
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 16 March 2023
Sec. General Cardiovascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1089304
Objective: This study aimed to investigate the correlation between serum laminin (LN) levels and clinical stages of heart failure in patients with chronic heart failure.
Methods: A total of 277 patients with chronic heart failure were selected from September 2019 to June 2020 in the Department of Cardiology, Second Affiliated Hospital of Nantong University. Based on stages of heart failure, the patients were divided into four groups: stage A, stage B, stage C, and stage D, with 55, 54, 77, and 91 cases, respectively. At the same time, 70 healthy people in this period were selected as the control group. Baseline data were recorded and serum Laminin (LN) levels were measured. The research compared, the differences in baseline data among the four groups of HF and normal controls, and analyzed the correlation between N-terminal pro-brain natriuretic peptide (NT-proBNP) and left ventricular ejection fraction (LVEF). The receiver operating characteristic (ROC) curve was used to evaluate the predictive value of LN in the C-D stage of heart failure. Logistic multivariate ordered analysis was applied to screen the independent related factors of clinical stages of heart failure.
Results: Serum LN levels in patients with chronic heart failure were significantly higher than those in healthy people, which were 33.2 (21.38, 101.9) ng/ml and 20.45 (15.53, 23.04) ng/ml, respectively. With the progression of clinical stages of HF, serum LN and NT-proBNP levels increased, while LVEF gradually decreased (P < 0.05). Correlation analysis showed that LN was positively correlated with NT-proBNP (r = 0.744, P = 0.000) and negatively correlated with LVEF (r = −0.568, P = 0.000). The area under the ROC curve of LN for predicting C and D stages of heart failure was 0.913, 95% confidence interval was 0.882–0.945, P = 0.000, specificity 94.97%, and sensitivity 77.38%. Multivariate Logistic analysis showed that LN, Total bilirubin, NT-proBNP and HA were all independent correlates of heart failure staging.
Conclusion: Serum LN levels in patients with chronic heart failure are significantly increased and are independently correlated with the clinical stages of heart failure. It could potentially be an early warning index of the progression and severity of heart failure.
Chronic heart failure (CHF) is one of the most common chronic diseases worldwide, and it has seen an increasing trend in its prevalence and morbidity (1). Ventricular remodeling is an important pathological basis of CHF, and inhibition of its process is beneficial to improve the prognosis of patients with heart failure (HF) (2). Myocardial fibrosis is one of the primary manifestations of ventricular remodeling. It has been found that myocardial fibrosis mainly features changes in the extracellular matrix (ECM) (3). Laminin (LN) is a vital component of ECM, which has significant biological functions, including adhesion, translocation, cell differentiation, cell growth, and inflammatory response (4). Many studies have shown that LN is closely related to the process of myocardial fibrosis (5–7). The 2022 AHA/ACC/HFSA guidelines (8) divide HF into four stages (stages A–D) according to HF progression, which objectively reflects the clinical progress of CHF. Early risk factor control and active drug treatment can prevent and delay HF progression to the greatest extent. Therefore, the identification of markers related to HF progression is of particular significance. To date, on study has focused on the correlation between LN and the clinical stage of CHF. This study aimed to explore the correlation between serum LN and HF severity of heart failure in patients with CHF by analyzing serum LN levels in patients with different stages of CHF.
We conducted an observational study from September 2019 to June 2020. 277 patients diagnosed as CHF in the Department of Cardiovascular Medicine of the Second Affiliated Hospital of Nantong University were selected. All patients met the diagnostic criteria of CHF, as shown in Figure 1 (8). Patients with rheumatic system diseases, liver fibrosis, malignant tumor and patients with incomplete clinical data were excluded. 70 healthy individuals who had no major physical illnesses and no chronic diseases such as cardiac/metabolic diseases at the time of physical examination at our medical examination center during the same period were selected as the control group. We collected relevant medical records of the patients, including past history and liver and kidney function indexes, and analyzed serum levels of LN, HA, and NT-proBNP, and detected LVEF values by echocardiography for the study, which was investigated in accordance with the principles in the Declaration of Helsinki. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Nantong University (IRB: No. 2020KN094), and informed consent was obtained from all patients.
Basic information of patients is recorded at admission, including gender, age, BMI, Medical history and family history, blood pressure and heart rate at admission, etc. cardiac troponin I (cTnI) were measured immediately after admission, and left ventricular ejection fraction (LVEF) was recorded by echocardiography. The levels of serum laminin (LN), hyaluronic acid (HA), N-terminal pro-brain natriuretic peptide (NT-proBNP) and alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (Tb), serum creatinine (Scr), urea nitrogen (UN), high-sensitivity C-reactive protein (CRP), Fasting glucose, Hemoglobin, Platelet, Triglycerides, Total cholesterol, high-density lipoprotein cholesterol (HDL-C), Low-density lipoprotein cholesterol (LDL-C) were detected on an empty stomach on the early morning of the second day of hospitalization.
Stage A: Pre-heart failure stage; Stage B: Pre-clinical heart failure stage; Stage C: Clinical heart failure stage; Stage D: Refractory end-stage heart failure stage (8). (Cardiac function staging is independently evaluated by two physicians. Once there is any inconsistency, it will be discussed and decided by another chief physician) Patients in the C/D stage of HF have an impact on their daily life and are often considered to be in a relatively severe stage.
On the second morning after admission, 4 ml fasting blood was taken from all patients to measure serum laminin. Fasting blood was centrifuged for 10 min at a rate of 2,500 r/min, and then serum laminin levels were measured by chemiluminescence immunoassay (MAGLUMI 2000). The test kit is provided by Fosun Diagnostics Company (Changsha, China). The determination of serum level of HA was performed with the same method. The normal reference range for the serum laminin kit in this study is 0.51–50 ng/ml, and the normal reference range for hyaluronic acid is 0–100 ng/ml. NT-pro BNP was measured using a chemiluminescence technique (on Cobas E. from Roche).
All subjects were examined with a Philips EPIQ 7C cardiovascular ultrasound S5–1 adult probe/frequency 1–5 MHz, performed and calibrated by an experienced sonographer. During the examination, patients were instructed to lie on the left side, breathe calmly, connect to the ECG, and acquire the left ventricular apical four-chamber and two-chamber views, respectively, and scan the left ventricular long-axis view and series of short-axis views, and calculate the left ventricular ejection fraction using the Simpson biplane method, which is a two-dimensional mode in which systolic and diastolic volumes are assessed in two different planes in the apical four-chamber and apical two-chamber hearts. The endocardial margin of the left ventricle, including the papillary muscle, was traced using the trackball tracing in both systole and diastole. LV ejection fraction = (LV end-diastolic volume − LV end-systolic volume)/LV end-diastolic volume × 100%.
Continuous variables of normal distribution are expressed as mean values ± standard deviation, while variables conforming to skewed distribution are expressed as median (25th percentile–75th percentile). Statistics are expressed as percentages or frequencies. Independent sample t-test was used for the measurement data of normal distribution, χ2 test was used for the counting data, and the MannWhitney U test was used for non-normally distributed measures between two groups, and the Kruskal Wallis rank sum test was used for non-normally distributed measures between multiple groups. The correlation between LN and NT-proBNP and LVEF was studied by Spearman's Rank Coefficient of Correlation, the predictive value of LN in C-D stage of HF was evaluated by ROC curve, and the independent factors influencing the severity of HF were studied by Logistic multivariate ordered analysis. The data were analyzed by SPSS 26.0 (SPSS Inc., Chicago, IL), P < 0.05 was considered statistically significant.
LN level in CHF group was significantly higher than that in healthy group, which were 33.2 (21.38, 101.9) ng/ml and 20.45 (15.53, 23.04) ng/ml respectively (P < 0.01, Figure 2). The data of NT-proBNP, HA, cTnI, Scr, Tb, AST, ALT, high-sensitivity CRP, fasting glucose, Systolic and male proportion of CHF group were significantly higher than those in healthy group, and LVEF value, Hemoglobin, HDL-C was lower than that in healthy group (P < 0.05). There was no significant difference in UN, age and sex (P > 0.05), as shown in Table 1.
LN gradually increased with the progress of HF stages (P < 0.01, Figure 3), age, ALT, AST, Tb, Scr, UN, TnI, NT-proBNP, HA also gradually increased with the increase of clinical stages, LVEF value tended to decrease with the progress of heart failure stages (P < 0.01), and there was no statistical difference in the history of type 2 diabetes, hypertension and gender (P > 0.01), as shown in Table 2.
The correlation analysis between LN and NT-proBNP and LVEF showed that LN was positively correlated with NT-proBNP (r = 0.744, P = 0.000, Figure 4) while negatively correlated with LVEF (r = −0.568, P = 0.000, Figure 5).
A total of 168 patients were enrolled in C-D stage of HF. The area of HF during C-D stage under ROC curve was predicted according to LN, which was 0.913, 95% confidence interval was 0.882–0.945, P = 0.000, cut-off value was 33.72 ng/ml, specificity 94.97% and sensitivity 77.38%. as shown in Figure 6 and Table 4
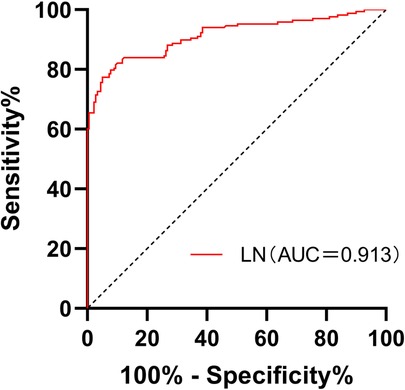
Figure 6. Receiver operating characteristic curves for laminin in the prediction of stages of C and D in CHF patients.
Multivariate ordered Logistic analysis to different clinical stages of heart failure showed that LN (OR: 1.021, 95% CI: 1.011–1.030, P = 0.000), HA (OR: 1.011, 95% CI: 1.003–1.019, P = 0.005), NT-proBNP (OR: 1.000, 95% CI: 1.000–1.000, P = 0.017), LVEF (OR: 0.893, 95% CI: 0.865–0.923, P = 0.000), age (OR: 1.033, 95% CI: 1.006–1.061, P = 0.016), AST (OR: 1.006, 95% CI: 1.000–1.012, P = 0.048), and Tb (OR: 1.061, 95% CI: 1.027–1.097, P = 0.000) are all independent risk factors of severity of heart failure (P < 0.05), as shown in Table 3.
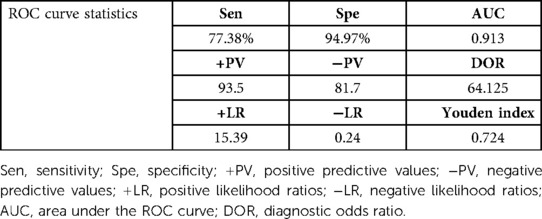
Table 4. Receiver operating characteristic curves for laminin in the prediction of stages of C and D in CHF patients.
HF (9) is a clinical syndrome with symptoms and signs caused by heart structure and/or function anomaly. It will ultimately lead to all chronic cardiovascular diseases and is one of the most harmful diseases to human health. During CHF progression, the whole myocardium undergoes extensive irreversible remodeling, and ventricular remodeling mainly includes myocardial cell injury and myocardial fibrosis (10, 11). In the past, researchers mainly focused on the study in the field of myocardial cell injury. At present, endomyocardial biopsy is used to diagnose myocardial fibrosis clinically, but it is difficult to carry out routinely due to the difficulties in obtaining materials and patients' acceptance (12). Therefore, biomarkers of myocardial fibrosis are widely accepted as alternative reference indicators clinically.
Myocardial fibrosis features by changes in ECM. Previous studies on ECM and myocardial fibrosis mainly focused on collagen types I and collagen type III, which were used as non-invasive monitoring methods for tissue and organ fibrosis (6, 13). Serum LN is an important part of ECM, which was first discovered in mouse tumor basement membrane by Timple and other researchers in 1979 (14), serum LN is one of the largest non-collagenous glycoproteins in the basement membrane and plays a key role in fibrosis formation. Studies have shown that various defective regenerative processes/unresolved inflammation and/or chronic injury endpoints include varying degrees of fibrosis (15). serum LN has been used to evaluate liver and pulmonary fibrosis in the past (16, 17).
Serum LN chain was confirmed to exist in the basement membrane area of the human embryo and fetal heart system by Roediger et al. (18) in 2010, and was proven to play a role in the development of the human circulatory system. Samura et al. found that serum LN is the most likely expressed cardiac LN, which can promote the differentiation of pluripotent human embryonic stem cells into myocardial cell lineage and promote cardiovascular progenitor cells to generate infarct human cardiac muscle in vivo (19). In 2016, Evelyn et al. (20) also discovered that serum LN participated in the periodic remodeling of cardiac basement membrane in rats with ischemic HF. HF could elevate the pressure in the central and peripheral veins, leading to congestion of passive organs in the abdominal cavity (21). The increase in sympathetic nerve activity further promoted the fibrosis process of the heart, kidney, and liver (22–24). Throughout these studies, it is evident that LN plays an important role in the fibrotic process in several organs, but no clinical studies have yet addressed the relevance of LN to the clinical staging of patients with CHF.
In recent decades, the prognosis of patients with HF has been greatly improved after standardized treatment. However, owing to the irreversibility of ventricular remodeling, the overall life quality still decreased significantly (25). The prognosis of patients with stages A-B of HF can be greatly improved with early intervention. Thus, this study aimed to explore the clinical significance of the changes in serum LN concentration in the during the development of different stages of CHF.
This study found that the serum LN level in patients with CHF was significantly higher than that in healthy people, and with the progress of clinical stages of HF, the serum LN level showed an increasing trend (P ≤ 0.001). LN was positively correlated with NT-proBNP (r = 0.744, P = 0.000) and negatively correlated with LVEF (r = −0.568, P = 0.000). The area under the ROC curve of LN for predicting C and D stages of HF was 0.913, 95% confidence interval was 0.882–0.945, P = 0.000, cut-off value was 33.72 ng/ml, specificity was 94.97%, and sensitivity was 77.38%. Multivariate logistic analysis proved that LN was an independent related factor in the stage of HF. Therefore, it is reasonable to speculate that serum LN is closely related to the process of myocardial fibrosis in CHF, and the concentration of serum LN could gradually increase with HF progression and continuous ventricular remodeling. Hawkes et al. (26) discovered that LN is abundant in the healthy heart, but fibronectin is increasingly expressed in the fibrotic heart. The traditional NT-proBNP is the most widely applied biomarker in the diagnosis and treatment of HF, which is of great significance in the process of diagnosis, treatment and prognosis evaluation of HF (8). However, NT-proBNP has been found to be higher or lower than the expected value of the disease state in clinics in recent years. It is susceptible to renal insufficiency, age, sex, atrial fibrillation and other factors, and could even show an abnormal “normalization” trend at the end stage of HF (27). Thus, the combination with LN, a fibrosis index, could more accurately evaluate the severity and clinical stage of HF when NT-proBNP is defective.
This study has some limitations. This study lacks data on the severity of fibrosis, and as a retrospective study, it lacks dynamic observation of LN levels at multiple time points, which is worthy of further large-scale clinical and basic research to verify its conclusions.
In summary, the serum LN level in patients with CHF is obviously increased, which is independently related to the clinical stage of HF, and could potentially become an early warning index of the severity of the HF process.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by The Ethics Committee of the Second Affiliated Hospital of Nantong University (IRB: No.2020KN094). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LX: writing manuscript. ZZ: processing data. H-XC and X-YY: critical review manuscript. J-QY and YJ: collecting data. LZ and QZ: writing and critical review manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Scientific Research Project of Nantong Municipal Health Commission (MB2021010), Project of Nantong Science and Technology Bureau (JCZ2022007), and Kangda College of Nanjing Medical University (KD2022KYCXTD007).
The authors acknowledge the support of the Cardiology and Department of General Medicine of the Second Affiliated Hospital of Nantong University. We would also like to thank all patients and volunteers who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1089304/full#supplementary-material.
1. Ewen S, Linicus Y, Böhm M. Update on therapy of chronic heart failure. Innovations and studies from last year. Herz. (2015) 40(8):1084–9. (Update zur Therapie der chronischen Herzinsuffizienz. Neue Erkenntnisse und Studien des letzten Jahres. Ger) . doi: 10.1007/s00059-015-4334-z
2. Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. (2015) 6(1):187–214. doi: 10.1002/cphy.c140055
3. Zannad F, Rossignol P, Iraqi W. Extracellular matrix fibrotic markers in heart failure. Heart Fail Rev. (2010) 15(4):319–29. doi: 10.1007/s10741-009-9143-0
4. Yao Y. Laminin: loss-of-function studies. Cell Mol Life Sci. (2017) 74(6):1095–115. doi: 10.1007/s00018-016-2381-0
5. Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. (2019) 73(7):795–806. doi: 10.1016/j.jacc.2018.11.042
6. Nikolov A, Popovski N. Extracellular matrix in heart disease: focus on circulating collagen type I and III derived peptides as biomarkers of myocardial fibrosis and their potential in the prognosis of heart failure: a concise review. Metabolites. (2022) 12(4):297. doi: 10.3390/metabo12040297
7. Xu LY, Xie L, Wang J, Chen HX, Cai HL, Tian LJ, et al. Correlation between serum laminin levels and prognosis of acute myocardial infarction. Front Cardiovasc Med. (2022) 9:936983. doi: 10.3389/fcvm.2022.936983
8. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(18):e895–1032. doi: 10.1161/cir.0000000000001063
9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol Pol. (2016) 74(10):1037–147. (Wytyczne ESC dotyczące diagnostyki i leczenia ostrej i przewlekłej niewydolności serca w 2016 roku. Pol). doi: 10.5603/KP.2016.0141
10. Schaper J, Kostin S, Hein S, Elsässer A, Arnon E, Zimmermann R. Structural remodelling in heart failure. Exp Clin Cardiol. (2002) 7(2–3):64–8.19649225
11. Liu T, Song D, Dong J, Zhu P, Liu J, Liu W, et al. Current understanding of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front Physiol. (2017) 8:238. doi: 10.3389/fphys.2017.00238
12. Ding Y, Wang Y, Zhang W, Jia Q, Wang X, Li Y, et al. Roles of biomarkers in myocardial fibrosis. Aging Dis. (2020) 11(5):1157–74. doi: 10.14336/AD.2020.0604
13. de Jong S, van Veen TA, de Bakker JM, Vos MA, van Rijen HV. Biomarkers of myocardial fibrosis. J Cardiovasc Pharmacol. (2011) 57(5):522–35. doi: 10.1097/FJC.0b013e31821823d9
14. Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin–a glycoprotein from basement membranes. J Biol Chem. (1979) 254(19):9933–7. doi: 10.1016/S0021-9258(19)83607-4
15. Muraine L, Bensalah M, Butler-Browne G, Bigot A, Trollet C, Mouly V, et al. Update on anti-fibrotic pharmacotherapies in skeletal muscle disease. Curr Opin Pharmacol. (2022) 68:102332. doi: 10.1016/j.coph.2022.102332
16. Parsian H, Rahimipour A, Nouri M, Somi MH, Qujeq D. Assessment of liver fibrosis development in chronic hepatitis B patients by serum hyaluronic acid and laminin levels. Acta Clin Croat. (2010) 49(3):257–65.21462814
17. Morales-Nebreda LI, Rogel MR, Eisenberg JL, Hamill KJ, Soberanes S, Nigdelioglu R, et al. Lung-specific loss of α3 laminin worsens bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. (2015) 52(4):503–12. doi: 10.1165/rcmb.2014-0057OC
18. Roediger M, Miosge N, Gersdorff N. Tissue distribution of the laminin beta1 and beta2 chain during embryonic and fetal human development. J Mol Histol. (2010) 41(2–3):177–84. doi: 10.1007/s10735-010-9275-5
19. Yap L, Wang JW, Moreno-Moral A, Chong LY, Sun Y, Harmston N, et al. In vivo generation of post-infarct human cardiac muscle by laminin-promoted cardiovascular progenitors. Cell Rep. (2020) 31(8):107714. doi: 10.1016/j.celrep.2020.107714
20. Kim EH, Galchev VI, Kim JY, Misek SA, Stevenson TK, Campbell MD, et al. Differential protein expression and basal lamina remodeling in human heart failure. Proteomics Clinical Applications. (2016) 10(5):585–96. doi: 10.1002/prca.201500099
21. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the acute heart failure committee of the heart failure association (HFA) of the European society of cardiology (ESC). Eur J Heart Fail. (2017) 19(7):821–36. doi: 10.1002/ejhf.872
22. Palazzuoli A, Evangelista I, Nuti R. Congestion occurrence and evaluation in acute heart failure scenario: time to reconsider different pathways of volume overload. Heart Fail Rev. (2020) 25(1):119–31. doi: 10.1007/s10741-019-09868-0
23. Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. (2018) 138(9):929–44. doi: 10.1161/CIRCULATIONAHA.117.028814
24. Cops J, Mullens W, Verbrugge FH, Swennen Q, De Moor B, Reynders C, et al. Selective abdominal venous congestion induces adverse renal and hepatic morphological and functional alterations despite a preserved cardiac function. Sci Rep. (2018) 8(1):17757. doi: 10.1038/s41598-018-36189-3
25. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
26. Hawkes W, Marhuenda E, Reynolds P, O'Neill C, Pandey P, Samuel Wilson DG, et al. Regulation of cardiomyocyte adhesion and mechanosignalling through distinct nanoscale behaviour of integrin ligands mimicking healthy or fibrotic extracellular matrix. Philos Trans R Soc B Biol Sci. (2022) 377(1864):20220021. doi: 10.1098/rstb.2022.0021
Keywords: serum laminin, chronic heart failure, degree of severity, stages of heart failure, correlations
Citation: Xie L, Zhou Z, Chen H-X, Yan X-Y, Ye J-Q, Jiang Y, Zhou L and Zhang Q (2023) Correlations between serum laminin level and severity of heart failure in patients with chronic heart failure. Front. Cardiovasc. Med. 10:1089304. doi: 10.3389/fcvm.2023.1089304
Received: 4 November 2022; Accepted: 24 February 2023;
Published: 16 March 2023.
Edited by:
Leonardo Roever, Federal University of Uberlandia, BrazilReviewed by:
Hui Hui Zhao, Beijing University of Chinese Medicine, China© 2023 Xie, Zhou, Chen, Yan, Ye, Jiang, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Zhang enpoYW5ncWluZzMyQHNpbmEuY24= Lei Zhou emhvdWxlaTcwMDVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to General Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.