 122.9 (±19.7) | 159.6 (±25.1) | 0.141 | <0.001 | 0.141 |
| Females | 77.1 (±16.6) | 113.1 (±16.6) | 145.0 (±20.0) | ||||
| Cardiac output, L/min | Males | 5.7 (±1.8) | 13.2 (±4.0) | 16.0 (±5.0) | 0.100 | <0.001 | 0.102 |
| Females | 5.6 (±1.1) | 11.0 (±2.5) | 12.6 (±4.2) | ||||
| VO2, L/min | Males | 0.4 (±0.1) | 1.4 (±0.5) | 2.5 (±0.78) | <0.001 | <0.001 | <0.001 |
| Females | 0.3 (±0.07) | 1.0 (±0.42) | 1.4 (±0.65) | ||||
| A- VO2 Diff, L/L | Males | 0.08 (±0.03) | 0.11 (±0.04) | 0.16 (±0.04) | 0.100 | <0.001 | 0.010 |
| Females | 0.06 (±0.01) | 0.09 (±0.02) | 0.10 (±0.02) | ||||
Echocardiographic and combined cardiopulmonary exercise- stress echocardiography parameters in the control group according to sex and stratified to the exercise phase.
RV, right ventricle; LVEDVi, left ventricle end diastolic volume index; BPM, beat per minute; VO2, rate of oxygen consumption.
Figure 1
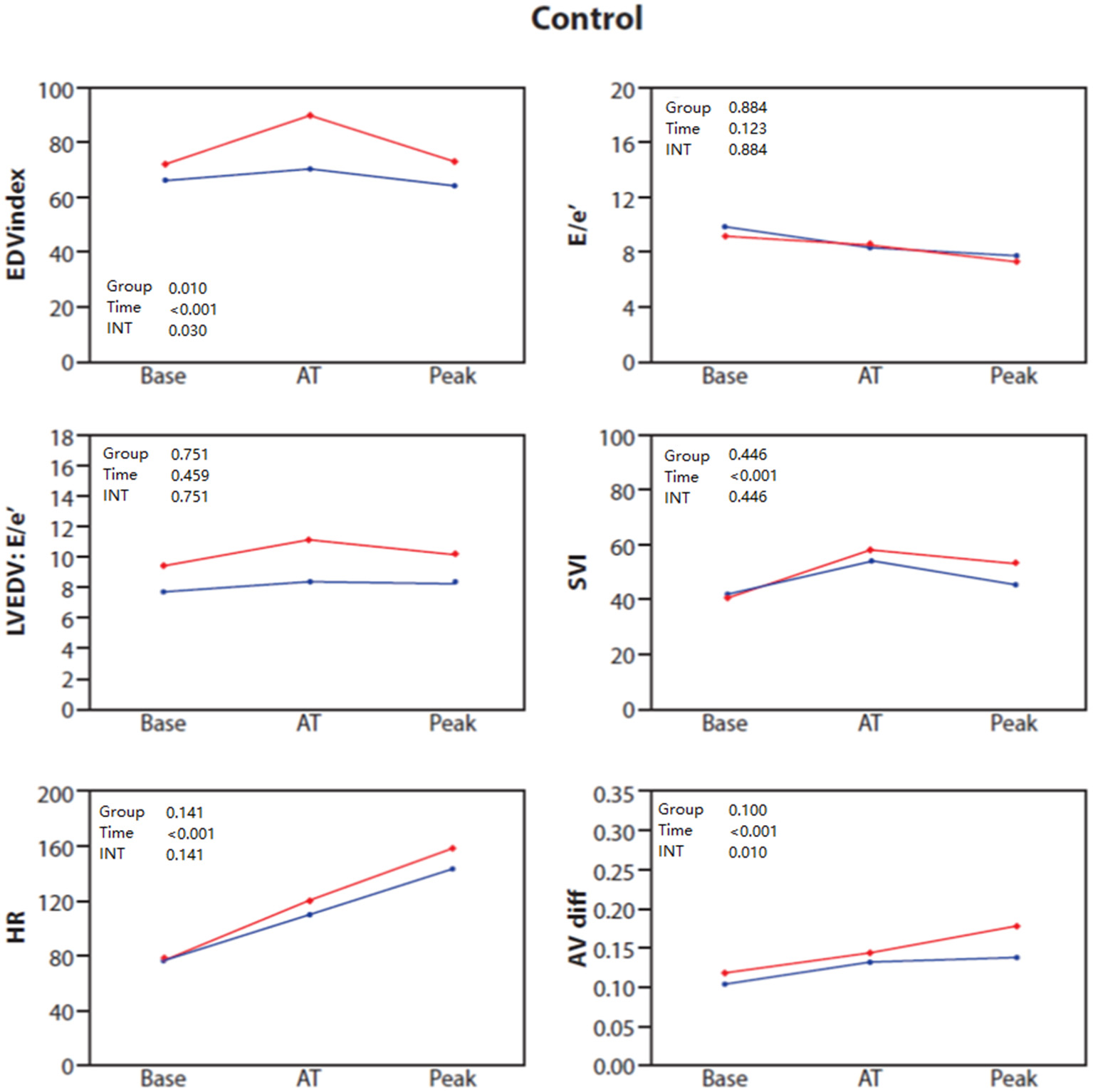
Baseline, anaerobic threshold (AT), and maximal cardiopulmonary exercise (Peak) test and stress echocardiography test for End diastolic volume (EDV) index, E/e', LVEDV: E/e' ratio, stroke volume index (SVI), heart rate (HR) and AVO2 difference in control patients stratified by sex: females (blue) and males (red).
3.3. Cardiovascular and peripheral responses in Patients with HFpEF
Clinical and baseline echocardiographic characteristics for HFpEF patients stratified by sex are shown in Supplementary Table 1. Echocardiographic and combined CPET-SE parameters for the sex groups in each of the exercise phases in the HFpEF group are presented in Table 4 and Figure 2A. The main differences are summarized by three main points. First, female patients showed attenuated increases in LVEDV index (P = 0.04 for sex × time interaction), but showed no difference in SV index or CO, compared to males. Second, female patients with HFpEF had a significantly increased measured E/e' throughout the exercise protocol (P < 0.001) and LVEDV:E/e ratio (P = 0.001), compared to males with HFpEF. Third female patients with HFpEF had significantly diminished increases in A-VO2 difference (P = 0.003 for sex × time interaction), compared to males with HFpEF.
Table 4
| Measurement control | Baseline | Anaerobic threshold | Maximal effort | Group | Time |
Time*group
interaction |
|
|---|---|---|---|---|---|---|---|
| End diastolic volume, ml | Males | 123.1 (±35.4) | 137.8 (±32.1) | 129.0 (±42.4) | <0.001 | 0.090 | 0.050 |
| Females | 103.9 (±22.5) | 107.9 (±32.3) | 99.6 (±26.7) | ||||
| End diastolic volume index | Males | 66.9 (±17.5) | 70.8 (±16.8) | 70.6 (±20.3) | 0.040 | 0.090 | 0.040 |
| Females | 62.8 (±14.5) | 63.1 (±17.0) | 57.7 (±15.0) | ||||
| End systolic volume, ml | Males | 43.9 (±18.5) | 51.7 (±27.0) | 48.4 (±25.9) | 0.010 | 0.060 | 0.173 |
| Females | 37.6 (±12.5) | 39.0 (±15.2) | 35.6 (±14.2) | ||||
| Ejection fraction, % | Males | 64.6 (±9.6) | 63.3 (±15.4) | 62.2 (±16.2) | 0.563 | 0.227 | 0.563 |
| Females | 63.9 (±8.6) | 62.4 (±14.7) | 63.3 (±14.5) | ||||
| Tissue Doppler RV S', cm/s | Males | 5.6 (±1.9) | 7.3 (±2.3) | 7.6 (±2.6) | 0.066 | <0.001 | 0.066 |
| Females | 4.9 (±1.5) | 6.5 (±2.1) | 6.7 (±2.6) | ||||
| Right ventricle end diastolic area, cm2 | Males | 21.1 (±5.0) | 24.5 (±4.5) | 24.6 (±5.1) | <0.001 | 0.010 | 0.276 |
| Females | 18.6 (±3.6) | 20.8 (±4.2) | 20.5 (±4.4) | ||||
| Right ventricle end systolic area, cm2 | Males | 12.3 (±3.4) | 13.2 (±3.7) | 12.8 (±4.1) | 0.050 | 0.199 | 0.383 |
| Females | 10.6 (±2.9) | 11.6 (±3.1) | 10.8 (±3.7) | ||||
| Right ventricle fractional area change, % | Males | 0.42 (±0.11) | 0.47 (±0.11) | 0.48 (±0.14) | 0.383 | 0.050 | 0.383 |
| Females | 0.43 (±0.12) | 0.44 (±0.14) | 0.48 (±0.11) | ||||
| Tissue Doppler e', cm/s | Males | 5.9 (±2.1) | 8.5 (±3.8) | 9.9 (±4.9) | 0.086 | <0.001 | 0.086 |
| Females | 5.7 (±2.5) | 8.6 (±4.9) | 9.1 (±5.4) | ||||
| E/e' | Males | 16.6 (±3.9) | 17.4 (±11.5) | 18.8 (±15.7) | <0.001 | 0.644 | 0.529 |
| Females | 30.4 (±11.8) | 35.0 (±19.0) | 34.1 (±17.7) | ||||
| Left ventricle end diastolic volume index: E/e' | Males | 5.2 (±6.1) | 6.1 (±7.6) | 5.9 (±7.1) | 0.001 | 0.443 | 0.040 |
| Females | 2.6 (±4.6) | 2.3 (±6.3) | 2.0 (±5.3) | ||||
| Stroke volume, ml | Males | 74.8 (±14.5) | 88.1 (±18.7) | 82.2 (±18.2) | 0.010 | <0.001 | 0.010 |
| Females | 65.5 (±15.5) | 79.5 (±18.6) | 71.2 (±22.7) | ||||
| Stroke volume index, ml/m | Males | 38.7 (±7.5) | 44.6 (±11.0) | 41.8 (±10.2) | 0.795 | <0.001 | 0.795 |
| Females | 40.2 (±10.1) | 45.2 (±11.4) | 41.3 (±12.5) | ||||
| Heart rate, BPM | Males | 78.7 (±13.7) | 104.1 (±20.5) | 121.3 (±27.1) | 0.817 | <0.001 | 0.817 |
| Females | 78.9 (±15.0) | 104.8 (±23.0) | 118.8 (±30.5) | ||||
| Cardiac output, L/min | Males | 5.8 (±1.3) | 8.9 (±2.8) | 9.7 (±2.8) | 0.100 | <0.001 | 0.449 |
| Females | 5.1 (±1.5) | 8.7 (±2.6) | 8.9 (±3.5) | ||||
| VO2, L/min | Males | 0.39 (±0.13) | 0.95 (±0.28) | 1.29 (±0.4) | <0.001 | <0.001 | 0.010 |
| Females | 0.33 (±0.07) | 0.75 (±0.25) | 0.95 (±0.36) | ||||
| A-VO2 Diff, L/L | Males | 0.07 (±0.02) | 0.11 (±0.03) | 0.13 (±0.04) | 0.015 | <0.001 | 0.003 |
| Females | 0.07 (±0.02) | 0.09 (±0.02) | 0.10 (±0.05) | ||||
Echocardiographic and combined cardiopulmonary exercise- stress echocardiography parameters in patients with heart failure and preserved ejection fraction according to sex and stratified to the exercise phase.
RV, right ventricle; BPM, beat per minute; VO2, rate of oxygen consumption.
Figure 2
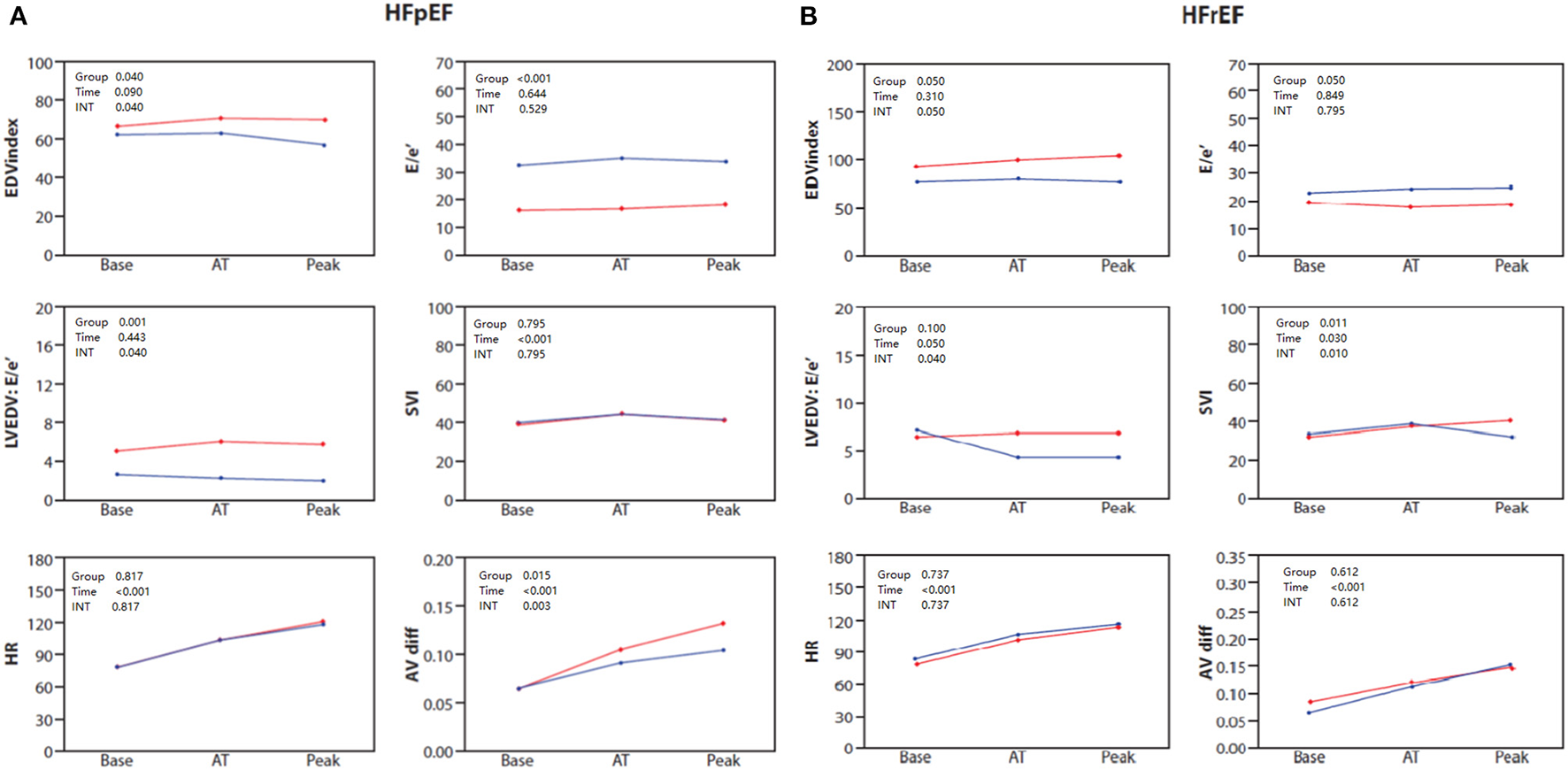
(A) Baseline, anaerobic threshold (AT), and maximal cardiopulmonary exercise (Peak) test and stress echocardiography test for End diastolic volume (EDV) index, E/e', LVEDV: E/e' ratio, stroke volume index (SVI), heart rate (HR) and AVO2 difference in HFpEF patients stratified by sex: females (blue) and males (red). (B) Baseline, anaerobic threshold (AT), and maximal cardiopulmonary exercise (Peak) test and stress echocardiography test for End diastolic volume (EDV) index, E/e', LVEDV: E/e' ratio, stroke volume index (SVI), heart rate (HR) and AVO2 difference in HFmrEF/HFrEF patients stratified by sex: females (blue) and males (red).
3.4. Cardiovascular and peripheral responses in patients with HFmrEF/HFrEF
Clinical and baseline echocardiographic characteristics of the patients with HFmrEF/HFrEF stratified by sex are shown in Supplementary Table 1. Echocardiographic and combined CPET-SE parameters of the sex groups in each of the exercise phases of HFmrEF/HFrEF patients are presented in Table 5 and Figure 2B. We found the main differences between the sexes in HFmrEF/HFrEF patients to be that females demonstrated attenuated increases in LVEDV (P = 0.05 for sex × time interaction), which resulted in a diminished increase in SV index (P = 0.010 for sex × time interaction) and CO (P = 0.05 for sex x time interaction), compared to males with HFmrEF/HFrEF. Although there were no significant differences in E/e', the LVEDV:E/e ratio was lower in the late stages of exercise in females. Interestingly, there was no difference between sexes in A-VO2 throughout the exercise protocol.
Table 5
| Measurement control | Baseline | Anaerobic threshold | Maximal effort | Group | Time |
Time*group
interaction |
|
|---|---|---|---|---|---|---|---|
| End diastolic volume, ml | Male | 181.9 (±72.9) | 206.7 (±91.8) | 193.4 (±61.4) | 0.010 | 0.286 | 0.050 |
| Female | 125.6 (±47.3) | 141.2 (±65.2) | 131.2 (±52.8) | ||||
| End diastolic volume index | Male | 93.6 (±32.5) | 100.7 (±41.2) | 105.0 (±30.1) | 0.050 | 0.31 | 0.050 |
| Female | 77.7 (±24.4) | 81.7 (±37.5) | 78.7 (±29.5) | ||||
| End systolic volume, ml | Male | 116.9 (±53.8) | 130.6 (±88.0) | 114.0 (±60.2) | 0.050 | 0.733 | 0.801 |
| Female | 79.3 (±34.4) | 78.4 (±43.9) | 82.7 (±34.7) | ||||
| Ejection fraction, % | Male | 36.4 (±9.1) | 39.1 (±18.1) | 42.9 (±14.9) | 0.356 | 0.050 | 0.356 |
| Female | 37.3 (±8.9) | 40.3 (±13.9) | 36.9 (±11.3) | ||||
| Tissue Doppler RV S', cm/s | Male | 5.2 (±2.1) | 5.9 (±2.2) | 5.8 (±2.6) | 0.201 | 0.010 | 0.201 |
| Female | 4.5 (±1.2) | 5.2 (±1.2) | 5.7 (±2.2) | ||||
| Right ventricle end diastolic area, cm2 | Male | 27.0 (±7.6) | 28.9 (±6.2) | 27.4 (±7.3) | 0.004 | 0.817 | 0.050 |
| Female | 20.7 (±5.8) | 21.3 (±4.8) | 23.7 (±4.6) | ||||
| Right ventricle end systolic area, cm2 | Male | 17.0 (±6.2) | 19.4 (±5.0) | 17.8 (±6.2) | 0.080 | 0.100 | 0.100 |
| Female | 13.4 (±4.3) | 15.7 (±3.9) | 16.2 (±3.1) | ||||
| Right ventricle fractional area change, % | Male | 0.36 (±0.1) | 0.32 (±0.1) | 0.33 (±0.1) | 0.449 | 0.009 | 0.449 |
| Female | 0.35 (±0.1) | 0.26 (±0.1) | 0.31 (±0.1) | ||||
| Tissue Doppler e', cm/s | Male | 5.5 (±2.3) | 8.8 (±4.3) | 8.7 (±4.3) | 0.646 | 0.003 | 0.646 |
| Female | 5.3 (±1.7) | 6.8 (±3.1) | 8.0 (±3.9) | ||||
| E/e' | Male | 18.2 (±12.7) | 16.7 (±11.6) | 17.9 (±9.9) | 0.050 | 0.849 | 0.795 |
| Female | 21.6 (±13.9) | 22.6 (±13.9) | 22.8 (±14.) | ||||
| Left ventricle end diastolic volume index:E/e' | Male | 6.6 (±3.4) | 6.9 (±6.5) | 6.9 (±4.0) | 0.100 | 0.050 | 0.040 |
| Female | 7.2 (±4.8) | 4.4 (±2.4) | 4.3 (±3.4) | ||||
| Stroke volume, ml | Male | 65.9 (±19.8) | 77.7 (±26.) | 81.6 (±35.0) | 0.036 | 0.050 | 0.030 |
| Female | 63.6 (±17.5) | 70.4 (±26.2) | 62.5 (±21.2) | ||||
| Stroke volume index, ml/m | Male | 33.2 (±9.1) | 39.0 (±14.5) | 41.7 (±17.0) | 0.011 | 0.030 | 0.010 |
| Female | 35.1 (±10.5) | 39.6 (±11.5) | 32.7 (±11.1) | ||||
| Heart rate, BPM | Male | 79.0 (±14.7) | 102.0 (±21.2) | 114.6 (±28.9) | 0.737 | <0.001 | 0.737 |
| Female | 84.1 (±19.2) | 106.5 (±19.9) | 115.2 (±26.1) | ||||
| Cardiac output, L/min | Male | 5.1 (±1.6) | 8.1 (±3.0) | 9.4 (±4.6) | 0.058 | <0.001 | 0.050 |
| Female | 5.1 (±1.2) | 7.7 (±3.2) | 7.0 (±2.7) | ||||
| VO2, L/min | Male | 0.4 (±0.1) | 0.9 (±0.31) | 1.2 (±0.47) | <0.001 | <0.001 | 0.007 |
| Female | 0.3 (±0.08) | 0.7 (±0.2) | 0.8 (±0.24) | ||||
| A-VO2 Diff, L/L | Male | 0.09 (±0.02) | 0.12 (±0.03) | 0.15 (±0.05) | 0.612 | <0.001 | 0.612 |
| Female | 0.07 (±0.02) | 0.11 (±0.04) | 0.14 (±0.06) | ||||
Echocardiographic and combined cardiopulmonary exercise- stress echocardiography parameters in patients with heart failure with mildly reduced and reduced ejection fraction according to sex and stratified to the exercise phase.
RV, right ventricle; BPM, beat per minute; VO2, rate of oxygen consumption.
4. Discussion
Our major findings are that: (1) the described combined CPET-SE protocol allows for detailed individualized non-invasive evaluation of exercise physiology throughout varying levels of effort for male and female patients; (2) Female patients with HFpEF have lower exercise capacity when compared with similar male patients due to poorer LV compliance, higher filling pressures, and attenuated peripheral oxygen extraction; and (3) Female patients with HFmrEF/HFrEF have lower exercise capacity when compared with similar male patients due to diminished increases in LV volumes at later stages of exercise induced stress resulting in attenuated increases in SV and CO.
Despite the fact that females represent around 50% of the total population of patients diagnosed with HF (13), they have been under-represented in currently published HF studies (14). CPET, with the measurement of peak VO2, has become the cornerstone tool for assessing functional capacity and predicting outcomes in patients with HF (8, 15). Surprisingly, the under-representation of females in CPET studies is even more apparent (15–17). These gaps in information limit our understanding of risk stratification, recommendations for physical therapy, and advanced HF intervention in females. Given the limited available data in CPET parameters for the clinical assessment of females with HF, we have devised our new combined CPET and SE protocol (5), which enables clinicians to non-invasively assess multiple responses and parameters related to dynamic exercise, and to define the mechanisms for difference in peak VO2 between male and female patients diagnosed with HFpEF or HFmrEF/HFrEF.
4.1. HFpEF
The main differences found between female and male patients with HFpEF were that female patients showed attenuated increases in LVEDV, increased E/e', and decreases in LVEDV:E/e ratio throughout the protocol. E/e' is the echocardiographic correlate of LV diastolic pressure and was higher in female patients throughout exercise. We have previously shown that LVEDV:E/e' ratio is an estimate of LV compliance (5) and estimates one's ability to utilize the Frank-Starling mechanism. Decreased LVEDV:E/e' ratio represent the failure of LV to appropriately increase its size proportionately to filling pressure. In this present study, we show that LVEDV:E/e' ratio was lower in female patients with HFpEF. This finding suggests that diastolic dysfunction assumes a role in the mechanism of exercise intolerance of female patients with HFpEF. The reduced ability of the stiffened LV to increase its size despite elevated filling pressure is exaggerated. The result is high pressure but relatively low volume, which in turn not only decreases SV but may also negatively impact LV contractility through the Frank-Starling mechanism. Interestingly, a recent hemodynamic study has shown that females with HFpEF exhibit a larger increase in pulmonary capillary wedge pressure in response to rapid saline loading compared to men (18). Another community-based study has shown greater age-related increase in LV stiffness in females compared to males, in concordance with our results (19, 20). Increases in LV diastolic stiffness elevate chamber filling pressures at similar chamber volumes, which may contribute to the lower effort capacity in female patients with HFpEF. Another important sex related difference in the HFpEF group was the diminished increases in A-VO2 difference likely suggesting the presence of less skeletal muscle mass or lower capillary to muscle fiber ratio (21). It may also suggest that lower metabolic efficiency in females with HFpEF contributes to their reduced effort capacity.
4.2. HFmrEF/HFrEF
The main sex related differences that we found between HFmrEF/HFrEF patients suggest that female patients demonstrated attenuated increases in LVEDV, which resulted in diminished increases in SV and CO. It is generally accepted that in healthy individuals during incremental exercise SV increases and plateaus at ~50% of VO2max mainly due to decreased diastolic filling time. Incremental exercise SV response of plateau with a drop has also been described (22). Attenuated increases in LVEDV may occur by decreased diastolic filling time as well as diastolic dysfunction and may result in a less effective Frank-Starling mechanism and subsequently decreased SV. Interestingly, although LV compliance was similar between males and females at the initiation of exercise, compliance lessened only in female patients upon reaching the anaerobic phase (Figures 2A, B), further reaching its nadir at peak exercise. This stress related reduction in compliance may be related to the innate load dependence physiology of LV compliance. LV stiffness may increase in proportion to filling volume changes and can be likened to the stiffness of a balloon, in which the amount of pressure required to cause a given increase in volume increases as the volume of the balloon is increased (5). The interesting disparity between females and males in this load dependent reduction in compliance may be related to the greater degree of concentric remodeling and LV diastolic elastance found in females (23). As explained by LaPlace law wall tension is maintained by the relation between pressure and radius, or left ventricle end-diastolic pressure (LVEDP) and LVEDV. A decrease in chamber size due to concentric remodeling, or an attenuated increase in LVEDV, may result in an increased LVEDP, thereby increasing LA pressure and contribute to exercise intolerance.
4.3. Combined cardio-pulmonary and echo exercise protocol
Prior cardiopulmonary exercise studies aimed at examining the determinants of exercise performance in females have mainly used protocols based on invasive tools for the assessment of CO. These studies are inherently limited by their invasive nature, which thereby limits their general clinical applicability, and may lead to selection bias (4, 24–26). A major strength of our protocol is the allowance for the simultaneous recording of CPET (peak VO2 VE/VCO2), echo-derived parameters (SV, CO, left and right ventricular function, filling pressures), and combined parameters (A-VO2 difference), which provides clinicians with a wealth of clinically significant, actionable information regarding individualized patient cardiovascular physiology summarized in a singular panel at the conclusion of one exam. Our study has several other advantages when compared to previous studies that used combined CPET and echocardiography methodology (27–30). First, we did not use a predetermined increase in work, we instead elected to calculate the expected work in watts after recording the height, weight, age, sex, and medical history of our patients. We then calculated the work rate increment necessary to reach the patient's estimated peak work in 8–12 min. The extended period of effort permitted the acquisition of comprehensive hemodynamic data (mitral inflow velocities, tissue Doppler annular signals, outflow tract velocity integral) in addition to LV volumes, thus, uniquely allowing for the calculation of LV compliance. Second, we obtained echocardiography images at individual stages of effort (rest, anaerobic threshold, maximal) instead of preselected power outputs, which may represent different stages of effort in different patients. Third, we did not stop β-blocker therapy prior to examination as we sought to determine the individual factors responsible for effort intolerance in the setting of “real-life” medical management, which is much more applicable and may allow for the administration of specific recommendations in individual subjects.
5. Limitations
Our study has several limitations. First, it is a single center study and thus generalization of our results is limited. Second, it is a retrospective study, and therefore our results are subject to the effects of possible confounders and may be biased by the nature of this design. Third, our imaging protocol was performed in the semi-supine position, which generates a somewhat different hemodynamic response than the more commonly used treadmill exercise. Last, SV and CO measurements may have been underestimated or overestimated because of the technical challenge of acquiring echocardiographic images during exercise. However, this technique has been used successfully and validated against radionuclide angiography and Fick SV with reported excellent day-to-day reproducibility and intraobserver and interobserver variability (28).
6. Conclusion
Based on previous evidence regarding the role of CPET in HF patients, current position papers (14) advocate for three ‘cut-point’ values (>18, 18–10, or <10 ml/kg/min) of peak VO2 for prognostication and risk stratification in patients with HF. However, there is still little data to aid in stratifying several large and clinically significant subgroups of patients: particularly females, or patients with HFpEF. Peak VO2 is significantly lower in females when compared to males with similar ventricular function. Our data suggest that the decrease in exercise capacity female HF (either HFmrEF/HFrEF or HFpEF) patients experience compared to male patients is related to a sex specific impairment in LV compliance. However, while in HFmrEF/HFrEF, the reduced compliance results in decreases in overall CO, in HFpEF, it results in elevated LV filling pressures without significant changes in SV. Another important difference is that in female patients with HFpEF, peripheral factors contribute significantly to the reduction in measured effort capacity, while in HFmrEF/HFrEF they do not. The acquisition of hemodynamic data obtained non-invasively by combined stress echocardiography and CPET improves clinician ability to measure comprehensive cardiac and respiratory function and may aid significantly in therapeutic decision-making. Furthermore, we believe that the clearer understanding of differences in exercise hemodynamics and peak oxygen consumption afforded by our new protocol may reveal differing sex-specific diagnostic strategies in males and females. However, further research is necessary to assess the potential advantages of these potential sex-specific approaches, which can lead to the development of new therapeutic targets that may reveal themselves following further elucidation of the underlying sex differences in myocardial structure and systemic vascular function.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Tel Aviv Sourasky 0346-13-TLV. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZR: contributed to conception and design of the work and acquisition, analysis and interpretation of data for the work, and drafting the work and revising it critically for important intellectual content. YG, BS, OH, JA, JS, MG, OB, YB, YS, and YT: contributed to acquisition and interpretation of data for the work and revising it critically for important intellectual content. GK: contributed to conception and design of the work and interpretation of data for the work and revising it critically for important intellectual content. ML-P: contributed to conception and design of the work and acquisition and drafting the work and revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1098395/full#supplementary-material
References
1.
Hsich E Chadalavada S Krishnaswamy G Starling RC Pothier CE Blackstone EH et al . Long-term prognostic value of peak oxygen consumption in women versus men with heart failure and severely impaired left ventricular systolic function. Am J Cardiol. (2007) 100:291–5. 10.1016/j.amjcard.2007.02.096
2.
Elmariah S Goldberg LR Allen MT Kao A . Effects of gender on peak oxygen consumption and the timing of cardiac transplantation. J Am Coll Cardiol. (2006) 47:2237–42. 10.1016/j.jacc.2005.11.089
3.
Pina IL Kokkinos P Kao A Bittner V Saval M Clare B et al . Baseline differences in the HF-ACTION trial by sex. Am Heart J. (2009) 158:S16–23. 10.1016/j.ahj.2009.07.012
4.
Kitzman DW Higginbotham MB Cobb FR Sheikh KH Sullivan MJ . Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. (1991) 17:1065–72. 10.1016/0735-1097(91)90832-T
5.
Shimiaie J Sherez J Aviram G Megidish R Viskin S Halkin A et al . Determinants of effort intolerance in patients with heart failure: combined echocardiography and cardiopulmonary stress protocol. JACC Heart Fail. (2015) 3:803–14. 10.1016/j.jchf.2015.05.010
6.
Rozenbaum Z Khoury S Aviram G Gura Y Sherez J Man A et al . Discriminating circulatory problems from deconditioning: echocardiographic and cardiopulmonary exercise test analysis. Chest. (2017) 151:431–40. 10.1016/j.chest.2016.09.027
7.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Euro Heart J. (2021) 42:3599–726. 10.1093/eurheartj/ehab368
8.
Rich MW Beckham V Wittenberg C Leven CL Freedland KE Carney RM et al . multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. (1995) 333:1190–5. 10.1056/NEJM199511023331806
9.
Guazzi M Adams V Conraads V Halle M Mezzani A Vanhees L et al . EACPR/AHA joint scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. (2012) 33:2917–27. 10.1093/eurheartj/ehs221
10.
American Thoracic Society; American College of Chest Physicians . ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. (2003) 167:211–7. 10.1164/rccm.167.2.211
11.
Lang RM Badano LP Mor-Avi V Afilalo J Armstrong A Ernande L et al . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. 10.1093/ehjci/jev014
12.
Nagueh SF Smiseth OA Appleton CP Byrd BF 3rd Dokainish H Edvardsen T et al . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2016) 17:1321–60.
13.
Benjamin EJ Muntner P Alonso A Bittencourt MS Callaway CW Carson AP et al . Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–528. 10.1161/CIR.0000000000000659
14.
Nguyen QD Peters E Wassef A Desmarais P Remillard-Labrosse D Tremblay-Gravel M . Evolution of age and female representation in the most-cited randomized controlled trials of cardiology of the last 20 years. Circ Cardiovasc Qual Outcomes. (2018) 11:e004713. 10.1161/CIRCOUTCOMES.118.004713
15.
Corra U Agostoni PG Anker SD Coats AJS Crespo Leiro MG de Boer RA et al . Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the committee on exercise physiology and training of the heart failure Association of the European Society of Cardiology. Eur J Heart Fail. (2018) 20:3–15. 10.1002/ejhf.979
16.
Ehrman JK Brawner CA Shafiq A Lanfear DE Saval M Keteyian SJ . Cardiopulmonary exercise measures of men and women with HFrEF differ in their relationship to prognosis: the henry ford hospital cardiopulmonary exercise testing (FIT-CPX) project. J Card Fail. (2018) 24:227–23. 10.1016/j.cardfail.2018.02.005
17.
Keteyian SJ Patel M Kraus WE Brawner CA McConnell TR Pina IL et al . Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol. (2016) 67:780–9. 10.1016/j.jacc.2015.11.050
18.
Fujimoto N Borlaug BA Lewis GD Hastings JL Shafer KM Bhella PS et al . Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. (2013) 127:55–62. 10.1161/CIRCULATIONAHA.112.111302
19.
Borlaug BA Redfield MM Melenovsky V Kane GC Karon BL Jacobsen SJ et al . Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. (2013) 6:944–52. 10.1161/CIRCHEARTFAILURE.113.000383
20.
Redfield MM Jacobsen SJ Borlaug BA Rodeheffer RJ Kass DA . Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. (2005) 112:2254–62. 10.1161/CIRCULATIONAHA.105.541078
21.
Karastergiou K Smith SR Greenberg AS Fried SK . Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. (2012) 3:13. 10.1186/2042-6410-3-13
22.
Vella CA Robergs RA . A review of the stroke volume response to upright exercise in healthy subjects. Br J Sports Med. (2005) 39:190–5. 10.1136/bjsm.2004.013037
23.
Ha JW Lee HC Park S Choi EY Seo HS Shim CY et al . Gender-related difference in left ventricular diastolic elastance during exercise in patients with diabetes mellitus. Circ J. (2008) 72:1443–8. 10.1253/circj.CJ-07-1028
24.
Borlaug BA Melenovsky V Russell SD Kessler K Pacak K Becker LC et al . Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. (2006) 114:2138–47. 10.1161/CIRCULATIONAHA.106.632745
25.
Higginbotham MB Morris KG Williams RS Coleman RE Cobb FR . Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol. (1986) 57:1374–9. 10.1016/0002-9149(86)90221-3
26.
Sullivan MJ Knight JD Higginbotham MB Cobb FR . Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. (1989) 80:769–81. 10.1161/01.CIR.80.4.769
27.
Haykowsky MJ Brubaker PH John JM Stewart KP Morgan TM Kitzman DW . Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. (2011) 58:265–74. 10.1016/j.jacc.2011.02.055
28.
Haykowsky MJ Brubaker PH Stewart KP Morgan TM Eggebeen J Kitzman DW . Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. (2012) 60:120–8. 10.1016/j.jacc.2012.02.055
29.
Haykowsky MJ Brubaker PH Morgan TM Kritchevsky S Eggebeen J Kitzman DW . Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. (2013) 68:968–75. 10.1093/gerona/glt011
30.
John JM Haykowsky M Brubaker P Stewart K Kitzman DW . Decreased left ventricular distensibility in response to postural change in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. (2010) 299:H883–9. 10.1152/ajpheart.00332.2010
Summary
Keywords
sex, heart failure, echocardiography, cardiopulmonary exercise, peak VO2
Citation
Rozenbaum Z, Granot Y, Sadeh B, Havakuk O, Arnold JH, Shimiaie J, Ghermezi M, Barak O, Ben Gal Y, Shacham Y, Keren G, Topilsky Y and Laufer-Perl M (2023) Sex differences in heart failure patients assessed by combined echocardiographic and cardiopulmonary exercise testing. Front. Cardiovasc. Med. 10:1098395. doi: 10.3389/fcvm.2023.1098395
Received
14 November 2022
Accepted
16 January 2023
Published
06 February 2023
Volume
10 - 2023
Edited by
Emma Louise Robinson, University of Colorado, United States
Reviewed by
Michinari Hieda, Kyushu University, Japan; Julie K. K. Vishram-Nielsen, University of Copenhagen, Denmark
Updates
Copyright
© 2023 Rozenbaum, Granot, Sadeh, Havakuk, Arnold, Shimiaie, Ghermezi, Barak, Ben Gal, Shacham, Keren, Topilsky and Laufer-Perl.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michal Laufer-Perl ✉ michalpela@gmail.com
This article was submitted to Sex and Gender in Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.