
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 30 January 2023
Sec. Cardiovascular Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1086978
This article is part of the Research Topic Diabetes Augmentation on Vascular Disease, Volume II View all 10 articles
Objectives: The triglyceride-glucose (TyG) index has been identified as a reliable and simple surrogate of insulin resistance. In this study, we sought to determine the association between TyG index and cardiac function among asymptomatic individuals with type 2 diabetes (T2DM) without history of any cardiovascular disease.
Materials and methods: The cross-sectional study enrolled 180 T2DM patients without cardiac symptoms. Heart failure with preserved ejection fraction (HFpEF) was defined as Heart Failure Association (HFA)-PEFF score ≥ 5 points.
Results: A total of 38 (21.1%) diabetic patients were identified with HFpEF. Compared with the low-TyG group (TyG index <9.47), patients in high-TyG group (TyG index ≥9.47) showed increased risk of metabolic syndrome and diastolic dysfunction (p < 0.05 for each). Furthermore, after adjustment of confounding variables, the TyG index showed positive correlation with risk factors of metabolic syndrome (including BMI, waist circumference, blood pressure, HbA1c, TG, TC, non-HDL-C, and fasting blood glucose, p < 0.05 for each) and parameters of diastolic dysfunction (E/e’ ratio, p < 0.0001) in patients with T2DM. Moreover, Receiver Operating Characteristic curve analysis showed that the TyG index could be better to predict the risk of suspected HFpEF than other indicators (AUC: 0.706, 95% CI: 0.612–0.801). According, on multiple regression analysis, TyG index was independently correlated with the incidence of HFpEF (odds ratio: 0.786, p = 0.0019), indicating that TyG index could be a reliable biomarker to predict the risk of HFpEF.
Conclusion: The TyG index showed a positive correlation with the risk of subclinical HFpEF in patients with T2DM, providing a new marker to predict and treat HFpEF in diabetes.
Diabetes mellitus (DM) can contribute to cardiac abnormalities both structurally and functionally, predisposing individuals to a heightened risk of cardiovascular disease (CVD) (1, 2). Diabetic cardiomyopathy (DCM) was initially described as a human pathological condition in which heart failure occurred independent of coronary artery disease (CAD), hypertension, and valvular heart disease (3, 4). DCM in early stage is characterized by asymptomatic cardiac dysfunction or described as heart failure (HF) at stage B (subclinical HF). Cardiac disorders include left atrial (LA) dilatation, concentric left ventricular (LV) remodeling, LV diastolic dysfunction, and reduced global longitudinal strain (5). Regardless of concomitant LV systolic dysfunction, mounting evidence from epidemiological studies imply that comparing to healthy individuals, patients with diastolic dysfunction impart poor prognostic implications, with an increased 3-fold risk of death in diabetic patients (6). Thus, in view of the large number of diabetic patients and related cardiac complications, it will be of great importance to identify high risk individuals prone to cardiac dysfunction through effective and simple diagnostic strategy at early stage.
Clinical evidence establishes that glycemic control correlates with elevated risk of DCM (7, 8). Moreover, higher glycosylated hemoglobin, type A1c (HbA1c) variability is identified to be correlated with heightened risk of all-cause and cardiovascular mortality in diabetic patients (9). However, the severity and progression of HF vary in diabetic patients with poor glycemic control, and HF may also occur in patients with well-controlled blood glucose levels. Therefore, in addition to hyperglycemia, other risk factors may also participate in the development of clinical manifestation of DCM.
Chronic hyperglycemia and insulin resistance (IR) are the major mechanisms involved in the pathology of diabetic complications (10). During diabetic and IR states, metabolic, structural, and functional alterations in the myocardium and vascular beds or vascular tissues lead to coronary artery disease (CAD), myocardial ischemia, and HF (5). Previous clinical studies demonstrated that homeostasis model of IR (HOMA-IR) was independently correlated with LV diastolic dysfunction (11). However, it is not clear whether IR predicts subclinical cardiac diastolic dysfunction in patients with diabetes.
At present, no specific methods are available for the accurate detection of IR. HOMA-IR is a validated and widely used surrogate by incorporating insulin concentrations and serum glucose level, but the clinical practice is limited due to atypical assessment of serum insulin levels (12). The triglyceride glucose (TyG) index, a product derived from fasting triglycerides (TG) and fasting blood pressure (FBG), has been proven to be superior to HOMA-IR in evaluating IR in individuals with or without diabetes (13). Therefore, the aim of this study was to investigate the association between TyG index and the risk of cardiac diastolic dysfunction in patients with type 2 diabetes (T2DM) and the predictive value of TyG index to provide novel clues for the early recognition and prevention of HF in diabetic patients.
A retrospective consecutive case series of T2DM patients hospitalized in the Department of Endocrinology at the Changzhou First People’s Hospital (Changzhou, Jiangsu, China) were recruited from April 2018 to May 2022. The Inclusion criteria were: (1) diagnosed T2DM according to the criteria of World Health Organization (14) and Chinese Diabetes Society (15) without cardiac symptoms; (2) aged from 18 to 70 years old independent of T2DM duration. The exclusion criteria were: (1) subjects with hypertension (Hypertension was diagnosed according to the following Chinese hypertension guidelines: a mean systolic blood pressure ≥ 140 mmHg and/or a mean diastolic blood pressure ≥ 90 mmHg and/or self-reported use of antihypertensive medication in the past 2 weeks) (16, 17), CAD, atrial fibrillation, structural heart disease or history of any cardiovascular-related disease; (2) subjects with diabetic complications including macro and microvascular diseases such as neuropathy, retinopathy, kidney disease, stroke and peripheral vascular disease; (3) pregnancy; (4) other serious comorbidities, including thyroid disturbances, malignant tumors, liver and renal insufficiency, rheumatic diseases or major mental illness. All the subjects signed written informed consent forms before the start of this study. The study was approved by the Institutional Review Committee and the Ethics Committee of the Third Affiliated Hospital of Soochow University.
Baseline characteristics including age, sex, weight, height, body mass index (BMI), waist circumference, diabetic duration and other complete medical history were recorded in detail on the day of admission. After fasting for at least 8 h, peripheral venous blood was collected before administration of hypoglycemic drugs on the morning after admission. Briefly, the concentration of HbA1c was evaluated through high performance liquid chromatography. Glutamic oxaloacetic transaminase (AST), alanine aminotransferase (ALT), creatinine (Cr), blood urea nitrogen (BUN), homocysteine, total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), FBG, and C peptide (0 min, 30 min, 60 min, 120 min, and 180 min) were analyzed by an automatic analyzer. Blood pressure including systolic (SBP) and diastolic blood pressure (DBP) were measured three times at 2-min intervals following at least 5 min of rest on the morning after admission.
The following parameters were measured and analyzed by echocardiography: the left atrial diameter (LAD), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), interventricular septal diameter (IVSD), left ventricular posterior wall thickness (LVPWT), left ventricular ejection fraction% (LVEF%), peak late diastolic trans-mitral flow velocity (MFV A), peak early diastolic trans-mitral flow velocity (MFV E), mitral valve septal velocity e, mitral valve lateral velocity e, and LA volume. e’ was defined as: (ventricular septal velocity e + mitral valve velocity e)/2. Left atrial volume index (LAVI) was defined as: LA volume/body surface area, where the body surface area was equal to 0.0128*weight (kg) + 0.006*height (cm) - 0.1529. Left ventricular mass index (LVMI) was defined as: 0.8*10.4 (IVSD+LVPWT+LVEDD). Relative ventricular wall thickness (RWT) was defined as: (LVPWT/LVEDD) *2.
The TyG index was calculated as: In [fasting TG (mg/dl) x fasting glucose (mg/dl)/2]. The Heart Failure Association (HFA)-PEFF score was originated from HFA-PEFF diagnostic algorithm, including functional, morphological, and biomarker domains (18). Data of the peak tricuspid velocity and global longitudinal strain were not available in this study. In the HFA-PEFF diagnostic algorithm, a total score ≥ 5 points was identified to be diagnostic of heart failure with preserved ejection fraction (HFpEF), while a score ≤ 1 was considered to be very unlikely of HFpEF. Patients with an intermediate PEFF score (2–4 points) required further functional and etiology assessment (18).
All data in this study were analyzed using SPSS statistical software 26.0. p value <0.05 was defined to be of statistical significance. The specific statistical analysis in this study were outlined as follows.
The baseline and echocardiographic data of diabetic patients were stratified based on binary TyG index. The differences between two groups were evaluated, continuous normal distribution variables were expressed as mean ± standard deviation (SD) by independent sample t-test, nonnormal distribution variables were expressed as median P50 (P25, P75) by Mann–Whitney U test, and the categorical variables were presented as number (percentage) and analyzed by χ2 test.
Pearson correlation analysis was used to analysis independent variables with the TyG index. Partial correlation analysis was used to correct suspicious confounding factors (make it/them a constant).
A logistic multivariable regression analysis with cardiac diastolic dysfunction categorized as HFA-PEFF score (≤1, 2–4, and ≥ 5 points) was used to determine the associations between the TyG index and HFpEF. The goodness of fit of the regression model was assessed by Hosmer-Lemeshow test (p > 0.05). In the logistic regression analysis, three models were set up, Model 1: adjusted by age and sex; Model 2: adjusted by BMI, waist circumference, and diabetic duration based on model 1; Model 3: adjusted by estimated glomerular filtration rate (eGFR), mean arterial pressure (MAP), TC and HbA1c based on model 2.
Receiver operating characteristic curve (ROC) analysis was constructed to evaluate the predictive value of TyG index, FBG, postprandial blood glucose (PBG), TG, TC, LDL-C, TG/HDL-C, and HbA1c for the subclinical HFpEF presence according to the value of the area under the ROC curve (AUC).
A stratified analysis was conducted based on sex, age, HbA1c and T2DM duration to eliminate the interference of confounding factors. Among them, means of age and HbA1c were defined as stratification criteria while the median of duration was used for the cut-off point since the latter does not conform to the normal distribution.
A total of 180 subjects with T2DM (102 men and 78 women), aged 53.82 ± 9.20 years old, with a median diabetic duration of 6 years (interquartile range 0.75–10 years) were included in this study. According to the mean value of TyG index, diabetic patients were separated into two groups as low-TyG group (TyG index <9.47, N = 88) and high-TyG group (TyG index ≥9.47, N = 92). Compared with the low-TyG group, patients in high-TyG group showed higher levels of metabolic syndrome-related risk factors, as indicated by elevated BMI, waist circumference, SBP, DBP, HbA1c, TG, TC, non-HDL-C, and FBG, and reduced HDL-C (p < 0.05 for each; Table 1). Accordingly, the TyG index was positively associated with these metabolic parameters (including BMI, waist circumference, MAP, SBP, DBP, TG, TC, non-HDL, LDL-C, and FBG; p < 0.05 for each) and negatively associated with HDL-C and eGFR (p < 0.05; Supplementary Table S1) after adjusting for age, sex, and duration of diabetes. In addition, Patients in high-TyG group were more likely to use biguanides, glucagon-like peptide 1 (GLP-1) receptor agonists, and statins (p < 0.05 for each; Table 1).
Compared with patients in low-TyG group, patients in high-TyG group showed cardiac diastolic disorder, as exhibited by elevated E/e’ ratio and LA volume (p < 0.05 for each; Table 2). Additionally, correlation analysis showed that the TyG index was positively associated with E/e’ ratio (r = 0.273, p = 0.0002) and negatively associated with septal e’ velocity (r = −0.245, p = 0.0010) and lateral e’ velocity (r = −0.339, p < 0.0001; Supplementary Table S2) after adjusting for age, sex, and duration of diabetes. However, no differences were detected in the systolic function and ventricular remodeling between two groups.
To confirm that TyG index is particularly well related to IR in patients with diabetes, we evaluated the association between other simultaneously measured IR or insulin sensitivity indices and TyG index. Previous studies have revealed that the lipid profile in T2DM patients with IR often manifested as a TG/HDL-C axis disorder, with elevations of TG and reductions of HDL-C. TG/HDL-C ratio was thus identified as one of the major risk factors for IR and CVD (19). Additionally, C-peptide is secreted from pancreatic β cells at an equimolar ratio to insulin, reflecting endogenous insulin secretion (20). In this study, patients with a higher TyG index had a higher TG/HDL-C ratio and C-peptide values at 0 min and 30 min (Table 3; p < 0.05 for each), suggesting that TyG index could be a reliable marker for IR in T2DM patients.
Next, we compared the significance of TyG index, TG/HDL-C ratio, FBG, and HbA1c to identify diabetic patients with subclinical cardiac dysfunction. First, previous studies have demonstrated that DM-related HF shifted from an asymptomatic stage to HFpEF, which was manifested by LV shrinkage but not LV dilatation, and finally developed to LV dilatation with reduced EF (HFrEF) (21). The HFA-PEFF score was a scoring system for suspected HFpEF assessing brain natriuretic peptide (BNP) and echocardiographic parameters (18). In this study, among 180 asymptomatic T2DM patients, 38 (21.1%) patients were identified with HFpEF as calculated by HFA-PEFF score ≥ 5 points, 33 (18,3%) patients were identified negative (HFA-PEFF score ≤ 1), and 109 (60.6%) patients were suspected to be positive (2 ≤ HFA-PEFF score ≤ 4). Compared to the negative group, the TyG index were higher in suspicious positive and positive HFpEF group (Figure 1). Furthermore, ROC analysis for detecting suspicious or positive HFpEF showed that AUC of TyG index was 0.706 (95% confidence interval (CI): 0.612–0.801), significantly higher than that of FBG, PBG, TG, TC, LDL-C, TG/HDL-C, and HbA1c (AUC < 0.5 not shown in the Figure 2). When the Youden Index reached the maximum, the optimal cut-off point of the TyG index was 9.0067. The corresponding sensitivity and specificity were 72.8 and 60.6%, respectively.
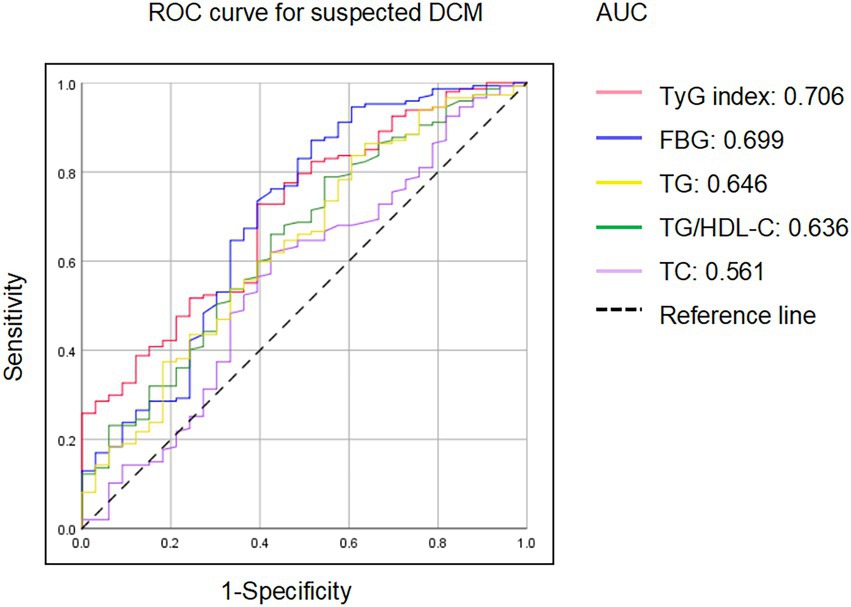
Figure 2. ROC analysis for the identification of diabetic patients with risk of HFpEF. TyG, triglyceride-glucose; FBG, fasting blood glucose; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol.
To further explore the relationship between TyG index and HFpEF in diabetic patients, multivariate logistic stepwise regression analysis was performed. Data showed that the TyG index was independently correlated with the risk of HFpEF (HFA-PEFF score ≥ 5) after adjusting for age, sex, BMI, waist circumference, MAP, diabetes duration, TC, eGFR, and HbA1c (Odds Ratio (OR): 0.786, 95% CI: 0.290–1.282, p = 0.0019; Table 4). Then we performed subgroup analyzes to evaluate the impact of other risk factors based on the following stratification variables: sex, age, HbA1c, and duration of diabetes (Table 5). An increased TyG index remained significantly correlated with the risk of HFpEF in the subgroups of age, sex, HbA1c, and duration of diabetes (p < 0.05 for each). Stronger correlations were found in the subgroups of male (OR: 0.877, p = 0.0174), age < 54 years (OR: 1.055, p = 0.0078), HbA1c ≥ 9.75% (OR: 1.084, p = 0.0032) and duration of diabetes after multivariable adjustment. However, no significant association was detected in female patients, patients aged ≥54, and patients with HbA1c < 9.75%. Clinical and metabolic characteristics stratified by HbA1c, and duration of DM were presented in Supplementary Tables S3 and S4, respectively.
This retrospective study demonstrated that among 180 asymptomatic patients with T2DM, 38 (21.1%) patients were identified with HFpEF as calculated by HFA-PEFF score ≥ 5 points. Elevated TyG index was positively corrected with metabolic syndrome-related risk factors (BMI, waist circumference, blood pressure, HbA1c, TG, TC, non-HDL-C, and FBG, p < 0.05 for each) and parameters of diastolic dysfunction (E/e’ ratio, p < 0.0001) after adjustment of confounding factors. Importantly, TyG index was independently correlated with greater risk of developing HFpEF as evaluated by HFA-PEFF. Our results suggested that in diabetic patients, TyG index might be considered as a reliable biomarker to identify asymptomatic patients with high HFpEF risk.
The TyG index, derived from FBG and TG, was proven to be a reliable and simple surrogate for metabolic syndrome and IR (22). Mounting evidence has proved the crucial value of TyG index in predicting diabetic complications in patients with T2DM (23–25). Study by Liu et al. showed a significant association between TyG index and the risk of diabetic nephropathy in 682 adult patients with T2DM (26). Pan et al. confirmed the predictive value of TyG index in distinguishing diabetic patients at an increased risk of lower limb vascular stenosis and nephric microvascular disorder (18). Furthermore, recent studies suggested that TyG index could be recognized as a risk factor for CVD even in asymptomatic patients. Lee et al. showed that higher level of TyG index was correlated with increased risk of coronary artery stenoses (CAS) in asymptomatic diabetic patients (27). Thai et al. confirmed this hypothesis and proposed that TyG index was positively associated with the number and severity of artery stenoses (28). However, the predictive value of TyG index in subclinical HF in diabetic patients has not been well evaluated. In accordance with prior studies, our findings showed that the TyG index had a strong association with metabolic syndrome and HFpEF in subjects with T2DM, including BMI, waist circumference, blood pressure, HbA1c, TG, TC, HDL-C, non-HDL-C, LDL-C and FBG.
As an indicator of IR, the relationship between TyG index and the occurrence of CVD in different groups, including non-diabetic and diabetic individuals, has been widely explored (22). However, few studies have investigated the association between TyG index and cardiac structure and hemodynamics evaluated by echocardiography, which may predict the risk of CVD. An observational study enrolled 823 general subjects found that high TyG index was correlated with elevated LA diameter and decreased LVEF (%) and ankle-branchial index (ABI) (29). These results were partly consistent with the data by Wang et al., in which TyG index was positively associated with cardiac hemodynamics such as LVESD, LVEDV, LVPW, IVS, and LV mass and negatively associated with LVEF. The latter study was conducted in 201 healthy controls and 446 asymptomatic patients with T2DM (30). Nevertheless, our results demonstrated that TyG index was positively correlated with E/e’ ratio and negatively correlated with septal e’ and lateral e’, but not correlated with parameters of cardiac systolic function. Of note, high TyG index was significantly positively correlated with increased risk of HFA-PEFF score ≥ 5 points, indicating a strong association between the TyG index and cardiac diastolic function. This inconsistence may be attributed to different recruited subjects, diverse diseases of enrolled population, and the potential impacts of drugs. Our study focused on exploring the diagnostic value of the TyG index to early detection of cardiac structural changes in diabetic patients, which may be of special significance for clinical cardiovascular risk assessment and secondary prevention.
Moreover, previous studies showed that myocardial dilatation defects are reported to be abnormal in patients with hyperglycemia and IR. Thus, the factors that determine TyG levels (high TG and high glucose at the baseline condition) related to the following conditions (1) hypo-insulinemia with hyperglycemia and (2) hyperinsulinemia and hyperglycemia with insulin resistance. To clarify the relationship between these conditions, we evaluated the association between TyG index and IR, or insulin deficiency. Our results showed that TyG index was positively related to HG/HDL-C value (IR biomarker) and C-peptide at 0 min and 30 min (insulin secretion marker). Additionally, subgroup analysis demonstrated that there was stronger association between TyG index and increased risk of HFpEF in patients with insufficient glycemic control (HbA1c ≥ 9.75%), suggesting that TyG index was mainly dependent on the second condition. Importantly, ROC analysis revealed that compared to sustained hyperglycemia status, TyG index preserved a higher predictive value for HFpEF in patients with T2DM, confirming the crucial role of IR in diabetic related cardiac dysfunction.
There are some limitations need to be emphasized in this study. Firstly, parameters evaluating cardiac diastolic function and HFA-PEFF score were incomplete, including tricuspid valve velocity and global longitudinal strain data. These missing data in the HFA-PEFF score may reduce statistical power and cause selection bias. More complete echocardiographic data in the future may improve the reliability and stability of TyG index in predicting diabetic patients with high HF risk. Secondly, HF is a series of dynamic and progressive disorders, the calculation of the baseline TyG index alone does not represent the longitudinal correlation between the TyG index and risk of diabetes-induced HF over time. Cumulative TyG index (the summation of average TyG index for each pair of consecutive assessments multiplied by the time between these two-consecutive inclusion in years) may be better than single TyG index at baseline in predicting HF or even other CVDs (31). Finally, the number of eligible patients was relatively limited, which may be due to the very specific population in this study. DM is often accompanied by different subtypes of CVDs involving multiple risk factors. Nevertheless, clinical studies of diabetic status itself (hyperglycemia with or without IR) on CVDs, especially on subclinical CVDs are limited. Thus, to simply the impact of diabetes on cardiac structure and function, we screened diabetic patients without any other risk factors to confirm the predictive value of TyG index in subclinical HF. Therefore, more sample size and multi-center studies are warranted to explore the crucial role of hyperglycemia and IR in diabetic complications. The diagnostic criteria for subclinical diabetic cardiac dysfunction also needs to be further refined.
In conclusion, we explored a significant correlation between TyG index and an increased risk of HFpEF in asymptomatic patients with T2DM. IR plays a crucial role in the pathophysiology of HFpEF and may be identified as a novel target for its prevention and treatment. Further studies are warranted to explore the correlation between the IR parameters, especially TyG index, and the risk of HF in patients with T2DM at different stages.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Third Affiliated Hospital of Soochow University. The patients/participants provided their written informed consent to participate in this study.
LT and XH designed the work. TW wrote the original version of this manuscript and analyzed the clinical data. LT performed the manuscript reviewing and editing. JX and HZ collected the clinical data. All authors read and approved the final manuscript.
This research was funded by the National Natural Science Foundation of China (NSFC) grants 82170356 and Changzhou Sci&Tech Program grant CJ20210091.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1086978/full#supplementary-material
TyG, triglyceride-glucose; T2DM, type 2 diabetes; HFpEF, Heart failure with preserved ejection fraction; HFA, Heart Failure Association; ROC, receive operating characteristic; OR, odds ratio; CI, confidence interval; DM, Diabetes mellitus; CVD, cardiovascular disease; HF, heart failure; LA, left atrial; LV, left ventricular; HbA1c, Hemoglobin type A1c.; IR, insulin resistance; CAD, coronary artery disease; HOMA-IR, homeostasis model of insulin resistance; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; ALT, glutamic oxaloacetic transaminase; AST, alanine aminotransferase; Cr, creatinine; BUN, blood urea nitrogen; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FBG, fasting blood glucose; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; IVSD, interventricular septal diameter; LVPWT, left ventricular posterior wall thickness; LVEF, left ventricular ejection fraction; MFV A, peak late diastolic trans-mitral flow velocity; MFV E, peak early diastolic trans-mitral flow velocity; LVMI, Left ventricular mass index; RWT, Relative ventricular wall thickness; SD, standard deviation; AUC, area under curve; CAS, coronary artery stenoses; ABI, ankle-branchial index.
1.Wang, Y, Yang, H, Huynh, Q, Nolan, M, Negishi, K, and Marwick, TH. Diagnosis of nonischemic stage B heart failure in type 2 diabetes mellitus: optimal parameters for prediction of heart failure. J Am Coll Cardiol Img. (2018) 11:1390–400. doi: 10.1016/j.jcmg.2018.03.015
2.Yancy, CW, Jessup, M, Bozkurt, B, Butler, J, Casey, DE Jr, Colvin, MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1016/j.jacc.2017.04.025
3.Dillmann, WH. Diabetic cardiomyopathy. Circ Res. (2019) 124:1160–2. doi: 10.1161/CIRCRESAHA.118.314665
4.Ritchie, RH, and Abel, ED. Basic mechanisms of diabetic heart disease. Circ Res. (2020) 126:1501–25. doi: 10.1161/CIRCRESAHA.120.315913
5.Jia, G, Whaley-Connell, A, and Sowers, JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. (2018) 61:21–8. doi: 10.1007/s00125-017-4390-4
6.Galderisi, M. Diastolic dysfunction and diastolic heart failure: diagnostic, prognostic and therapeutic aspects. Cardiovasc Ultrasound. (2005) 3:9. doi: 10.1186/1476-7120-3-9
7.Mozaffarian, D, Benjamin, EJ, Go, AS, Arnett, DK, Blaha, MJ, Cushman, M, et al. Heart disease and stroke Statistics-2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38–e360. doi: 10.1161/CIR.0000000000000350
8.Iribarren, C, Karter, AJ, Go, AS, Ferrara, A, Liu, JY, Sidney, S, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. (2001) 103:2668–73. doi: 10.1161/01.cir.103.22.2668
9.Lee, S, Liu, T, Zhou, J, Zhang, Q, Wong, WT, and Tse, G. Predictions of diabetes complications and mortality using hba 1c variability: a 10-year observational cohort study. Acta Diabetol. (2021) 58:171–80. doi: 10.1007/s00592-020-01605-6
10.Paolillo, S, Marsico, F, Prastaro, M, Renga, F, Esposito, L, De Martino, F, et al. Diabetic cardiomyopathy: definition, diagnosis, and therapeutic implications. Heart Fail Clin. (2019) 15:341–7. doi: 10.1016/j.hfc.2019.02.003
11.Peterson, V, Norton, GR, Raymond, A, Libhaber, CD, Millen, AM, Majane, OH, et al. Insulin resistance-associated decreases in left ventricular diastolic function are strongly modified by the extent of concentric remodeling in a community sample. Int J Cardiol. (2016) 220:349–55. doi: 10.1016/j.ijcard.2016.06.206
12.Minh, HV, Tien, HA, Sinh, CT, Thang, DC, Chen, CH, Tay, JC, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). (2021) 23:529–37. doi: 10.1111/jch.14155
13.Simental-Mendía, LE, Rodríguez-Morán, M, and Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304. doi: 10.1089/met.2008.0034
14.Alberti, KG, and Zimmet, PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
15.Chinese Diabetes Society; National Office for Primary Diabetes Care. National guidelines for the prevention and control of diabetes in primary care (2022). Zhonghua Nei Ke Za Zhi. (2022) 61:249–62. doi: 10.3760/cma.j.cn112138-20220120-000063
16.Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision Committee of Chinese Guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. (2019) 16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014
17.Liu, LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. (2011) 39:579–615. doi: 10.3760/cma.j.cn501113-20220824-00436
18.Pieske, B, Tschöpe, C, de Boer, RA, Fraser, AG, Anker, SD, Donal, E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40:3297–317. doi: 10.1093/eurheartj/ehz641
19.Szapary, PO, and Rader, DJ. The triglyceride-high-density lipoprotein axis: an important target of therapy? Am Heart J. (2004) 148:211–21. doi: 10.1016/j.ahj.2004.03.037
20.Saisho, Y. Postprandial C-peptide to glucose ratio as a marker of β cell function: implication for the Management of Type 2 diabetes. Int J Mol Sci. (2016) 17:744. doi: 10.3390/ijms17050744
21.Seferović, PM, and Paulus, WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. (2015) 36:1718–27. doi: 10.1093/eurheartj/ehv134
22.Tao, LC, Xu, JN, Wang, TT, Hua, F, and Li, JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. (2022) 21:68. doi: 10.1186/s12933-022-01511-x
23.Wang, L, Cong, HL, Zhang, JX, Hu, YC, Wei, A, Zhang, YY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. (2020) 19:80. doi: 10.1186/s12933-020-01054-z
24.Pan, Y, Zhong, S, Zhou, K, Tian, Z, Chen, F, Liu, Z, et al. Association between diabetes complications and the triglyceride-glucose index in hospitalized patients with type 2 diabetes. J Diabetes Res. (2021) 2021:8757996. doi: 10.1155/2021/8757996
25.Srinivasan, S, Singh, P, Kulothungan, V, Sharma, T, and Raman, R. Relationship between triglyceride glucose index, retinopathy and nephropathy in type 2 diabetes. Endocrinol Diabetes Metab. (2021) 4:e00151. doi: 10.1002/edm2.151
26.Liu, L, Xia, R, Song, X, Zhang, B, He, W, Zhou, X, et al. Association between the triglyceride-glucose index and diabetic nephropathy in patients with type 2 diabetes: a cross-sectional study. J Diabetes Investig. (2021) 12:557–65. doi: 10.1111/jdi.13371
27.Lee, EY, Yang, HK, Lee, J, Kang, B, Yang, Y, Lee, SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. (2016) 15:155. doi: 10.1186/s12944-016-0324-2
28.Thai, PV, Tien, HA, Van Minh, H, and Valensi, P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. (2020) 19:137. doi: 10.1186/s12933-020-01108-2
29.Chiu, TH, Tsai, HJ, Chiou, HC, Wu, PY, Huang, JC, and Chen, SC. A high triglyceride-glucose index is associated with left ventricular dysfunction and atherosclerosis. Int J Med Sci. (2021) 18:1051–7. doi: 10.7150/ijms.53920
30.Wang, C, Zhao, Z, Deng, X, Cai, Z, Gu, T, Li, L, et al. Association of triglyceride-glucose with cardiac hemodynamics in type 2 diabetes. Diab Vasc Dis Res. (2022) 19:14791641221083396. doi: 10.1177/14791641221083396
Keywords: triglyceride-glucose index, heart failure with preserved ejection fraction, type II diabetes, insulin resistance, HbA1c - hemoglobin A1c
Citation: Wang T, Xu J, Zhang H, Tao L and Huang X (2023) Triglyceride-glucose index for the detection of subclinical heart failure with preserved ejection fraction in patients with type 2 diabetes. Front. Cardiovasc. Med. 10:1086978. doi: 10.3389/fcvm.2023.1086978
Received: 01 November 2022; Accepted: 11 January 2023;
Published: 30 January 2023.
Edited by:
Catherine A. Reardon, The University of Chicago, United StatesReviewed by:
Alfredo Caturano, University of Campania Luigi Vanvitelli, ItalyCopyright © 2023 Wang, Xu, Zhang, Tao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lichan Tao, ✉ c2hlcnJ5MDAxOUAxMjYuY29t; Xiaolin Huang, ✉ aHhsNDAzOEBjemZwaC5jb20=
†These authors have contributed equally to this work
‡ORCID: Jiani Xu https://orcid.org/0000-0002-3558-4582
Hong Zhang https://orcid.org/0000-0002-7820-4201
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.