
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 05 May 2023
Sec. Structural Interventional Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1082663
This article is part of the Research Topic Imaging in Structural Heart Interventions View all 8 articles
Background: Transcatheter closure of atrial septal defect (ASD) and patent foramen ovale (PFO) is an established practice, and it requires monitoring and guidance. Both transoesophageal echocardiography (TEE) and intracardiac echocardiography (ICE) can be used as guidance tools. However, the use of ICE and TEE in structural heart disease is controversial and the advantages and disadvantages of both for ASD and PFO closure need to be investigated. We did a systematic review and meta-analysis to compare the efficacy and safety of TEE and ICE for guiding transcatheter closure of ASD and PFO.
Methods: A systematic search of Embase, PubMed, Cochrane library, Web of Science was conducted from inception to May 2022. The outcomes of this study included average time for both fluoroscopy and the procedure, complete closure, length of stay at hospital and adverse events. This study was performed using mean difference (MD), relative risk (RR) and 95% confidence interval (CI).
Results: The meta-analysis was conducted with a total of 11 studies, involving 4,748 patients were included in meta-analysis, including 2,386 patients in the ICE group and 2,362 patients in the TEE group. The results of the meta-analysis showed that compared with TEE, ICE was shorter in time both fluoroscopy [MD: −3.72 (95%CI: −4.09 to −3.34) minutes, P < 0.00001] and the procedure [MD: −6.43 (95%CI: −7.65 to −5.21) minutes, P < 0.00001], shorter length of stay at hospital [MD = −0.95 (95% CI = −1.21 to −0.69) days, P < 0.00001], lower incidence of adverse events (RR = 0.72, 95%CI: 0.62 to 0.84, P < 0.0001), and the arrhythmia (RR = 0.50, 95% CI = 0.27 to 0.94, P = 0.03) and vascular complications (RR = 0.52, 95%CI = 0.29 to 0.92, P = 0.02) in ICE group were lower than those in TEE group. No significant difference in complete closure was found between ICE and TEE (RR = 1.00, 95% CI = 0.98 to 1.03, P = 0.74).
Conclusion: Under the premise of ensuring successful rate of complete closure, ICE can shorten time between fluoroscopy and procedure and length of stay at hospital, and there was no increase in adverse events. However, more high-quality studies are needed to confirm the benefits of using ICE in ASD and PFO closure.
Atrial septal defect (ASD), one of the most common congenital heart diseases (CHDs) in adulthood, accounts for 25%–30% of newly diagnosed CHDs, which can cause systemic circulation blood to flow into the pulmonary circulation (1). However, the majority of ASD patients remain asymptomatic through infancy and early childhood. Even those with a large left-to-right shunt might not go undiagnosed until the onset of significant symptoms in adulthood (2). Patients with ASD are at risk of developing a range of complications as time goes on, such as arrhythmias, right heart failure, paradoxical embolism, occasionally pulmonary hypertension and even higher mortality rate compared to normal population (3, 4). The first catheter closure of ASD was reported in 1976 (5). Transcatheter intervention is the common treatment option when technically feasible. Like ASD, patent foramen ovale (PFO) is common cardiovascular disease. Although the relationship between PFO and cryptogenic stroke remains controversial, some studies have confirmed the efficacy and safety of PFO closure (6–9).
Both ASD and PFO closure are increasingly common interventional procedures, which are well-established option for both ASD and PFO, while a certain risk of severe complications remains (10). The choice of echocardiographic monitoring is crucial to avoid several complications of transcatheter closure. During the procedure, interatrial communications could be assessed by transesophageal echocardiography (TEE) with defect size and position, proximity to surrounding structures, rim morphology, and final device positioning (11). However, some preparations during TEE examination, such as fasting and drinking forbidden, may cause discomfort to the patient, and potential risks should be considered, including esophageal injury, general anesthesia (GA) and tracheal intubation, which may lead to death when complications occur. In addition, the imaging of TEE is suboptimal for the lower part of the atrial septum (12, 13). With the continuous updating and rapid development of interventional equipment, intracardiac echocardiography (ICE) has been used as guidance during transcatheter closure. The clinical application of ICE dates back to the 1960s (14). Compared with TEE, ICE does not need GA and does not cause esophageal injury, which can avoid the occurrence of possible complications (15, 16). ICE has been used to guide numerous invasive cardiac procedures and interventions, especially in ASD and PFO (17–20). However, some studies concluded that ICE was used to patients with complex lesions and should not be used routinely (20–22). Therefore, we performed a meta-analysis to evaluate if the use of ICE is superior to TEE during ASD and PFO closure.
A systematic search of Web of Science, Embase, PubMed and Cochrane library was conducted from inception to May 2022. Cohort studies on the comparison of ICE and TEE in transcatheter closure of ASD and PFO were collected. The search was conducted by combining subject words and free words, and adjusted according to the characteristics of each database. The articles' reference lists were retrieved to supplement the relevant information. No filters or language restrictions used. The search strategy included the main search terms “Heart Defects, Congenital”, “Atrial septal defect”, “Patent Foramen Ovale”, “intracardiac echocardiography”. Articles were subjected to review by two researchers indepen dently after the title and abstract screening. Disagreements were resolved through adjudication by a third researcher.
Studies which met the inclusion criteria were eligible for the meta-analysis: (1) Study type was cohort study; (2) Patients with a confirmed diagnosis of ASD or PFO were included; (3) Comparison of percutaneous transcatheter closure of ASD or PFO with different guidance tools; (4) One or more of the following outcome indicators were in the included article: average time for both fluoroscopy and the procedure, complete closure, length of stay at hospital and adverse events.
Exclusion criteria are as follows: (1) Duplicated articles, reviews, case reports, commentaries, editorials and conference abstract; (2) No data, incomplete or incorrect data; (3) Non- relevant outcomes.
We extracted the following data: (1) The first author, year of publication, country, type of study, participation, sample size, gender, age, assistance by fluoroscopy, follow-up, outcome assessment; (2) Intervention measures: the guidance tools of different groups; (3) Outcomes: average time for both fluoroscopy and the procedure, complete closure, length of stay at hospital and adverse events. Two researchers accessed the included studies and the quality of the articles, and another researcher would resolve by discussion to reach consensus if there were disagreement. We used Newcastle-Ottawa Quality Assessment Scale (NOS) to evaluate the risk of deviation of the included studies. NOS is composed of three aspects (selection of participants, comparability of research groups and results) with a maximum score of 9 points. NOS score of six or higher was high-quality studies.
Statistical analysis was performed using Review Manager software. Dichotomous data were present as relative risks (RR) with 95% confidence intervals (CI) and continuous data were expressed as mean difference (MD) with 95% CI. All effective outcomes were evaluated for heterogeneity by chi-square test and it was quantified by I-squares values. I-squared <50% indicates the absence of heterogeneity and a fixed-effects model was used. Otherwise, the random effect model was applied for meta-analysis. The results were analysed by funnel plot to assess publication bias. Sensitivity analyses were performed to find sources of heterogeneity by removing each study from include studies to access the robustness of the results. P-value < 0.05 was considered statistically significance.
Two individual researchers retrieved for a total of 1,239 studies from Embase, Cochrane library, PubMed and Web of Science through the search strategy. There were 805 studies left after removing duplicate literature and 122 studies left after reading abstract and title. According to inclusion and exclusion criteria, 11 studies were finally included for meta-analysis. The NOS score of the included studies is 5–8, and there are 8 high-quality studies. Figure 1 shows the flow chart of screening.
A total of 11 studies (23–33) including 4,748 patients were included for this meta-analysis. There were 2,386 patients in the ICE group and 2,362 patients in the TEE group (Table 1). All included studies counted their sample size, age, female ratio, country and duration of follow-up. At the same time, assistance by fluoroscopy of the included studies was recorded in detail. All contents were summarized in Table 1.
Six studies (23, 25–27, 29, 33) reported fluoroscopy time (FT) with a total of 623 patients and four studies (23, 25–27) reported procedure time (PT) with a total of 598 patients. Due to the high heterogeneity (FT: I2 = 70%, P = 0.0004), we conducted sensitivity analysis in order to explore the sources of heterogeneity. After we removed the Schimizu 2013 (29) study, heterogeneity across the studies was relatively low (FT: I2 = 0%, P = 0.76, PT: I2 = 37%, P = 0.14). Results indicated that times for both fluoroscopy [MD: −3.72 (95%CI: −4.09 to −3.34) minutes, P < 0.00001, Figure 2] and the procedure [MD: −6.43 (95%CI: −7.65 to −5.21) minutes, P < 0.00001, Figure 3] were shorter in ICE group than in TEE group. The subgroup analysis results of ASD patients were consistent with the overall results (FT (total): MD: −3.73 [95%CI: −4.26 to −3.20] minutes, P < 0.00001, FT(PFO&ASD): MD: −3.70 [95%CI: −4.27 to −3.12] minutes, P < 0.00001, FT(ASD): MD: −3.78 [95%CI: −5.24 to −2.31] minutes, P < 0.00001, Figure 2; PT (total): MD: −6.43 [95%CI: −8.16 to −4.71] minutes, P < 0.00001, PT(PFO&ASD): MD: −5.93 [95%CI: −7.83 to −4.02] minutes, P < 0.00001, PT(ASD): MD: −8.74 [95%CI: −12.81 to −4.67] minutes, P < 0.00001, Figure 3). In addition, we would like to conduct further subgroup analysis on patients with PFO, but the data were limited for subgroup analysis.
Four studies (25, 28–30) including 825 patients evaluated complete closure. No statistical heterogeneity was found among the studies (I2 = 0%, P = 0.63). There was no difference in complete closure between ICE group and TEE group in the pooled results (RR = 1.00, 95% CI = 0.98 to 1.03, P = 0.74). A subgroup analysis was performed and no significant difference was found between ICE group and TEE group in complete closure at discharge (RR = 1.01, 95% CI = 0.95 to 1.07; P = 0.74) and follow-up (RR = 1.00, 95% CI = 0.98 to 1.02; P = 0.99, Figure 4). All included patients were all ASD patients, and subgroup analysis by disease type was not available.
Two studies (31, 33) including 3,392 patients reported the length of stay at hospital, heterogeneity among the studies was relatively low (I2 = 39%, P = 0.20). Compared to TEE for ASD and PFO, ICE can reduce the length of stay at hospital [MD = −0.95 (95% CI = −1.21 to −0.69) days; P < 0.00001, Figure 5].
Eight studies (23, 24, 27–32) including 4,510 patients reported adverse events, we selected a fixed-effect model for meta-analysis because I-squared was less than 50% (I2 = 0%, P = 0.92). The adverse events of TEE were significantly higher than that in the ICE (RR = 0.72, 95%CI:0.62 to 0.84, P = 0.002, Figure 6). We further conducted subgroup analysis on adverse events and we found that the arrhythmia (RR = 0.50, 95%CI = 0.27 to 0.94, P = 0.03, Figure 6) and vascular complications (RR = 0.52, 95%CI = 0.29 to 0.92, P = 0.02, Figure 6) in ICE group were lower than those in TEE group.
We drew the funnel plots to detect the potential risk of publication bias, and the funnel plots showed that the scattered points distribution were symmetrical from the point of view of the geometry, indicating that the risk of bias was low for the studies included in our meta-analysis (Figure 7).
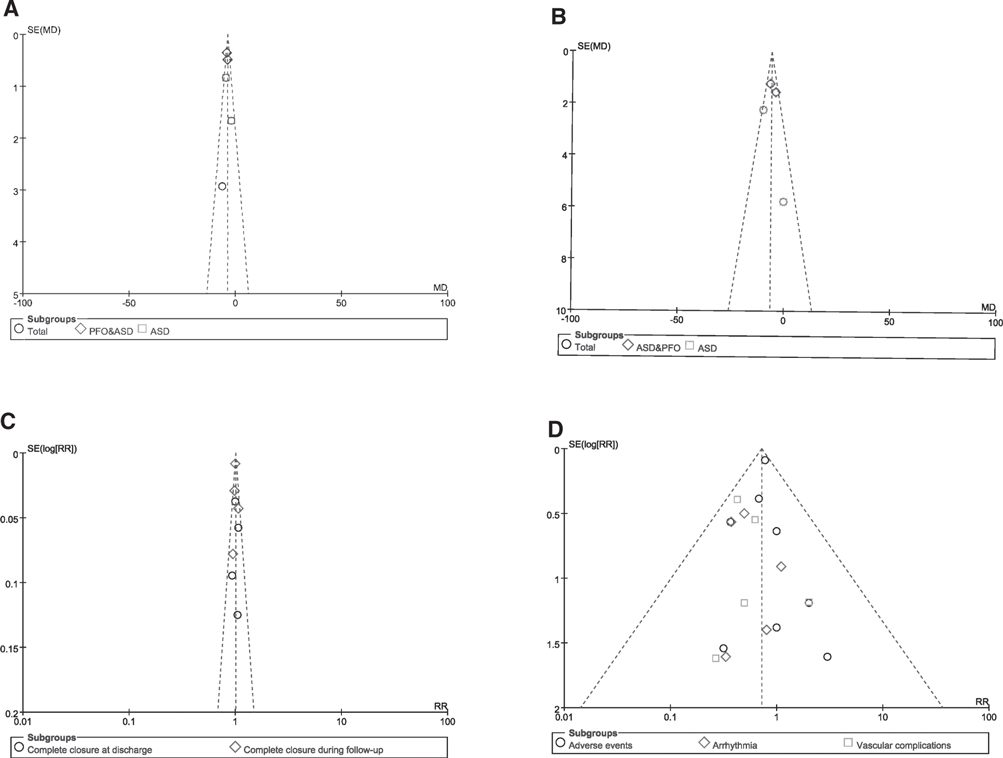
Figure 7. Funnel plot of publication bias. (A) Funnel plots for the meta-analysis of the FT; (B) Funnel plots for the meta-analysis of the PT; (C) Funnel plots for the meta-analysis of complete closure; (D) Funnel plots for the meta-analysis of adverse events.
We evaluated the efficacy and safety of ICE as a guidance tool for ASD and PFO closure. On this basis, we further clarified the impact of ICE guidance on FT, PT and the length of hospitalization. The findings of this article are as follows: ① Compared with TEE, the success rate of complete occlusion using ICE as guidance tool is similar to that of TEE. Further subgroup analysis showed that there was no difference between the two groups at discharge and follow-up; ② Transcatheter closure of ASD and PFO under ICE guidance can shorten FT, PT and the length of stay at hospital; ③ The use of ICE may decrease the incidence of adverse reactions compared with TEE.Although our subgroup analysis did not significantly increase the incidence of arrhythmia and vascular related adverse events, the use of a second catheter may lead to the increase of catheter-related complications.
With the use of safe and effective sealing devices and the advances in interventional tools, the non-surgical treatment of ASD and PFO has become possible. Percutaneous closure of interatrial communications has become the preferred treatment for most patients. Compared with surgery, interventional treatment has faster recovery and less trauma, but it is under the guidance of fluoroscopy during procedure. Radiation can cause plenty of inevitable side effects to physiological functions of patients and physicians, which results in damage to the hematopoietic function of bone marrow, interference with normal function of thyroid and potential risks of developing various cancers (12). Therefore, FT and PT should be as short as possible. It is presented in this study that FT and PT can be shortened under ICE guidance during transcatheter closure of ASD and PFO. The main reasons for the time reduction are as follows: GA not being used can significantly reduce PT. As for the FT, some studies point to higher image quality with ICE than TEE, fluoroscopy and transthoracic echocardiography for accurate evaluation of intracardiac structures, which might save time (27, 34).
Classically, TEE has been used in ASD and PFO closure with fluoroscopy for procedural guidance. However, TEE guidance requires the patient to be under GA with or without endotracheal intubation (12). Complications caused by GA are less, but it is more serious when it occurs. While ICE not only eliminates the need for GA, but also provides clearer and more accurate in assessing defects and the observation of adjacent structures (35). However, we did not find the advantage of using ICE guidance in the success rate of closure. Compared with TEE, some scholars are worried that the use of ICE will increase the hospitalization cost, but ICE guidance to ASD and PFO closure did not increase the global cost (29). We consider the following reasons: first, during GA, anesthesiologists are required to monitor the fluctuation of heart rate, blood pressure, oxygen saturation continuously, however, if ICE is used as guidance tool during operation, GA is not required and relevant physician, drugs and device support for GA is no longer needed; Second, ICE guidance does not require an additional echocardiographer and only cardiologist can perform the procedure (12, 36, 37); Finally, it is found in this study that the length of hospitalization in the ICE group is shortened. The cost of TEE can offset the additional cost of the ICE catheter. Previous studies showed that similar costs for hospital and physician charges using ICE or TEE (US dollars 34,861 ± 43,759 vs. US dollars 32,812 ± 2,656, P = 0.107) (38). Taking into account labour, additional individual requirements and anaesthetic costs, the total costs of ICE and TEE might be comparable (39). Although the relevant studies in Europe and the United States mentioned above have not found the cost increase caused by the use of ICE, reusability of the catheter is limited and health insurance agencies in many countries do not cover the costs of ICE catheters, which is a heavy burden for patients. In addition, ICE as a monitoring tool will increase the need for an additional venous sheath,and it can cause the danger of potentially provoking transient atrial arrhythmias (40, 41).This is not consistent with the results of this article.
The limitations of this study include: ① The relatively small sample size of included studies may affect the accuracy of the results; ② Different studies include different patients among the included articles. For example, some studies only include ASD patients, some studies only include PFO patients, while some studies include both ASD and PFO patients; ③ Different research institutes use different equipment. For example, some research centers, like Zhao 2015 (30), used Philips echocardiography equipment, while others use vivid I (32). The difference may lead to the possibility of bias.
In conclusion, our study suggests that ICE can be used safely and effectively in transcatheter closure of ASD and PFO, which provides shorter FT and PF, shorter hospital stay, and decrease in adverse events and no significant difference in complete closure. In the future, we still require more large-scale high-quality studies in order to further confirm the safety and efficacy of ICE for image guidance in ASD and PFO.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
QL and JZ contributed to the conception of the work; QL and FW conducted databases search; QL, SW and XY performed data extraction and quality assessment, QL and XY explained the data. QL drafted the manuscript and QL and JZ revised it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
This study was supported by Qingdao Key Health Discipline Development Fund; National Natural Science Foundation of China (81970282).
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fcvm.2023.1082663/full#supplementary-material.
1. Lindsey JB, Hillis LD. Clinical update: atrial septal defect in adults. Lancet. (2007) 369:1244–6. doi: 10.1016/S0140-6736(07)60576-5.17434386
2. Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet. (2014) 383:1921–32. doi: 10.1016/S0140-6736(13)62145-5.24725467
3. Nashat H, Montanaro C, Li W, Kempny A, Wort SJ, Dimopoulos K, et al. Atrial septal defects and pulmonary arterial hypertension. J Thorac Dis. (2018) 10:S2953–s2965. doi: 10.21037/jtd.2018.08.92.30305956
4. Murphy JG, Gersh BJ, McGoon MD, Mair DD, Porter CJ, Ilstrup DM, et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med. (1990) 323:1645–50. doi: 10.1056/NEJM199012133232401.2233961
5. King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. Jama. (1976) 235:2506–9. doi: 10.1001/jama.1976.03260490024013.946659
6. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, et al. Long-Term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. (2017) 377:1022–32. doi: 10.1056/NEJMoa1610057.28902590
7. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. (2017) 377:1033–42. doi: 10.1056/NEJMoa1707404.
8. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. Antiplatelets after stroke. N Engl J Med. (2017) 377:1011–21. doi: 10.1056/NEJMoa1705915.28902593
9. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol. (2018) 71:2335–42. doi: 10.1016/j.jacc.2018.02.046.29544871
10. Chessa M, Carminati M, Butera G, Bini RM, Drago M, Rosti L, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. (2002) 39:1061–5. doi: 10.1016/S0735-1097(02)01711-4.11897451
11. Mullen MJ, Dias BF, Walker F, Siu SC, Benson LN, McLaughlin PR. Intracardiac echocardiography guided device closure of atrial septal defects. J Am Coll Cardiol. (2003) 41:285–92. doi: 10.1016/S0735-1097(02)02616-5.12535824
12. Hijazi Z, Wang Z, Cao Q, Koenig P, Waight D, Lang R. Transcatheter closure of atrial septal defects and patent foramen ovale under intracardiac echocardiographic guidance: feasibility and comparison with transesophageal echocardiography. Catheter Cardiovasc Interv. (2001) 52:194–9. doi: 10.1002/1522-726X(200102)52:2%3C194::AID-CCD1046%3E3.0.CO;2-4.11170327
13. Koenig P, Cao QL. Echocardiographic guidance of transcatheter closure of atrial septal defects intracardiac echocardiography better than transesophageal echocardiography? Pediatr Cardiol. (2005) 26:135–9. doi: 10.1007/s00246-004-0952-6.15868327
14. Hijazi ZM, Shivkumar K, Sahn DJ. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation. (2009) 119:587–96. doi: 10.1161/CIRCULATIONAHA.107.753046.19188519
15. Maloney JD, Burnett JM, Dala-Krishna P, Glueck R. New directions in intracardiac echocardiography. J Interv Card Electrophysiol. (2005) 13(Suppl 1):23–9. doi: 10.1007/s10840-005-1097-0.16133852
16. Barker PC. Intracardiac echocardiography in congenital heart disease. J Cardiovasc Transl Res. (2009) 2:19–23. doi: 10.1007/s12265-009-9088-7.20559965
17. Zanchetta M, Rigatelli G, Pedon L, Zennaro M, Carrozza A, Onorato E. Catheter closure of perforated secundum atrial septal defect under intracardiac echocardiographic guidance using a single amplatzer device: feasibility of a new method. J Invasive Cardiol. (2005) 17:262–5. 15879606.15879606
18. Seca L, Cação R, Silva J, Providência R, Mota P, Costa M, et al. Intracardiac echocardiography imaging for device closure of atrial septal defects–a single-center experience. Rev Port Cardiol. (2012) 31:407–12. doi: 10.1016/j.repc.2011.11.009.22579835
19. Assaidi A, Sumian M, Mauri L, Mancini J, Ovaert C, Salaun E, et al. Transcatheter closure of complex atrial septal defects is efficient under intracardiac echocardiographic guidance. Arch Cardiovasc Dis. (2014) 107:646–53. doi: 10.1016/j.acvd.2014.06.010.25241222
20. Ponnuthurai FA, van Gaal WJ, Burchell A, Mitchell AR, Wilson N, Ormerod OJ. Safety and feasibility of day case patent foramen ovale (PFO) closure facilitated by intracardiac echocardiography. Int J Cardiol. (2009) 131:438–40. doi: 10.1016/j.ijcard.2007.07.141.18037512
21. Barker M, Muthuppalaniappan AM, Abrahamyan L, Osten MD, Benson LN, Bach Y, et al. Periprocedural outcomes of fluoroscopy-guided patent foramen Ovale closure with selective use of intracardiac echocardiography. Can J Cardiol. (2020) 36:1608–15. doi: 10.1016/j.cjca.2019.12.032.32610094
22. Hildick-Smith D, Behan M, Haworth P, Rana B, Thomas M. Patent foramen ovale closure without echocardiographic control: use of “standby” intracardiac ultrasound. JACC Cardiovasc Interv. (2008) 1:387–91. doi: 10.1016/j.jcin.2008.05.006.19463334
23. Bartel T, Konorza T, Arjumand J, Ebradlidze T, Eggebrecht H, Caspari G, et al. Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation. (2003) 107:795–7. doi: 10.1161/01.CIR.0000057547.00909.1C.12591745
24. Boccalandro F, Muench A, Salloum J, Awadalla H, Carter C, Barasch E, et al. Interatrial defect sizing by intracardiac and transesophageal echocardiography compared with fluoroscopic measurements in patients undergoing percutaneous transcatheter closure. Catheter Cardiovasc Interv. (2004) 62:415–20. doi: 10.1002/ccd.20082.15224315
25. Zanchetta M. On-line intracardiac echocardiography alone for amplatzer septal occluder selection and device deployment in adult patients with atrial septal defect. Int J Cardiol. (2004) 95:61–8. doi: 10.1016/j.ijcard.2003.04.022.15159040
26. Bartel T, Konorza T, Neudorf U, Ebralize T, Eggebrecht H, Gutersohn A, et al. Intracardiac echocardiography: an ideal guiding tool for device closure of interatrial communications. Eur J Echocardiogr. (2005) 6:92–6. doi: 10.1016/j.euje.2004.07.007.15760685
27. Chien JC, Hwang B, Fu YC, Lee PC, Hsieh KS, Jan SL. Sizing of atrial septal defects by intracardiac echocardiography for device closures. J Chin Med Assoc. (2008) 71:399–405. doi: 10.1016/S1726-4901(08)70090-X.18772119
28. Kim NK, Park SJ, Shin JI, Choi JY. Eight-French intracardiac echocardiography—safe and effective guidance for transcatheter closure in atrial septal defects. Circ J. (2012) 76:2119–23. doi: 10.1253/circj.CJ-11-1286.22975637
29. Shimizu S, Kawamura A, Arai T, Ohno Y, Mogi S, Kodaira M, et al. Intracardiac echocardiography for percutaneous closure of atrial septal defects: initial experiences in Japan. Cardiovasc Interv Ther. (2013) 28:368–73. doi: 10.1007/s12928-013-0187-7.23709325
30. Zhao J, Li L, Peng L. Safe and effective guidance by intracardiac echocardiography for transcatheter closure in atrial septal defects. Int J Clin Exp Med. (2015) 8:9815–9. 4537986.26309662
31. Alqahtani F, Bhirud A, Aljohani S, Mills J, Kawsara A, Runkana A, et al. Intracardiac versus transesophageal echocardiography to guide transcatheter closure of interatrial communications: nationwide trend and comparative analysis. J Interv Cardiol. (2017) 30:234–41. doi: 10.1111/joic.12382.28439973
32. Yamano M, Yamano T, Nakamura T, Zen K, Shiraishi H, Shirayama T, et al. Appropriate selection of echocardiographic guidance for transcatheter atrial septal defect closure. Int J Cardiovasc Imaging. (2020) 36:855–63. doi: 10.1007/s10554-020-01778-9.32040685
33. Moon J, Park Y, Park SJ, Oh PC, Jang AY, Chung WJ, et al. Comparison of intracardiac echocardiography and transesophageal echocardiography for image guidance in percutaneous patent foramen Ovale closure. Medicina (Kaunas). (2020) 56(8):401. doi: 10.3390/medicina56080401.
34. Ali S, George LK, Das P, Koshy SK. Intracardiac echocardiography: clinical utility and application. Echocardiography. (2011) 28:582–90. doi: 10.1111/j.1540-8175.2011.01395.x.21564275
35. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13.15840177
36. Jingquan Z, Deyong L, Huimin C, Hua F, Xuebin H, Chenyang J, et al. Intracardiac echocardiography Chinese expert consensus. Front Cardiovasc Med. (2022) 9:1012731. doi: 10.3389/fcvm.2022.1012731.36277762
37. George JC, Varghese V, Mogtader A. Intracardiac echocardiography: evolving use in interventional cardiology. J Ultrasound Med. (2014) 33:387–95. doi: 10.7863/ultra.33.3.387.24567449
38. Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am J Cardiol. (2004) 94:690–2. doi: 10.1016/j.amjcard.2004.05.048.15342315
39. Budts W, Troost E, Voigt JU, Gewillig M. Intra-cardiac echocardiography in atrial septal interventions: impact on hospitalization costs. Acta Cardiol. (2010) 65:147–52. doi: 10.2143/AC.65.2.2047047.20458821
40. Bartel T, Müller S, Biviano A, Hahn RT. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J. (2014) 35:69–76. doi: 10.1093/eurheartj/eht411.24144789
Keywords: atrial septal defect, patent foramen ovale, transesophageal echocardiography, intracardiac echocardiography, congenital, congenital heart diasease
Citation: Lan Q, Wu F, Ye X, Wang S and Zhong J (2023) Intracardiac vs. transesophageal echocardiography for guiding transcatheter closure of interatrial communications: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1082663. doi: 10.3389/fcvm.2023.1082663
Received: 28 October 2022; Accepted: 20 April 2023;
Published: 5 May 2023.
Edited by:
Stamatios Lerakis, Mount Sinai Hospital, United StatesReviewed by:
Daniel De Wolf, Ghent University Hospital, Belgium© 2023 Lan, Wu, Ye, Wang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingquan Zhong MTk4NzYyMDAwNzc4QGVtYWlsLnNkdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.