- Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Background: Coronary computed tomographic angiography (CCTA) has emerged as a powerful imaging modality for the detection and prognostication of individuals with suspected coronary artery disease (CAD). High amounts of coronary artery calcium (CAC) significantly obscure the interpretation of CCTA. Clinical risk assessment tools and data specific to predictors of high CAC in symptomatic patients are limited.
Methods: Consecutive patients who underwent CAC scan and CCTA to diagnose CAD during 2016–2020 were included. A high CAC score was defined as >400 by Agatston method. Univariate and multivariate analyses were performed to determine the predictors of high CAC. The clinical risk score was derived from factors independently associated with high CAC. The derivation cohort was composed of 465 patients; this score was validated in 98 patients.
Results: The mean age was 63 ± 11 years, 53% were female, and 15.9% had high CAC scores. The independent predictors of high CAC scores were age >65 years (odds ratio [OR] 3.02, 95% confidence interval (95%CI) 1.56–5.85, p = 0.001), chronic kidney disease (CKD) (OR 11.09, 95%CI 3.38–36.38, p < 0.001), heart failure (OR 6.52, 95%CI 2.23–19.09, p = 0.001), hypertension (OR 26.44, 95%CI 9.02–77.44, p < 0.001), and vascular diseases, including ischemic stroke/transient ischemic attack and peripheral arterial disease (OR 20.96, 95%CI 4.19–104.86, p < 0.001). The H2VK-65 (Hypertension, Heart failure, Vascular diseases, CKD, and Age > 65) score allocates 1 point for age >65, 2 points for CKD or heart failure, and 3 points for hypertension or vascular diseases. Using a threshold of ≥4 points, the sensitivity and specificity to detect high CAC was 81% and 80%, respectively. The area under the curve was 0.88 and 0.85 in the derivation and validation cohorts, respectively.
Conclusion: The novel H2VK-65 score demonstrated good performance for predicting high CAC scores in symptomatic patients referred for CCTA.
Introduction
Coronary artery disease (CAD) is currently the leading cause of death worldwide and is predicted to remain so for the next 10 years (1). Coronary computed tomography angiography (CCTA) is being increasingly used to assess symptomatic patients for CAD (2). CCTA detects subclinical coronary atherosclerosis and can also accurately rule out anatomy and functionally significant CAD (3). CCTA also yields prognostic information that can be used to guide preventive therapy in patients with stable chest pain (4).
CCTA is the preferred test for patients with a low-intermediate clinical likelihood of obstructive CAD, no previous diagnosis of CAD, and having characteristics associated with a high likelihood of good image quality. However, despite improved computed tomography (CT) imaging systems and proper preparation, a high burden of coronary artery calcium (CAC) adversely impacts the interpretation of CCTA diagnostic imaging due to the presence of beam hardening artifacts and the partial volume effect (5–8). The prevalence of high CAC (Agatston score >400–1,000) in patients with known or suspected CAD who underwent CCTA was reported to range from 10% to 20% (4, 9, 10), which decreases the accuracy of CCTA for diagnosing obstructive CAD (11–14). Hence, the current European Society of Cardiology guideline does not recommend using CCTA in patients with extensive coronary calcification (2).
The development of a simple clinical risk prediction tool to identify patients at high risk for having high CAC would be useful for identifying patients who may not be suitable for CCTA. Accordingly, the aim of this study was to identify the independent clinical predictors of high CAC in symptomatic patients who underwent clinically indicated CAC scan and CCTA, and to develop a clinical risk assessment tool for predicting high CAC in symptomatic patients.
Materials and methods
Study population
Consecutive patients aged ≥18 years who underwent both a CAC scan and CCTA using a dual-source CT scanner for diagnosis of CAD between 2016 and 2020 in a tertiary hospital were included. Patients with a previous history of CAD, including myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary bypass surgery, were excluded. Patients with incomplete CT examinations were also excluded. The protocol for this study was approved by the institutional review board. The requirement to obtain written informed consent was waived due to the retrospective design of our study.
Clinical symptoms, CAD risk factors, and current medications were obtained from electronic medical records. Chest pain was classified according to the methods published by Diamond and Forrester (15). The pre-test probability of obstructive CAD was calculated using age, gender, and symptoms (2). Hypertension was defined as a self-reported history of hypertension, the use of antihypertensive medication, or a blood pressure of ≥140/90 mmHg. Diabetes was defined as a self-reported history of diabetes and/or receiving anti-diabetic treatment, or a fasting glucose of ≥126 mg/dl. Dyslipidemia was defined as a total cholesterol level of ≥240 mg/dl, a low-density lipoprotein (LDL) cholesterol level of ≥130 mg/dl, a high-density lipoprotein (HDL) cholesterol level of <40 mg/dl, a triglyceride level of ≥200 mg/dl, and/or treatment with a lipid-lowering agent. Vascular diseases included ischemic stroke/transient ischemic attack (TIA) and peripheral arterial disease (PAD). Chronic kidney disease (CKD) was defined as structural or functional kidney damage for ≥3 months with or without decreased estimated glomerular filtration rate (eGFR) (eGFR <60 ml/min/1.73 m2) (16). Blood pressure and heart rate data were obtained before CCTA. Laboratory results, including serum creatinine, eGFR, hematocrit, fasting plasma glucose, total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride, were obtained from the medical records within 3 months of CCTA. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (17).
CAC and CCTA protocols
CAC and CCTA scans were performed using a 256-slice scanner (SOMATOM Definition Flash; Siemens Healthcare, Erlangen, Germany) according to established guidelines (18) and institutional protocol at the time of the scan. To reduce the heart rate of patients with a heart rate >65 beats per minute, an oral beta blocker was administrated 1 h before imaging. A 0.3 mg sublingual dose of nitroglycerin was administered before initiation of the CCTA scan. Prior to CCTA, a non-enhanced prospective electrocardiography (ECG)-gated sequential scan was performed to determine CAC scoring using the following parameters: rotation time of 280 ms, slice collimation of 0.6 mm, slice width of 3.0 mm, tube voltage of 120 kV, and tube current of 50 mAs. CCTA was then performed using prospective ECG gating and the following parameters: rotation time of 330 ms and slice collimation of 0.6 mm. The tube voltage and tube current were determined by the scanner software (CARE kV and CARE Dose4D, respectively—both Siemens Healthcare). CCTA acquisition was performed using 50–70 ml of iodinated contrast (iopamidol 370 mg iodine per ml) injected intravenously at 5.0–6.0 ml per second followed by normal saline solution. Automated bolus tracking or timing bolus was used to trigger acquisition.
Image analysis
Analysis of CAC and CCTA images was performed following the standard protocol on a separate workstation (Syngovia; Siemens Healthineers, Erlangen, Germany). CAC scans were interpreted according to the Agatston method (19), with a high CAC score defined as >400. CCTA images were interpreted in accordance with the Society of Cardiovascular Computed Tomography guidelines (20). Overall CCTA image quality was assessed on a four-point ranking scale, as follows: 4, excellent (no artifacts, unrestricted evaluation); 3, good (minor artifacts, good diagnostic quality); 2, adequate (moderate artifacts, still acceptable and diagnostic); and 1, not assessable (severe artifacts impairing accurate evaluation). Coronary atherosclerotic lesions were quantified for lumen diameter stenosis by visual inspection with two-observer consensus using categories of 0%, 1%–24%, 25%–49%, 50%–69%, 70%–99%, and 100% stenosis or uninterpretable. Obstructive CAD was defined when coronary artery segments exhibited plaque with luminal diameter stenosis ≥70% or ≥50% in the left main (LM) coronary artery. Nonobstructive CAD was defined when coronary artery segments exhibited plaque with luminal diameter stenosis 1%–70% or 1%–50% in the LM coronary artery. Vessels smaller than 2 mm were not evaluated. The extent of CAD was also assessed according to the number of vessels with CAD as 1-vessel, 2-vessel, or 3-vessel/LM disease. Uninterpretable segments were coded as being due to calcification, significant motion artifact, or not well visualized.
Statistical analysis
All statistical analyses were performed using SPSS Statistics for Windows version 20.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables with normal distribution were presented as mean ± standard deviation, and continuous variables with non-normal distribution were presented as median and interquartile ranges (IQR). The normality of the distribution of variables was examined by the Kolmogorov-Smirnov test. Categorical variables were present as absolute numbers and percentages. Differences between patients with CAC ≤ and >400 in terms of baseline and image characteristics were compared using the Student’s unpaired t-test or the Mann-Whitney U test for continuous variables, while the chi-square test or Fisher’s exact test was used for categorical variables, as appropriate.
Binary logistic regression analysis was performed to identify significant predictors of high CAC from baseline characteristics. Variables with a p-value <0.05 from univariate analysis were entered into multivariate analysis. The results of the univariate and multivariate analyses are given as odds ratios along with their respective 95% confidence intervals. A p-value less than 0.05 was considered statistically significant for all tests.
The factors found to be independently associated with high CAC from the multivariate analysis were used to develop a clinical risk assessment tool for predicting high CAC. A risk score was generated using the sum of assigned points, defined as the exponential of the estimated coefficient and rounded to the nearest integer. We performed receiver operating characteristic (ROC) curve analysis to evaluate the diagnostic performance of our score and to determine the best cutoff value for predicting a high CAC score. The accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. A validation cohort was used to evaluate the accuracy of the developed risk assessment tool.
Results
Patient and image characteristics
A total of 487 patients were studied. Twenty were excluded due to having known CAD, while two had incomplete CT examinations, resulting in a final study population of 465 patients in the derivation cohort. The mean age of patients was 63.1 ± 11.3 years, and 53.3% were female. The majority of patients presented to the hospital with atypical angina or dyspnea, and most patients had intermediate CAD pre-test probability (mean: 14.4 ± 9.5%). Seventy-four patients (15.9%) had a high CAC score (>400). Baseline characteristics of patients in the derivation cohort stratified according to CAC scores are shown in Table 1. Patients with high CAC scores were older, had more CAD risk factors, and had a significantly higher pre-test CAD probability than those with lower CAC scores. Patients with high CAC scores also had a significantly higher prevalence of heart failure, vascular diseases, and CKD, and were significantly more likely to be taking antithrombotics, antihypertensives, or statins.
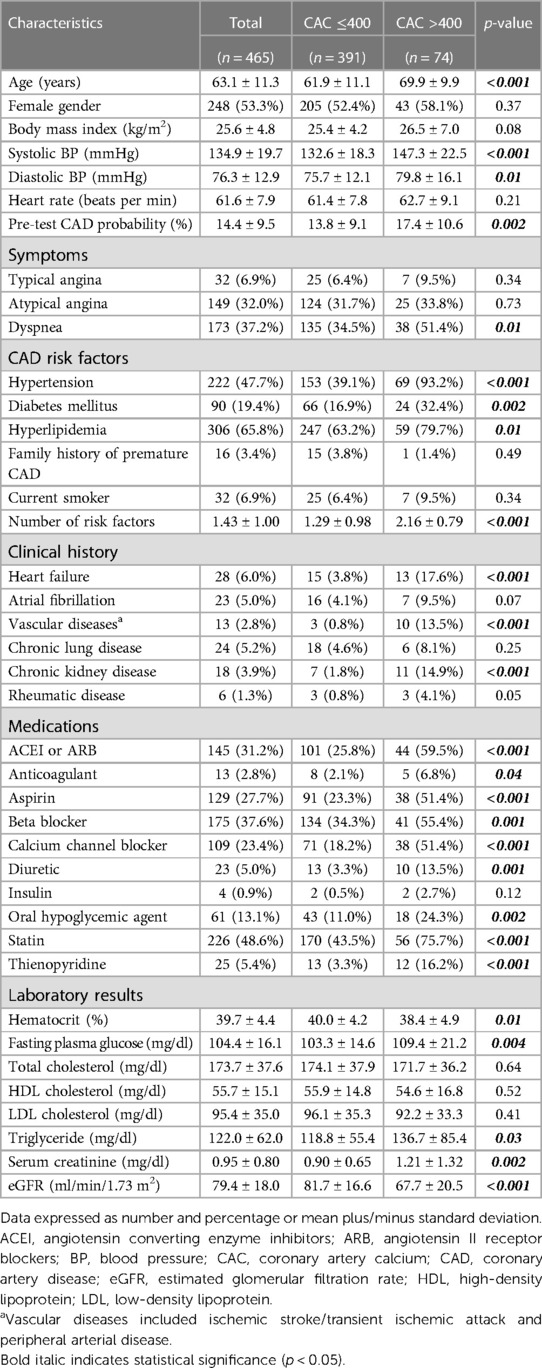
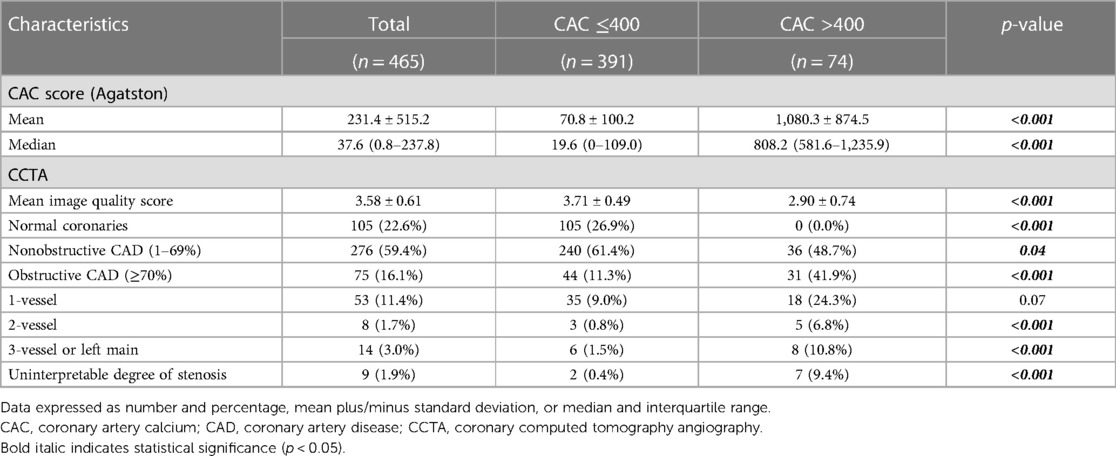
Table 1. Baseline characteristics of patients in the derivation cohort stratified according to CAC scores.
Table 2 shows the CAC and CCTA characteristics for all patients in the derivation cohort and compared between the low and high CAC groups. The mean and median CAC scores were 231.4 ± 515.2 and 37.6 (IQR: 0.8–237.8), respectively. Patients with high CAC scores had significantly lower mean CCTA image quality scores (2.90 ± 0.74 vs. 3.71 ± 0.49, respectively; p < 0.001), and 7 (9.4%) patients had uninterpretable images due to dense calcification. Patients with high CAC scores also had a significantly higher prevalence of obstructive CAD and multivessel or left main disease.
Predictors of high CAC score and development of the H2VK-65 tool
Table 3 shows the results of univariate and multivariate logistic regression analysis to identify factors independently associated with high CAC scores. The significant predictors of high CAC identified by univariate analysis were age, dyspnea, hypertension, diabetes mellitus, hyperlipidemia, history of heart failure, vascular diseases, CKD, and rheumatic disease. Subsequent multivariate analysis revealed age, hypertension, history of heart failure, vascular diseases, and CKD as independent predictors of high CAC. The age threshold with the highest AUC using a CAC score >400 as the reference was 65 years (AUC: 0.71, p < 0.001).
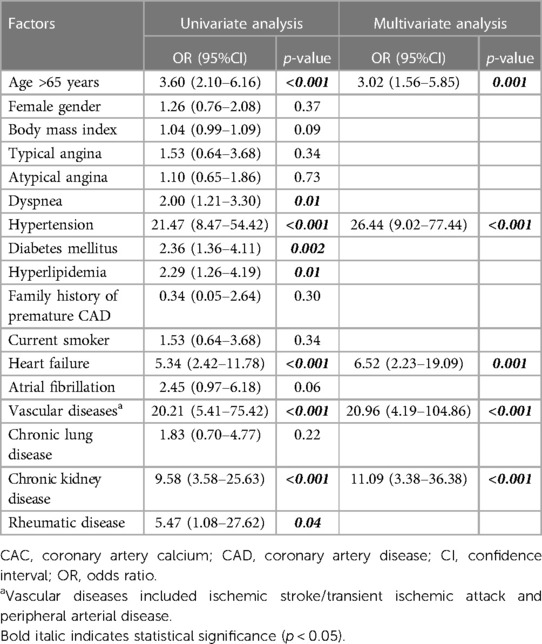
Table 3. Univariate and multivariate logistic regression analysis to identify factors associated with high CAC scores.
We developed a clinical risk assessment tool to predict high CAC using an estimated coefficient (B) for each independent predictor, as follows: age >65 years (B: 1.11); hypertension (B: 3.28); history of heart failure (B: 1.88): vascular disease (B: 3.04); and CKD (B: 2.41). The H2VK-65 score (Hypertension, Heart failure, Vascular disease, CKD, and Age >65), which ranges from 0 to 11, allocates 1 point for age >65 years, 2 points for CKD or heart failure, and 3 points for hypertension or vascular diseases. Figure 1 shows the prevalence of patients with high CAC scores relative to H2VK-65 scoring compared between the derivation and validation cohorts. As demonstrated in the figure, the number of patients with high CAC scoring increases as the H2VK-65 score increases. No patients in the derivative cohort with an H2VK-65 score of 0 or 1 had a CAC score >400 (0 from 225 patients).
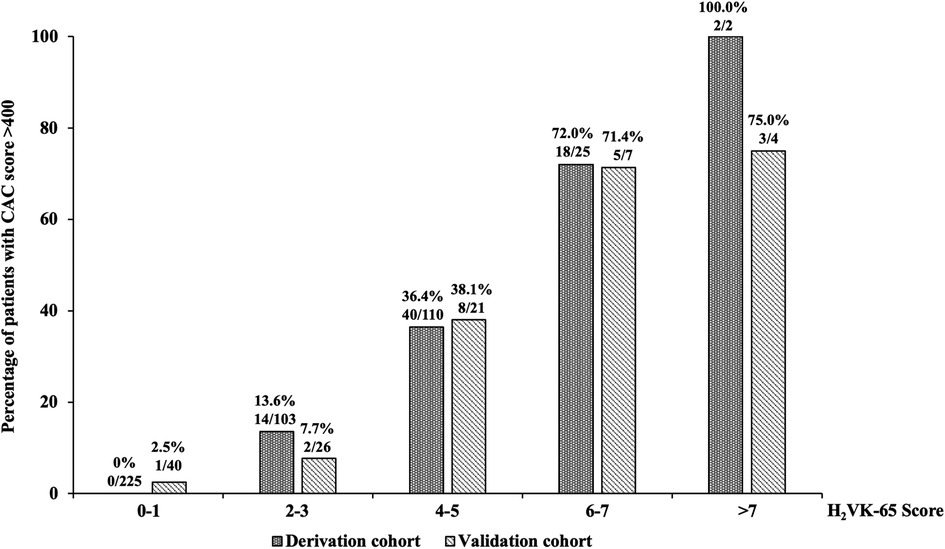
Figure 1. The prevalence of patients with high coronary artery calcium (CAC) scores relative to H2VK-65 scoring compared between the derivation and validation cohorts.
An H2VK-65 cutoff value of ≥4 was shown to have an AUC of 0.88 (95%CI: 0.85–0.92) (Figure 2A and Table 4), and a sensitivity of 0.81 (95%CI: 0.70–0.89), specificity of 0.80 (95%CI: 0.76, 0.84), positive predictive value (PPV) of 0.44 (95%CI: 0.38–0.49), and negative predictive value (NPV) of 0.95 (95%CI: 0.93–0.97) for predicting the presence of high CAC in symptomatic patients (Table 4). Therefore, an H2VK-65 score <4 is considered negative for high CAC, and a score ≥4 is considered positive for high CAC in symptomatic patients. Furthermore, we assessed the H2VK-65 score with a cutoff of ≥4 to predict a CAC score >1,000. Among the 28 patients with a CAC score >1,000, the AUC was 0.85 (95%CI: 0.80–0.90), sensitivity was 0.89 (95%CI: 0.72–0.98), specificity was 0.74 (95%CI: 0.70–0.78), accuracy was 0.75 (0.71, 0.79), PPV was 0.18 (0.12, 0.26), and NPV was 0.99 (0.97, 0.99).
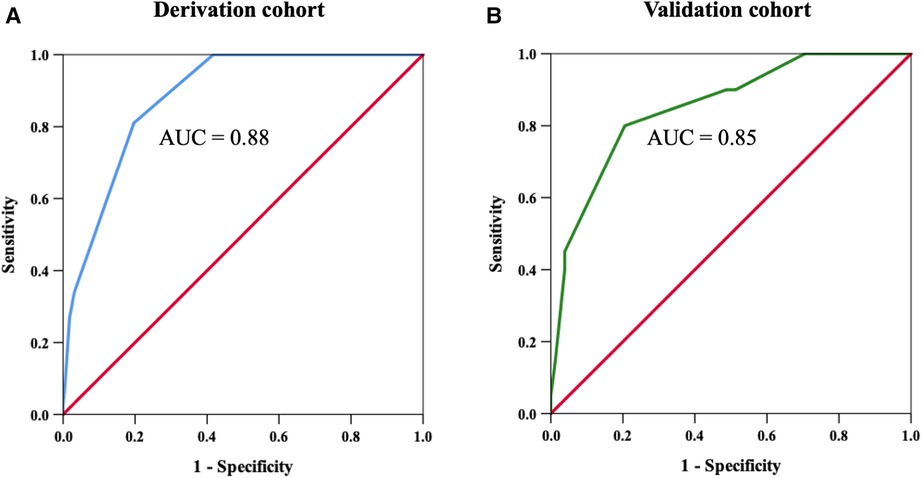
Figure 2. Receiver operating characteristic curves to determine the H2VK-65 score cutoff with the highest sensitivity and specificity for predicting high coronary artery calcium (CAC) score in the derivation cohort (A), and to confirm the identified cutoff value in the validation cohort (B). Using a cutoff threshold of 4 points or above, the sensitivity and specificity for detecting high CAC was 81% and 80%, respectively, in the derivation cohort. The area under the curve was 0.88 and 0.85 in the derivation cohort and validation cohort, respectively.
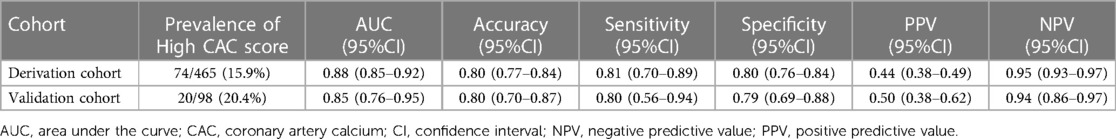
Table 4. Diagnostic performance of the H2VK-65 scores for identifying symptomatic patients with high CAC in the derivation and validation cohorts.
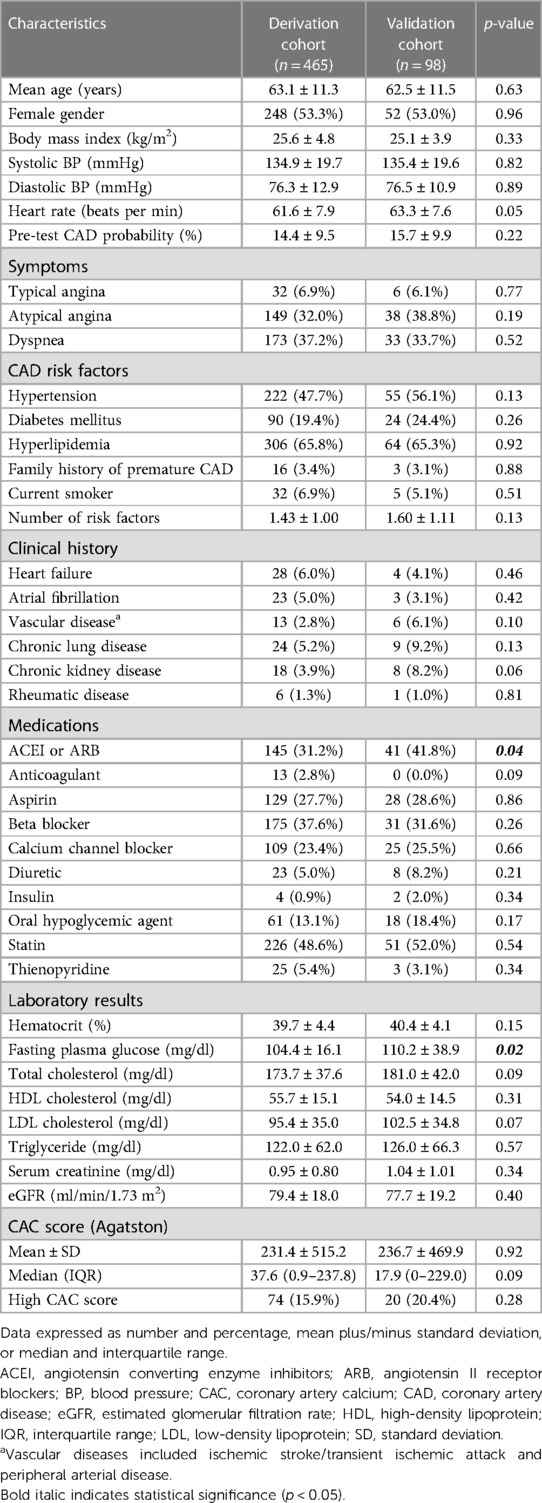
Validation of the H2VK-65 score
The developed H2VK-65 clinical assessment tool was validated in a separate and different group of 98 patients (validation cohort) that were referred for both CAC and CCTA to diagnose CAD. Baseline patient characteristics compared between the derivation and validation cohorts are shown in Table 5. Most variables, including CAD risk factors, symptoms, and pre-test CAD probability, were non-significantly different between the validation cohort and derivation cohort. The prevalence of high CAC scores and mean and median CAC scores were also non-significantly different between the two cohorts. The diagnostic performance of the H2VK-65 scores for identifying symptomatic patients with high CAC compared between the derivation and validation cohorts is shown in Table 4. The AUC of the H2VK-65 score for predicting high CAC in the validation cohort was 0.85 (95%CI: 0.76–0.95) (Figure 2B).
Discussion
Our results demonstrated that 15.9% of symptomatic patients who underwent CCTA for diagnosis of CAD had a high CAC score (>400 by the Agatston method). Age, hypertension, history of heart failure, vascular diseases, and CKD were identified as independent predictors of high CAC scores. The H2VK-65 scoring system is a new and simple clinical assessment score that was designed to predict high CAC with high sensitivity and specificity. The H2VK-65 score was also internally validated in another group of patients, and diagnostic performance very similar to that found in the derivation cohort was observed.
CAD is one of the most common types of heart disease in both developing and developed countries, and it was the cause of more than 380,000 deaths globally in 2020 (21). Prevention and early detection of CAD are, therefore, essentially important. CAC is now established as a reliable tool for estimating the risk of MI, coronary death, and all-cause mortality (22, 23). Current guidelines endorse the use of non-contrast CT to assess CAC in suitable asymptomatic patients to improve clinical risk evaluation (23, 24). CCTA is considered a recommended modality in patients with suspected CAD. Given its excellent negative predictive value for excluding CAD in patients with low-intermediate pre-test probability (2), the use of CCTA is now rapidly increasing. However, CCTA is not recommended for patients with extensive CAC due to beam hardening and blooming artifacts caused by dense calcifications (2, 25). Multiple studies have reported the limited specificity, accuracy, and PPV of CCTA in patients with high CAC (7, 8, 11–14). Moreover, during earlier use of CCTA, patients with very high CAC scores (400–1,000) often had their CCTA studies canceled.
The CAC score was previously reported to be an independent predictor of cardiovascular risk compared to conventional epidemiological scores, and an increasing calcium burden was found to be associated with an increased burden of atherosclerosis and stenosis (26). In our study, the prevalence of CAC scoring >400 was 15.9%, which is similar to previously reported rates (4, 9, 10, 27). Patients with high CAC scores had significantly lower image quality, and a higher prevalence of uninterpretable images. Moreover, patients with a high CAC score were more likely to be older, to have a higher pre-test probability of obstructive CAD, and to have multiple CAD risk factors, including hypertension, diabetes mellitus, and dyslipidemia. Patients with high CAC scores also had a higher prevalence of CKD and lower eGFR. These observed characteristics concur with those from previous studies (26, 28–31). Age was reported to be one of the strongest predictors of CAC scoring in both men and women (32). Alison et al. found that asymptomatic patients aged >65 years had CAC scores at least six times higher than those observed in patients aged <45 years (32). CAC scores were reported to increase in patients with high blood pressure, and were also found to predict new-onset hypertension (33). Leening et al. demonstrated clear association between the extent of CAC and the risk of heart failure, independent of overt CAD (34).
Until now, no risk score has been developed to predict high CAC in symptomatic patients referred to undergo CCTA. As previously described, 10%–20% of this population had high CAC scores (including 15.9% in the present study) that significantly adversely affected CCTA image quality. In this study, we developed a clinical scoring system using independent predictors of a high CAC score, including age, hypertension, history of heart failure, vascular diseases, and CKD. The H2VK-65 score is a new and easy-to-use clinical score to detect high CAC in symptomatic patients. This score reliably ruled out clinically significant high CAC with an NPV of 95% and 94% in the derivation and validation cohorts, respectively. An ideal screening score should have high sensitivity to avoid false-negative results, but also be specific enough to avoid referral of low-risk patients for costly and time-consuming investigations. The H2VK-65 score also demonstrated high sensitivity and specificity (>80% for both); however, the PPV was modest due to the low-intermediate prevalence of obstructive CAD in this population. The H2VK-65 score was internally validated in another group of patients, and similar diagnostic performance was observed in both the derivation and validation cohorts.
This study has some mentionable limitations. First, although a widely acknowledged weakness of retrospective studies is their vulnerability to missing or incomplete data, we were careful to include only patients with complete data in this study. Second, all included patients were referred for CCTA, so some selection bias cannot be excluded. Patients with a very low or high likelihood of obstructive CAD are not normally referred for diagnostic testing. Moreover, patients with atrial fibrillation or severe kidney disease may be under-represented because all patients had to be eligible for CCTA. Third, there are multiple definitions of high CAC score, and there is no consensus regarding the most suitable definition. Accordingly, our score may not correspond with or should be considered to be validated for all definitions. Fourth, the validation analysis was performed in our center without external validation, and it lacked data specific to other races or ethnicities. Previous studies found some differences in the prevalence and severity of CAC scores according to race and ethnicity (35, 36), and that CAC predicts clinical outcomes in all race/ethnicity groups, including Asians (37, 38). Finally, this study was conducted in a single center that is a large university-based national tertiary referral center. Therefore, further multicenter study in a much larger and more racially diverse study population is needed to validate our results.
Conclusion
The newly developed H2VK-65 clinical assessment tool demonstrated good performance for predicting high CAC scores in symptomatic patients referred for CCTA. This tool will help to identify symptomatic patients who may not be suitable for CCTA due to the high risk of poor/uninterpretable image quality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Siriraj Institutional Review Board, Faculty of Medicine Siriraj Hospital, Mahidol University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YK – Conception and design, research operation, data collecting, analysis and interpretation of data, discussion of the results, drafting of the manuscript or revising it critically for important intellectual content, and final approval of the manuscript submitted. NP and SP – Research operation, data collecting, discussion of the results, drafting of the manuscript or revising it critically for important intellectual content, and final approval of the manuscript submitted. TB – Conception and design, research operation, analysis and interpretation of data, discussion of the results, drafting of the manuscript or revising it critically for important intellectual content, and final approval of the manuscript submitted. All authors contributed to the article and approved the submitted version.
Funding
Faculty of Medicine Siriraj Hospital, Mahidol University.
Acknowledgments
The authors gratefully acknowledge Khemajira Karaketklang and Dittapol Muntham for their assistance with statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; AUC, area under the curve; CAC, coronary artery calcium; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CI, confidence interval; CKD, chronic kidney disease; ECG, electrocardiography; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
References
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3(11):e442. doi: 10.1371/journal.pmed.0030442
2. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European society of cardiology (ESC). Eur Heart J. (2019) 41(3):407–77. doi: 10.1093/eurheartj/ehz425
3. Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. (2018) 39(35):3322–30. doi: 10.1093/eurheartj/ehy267
4. Investigators S-H. CT Coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. (2015) 385(9985):2383–91. doi: 10.1016/S0140-6736(15)60291-4
5. Cheng V, Gutstein A, Wolak A, Suzuki Y, Dey D, Gransar H, et al. Moving beyond binary grading of coronary arterial stenoses on coronary computed tomographic angiography: insights for the imager and referring clinician. JACC Cardiovasc Imaging. (2008) 1(4):460–71. doi: 10.1016/j.jcmg.2008.05.006
6. Kalisz K, Buethe J, Saboo SS, Abbara S, Halliburton S, Rajiah P. Artifacts at cardiac CT: physics and solutions. RadioGraphics. (2016) 36(7):2064–83. doi: 10.1148/rg.2016160079
7. Gueret P, Deux JF, Bonello L, Sarran A, Tron C, Christiaens L, et al. Diagnostic performance of computed tomography coronary angiography (from the prospective national multicenter multivendor EVASCAN study). Am J Cardiol. (2013) 111(4):471–8. doi: 10.1016/j.amjcard.2012.10.029
8. Schuhbaeck A, Schmid J, Zimmer T, Muschiol G, Hell MM, Marwan M, et al. Influence of the coronary calcium score on the ability to rule out coronary artery stenoses by coronary CT angiography in patients with suspected coronary artery disease. J Cardiovasc Comput Tomogr. (2016) 10(5):343–50. doi: 10.1016/j.jcct.2016.07.014
9. Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, et al. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry). Circulation. (2012) 126(3):304–13. doi: 10.1161/CIRCULATIONAHA.111.081380
10. Kim YJ, Hur J, Lee HJ, Chang HJ, Nam JE, Hong YJ, et al. Meaning of zero coronary calcium score in symptomatic patients referred for coronary computed tomographic angiography. Eur Heart J Cardiovasc Imaging. (2012) 13(9):776–85. doi: 10.1093/ehjci/jes060
11. Abdulla J, Pedersen KS, Budoff M, Kofoed KF. Influence of coronary calcification on the diagnostic accuracy of 64-slice computed tomography coronary angiography: a systematic review and meta-analysis. Int J Cardiovasc Imaging. (2012) 28(4):943–53. doi: 10.1007/s10554-011-9902-6
12. Cademartiri F, Mollet NR, Lemos PA, Saia F, Runza G, Midiri M, et al. Impact of coronary calcium score on diagnostic accuracy for the detection of significant coronary stenosis with multislice computed tomography angiography. Am J Cardiol. (2005) 95(10):1225–7. doi: 10.1016/j.amjcard.2005.01.051
13. Vavere AL, Arbab-Zadeh A, Rochitte CE, Dewey M, Niinuma H, Gottlieb I, et al. Coronary artery stenoses: accuracy of 64-detector row CT angiography in segments with mild, moderate, or severe calcification–a subanalysis of the CORE-64 trial. Radiology. (2011) 261(1):100–8. doi: 10.1148/radiol.11110537
14. Ahn SJ, Kang DK, Sun JS, Yoon MH. Accuracy and predictive value of coronary computed tomography angiography for the detection of obstructive coronary heart disease in patients with an Agatston calcium score above 400. J Comput Assist Tomogr. (2013) 37(3):387–94. doi: 10.1097/RCT.0b013e318282d61c
15. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. (1979) 300(24):1350–8. doi: 10.1056/NEJM197906143002402
16. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. (2005) 67(6):2089–100. doi: 10.1111/j.1523-1755.2005.00365.x
17. Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Thienpont LM, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. (2005) 129(3):297–304. doi: 10.5858/2005-129-297-CMSOTA
18. Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, et al. SCCT Guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee: endorsed by the North American society for cardiovascular imaging (NASCI). J Cardiovasc Comput Tomogr. (2016) 10(6):435–49. doi: 10.1016/j.jcct.2016.10.002
19. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. (1990) 15(4):827–32. doi: 10.1016/0735-1097(90)90282-T
20. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT Guidelines for the interpretation and reporting of coronary CT angiography: a report of the society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr. (2014) 8(5):342–58. doi: 10.1016/j.jcct.2014.07.003
21. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American heart association. Circulation. (2022) 145(8):e153–e639. doi: 10.1161/CIR.0000000000001052
22. Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. (2015) 239(1):109–17. doi: 10.1016/j.atherosclerosis.2014.12.040
23. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American college of cardiology foundation appropriate use criteria task force, the society of cardiovascular computed tomography, the American college of radiology, the American heart association, the American society of echocardiography, the American society of nuclear cardiology, the north American society for cardiovascular imaging, the society for cardiovascular angiography and interventions, and the society for cardiovascular magnetic resonance. J Am Coll Cardiol. (2010) 56(22):1864–94. doi: 10.1016/j.jacc.2010.07.005
24. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 140(11):e596–e646. doi: 10.1161/CIR.0000000000000678
25. Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American heart association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation. (2006) 114(16):1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458
26. Palumbo AA, Maffei E, Martini C, Tarantini G, Di Tanna GL, Berti E, et al. Coronary calcium score as gatekeeper for 64-slice computed tomography coronary angiography in patients with chest pain: per-segment and per-patient analysis. Eur Radiol. (2009) 19(9):2127–35. doi: 10.1007/s00330-009-1398-2
27. Meng L, Cui L, Cheng Y, Wu X, Tang Y, Wang Y, et al. Effect of heart rate and coronary calcification on the diagnostic accuracy of the dual-source CT coronary angiography in patients with suspected coronary artery disease. Korean J Radiol. (2009) 10(4):347–54. doi: 10.3348/kjr.2009.10.4.347
28. Husmann L, Schepis T, Scheffel H, Gaemperli O, Leschka S, Valenta I, et al. Comparison of diagnostic accuracy of 64-slice computed tomography coronary angiography in patients with low, intermediate, and high cardiovascular risk. Acad Radiol. (2008) 15(4):452–61. doi: 10.1016/j.acra.2007.12.008
29. Lo-Kioeng-Shioe MS, Vavere AL, Arbab-Zadeh A, Schuijf JD, Rochitte CE, Chen MY, et al. Coronary calcium characteristics as predictors of Major adverse cardiac events in symptomatic patients: insights from the CORE320 multinational study. J Am Heart Assoc. (2019) 8(6):e007201. doi: 10.1161/JAHA.117.007201
30. Barraclough KA, Stevens LA, Er L, Rosenbaum D, Brown J, Tiwari P, et al. Coronary artery calcification scores in patients with chronic kidney disease prior to dialysis: reliability as a trial outcome measure. Nephrol Dial Transplant. (2008) 23(10):3199–205. doi: 10.1093/ndt/gfn234
31. Kälsch H, Lehmann N, Möhlenkamp S, Neumann T, Slomiany U, Schmermund A, et al. Association of coronary artery calcium and congestive heart failure in the general population: results of the Heinz Nixdorf Recall study. Clin Res Cardiol. (2010) 99(3):175–82. doi: 10.1007/s00392-009-0104-3
32. Allison MA, Wright CM. Age and gender are the strongest clinical correlates of prevalent coronary calcification (R1). Int J Cardiol. (2005) 98(2):325–30. doi: 10.1016/j.ijcard.2004.03.015
33. Weinberg RL, Rubenfire M, Brook RD. Coronary artery calcium scoring in patients with hypertension. J Hum Hypertens. (2020) 34(9):609–16. doi: 10.1038/s41371-020-0350-4
34. Leening MJ, Elias-Smale SE, Kavousi M, Felix JF, Deckers JW, Vliegenthart R, et al. Coronary calcification and the risk of heart failure in the elderly: the Rotterdam study. JACC Cardiovasc Imaging. (2012) 5(9):874–80. doi: 10.1016/j.jcmg.2012.03.016
35. Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the multi-ethnic study of atherosclerosis (MESA). Circulation. (2005) 111(10):1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B
36. Budoff MJ, Nasir K, Mao S, Tseng PH, Chau A, Liu ST, et al. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. (2006) 187(2):343–50. doi: 10.1016/j.atherosclerosis.2005.09.013
37. Orimoloye OA, Budoff MJ, Dardari ZA, Mirbolouk M, Uddin SMI, Berman DS, et al. Race/ethnicity and the prognostic implications of coronary artery calcium for all-cause and cardiovascular disease mortality: the coronary artery calcium consortium. J Am Heart Assoc. (2018) 7(20):e010471. doi: 10.1161/JAHA.118.010471
Keywords: coronary artery disease, coronary calcium score, cardiac computed tomographic (CT) imaging, risk prediction, chest pain
Citation: Kaolawanich Y, Prapan N, Phoopattana S and Boonyasirinant T (2023) The novel H2VK-65 clinical risk assessment tool predicts high coronary artery calcium score in symptomatic patients referred for coronary computed tomography angiography. Front. Cardiovasc. Med. 10:1096036. doi: 10.3389/fcvm.2023.1096036
Received: 11 November 2022; Accepted: 19 June 2023;
Published: 3 July 2023.
Edited by:
Alexander Van Rosendael, Leiden University Medical Center (LUMC), NetherlandsReviewed by:
Yousef Shahin, The University of Sheffield, United KingdomHenrique Doria De Vasconcellos, Johns Hopkins Medicine, United States
© 2023 Kaolawanich, Prapan, Phoopattana and Boonyasirinant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thananya Boonyasirinant ZHJ0aGFuYW55YWFAeWFob28uY29t
 Yodying Kaolawanich
Yodying Kaolawanich Natthaporn Prapan
Natthaporn Prapan Thananya Boonyasirinant
Thananya Boonyasirinant