- Department of Cardiac Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
Background: We studied acute lung injury (ALI) in thoracic aortic disease (TAD) patients and investigated the predictive effect of interleukin-6 (IL-6) in acute lung injury after thoracic aortic disease.
Methods: Data on 188 TAD patients, who underwent surgery between January 2016 to December 2021 at our hospital, were enrolled in. We analyzed acute lung injury using two patient groups. Patients with No-ALI were 65 and those with ALI were 123. Univariate logistic, LASSO binary logistic regression model and multivariable logistic regression analysis were performed for acute lung injury.
Results: Preoperative IL-6 level was lower (15.80[3.10,43.30] vs. 47.70[21.40,91.60] pg/ml, p < 0.001) in No-ALI group than in ALI group. The cut-off points, determined by the ROC curve, were preoperative IL-6 > 18 pg/ml (area under the curve: AUC = 0.727). Univariate logistic regression analysis showed 19 features for TAD appeared to be early postoperative risk factors of acute lung injury. Using LASSO binary logistic regression, 19 features were reduced to 9 potential predictors (i.e., Scrpost + PLTpost + CPB > 182 min + D-dimerpost + D-dimerpre + Hypertension + Age > 58 years + IL6 > 18 pg/ml + IL6). Multivariable logistic regression analysis showed that Postoperative creatinine, CPB > 182 min and IL-6 > 18 pg/ml were early postoperative risk factors for ALI after TAD, and the odds ratios (ORs) of postoperative creatinine, CPB > 182 min and IL-6 > 18 pg/ml were 1.006 (1.002–1.01), 4.717 (1.306–19.294) and 2.96 (1.184–7.497), respectively. When postoperative creatinine, CPB > 182 min and IL-6 > 18 pg/ml (AUC = 0.819), the 95% confidence interval [CI] was 0.741 to 0.898. Correction curves were nearly diagonal, suggesting that the nomogram fit well. The DCA curve was then drawn to demonstrate clinical applicability. The DCA curve showed that the threshold probability of a patient is in the range of 30% to 90%.
Conclusions: The inclusion of interleukin-6 demonstrated good performance in predicting ALI after TAD surgery.
Introduction
Thoracic aortic disease (TAD) contains aneurysms and acute aortic syndromes (intramural hematomas, dissections, penetrating atherosclerotic ulcers). Thoracic aortic disease (TAD) is a type of vascular disease, which if left untreated, could cause fatal complications. Surgery is an important treatment modality for TAD. Aortic dissection is the worst complication of thoracic aortic disease (1). Acute aortic dissection is commonly reported to be accompanied by acute lung injury (ALI), where oxygenation of the lungs has been impaired severely (2). Acute lung injury (ALI) is a devastating and potentially life-threatening postoperative complication that can also prolong the duration of ventilator support and hospital stay. In large series of patients undergoing open surgery for thoracoabdominal aortic aneurysm, serious lung damage was reported at 60% (3, 4), and in recent publications, it was reported that it was between 40% and 50% (5). Acute hypoxic respiratory insufficiency caused by ALI is caused by a variety of factors that damage alveolar epithelial cells and capillary endothelial cells. Due to these pathological changes, ventilation is reduced, gas exchange is impaired, the ventilation-perfusion imbalance is severe, hypoxia is experienced, and pulmonary compliance is poor (6).
A significant role is played by inflammation in TAD development. A significant association between TAD and elevated plasma inflammatory markers has been reported (7, 8). On the one hand, the lung contains a considerable number of monocytes and macrophages in the cytoplasm to perform a protective function since the trachea is in direct touch with the outside environment; On the other hand, large and slow amount of circulating blood is needed to exchange gas between erythrocytes and alveoli. Lungs are therefore susceptible to inflammatory damage due to this feature (9). The role of inflammatory biomarkers as prognostic indicators after cardiac surgery has gained increasing attention. The impact of perioperative inflammatory biomarkers on clinical outcomes has been understudied in patients undergoing surgery for TAD.
There is a close correlation between cardiovascular disease and intercellular IL-6, which is an inflammatory cytokine that plays an important role in inflammation and immune response (10). The association between interleukin-6 (IL-6) and the pathology of aortic dissection has been demonstrated in numerous basic studies. As well, clinical studies have shown that elevated IL-6 can lead to complications after cardiovascular surgery (11–13). Therefore, to explore if IL-6 plays a predictive role in the occurrence of postoperative ALI in TAD patients undergoing surgery, we collected data on TAD patients from January 2016 to December 2021 who had IL-6 measurement.
Methods
Patient population
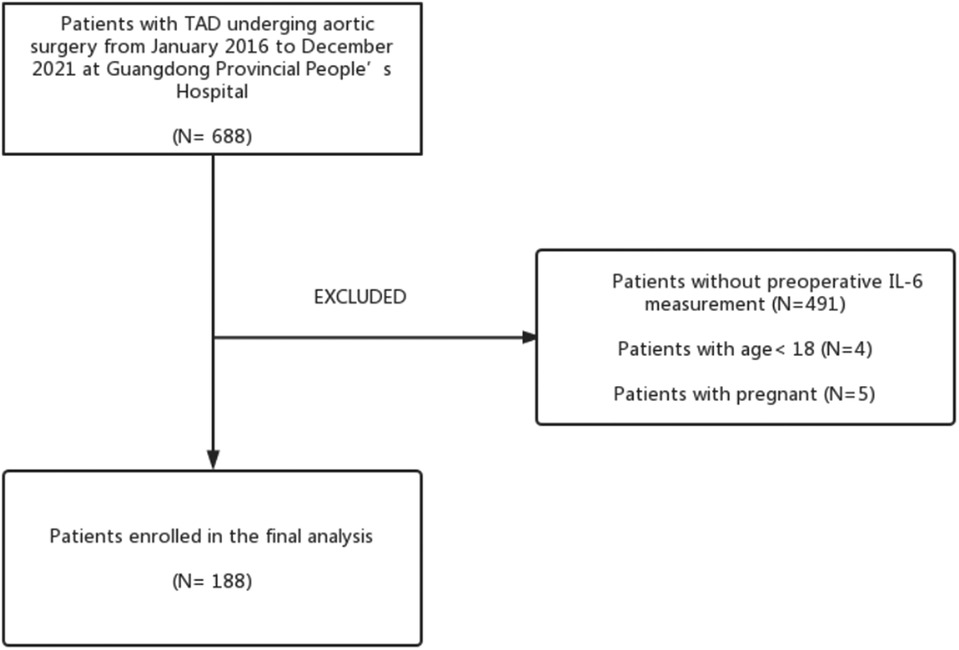
The cohort of this retrospective study consisted of 188 patients with surgical treatment for TAD who had preoperative levels of IL-6 measured from Guangdong Provincial People's Hospital (Guangzhou, China) from January 2016 to December 2021. Figure 1 shows the patient screening process. ALI and Non-ALI are classified according to the presence or absence of acute lung injury after surgery. In our study, we included patients whose CT angiography or magnetic resonance angiography (MRA) confirmed the presence of TAD. The exclusion criteria were as follows: (1) No preoperative IL-6 measurement; (2) Age less than 18 years; (3) Death before surgery; (4) Malignant tumors. An overview of the clinical characteristics of the TAD patients included in the study can be found in Table 1. This study protocol was approved by the Institutional Ethics Committee of Guangdong Provincial People's Hospital (KY-Q-2021-183-02) and conformed to the Declaration of Helsinki.
Definitions
ALI severity was put into the following categories based on the Berlin definition: mild ALI [200 mmHg < oxygen index (OI) ≤ 300 mmHg], moderate ALI (100 mmHg < OI ≤ 200 mmHg), and severe ALI (OI ≤ 100 mmHg) (14). Early mortality was defined as all-cause mortality in-hospital. Stroke was defined as a permanent neurologic injury with clinical or radiographic evidence, such as CT or MRI. The term MODS refers to the acute and potentially reversible dysfunction of two or more organ systems resulting from a variety of clinical factors (15).
Data collection
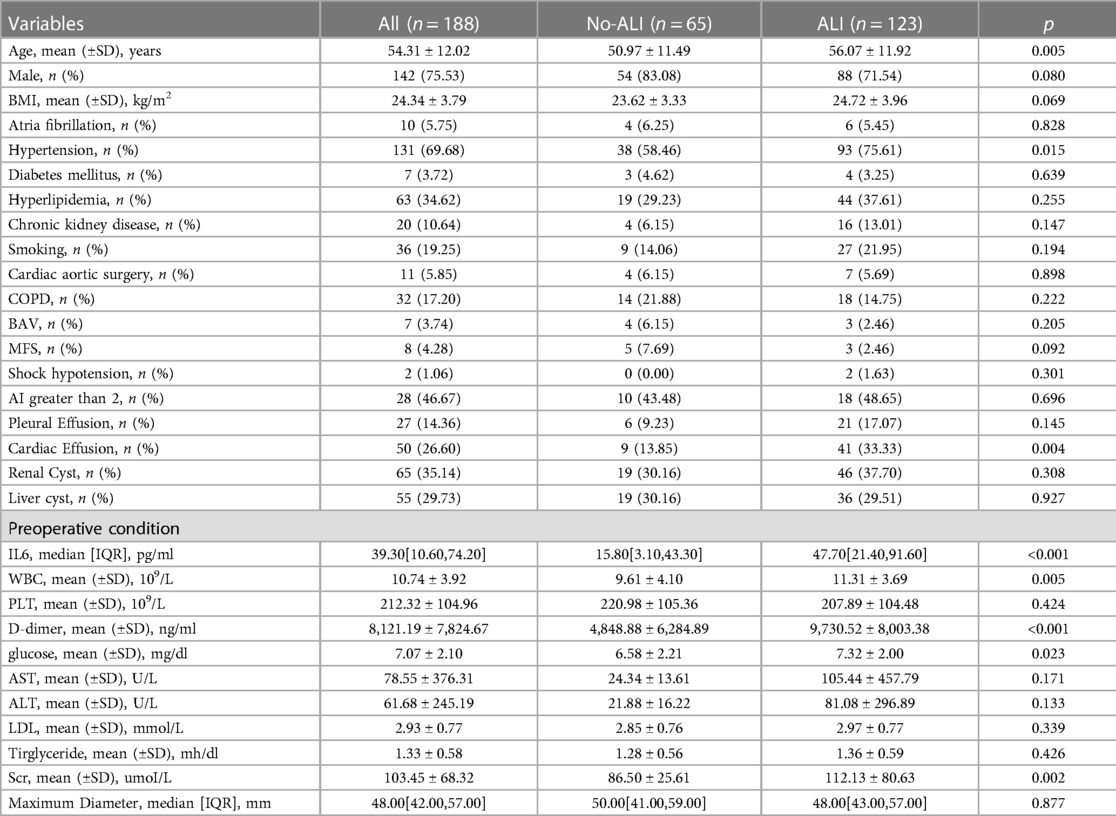
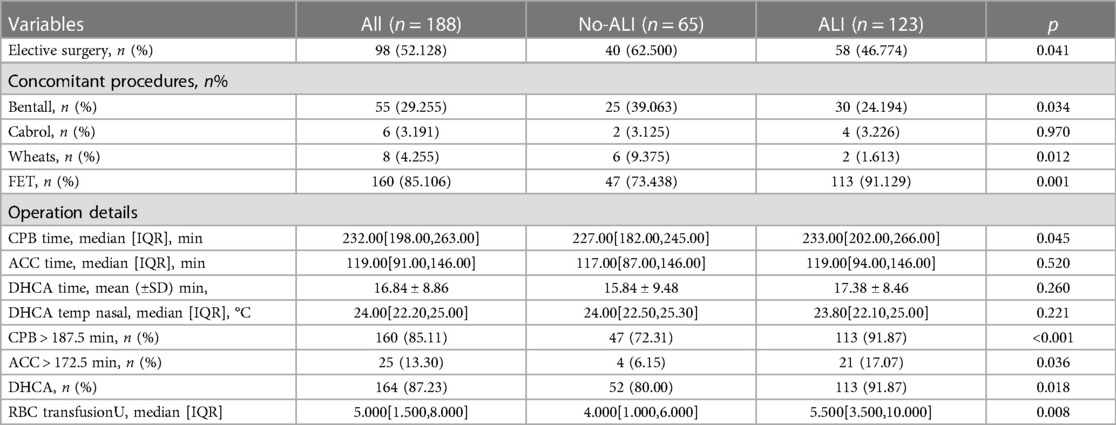
Before surgery, venous blood was extracted from all patients (within 48 h of the onset of symptoms). The preoperative indices of all patients were observed and summarized in Table 1. Preoperative indicators included age, gender, body mass index (BMI), IL-6, D-dimer, serum creatinine (SCR), triglyceride, low density lipoprotein (LDL), alanine aminotransferase (ALT), aspartic transaminase (AST), glucose, white blood cells (WBC), platelets (PLT), cardiac effusion, hypertension, and other previous history. Intraoperative and postoperative indicators were summarized in Table 2. Intraoperative and postoperative indicators included cardiopulmonary bypass (CPB), aortic cross-clamp time (ACC), deep hypothermic circulation arrest (DHCA), WBC, PLT, D-dimer, glucose, SCR, AST, ALT, mediastinitis, and other postoperative complications.
Statistical analysis
Continuous variables were reported with mean ± standard deviation (SD) and were compared using Student's independent t-test. If normality was not assumed (Kolmogorov-Smirnov test), median (interquartile range, IQR) and Mann-Whitney U test would be used. Categorical variables were presented as numbers and percentages and were compared using the Chi-square test or Fisher's exact test (if expected value ≤5 was found). Univariate and multivariate logistic regression models and estimated odds ratio (OR) were used to investigate the association between independent variables and in-hospital mortality. Partial nonbinary variables were dichotomized via univariable logistic regression and optimal cut-off points were estimated via receiver operating characteristic (ROC) curve analysis and determined based on the maximum Youden index.
We selected the most useful predictive features from the primary data set by using the least absolute shrinkage and selection operator (LASSO) method. This method is suitable for reducing high-dimensional data. With the LASSO binary logistic regression model, all the clinicopathologic variables in the cohort were reduced to a limited set of potential predictors. If the penalization coefficient lambda (λ) is high, the predicted regression parameters are unaffected; but, as the coefficients decrease, certain coefficients may be reduced to zero. We then selected the optimal λ in the LASSO model by using 10-fold cross-validation via minimum criteria and one standard error of the minimum criteria (the 1-SE criterion). Lastly, the Lasso method was used to select all coefficients that were not zero (Figure 1) to re-fit the model. The variables that were also significant in multivariate results would be recognized as associated factors in patients' ALI. A p < 0.05 would be recognized as reaching significance for each test, two-tailed. All analyses were performed using IBM SPSS Version 25 (SPSS Statistics V25, IBM Corporation, Somers, New York) and R software (version 4.2.1).
The model was assessed using the area under the ROC curve. The AUC (area under the receiver operating characteristic curve) was measured to quantify the discrimination performance of the model. Based on the cohort, we conducted decision curve analyses to determine the clinical usefulness of the model. This model's calibration was assessed using calibration plots.
Results
Clinical characteristics
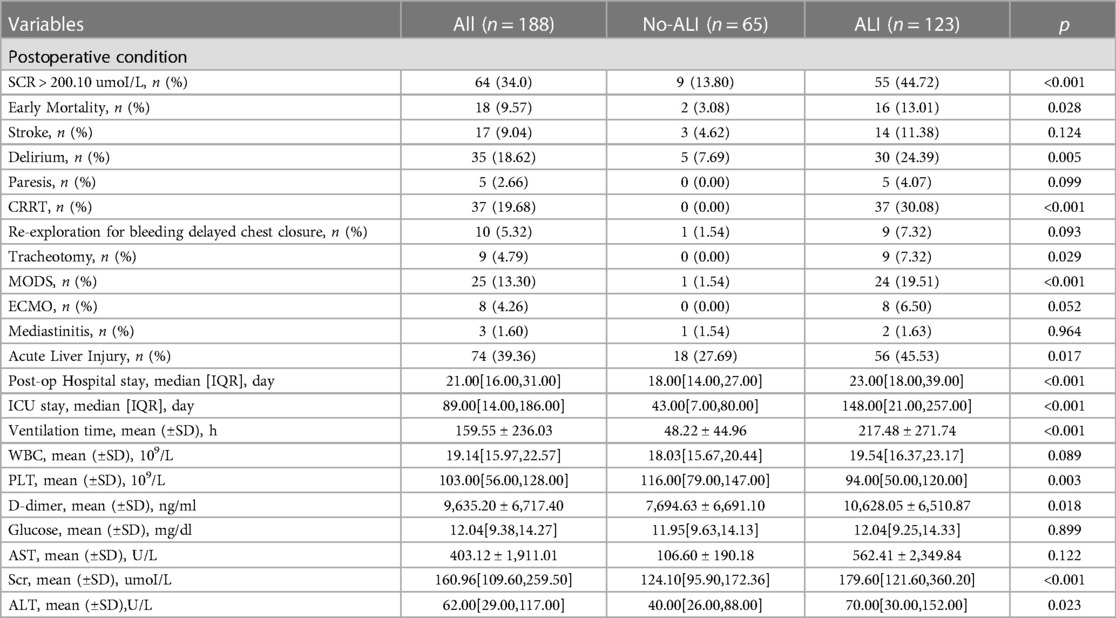
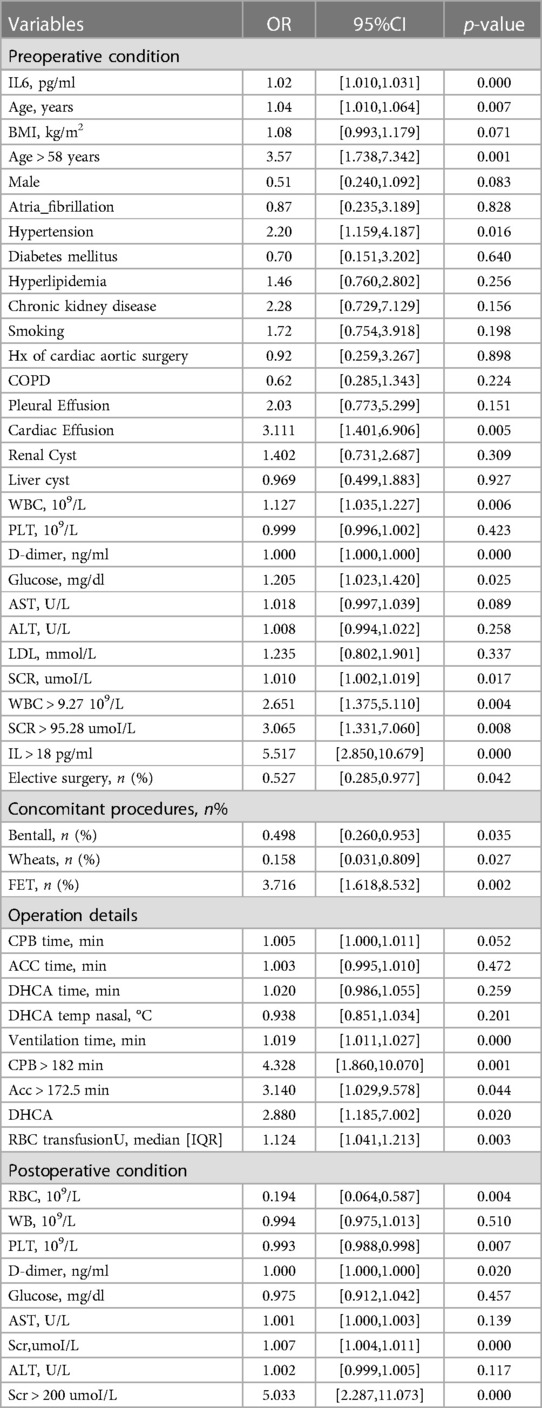
In this study, 188 patients undergoing TAD surgery were included after inclusion and exclusion criteria were met. The mean age of patients was 54.31 ± 12.02 years. In addition, 75.53% of patients were male. According to the postoperative OI, ALI was identified in 123 patients and the incidence of preoperative ALI was 65.4%. As shown in Table 1, patients who have and do not have postoperative ALI are compared in terms of their basic characteristics. The ALI patients were older (56.07 ± 11.92 vs. 50.97 ± 11.49; p = 0.005), had a higher IL6 (15.8 VS. 47.7; p < 0.001), a higher WBC (9.61 ± 4.10 VS. 11.31 ± 3.69; p = 0.005), a higher D-dimer (4,848.88 ± 6,284.89 vs. 9,730.52 ± 8,003.38; p < 0.001), a higher glucose (6.58 ± 2.21 vs. 7.32 ± 2.00; p = 0.023) and SCR (86.50 ± 25.61 vs. 112.13 ± 80.63; p = 0.002). Compared to no-ALI, a greater percentage of ALI were hypertensive (75.61% vs. 58.46%; p = 0.015). There was no statistically significant difference between the ALI and no-ALI groups in other factors. Patients with lung injury have a relatively worse postoperative situation (Table 3).
Feature selection
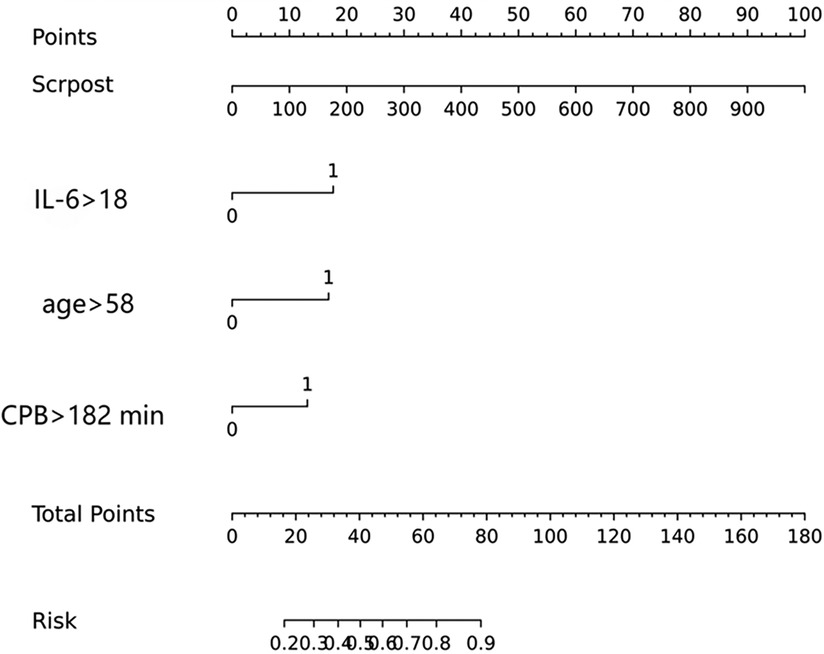
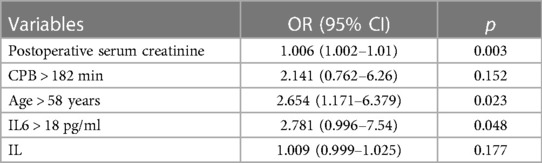
The model was built using LASSO binary logistic regression because the sample size in this study was insufficient to satisfy the advised number of events per variable. The λ value was 0.034. Of all the relevant variables, 24 features were reduced to 10 potential predictors on the basis of the cohort. The 10 variables with non-zero coefficients in the LASSO logistic regression model (i.e., Scrpost + RBC transfusionU + CPB > 182mi + D-dimerpre + Hypertension + Age > 58 years + Wheats + FET + IL6 > 18 pg/ml + IL6) were used in the final model (in Figures 2, 3). Next, the above mentioned 10 variables were included in the multivariable logistic regression analysis to determine the risk factors associated with postoperative ALI. Finally, postoperative creatinine, age > 58 and IL-6 > 18 pg/ml were as ALI risk factors (Tables 4, 5 and Figure 4). And ROC curve was shown in Figure 5.
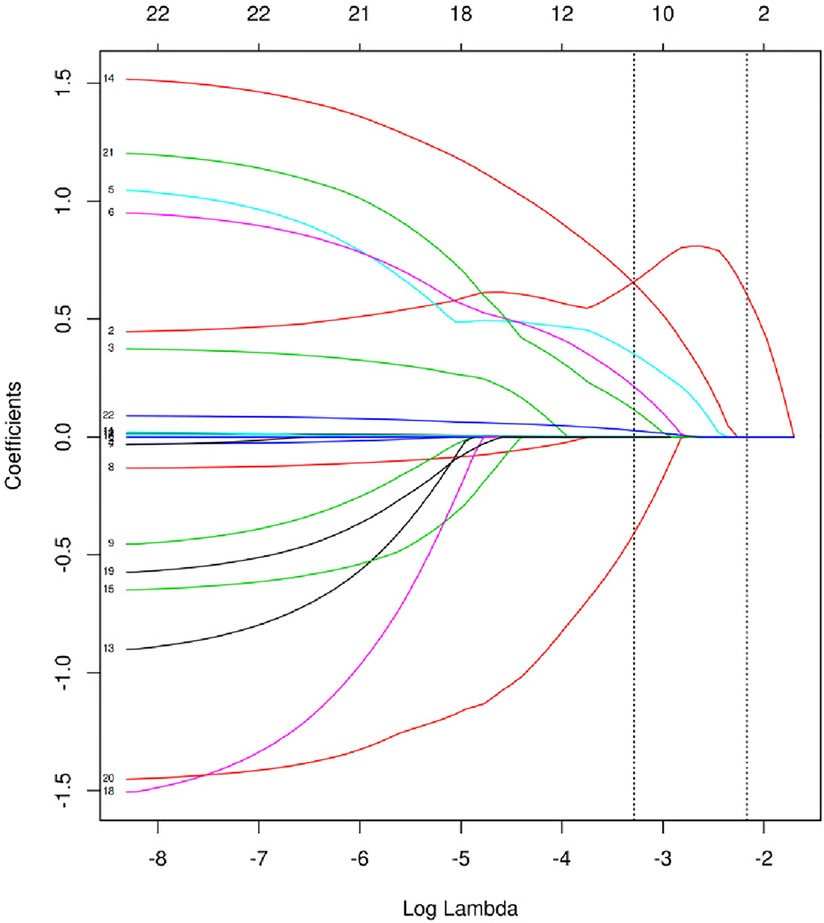
Figure 2. Texture feature selection using the least absolute shrinkage and selection operator (LASSO) binary logistic regression model.
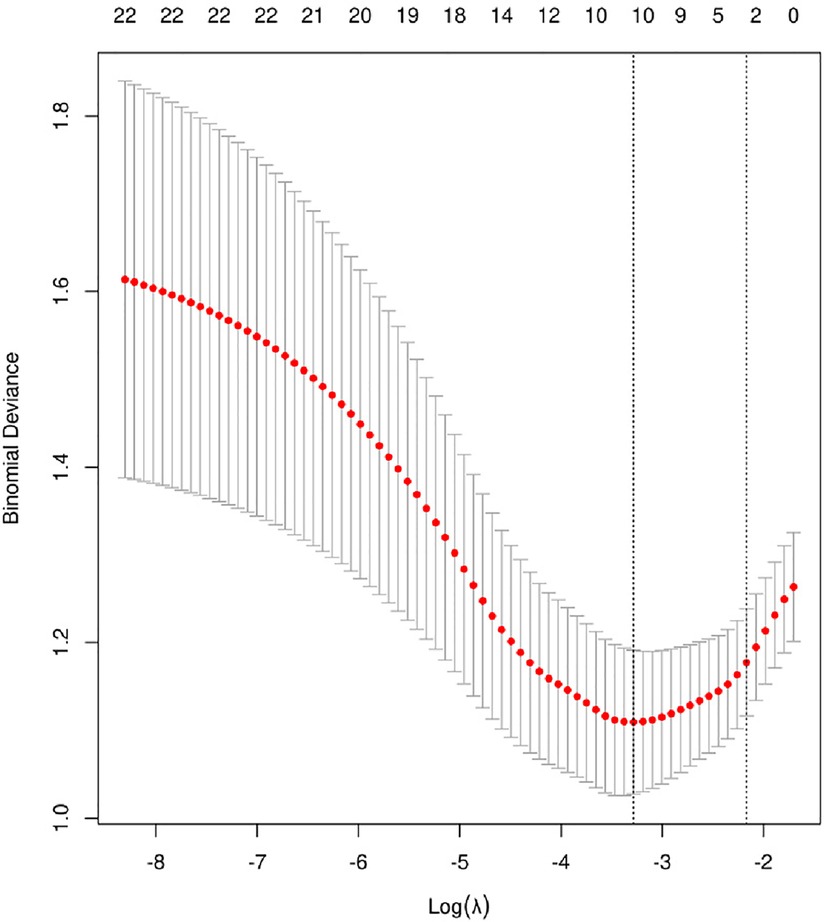
Figure 3. Cross-validation of the LASSO regression model. Lasso coefficient profiles of the 24 features. A coefficient profile plot was produced against the log (λ) sequence. The vertical line was drawn at the value selected using 10-fold cross-validation, where optimal resulted in 10 features with non-zero coefficients.
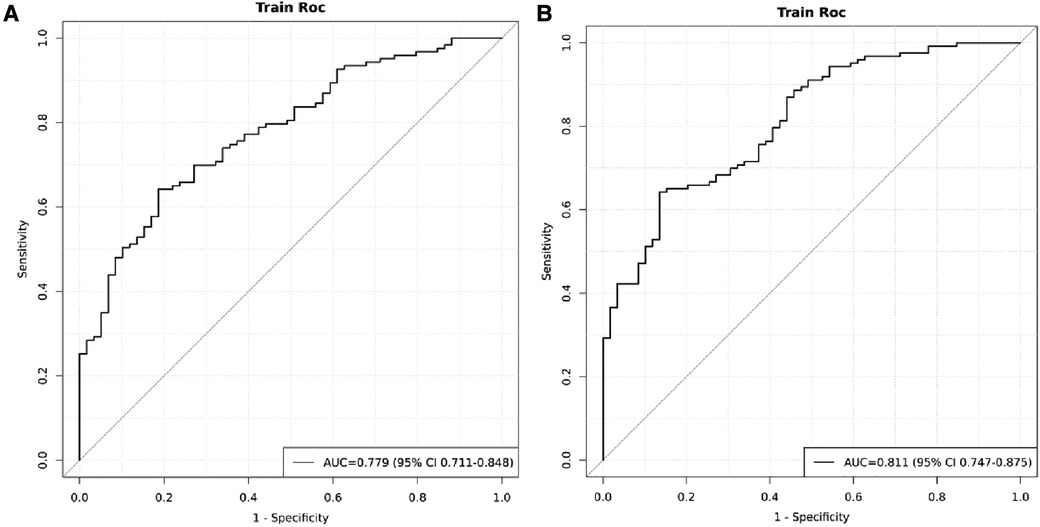
Figure 5. ROC of the cohort. (A) ROC curves when IL > 18 was excluded. (B) ROC curves at inclusion of postoperative creatinine, age > 58 and IL-6 > 18 pg/ml.
Nomogram construction
The above-mentioned variables were screened using logistic regression backwards selection. Postoperative creatinine, age > 58 and IL-6 > 18 pg/ml were identified as ALI risk factors. The ALI risk was 2.78-fold higher in IL > 18 than in IL < 18 patients (95% CI = 1.0–7.54; p = 0.048), postoperative creatinine (OR for ALI: 1.006, 95% CI: 1.002–1.01, p = 0.005) and Age > 58 years (OR for ALI: 2.654, 95% CI: 1.171–6.379, p = 0.023) were independent risk factors for postoperative ALI (Table 4). These results were used to construct a nomogram for estimating the postoperative ALI risk in TAD patients during hospitalization (Figure 4). Three independent predictors of postoperative ALI were used. The three factors used to estimate the postoperative risk of ALI each received a score based on their value, and the total score was calculated by summing the scores.
Nomogram validation
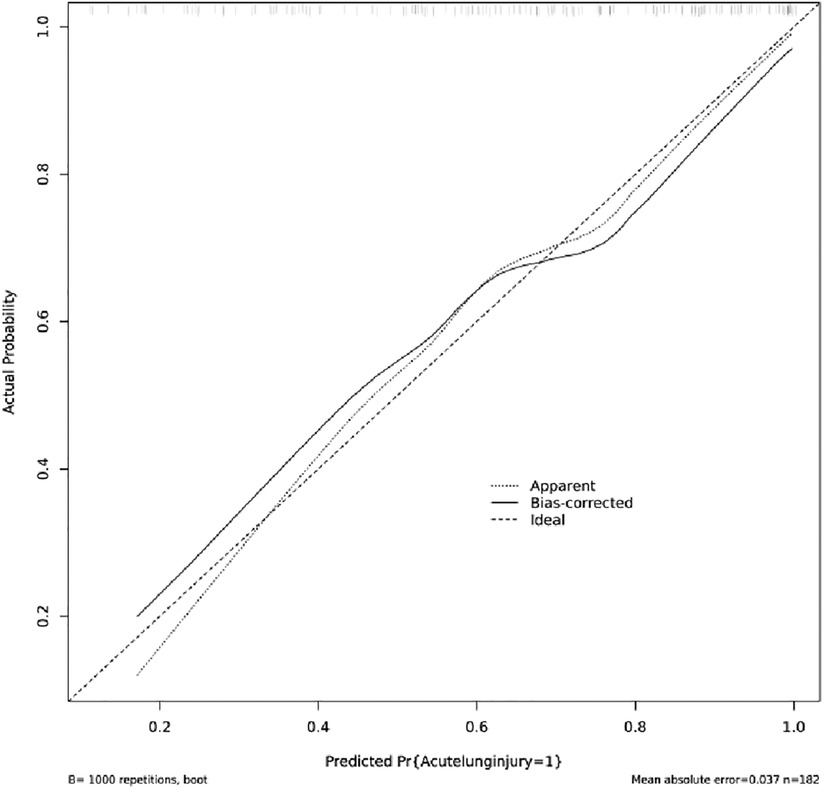
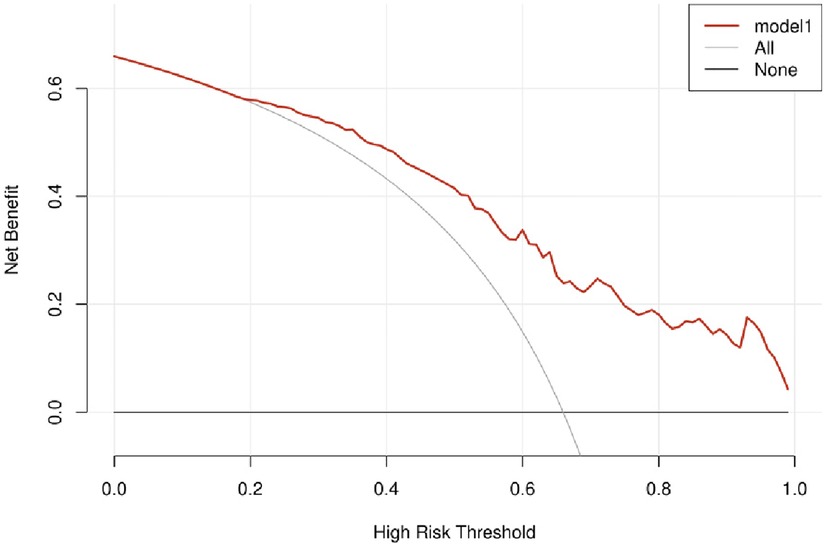
Figure 4 indicated that the AUC values of the nomogram were 0.811(95% CI = 0.747–0.875) for the cohort. Figure 6 displayed the calibration curves of the nomogram. The Hosmer–Lemeshowchi-square statistic was 4.24 and the p value was 0.8348, demonstrating good calibration. In the cohort, correction curves were nearly diagonal, suggesting that the nomogram fit well. The DCA curve was then drawn to demonstrate clinical applicability (Figure 7). The DCA curve showed that the threshold probability of a patient is in the range of 30% to 90%, the use of a nomogram to predict postoperative ALI in patients with TAD is more beneficial than either the treat-all-patients or treat-none scheme.
Discussion
We looked into whether adding IL-6 could predict occurrence of ALI after TAD surgery. Association of interleukin-6 with postoperative complications is an area of ongoing research. This study explores the predictive role of interleukin-6 in the occurrence of postoperative ALI after surgery for TAD and demonstrates that the inclusion of interleukin-6 improves the performance of predictive model for ALI after TAD surgery. We developed a diagnostic nomogram for the postoperative individualized prediction of ALI in patients with TAD during hospitalization. The nomogram incorporated three items: IL-6 > 18 pg/ml, ventilation time and postoperative serum creatinine. We selected an optimum IL-6 cutoff value based on the Youden index (Youden index = sensitivity + specificity-100%) for the prediction of patients with postoperative ALI, and also considered the sensitivity and specificity. In practice, the choice of cutoff value might be dependent on the risk of misdiagnosis and missed diagnosis. Our study indicated that IL-6 > 18 pg/ml, ventilation time and postoperative serum creatinine are independent risk factors for postoperative ALI in TAD patients. The validity of our nomogram model was determined using multiple indicators, including AUC, correction curve, Hosmer-Lemeshow test and DCA. Through shrinking the regression coefficients that represent the correlation between predictor and outcome, 24 candidate features were reduced to 10 potential predictors for the construction of the model. This strategy outperforms the method of selecting predictors based on the strength of their univariable correlation with the result.
A number of studies found that blood or lung tissues from aortic dissection and thoracic aortic aneurysm patients with postoperative ALI showed significant increases in C-reactive protein (CRP), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1). As a result of these reactions, pulmonary microvascular endothelial cells (PMVECs) are subject to apoptosis and barrier dysfunction, which lead to the elevation of endothelial permeability and the development of ALI (5, 16). Patients with ALI had higher levels of blood IL-6. Experimental evidence suggested aortic aneurysms can secrete IL-6 (17–19). In basic experimental research, Paige et al. found aortic rupture and death can be decreased in AAA mouse models by selectively inhibiting the IL-6 trans-signaling pathway, which suggested a possible therapeutic target (20). The expression of IL-6 was increased in AD rat models. MMP-2 expression may be enhanced by IL-6, which may promote extracellular matrix degradation, which promoted AD development (19). In clinical studies, the IL-6 level was found to be elevated in postoperative delirium (POD) patients following aortic dissection surgery by Lv Xiaochai et al. In this way, plasma IL-6 levels could be used to evaluate the outcomes of POD in AAD patients (21). Furthermore, high levels of IL-6 and D-dimer have predictive significance in predicting poor prognosis following acute Stanford type A aortic dissection surgery (13). Researchers have looked into the link between IL-6 (22), but it is not clear if IL-6 also affects ALI after TAD surgery.
In our study, patients with postoperative ALI had higher levels of IL-6 than those who did not have ALI, and the difference was statistically significant. After using logistic regression, it was found that IL-6 > 18 was a risk factor for postoperative ALI. Moreover, the AUC in the development cohort was 0.811 and correction curves were nearly diagonal. Compared with other studies, we selected the cutoff value, but whether this cutoff value can be applied to the subsequent validation still needs to be explored by expanding the sample size.
In cardiac surgery, a serious problem associated with cardiopulmonary bypass (CPB) was that it triggers an inflammatory response that contributed to the dysfunction of various organs, including the lungs (23, 24). The complement system in the blood and many different body cells were broadly stimulated in the particular cardiopulmonary bypass environment, and their functions were progressively damaged. During the postoperative period, their own inflammatory response was amplified, leading to SIRS and further causing organ dysfunction, including ALI. Lung immunological and anatomical characteristics contribute directly to the poor prognosis of ALI, which makes it one of the main targets of inflammation (25). A prolonged cardiopulmonary bypass will, therefore, result in postoperative ALI in ATAAD surgery.
The kidneys were responsible for eliminating creatinine from the body. Increased creatinine levels suggest renal disease. However, a statistically significant difference was found in postoperative serum creatinine levels higher in the ALI group in our study. Despite being different organs with their own location, structure, and function, lung and kidney tissue could be damaged simultaneously in the course of systemic diseases since they were not completely independent from one another (26). It has been proposed that renal ischemia/reperfusion could cause the systemic release of injurious factors that activated pulmonary capillary endothelial cells, resulting in increasing permeability and expression of adhesion molecules in the cells (27). Previous studies have found in the univariate analysis, increased creatinine levels were a significant risk factor for hypoxemia at 1 and 12 h after surgery (28). A possible explanation was that this group of patients was more susceptible to fluid overload during surgery due to their increased sensitivity to fluids. Some investigators have identified excessive hemodilution during CPB as an important factor in the development of lung injury (28). We were unable to determine hemodilution and fluid load because fluid intake and output were not counted in our data.
The shortcoming of our study is that relatively few previous tests for IL-6 have been performed, so the number of patients included in the study is relatively insufficient, which may make the results less perfect. Therefore, for follow-up studies, preoperative testing of IL-6 in patients should be increased. IL-6 testing is not included as a mandatory preoperative test, but since studies have found that IL-6 has a predictive effect on lung injury, it should be recommended that patients be tested for IL-6 prior to surgery in order to better prevent postoperative lung injury. We also hope to explore the cut-off value of IL-6 in a follow-up study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The study protocol was approved by the institutional Ethics Committee of Guangdong Provincial People’s Hospital (KY-Q-2021-183-02) and written informed consent was not required in accordance with the national legislation.
Author contributions
JW and HL: contributed to the study design. HL and WF: participated in the data measurement and analysis. QW and CL: conducted the statistical analysis. HL, WF, and JZ: drafted the manuscript. TS and JW: revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1093616/full#supplementary-material
References
1. Aldridge A, Desai A, Owston H, Jennings LM, Fisher J, Rooney P, et al. Development and characterisation of a large diameter decellularised vascular allograft. Cell Tissue Bank. (2018) 19:287–300. doi: 10.1007/s10561-017-9673-y
2. Kurabayashi M, Okishige K, Azegami K, Ueshima D, Sugiyama K, Shimura T, et al. Reduction of the PaO2/FiO2 ratio in acute aortic dissection—relationship between the extent of dissection and inflammation. Circ J. (2010) 74:2066–73. doi: 10.1253/circj.CJ-10-0336
3. Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg. (2007) 83:S856–61; discussion S890–2. doi: 10.1016/j.athoracsur.2006.10.096
4. Rigberg DA, McGory ML, Zingmond DS, Maggard MA, Agustin M, Lawrence PF, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. (2006) 43:217–22; discussion 223. doi: 10.1016/j.jvs.2005.10.070
5. Estrera AL, Sandhu HK, Charlton-Ouw KM, Afifi RO, Azizzadeh A, Miller CC 3rd, et al.. A quarter century of organ protection in open thoracoabdominal repair. Ann Surg. (2015) 262:660–8. doi: 10.1097/SLA.0000000000001432
6. Liu K, Liu J, Wu S. Association of dynamic changes in serum cytokine levels with the severity of injury in patients suffering from closed chest traumas complicated with pulmonary contusions. Exp Ther Med. (2011) 2:563–7. doi: 10.3892/etm.2011.241
7. Zhang J, Liu J, Zhao M, Ye J, Xu Y, Wang Z, et al. The expression of interleukin 20 increases in plasma and aortic tissues from patients with acute aortic dissection. Clin Chim Acta. (2020) 510:373–80. doi: 10.1016/j.cca.2020.07.049
8. Ramprasath T, Han YM, Zhang D, Yu CJ, Zou MH. Tryptophan catabolism and inflammation: a novel therapeutic target for aortic diseases. Front Immunol. (2021) 12:731701. doi: 10.3389/fimmu.2021.731701
9. Duan XZ, Xu ZY, Lu FL, Han L, Tang YF, Tang H, et al. Inflammation is related to preoperative hypoxemia in patients with acute Stanford type A aortic dissection. J Thorac Dis. (2018) 10:1628–34. doi: 10.21037/jtd.2018.03.48
10. Nagareddy P, Smyth SS. Inflammation and thrombosis in cardiovascular disease. Curr Opin Hematol. (2013) 20:457–63. doi: 10.1097/MOH.0b013e328364219d
11. Ju X, Ijaz T, Sun H, Ray S, Lejeune W, Lee C, et al. Interleukin-6-signal transducer and activator of transcription-3 signaling mediates aortic dissections induced by angiotensin II via the T-helper lymphocyte 17-interleukin 17 axis in C57BL/6 mice. Arterioscler Thromb Vasc Biol. (2013) 33:1612–21. doi: 10.1161/ATVBAHA.112.301049
12. Brocca A, Virzi GM, de Cal M, Giavarina D, Carta M, Ronco C. Elevated levels of procalcitonin and interleukin-6 are linked with postoperative complications in cardiac surgery. Scand J Surg. (2017) 106:318–24. doi: 10.1177/1457496916683096
13. Wu Q, Li J, Chen L, Yan LL, Qiu Z, Shen Y, et al. Efficacy of interleukin-6 in combination with D-dimer in predicting early poor postoperative prognosis after acute Stanford type A aortic dissection. J Cardiothorac Surg. (2020) 15:172. doi: 10.1186/s13019-020-01206-y
14. Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
15. Gourd NM, Nikitas N. Multiple organ dysfunction syndrome. J Intensive Care Med. (2020) 35:1564–75. doi: 10.1177/0885066619871452
16. Wu Z, Wang Z, Dai F, Liu H, Ren W, Chang J, et al. Dephosphorylation of Y685-VE-cadherin involved in pulmonary microvascular endothelial barrier injury induced by angiotensin II. Mediat Inflamm. (2016) 2016:8696481. doi: 10.1155/2016/8696481
17. Dawson J, Cockerill GW, Choke E, Belli A-M, Loftus I, Thompson MM. Aortic aneurysms secrete interleukin-6 into the circulation. J Vasc Surg. (2007) 45:350–6. doi: 10.1016/j.jvs.2006.09.049
18. Dawson J, Cockerill G, Choke E, Loftus I, Thompson MM. Aortic aneurysms as a source of circulating interleukin-6. Ann N Y Acad Sci. (2006) 1085:320–3. doi: 10.1196/annals.1383.009
19. Cai YL, Wang ZW. The expression and significance of IL-6, IFN-γ, SM22α, and MMP-2 in rat model of aortic dissection. Eur Rev Med Pharmacol Sci. (2017) 21:560–8.28239811
20. Paige E, Clement M, Lareyre F, Sweeting M, Raffort J, Grenier C, et al. Interleukin-6 receptor signaling and abdominal aortic aneurysm growth rates. Circ Genom Precis Med. (2019) 12:e002413. doi: 10.1161/CIRCGEN.118.002413
21. Lv XC, Lin Y, Wu QS, Wang L, Hou YT, Dong Y, et al. Plasma interleukin-6 is a potential predictive biomarker for postoperative delirium among acute type a aortic dissection patients treated with open surgical repair. J Cardiothorac Surg. (2021) 16:146. doi: 10.1186/s13019-021-01529-4
22. Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. (2016) 140:345–50. doi: 10.5858/arpa.2015-0519-RA
23. Deng Y, Hou L, Xu Q, Liu Q, Pan S, Gao Y, et al. Cardiopulmonary bypass induces acute lung injury via the high-mobility group box 1/toll-like receptor 4 pathway. Dis Markers. (2020) 2020:8854700. doi: 10.1155/2020/8854700
24. Ge J, Tian J, Yang H, Hou L, Wang Z, He Z, et al. Alpha7 nicotine acetylcholine receptor agonist PNU-282987 attenuates acute lung injury in a cardiopulmonary bypass model in rats. Shock. (2017) 47:474–9. doi: 10.1097/SHK.0000000000000744
25. Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. (2014) 346:1234–8. doi: 10.1126/science.1256478
26. Visconti L, Santoro D, Cernaro V, Buemi M, Lacquaniti A. Kidney-lung connections in acute and chronic diseases: current perspectives. J Nephrol. (2016) 29:341–8. doi: 10.1007/s40620-016-0276-7
27. Karimi Z, Ketabchi F, Alebrahimdehkordi N, Fatemikia H, Owji SM, Moosavi SM. Renal ischemia/reperfusion against nephrectomy for induction of acute lung injury in rats. Ren Fail. (2016) 38:1503–15. doi: 10.1080/0886022X.2016.1214149
Keywords: interleukin-6, acute lung injury, thoracic aortic disease, postoperative, predict
Citation: Li H, Feng W, Wang Q, Li C, Zhu J, Sun T and Wu J (2023) Inclusion of interleukin-6 improved the performance of postoperative acute lung injury prediction for patients undergoing surgery for thoracic aortic disease. Front. Cardiovasc. Med. 10:1093616. doi: 10.3389/fcvm.2023.1093616
Received: 9 November 2022; Accepted: 24 July 2023;
Published: 11 August 2023.
Edited by:
Massimo Bonacchi, University of Florence, ItalyReviewed by:
Xiao-Qiao Dong, Zhejiang Chinese Medical University, ChinaShahzad Raja, Harefield Hospital, United Kingdom
© 2023 Li, Feng, Wang, Li, Zhu, Sun and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huili Li bGhsMTAwNzYxQDE2My5jb20= Jinlin Wu d3VqaW5saW5AZ2RwaC5vcmcuY24=
†These authors share co-first authorship
 Huili Li
Huili Li Weiqi Feng
Weiqi Feng Qiuji Wang
Qiuji Wang Chenxi Li
Chenxi Li Jiade Zhu
Jiade Zhu Tucheng Sun
Tucheng Sun Jinlin Wu
Jinlin Wu