
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 09 February 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1089636
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2022 View all 39 articles
Primary cardiac intimal sarcoma, an extremely rare cardiac tumor subtype, is often mis-diagnosed owing to its rarity and non-specific clinical and radiological features. We report a case of cardiac intimal sarcoma mimicking atrial myxoma in which the clinical presentation and multimodality imaging are described in detail, and diagnostic challenges are highlighted.
Primary cardiac intimal sarcoma is an extremely rare subtype of cardiac tumor, with only a few such cases having been reported (1–8). It often occurs in the left atrium, whereas it seldom originates in the right atrium and mitral valve (6, 8). Owing to its rarity and non-specific characteristics, cardiac intimal sarcomas are often mistaken for myxomas or thrombi. We report a case of cardiac intimal sarcoma of the left atrium and provide a detailed description of multimodality imaging aimed at highlighting pre-operative diagnostic challenges.
A 52-year-old woman was admitted to our hospital with a 3-month history of progressive cough and shortness of breath. On admission, laboratory findings and non-contrast thoracic computed tomography (CT) scan results were negative for infectious diseases. Her electrocardiogram was normal (heart rate, 76 bpm); however, transthoracic echocardiography revealed an irregularly shaped 4.8 × 6.7 mm hypoechoic mass in the left atrium that appeared broadly based and originating from the lateral atrial wall (Figure 1). The mass induced significant mitral stenosis through prolapsing toward the left ventricle during diastole.
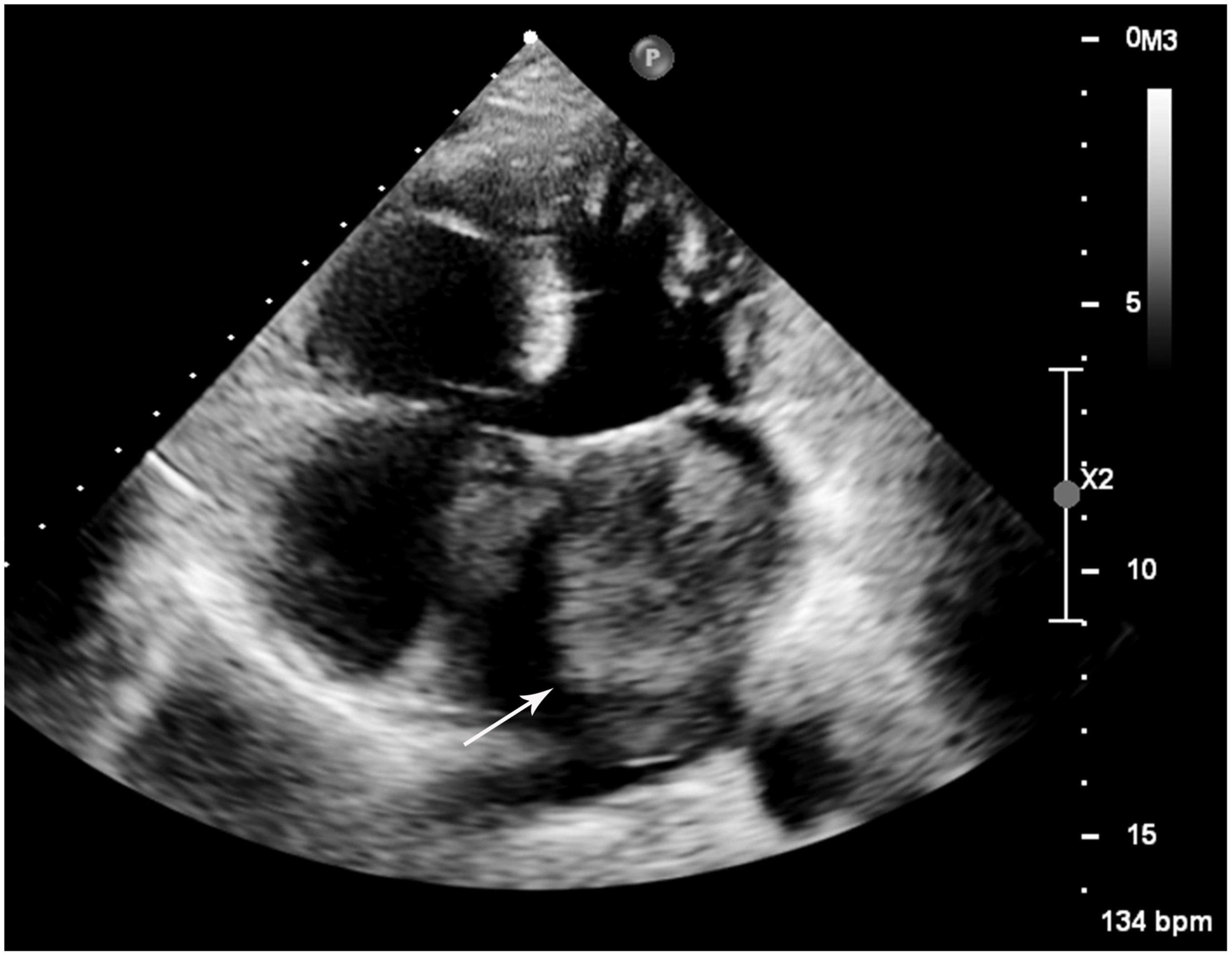
Figure 1. Transthoracic echocardiogram of the left mass. A large hypoechoic mass (white arrow) was revealed in the left atrium attaching to the lateral atrial wall via a broad base.
The patient had undergone cervical cancer resection in 2014. No mass or mass-like lesion of the heart had been observed on contrast-enhanced thoracic CT during the follow-up period in 2019. However, possible cardiac metastasis was now suspected. Contrast-enhanced CT and cardiovascular magnetic resonance (CMR) imaging were performed for further characterization. CT images showed that the lobulated mass with homogeneous hypodensity almost occupied the left atrium and the left atrial appendage, extending to the orifice of the left superior pulmonary vein, and showing significantly inhomogeneous enhancement in the arterial phase. Contrast uptake decreased with a longer delay time (almost into the portal venous phase) (Figure 2).
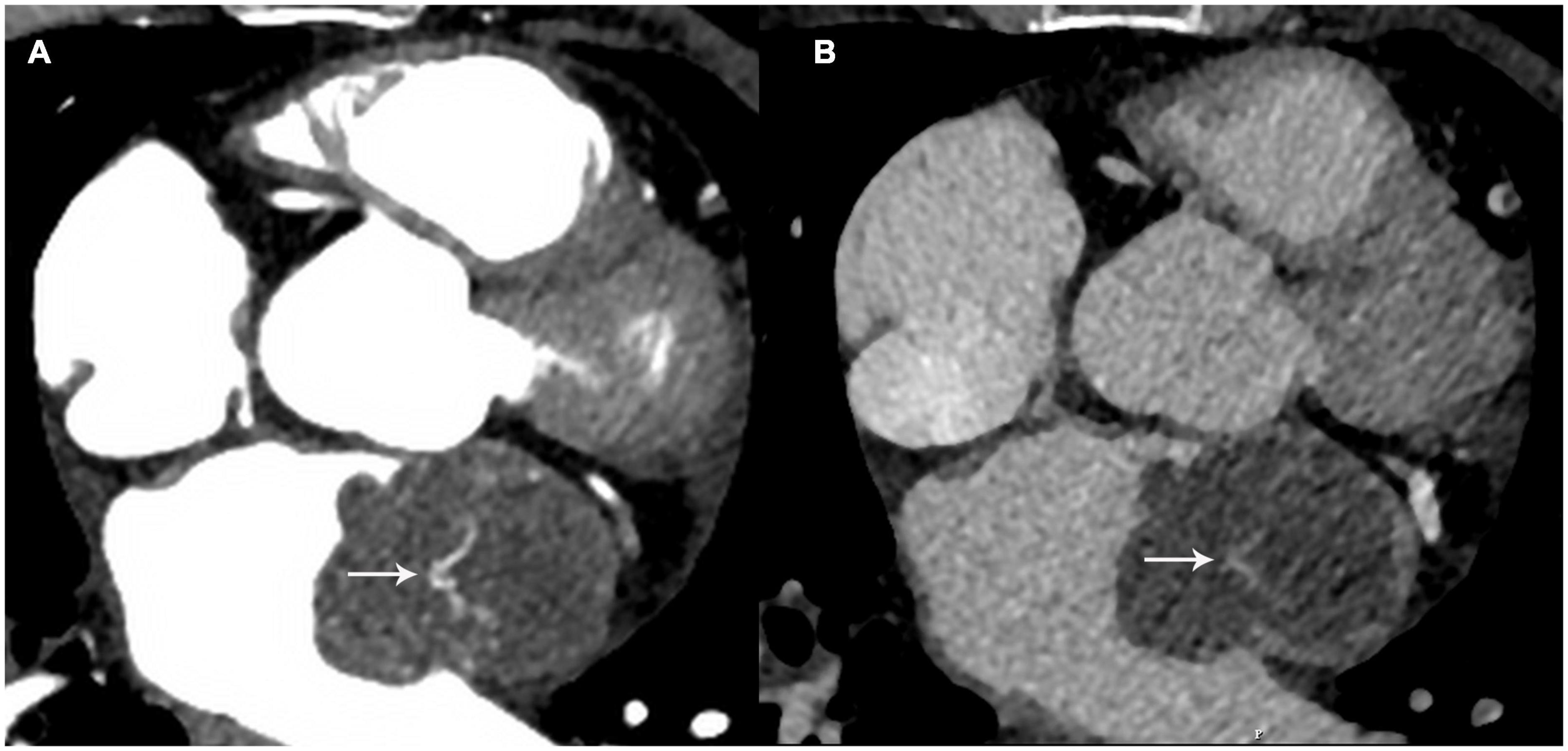
Figure 2. The mass showed heterogeneous enhancement at the arterial phase (A) white arrow and the contrast uptake decreased with a longer delay time (B) white arrow.
Regarding CMR imaging, compared with the myocardium, the lesion showed significantly elevated native T1 and T2 values on T1 and T2 mapping (Figure 3). Mildly increased regional perfusion was observed in the mass during first-pass perfusion imaging, as well as heterogeneous enhancement on late gadolinium-enhanced (LGE) imaging (Figure 4). No adjacent tissue infiltration was observed. Radiological findings suggested a possible diagnosis of atrial myxoma.
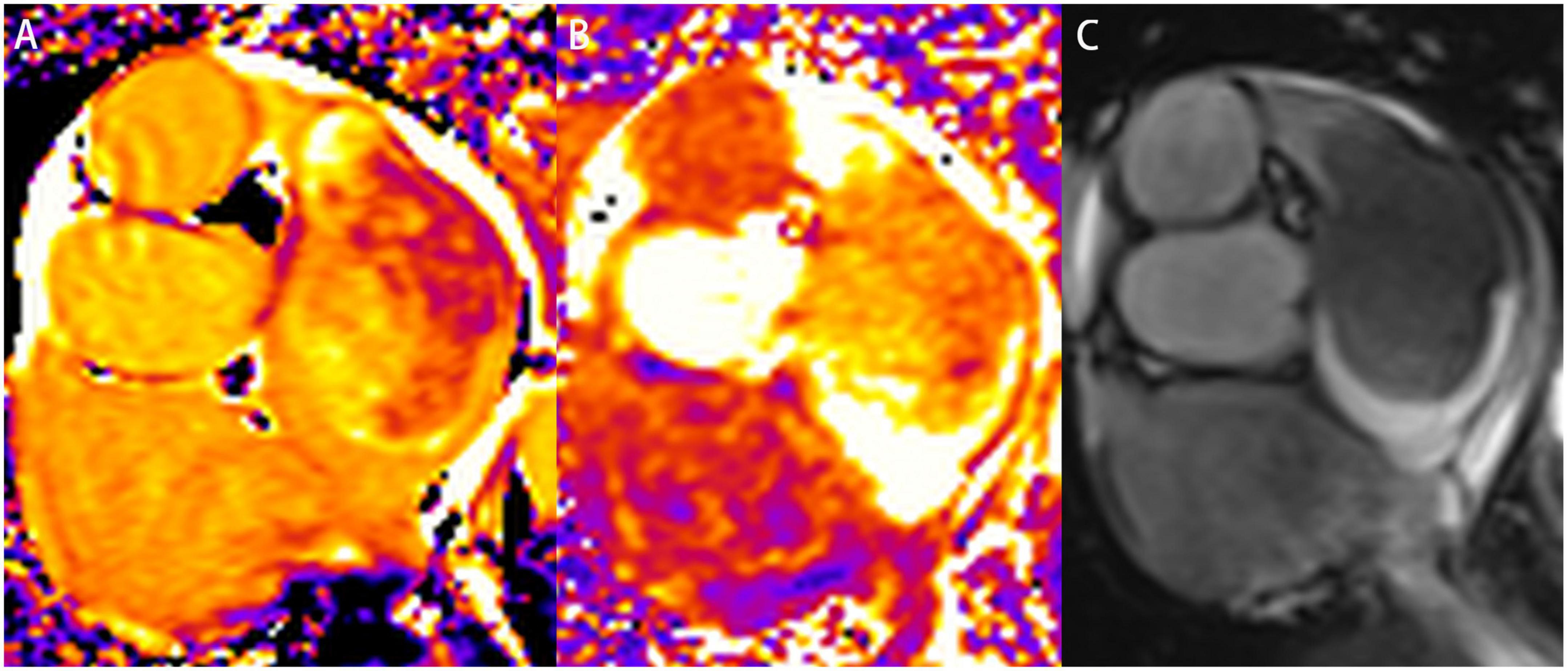
Figure 3. Characterization of the left atrial mass on cardiovascular magnetic resonance imaging. A slice of a short-axis view of left atrium was acquired on native T1 mapping (A), T2 mapping (B), and cine imaging (C), respectively. The mass was heterogeneous on native T1 mapping and T2 mapping. The native T1 value ranged from 1,661 to 2,109 ms. The T2 value was also significantly elevated.
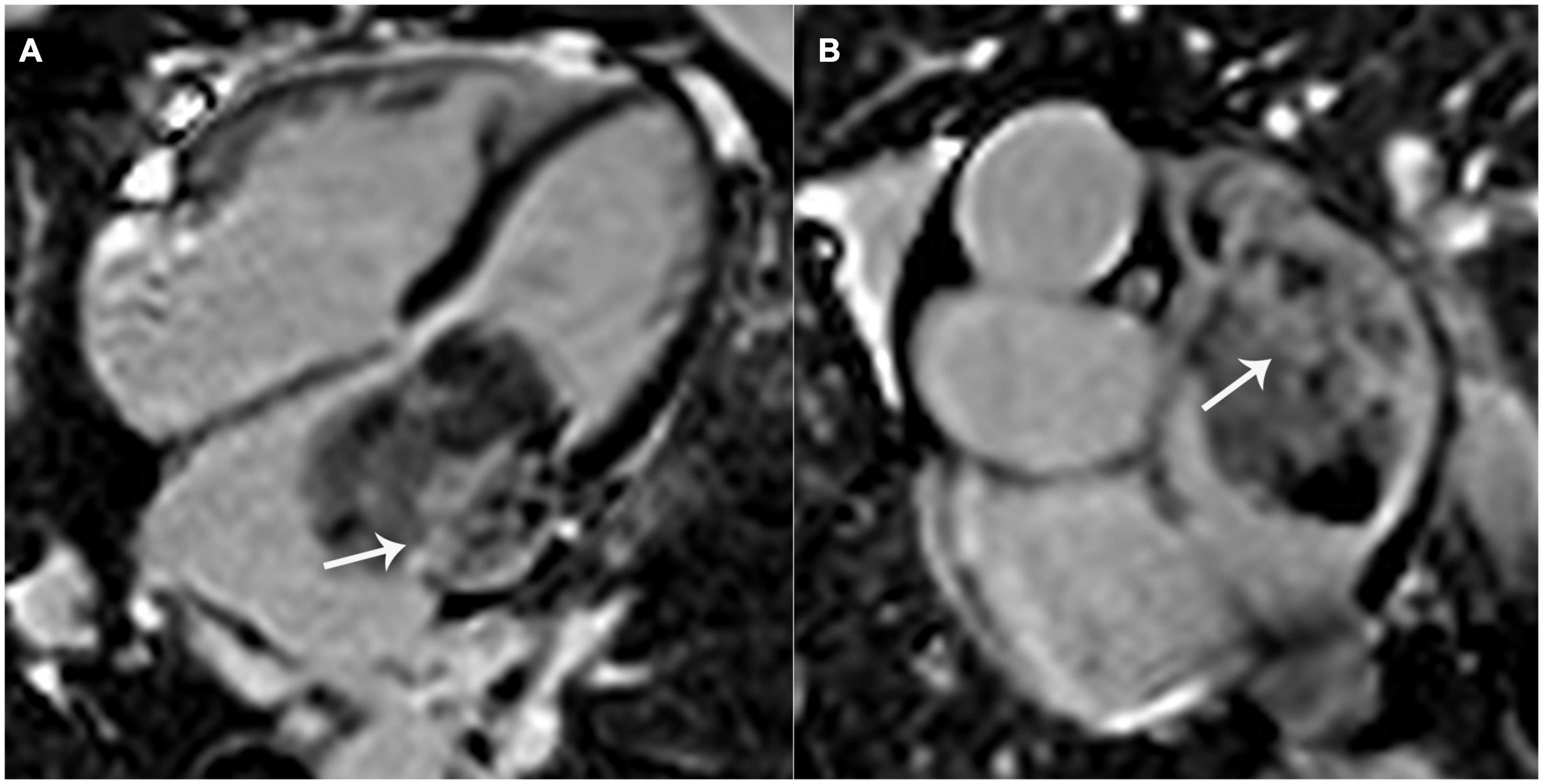
Figure 4. Left atrial mass on late gadolinium-enhanced (LGE) imaging. Significant patchy enhancement (white arrows) was observed on LGE images. (A) 4-chamber view; (B) a shot-axis view of left atrium.
Surgical resection was then performed. Histopathological examination revealed spindle-shaped tumor cells with prominent atypia. Mitotic activity was frequent. Some stromal myxoid changes could also be observed in the tumor (Figure 5). Extensive immunohistochemical analyses showed that the cells were positive for vimentin, caldesmon, CDK4, Bcl-2, CD34 (focal), CK (focal), SMA (focal), TLE1 (focal), CD99 (focal) and negative for desmin, EMA, S-100, CD31, ERG, MDM-2, P16, SOX-10, MyoD1, myogenin, STAT6. The Ki-67 proliferative index was 40%. Pathological results supported the diagnosis of high-grade intimal sarcoma. At 4 months post-operatively, the patient was re-hospitalized and underwent systemic chemotherapy for a metastatic lesion of the left femur.
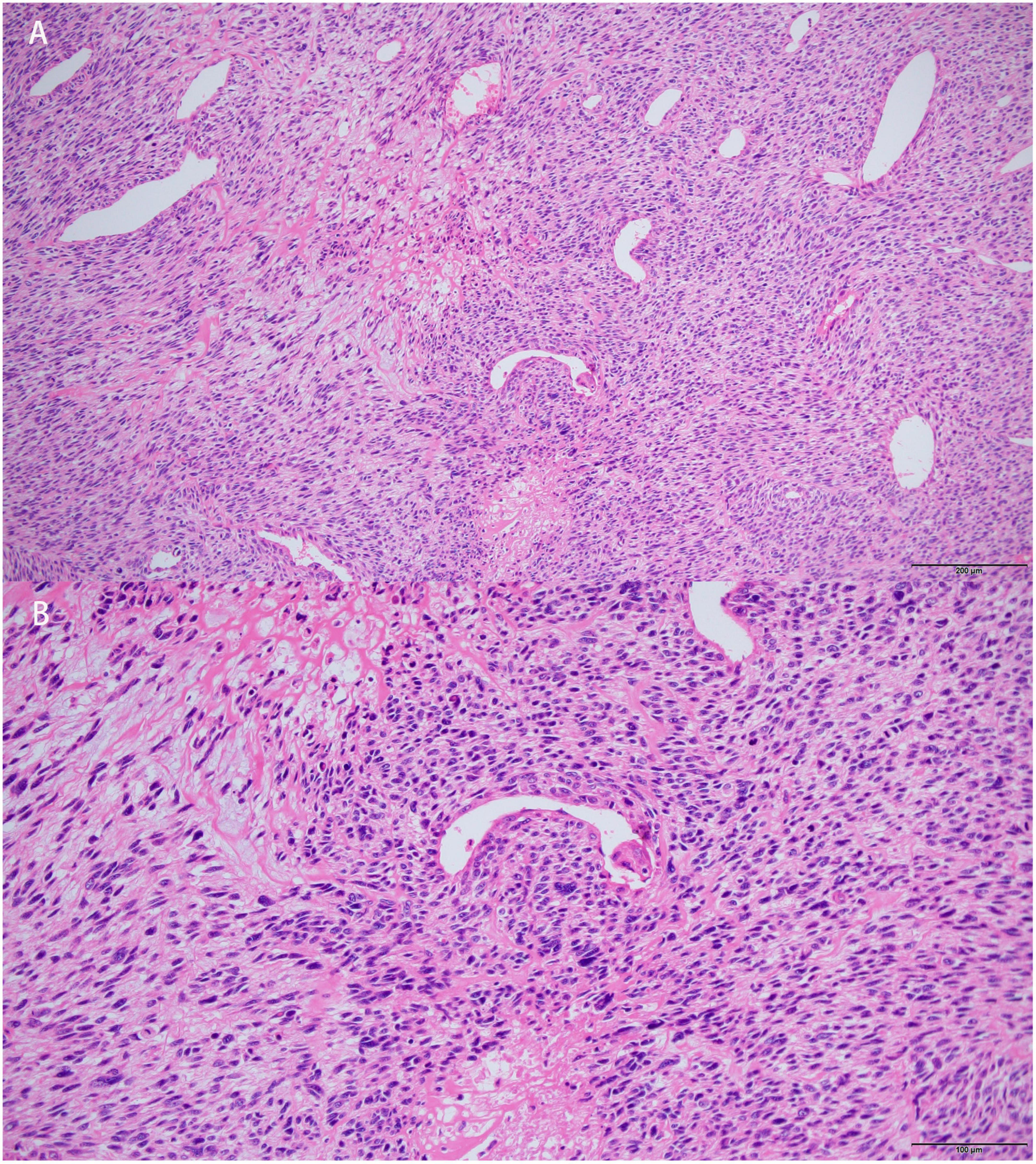
Figure 5. Histopathological features of the left atrial mass. Hematoxylin-eosin (HE) stained section showed that tumors cells were spindle-shaped, prominent atypical. Mitotic activity was frequent. Myxoid changes could be observed in stroma. [Bars: (A) 200 μm; (B) 100 μm].
Non-invasive pre-operative assessment of cardiac tumors is vital for further management and could be an important predictor of prognosis. Echocardiography, CT, and magnetic resonance imaging (MRI) are common techniques used for diagnosis and monitoring. Owing to its wide availability and convenience, echocardiography is often used as the first imaging modality to confirm the presence of a cardiac mass and quantify the morphological characteristics, attachment, and mobility of the tumor. CT and MRI are optimal approaches for assessing tissue characterization, vascularity, adjacent infiltration, and extracardiac metastases (9–11). Considering the limitations of different imaging techniques, multimodality imaging of cardiac masses is required for determining treatment strategies.
Intimal sarcoma is a rare subtype of mesenchymal sarcoma that commonly originates in the great vessels with a predilection for the pulmonary arteries (12). The heart is rarely involved in intimal sarcomas, and only a few such case reports have been published (1–4). Cardiac intimal sarcomas are frequently misdiagnosed as either atrial myxomas or thrombi. In this case, the diagnostic challenge has also been highlighted. Typical myxomas are well-defined, often located in the left atrium, and attached to the interatrial septum (9, 10, 13). The mass in our case originated from the atrial free wall, which was the less likely origin of myxomas, as shown in some reported cases (14). In terms of tissue characterization, regarding the similarity of histological components of myxomas and intima sarcomas (4), both may be characterized using isointense/hypointensity on T1-weighted imaging, hyperintensity on T2-weighted imaging and cine imaging, through comparison with the myocardium. Heterogeneous enhancement is often observed in LGE images (9, 10). One point of note is that on CT images, contrast uptake of myxomas is hardly observed in the arterial phase, whereas it significantly increases with a longer delay time (9, 15, 16). The mass in the reported case showed significant heterogeneous enhancement in the arterial phase, and the density decreased with a longer delay time, which is concerning for rich vascularity, an important characteristic of malignant lesions. In addition, a large mass with multiple separated sites of attachment should be considered as a sign of malignancy (1), although this was not observed in our case. Imaging characteristics of the two diseases were summarized in Table 1.
The mean survival of patients with cardiac intimal sarcoma ranges from 3 months to 1 year (11). Surgical resection is the only management strategy that is associated with prolonged survival (17). Chemotherapy is indicated in patients who cannot undergo surgery, although its benefits are limited.
Cardiac intimal sarcoma is a rare disease with specific characteristics. This case emphasizes the challenges of differential diagnosis and the importance of multimodality imaging for preoperative assessment.
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Zhongnan Hospital of Wuhan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LL conducted the draft writing and data analysis. NY was responsible for literature research and data acquisition. HH contributed to image postprocessing. HX and JL supervised the activities. All authors participated in concept development, revision of the manuscript, and read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rehman M, El-Dabh A, Mandal S, Sattur S. A case report of a massive cardiac intimal sarcoma manifesting as syncope during a stress test. Eur Heart J Case Rep. (2021) 5:ytab258. doi: 10.1093/ehjcr/ytab258
2. Ho K, Yatham K, Seno R, Sultan O. A case report of primary cardiac intimal sarcoma presenting with atrial fibrillation and a left atrial mass. Eur Heart J Case Rep. (2021) 5:ytab410. doi: 10.1093/ehjcr/ytab410
3. Braams N, Kaffka Genaamd Dengler S, Rutten E, de Boer K. Left atrial spindle cell sarcoma: a case report. Eur Heart J Case Rep. (2019) 3:ytz005. doi: 10.1093/ehjcr/ytz005
4. Durieux R, Tchana-Sato V, Lavigne J, Radermecker M, Moonen M, Scagnol I, et al. Recurrent cardiac intimal sarcoma misdiagnosed as a myxoma or malignant transformation of a cardiac myxoma? J Card Surg. (2021) 36:357–62. doi: 10.1111/jocs.15200
5. Rahmouni K, Al Abri Q, Janelle M, Nguyen V, Sabapathy C, Bernier P. Intimal cardiac sarcoma in an adolescent presenting with sudden cardiogenic shock. Pediatr Blood Cancer. (2021) 68:e29083. doi: 10.1002/pbc.29083
6. Chen Y, Li Y, Lee J, Chen J. Staged surgery for advanced cardiac intimal sarcoma involving the right atrium and the inferior vena cava. J Card Surg. (2021) 36:3973–5. doi: 10.1111/jocs.15885
7. Muturi A, Kotecha V, Ruturi J, Muhinga M, Waweru W. High-grade spindle cell sarcoma of the heart: a case report and review of literature. J Cardiothorac Surg. (2015) 10:46. doi: 10.1186/s13019-015-0245-6
8. Todo S, Toba T, Okada K, Hirata KI. A rare manifestation of primary cardiac intimal sarcoma: mimicking non-bacterial thrombotic endocarditis with severe mitral regurgitation. Eur Heart J Cardiovasc Imaging. (2021) 22:e8. doi: 10.1093/ehjci/jeab026
9. Tyebally S, Chen D, Bhattacharyya S, Mughrabi A, Hussain Z, Manisty C, et al. Cardiac tumors: JACC CardioOncology state-of-the-art review. JACC CardioOncol. (2020) 2:293–311. doi: 10.1016/j.jaccao.2020.05.009
10. Motwani M, Kidambi A, Herzog B, Uddin A, Greenwood J, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology. (2013) 268:26–43. doi: 10.1148/radiol.13121239
11. Butany J, Nair V, Naseemuddin A, Nair G, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. (2005) 6:219–28. doi: 10.1016/S1470-2045(05)70093-0
12. Assi T, Kattan J, Rassy E, Moussa T, Nassereddine H, Honore C, et al. A comprehensive review on the diagnosis and management of intimal sarcoma of the pulmonary artery. Crit Rev Oncol Hematol. (2020) 147:102889. doi: 10.1016/j.critrevonc.2020.102889
13. Griborio-Guzman A, Aseyev O, Shah H, Sadreddini M. Cardiac myxomas: clinical presentation, diagnosis and management. Heart. (2022) 108:827–33. doi: 10.1136/heartjnl-2021-319479
14. Wang J, Wang B, Hu Y, Liu J, Liu B, Liu H, et al. Clinicopathologic features and outcomes of primary cardiac tumors: a 16-year-experience with 212 patients at a Chinese medical center. Cardiovasc Pathol. (2018) 33:45–54. doi: 10.1016/j.carpath.2018.01.003
15. Scheffel H, Baumueller S, Stolzmann P, Leschka S, Plass A, Alkadhi H, et al. Atrial myxomas and thrombi: comparison of imaging features on CT. AJR Am J Roentgenol. (2009) 192:639–45. doi: 10.2214/AJR.08.1694
16. Grebenc M, Rosado-de-Christenson M, Green C, Burke A, Galvin J. Cardiac myxoma: imaging features in 83 patients. Radiographics. (2002) 22:673–89. doi: 10.1148/radiographics.22.3.g02ma02673
Keywords: intimal sarcoma, myxoma, cardiovascular magnetic resonance, CMR, multimodality imaging
Citation: Ye N, Lan L, Hu H, Liu J and Xu H (2023) Case report: The diagnostic challenge of primary cardiac intimal sarcoma. Front. Cardiovasc. Med. 10:1089636. doi: 10.3389/fcvm.2023.1089636
Received: 04 November 2022; Accepted: 27 January 2023;
Published: 09 February 2023.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Abdallah Al-Mohammad, Sheffield Teaching Hospitals NHS Foundation Trust, United KingdomCopyright © 2023 Ye, Lan, Hu, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Xu,  eHVoYWlib0B3aHUuZWR1LmNu; Jinping Liu,
eHVoYWlib0B3aHUuZWR1LmNu; Jinping Liu,  bGl1amlucGluZ0B6bmhvc3BpdGFsLmNu
bGl1amlucGluZ0B6bmhvc3BpdGFsLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.