- 1Heart Transplant Program, St Vincent’s Hospital, Sydney, NSW, Australia
- 2School of Medicine, University of Notre Dame, Sydney, NSW, Australia
- 3School of Medicine, University of New South Wales, Sydney, NSW, Australia
- 4Infiltrative Cardiomyopathy Laboratory, Victor Chang Cardiac Research Institute, Sydney, NSW, Australia
- 5Centre for Healthy Brain Ageing, School of Psychiatry, Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
- 6Department of Neurology, Macquarie University Hospital, Sydney, NSW, Australia
Frailty is a complex, multi-system condition often associated with multimorbidity. It has become an important prognostic maker across a range of conditions and is particularly relevant in patients with cardiovascular disease. Frailty encompasses a range of domains including, physical, psychological, and social. There are currently a range of validated tools available to measure frailty. It is an especially important measurement in advanced HF, because frailty occurs in up to 50% of HF patients and is potentially reversible with therapies such as mechanical circulatory support and transplantation. Moreover, frailty is dynamic, and therefore serial measurements are important. This review delves into the measurement of frailty, mechanisms, and its role in different cardiovascular cohorts. Understanding frailty will help determine patients that will benefit from therapies, as well as prognosticate outcomes.
Introduction
Frailty is an age-related clinical syndrome and describes a reduced capacity of multiple organ systems with increased susceptibility to stressors and has become an important prognostic indicator across a range of medical conditions including heart failure (HF). The dual pressures of an increasingly older population and need to meaningfully risk stratify patients with chronic illness has led to an explosion in research (1). There are difficulties with estimating frailty prevalence due to a lack of standardisation of concepts and measures in a continually evolving field (2). However, frailty prevalence increases with age and is higher in women than men (3). Higher rates are also seen in ethnic minority groups and those in lower socioeconomic groups (3). Frailty is associated with increased mortality, hospitalisations, worsening mobility, nursing home admissions and lower quality of life (3–5).
There is a clear association between increased health care utilisation and health care costs with a higher degree of frailty (6, 7). Frailty worldwide is estimated to be as prevalent as 15.7% in those aged 80–84 years to be and 26% in those aged >85 years (8). The World Health Organisation has estimated that by 2050, the world's population of people aged 60 years and above will double. With frailty predisposing to healthcare dependency (9), it is a major contributor to global health burden.
History of cardiac disease and frailty
Ever since research into the definition and clinical implications of frailty began, there has been research into the associations between cardiovascular disease and frailty and its impact (10, 11). Bidirectional relationships were identified early with the prevalence of HF increasing six to sevenfold with increasing frailty severity (10, 12) and the prevalence of frailty two to threefold higher in older individuals with cardiovascular disease (13). An increasing literature has addressed the relationship between frailty, cardiovascular disease, and HF over the past two decades.
Association between specific pathologies and frailty
There is a strong evidence base for an association between multimorbidity and frailty with a recent meta-analysis identifying that approximately three-quarters of those with frailty had two or more diseases (14). Specifically, there is robust evidence for a greater prevalence and prognostic impact of frailty in coronary artery disease and revascularisation, HF, aortic stenosis and valve replacement, cancer and chemotherapy or cancer surgery, peripheral vascular disease and surgery, general surgery, chronic kidney disease and dialysis, cirrhosis and liver transplantation and critical illness and intensive care unit admission (2).
The overall prevalence of frailty is higher in females (6, 10) In the HF population, women are more likely to have HF with preserved ejection fraction, be older and have a higher comorbidity burden (12).
Although frailty is more prevalent with increasing chronological age, it also occurs independent of age. Frailty is a clinical proxy for biological age. A key goal of ageing research is to determine what factors narrow the gap between chronological and biological age. Another way of expressing this is for health span (those years of life when individuals are functioning well and chronic disease free) to approximate lifespan (15). It follows that frailty is also more prevalent in chronic illness, particularly in the context of long-term inflammation, immune dysregulation, or immune suppression (16, 17). The frailty tools currently in use are modified from their initial application in a geriatric population, and therefore may not address the diverse array of biopsychosocial factors.
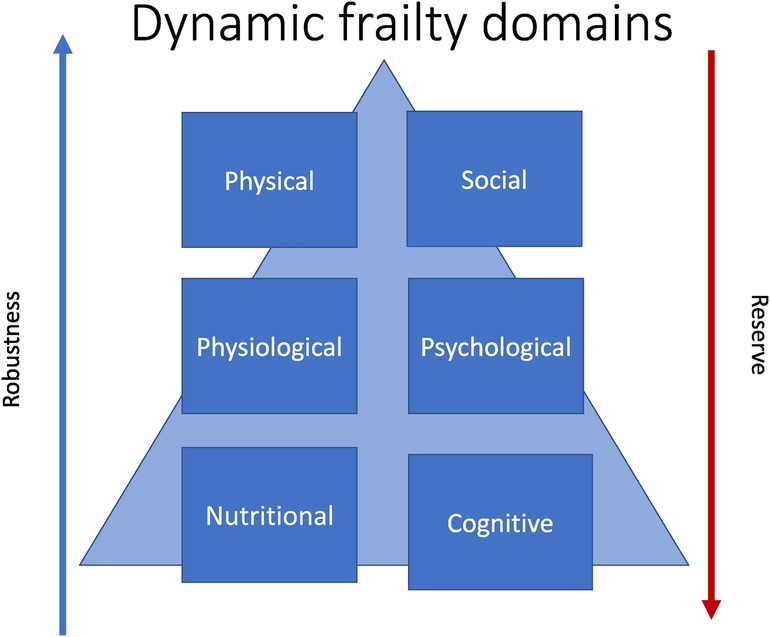
Frailty is multisystem process that is dynamic and potentially preventable. If appropriately recognised and addressed, reversibility of the overall frailty phenotype is also possible. The reverse of frailty is robustness, or a resilience in the face of biopsychosocial stressor (Figure 1). Robustness is related to intrinsic capacity which is defined as all the individual characteristics that contribute to a person's ability to be and to do what they have reason to value (18). This has cognitive, locomotor, vitality, sensory and psychological domains (19).
As will be explored in this review, frailty as a phenotype is a powerful prognostic, but also therapeutic target.
Definition and communication of frailty
Frailty is defined as reduced reserve in individual with a resultant reduction in ability to tolerate minor or major stressors. The reduced reserve was initially thought to be isolated to the physical domain (10). It is now understood to be the intersection of physical, physiological, immune, cognitive, and social domains. The World Health Organisation on aging broaden the definition to include vulnerability of several organ systems (18). It is important to note, that given the complexity of frailty, there is no universal definition for clinical practice at present (20).
The term “frailty” appeared in the medical literature from the 1950s onwards and was used to describe older people who required health services due to multimorbidity (21). In the 1990s, it was discovered that the incorporation of multiple frailty manifestations better predicted clinical outcomes than any single component alone (22). However, the introduction of the frailty phenotype by Fried and colleagues in 2001 is generally considered the birth of modern frailty research (23). Fried et al. described frailty as a physical phenotype (10) while Mitnitski and Rockwood introduced a frailty index based upon the accumulation of age-related deficits (24). The concept of frailty has evolved over the past two decades with ongoing debate over how it is best defined.
The World Health Organisation (WHO) developed the Integrated Care for Older People (ICOPE) package in 2017 as part of their strategy to implement the Decade of Healthy Ageing Healthy ageing is defined by the WHO as “the process of developing and maintaining the functional ability that enables wellbeing in older age”. The ICOPE program was also designed to maximise intrinsic capacity which is essentially the opposite of frailty and comprises all the mental and physical capacities upon which a person relies to function.
For reference, in this review cognitive frailty refers to cognitive decline in the absence of dementia (25), social frailty refers to loneliness and lack of social networks, and psychological frailty refers to psychological factors that diminish reserve in the event of a stressor, and is sometimes also called “depressive frailty” (9, 26). Finally nutritional frailty (27) is “rapid, unintentional loss of body weight and accompanying disability that often signals the beginning of a terminal decline in an older individual.”
Frailty is a way of communicating with families to help them understand the vulnerabilities of their loved one. A geriatrician popularised the communication of frailty by explaining it as a beautiful “paper boat” to patients and their families (28). If they were to envisage their loved one as a delicate paper boat floating on a pond, then when the pond was quiet and calm, they could sail with no issues. However high winds or storms, which could be a major infection or operation, the boat would no longer be able to sail. This analogy teaches clinicians that they need to assess for, and communicate frailty to patients, especially in the peri-operative or peri-procedural setting. It helps the concept of “shared decision making” and explaining risks (29). Finally, given frailty is dynamic and multi-dimensional, domains such as social networks and nutrition can be supported by families and loved ones.
Measurement tools
Multiple frailty measurement tools exist in the clinical and research space and although multiple reviews have highlighted the need for a standard measurement, no such tool exists. Moreover, approaches differ between medical specialties. This likely reflects the complexities of frailty and our evolving understanding.
One of the early and best validated tools is the Fried's Frailty Phenotype, also known as the Physical Frailty Phenotype (PFP) (10). It incorporates five domains: unintentional weight lost, self-reported exhaustion, low physical activity level, slow gait speed and weak grip strength with a maximum score of five. A score of 0/5 indicates no frailty (or robustness), 1–2/5 indicates that the patient is prefrail and a score of 3/5 indicates frailty. Limitations of this phenotype include inclusion of grip strength which is not routinely measured in clinical practice and a focus on physical measures alone.
The Clinical Frailty Scale relies on clinician assessment of a patient's level of activity and functional status scored between 1 (very fit) and 9 (terminally ill). The Clinical Frailty Scale (CFS) is very straightforward and widely used in clinical practice and research with an advantage being that scores can be extracted from medical record review. One study evaluated rates of successful resuscitation based on baseline CFS, with no patients with a CFS of 4 or more surviving (30, 31).
Another well validated tool is the deficit accumulation index (DAI), which uses 70 domains with a greater number of health deficits indicating higher frailty (32). Though this score may be more representative of the complexity of frailty domains, it can be clinically cumbersome and time-consuming and is therefore less practical as a repeated measure.
All full discussion of all published frailty tools is beyond the scope of this review. However, Dent et al. have identified 29 different frailty measures and comprehensively reviewed those in common use as well as individual factors such as gait speed (31). Macdonald et al. have also summarised those tools relevant to heart transplantation and failure (33). A new clinical measure called the “Frail Trait Scale” (FTS) is currently being evaluated as part of the FRAILTOOLs project and has been shown to be one of the best tools for predicting a range of adverse outcomes in older people across clinical settings (34).
The American Society of Transplant Surgeons and the American Society of Transplantation, have recommended the modified version of the PFP for measurement of frailty in cardiovascular patients, particularly those referred for transplantation, and that grip strength is a suitable individual measure in non-ambulant patients (35).
Common pathways for frailty and cardiovascular disease
Moreover, several risk factors for frailty are also risk factors for cardiovascular disease. For example, reduced exercise capacity, and endocrine dysfunction such as insulin resistance increase the rate of cardiovascular disease and frailty (37). Frailty itself may be a risk factor for cardiovascular disease. The inverse is also true, with cardiovascular symptomology and pathophysiology often accelerating the frailty syndrome.
One important common pathway is low-grade inflammation, with both frail patients and those with cardiovascular disease demonstrating higher levels of C-reactive protein (CRP) and IL-6 (38). Inflammation is a hallmark of several types of cardiovascular disease including ischaemic heart disease, and HF (HF). Recent clinical trials have shown that modulating inflammation can prevent cardiovascular diseases (39). For example, the landmark CANTOS study (40) showed than an anti-inflammatory monoclonal antibody could reduce inflammatory biomarkers such as C-reactive protein, and significantly lowered recurrent cardiovascular events. In addition, a recent concept of “inflammageing” (41) has emerged, which is characterized by high levels of inflammatory markers as individuals age. This is strongly correlated with frailty, heart disease and multi-morbidity (42, 43). For example, in the FRAXI study (44), frailty and inflammation were correlated with arterial stiffness. The correlation is explained by a dysfunctional immune state being a common mechanism for each of these conditions (44, 45).
One commonality is nutrition and sarcopenia, which is the involuntary loss of muscle mass and strength. In advanced HF, patients often develop cardiac cachexia, which is a catabolic/anabolic imbalance seen in over 10% of advanced HF patients where nutritional deficiency and sarcopenia is common. This state is also characterized by abnormalities in blood markers such as haemoglobin and albumin (which are also scored on some frailty tools) (13, 46). Interestingly, one of underlying causes of this is chronic inflammation, with these patients having high circulating levels of marker such as IL-6 and TNF-alpha, and high C-reactive protein levels (46). HF patients also show hormonal dysregulation, which can occur before metabolic changes, with impairments in hormones such as leptin, ghrelin, adiponectin (47, 48).
Immune dysregulation has also been implicated in both frailty and cardiovascular disease. Ischaemic heart disease is the leading cause of cardiovascular morbidity worldwide. When myocardial tissue is ischemic both the innate and adaptive immune system is activated (49). With cellular necrosis, and inflammatory cascade is activated. Dysregulation of both the innate and adaptive immune system is also integral to the pathogenesis of frailty (41).
Finally, psychological status affects frailty and cardiovascular outcomes. Comorbid depression and anxiety are common in HF and contributes to frailty. In one prospective study, depression independently increased the risk of HF by 18% over seven years (50). Poor end-organ perfusion, particularly cerebral perfusion is common in the low output state of HF and can be difficult to differentiate from psychomotor motor slowing associated with depression and can also exist concurrently (51). Moreover, HF itself can lead to depression, which then increases the number of HF exacerbations, hospitalisation, and overall risk of death (51).
Frailty is associated with poor outcomes across the spectrum of cardiovascular disease One meta-analysis found that both frailty and pre-frailty was associated with cardiovascular disease, and that frailty was associated with a 3-fold increase in cardiovascular death (52). This is reinforced in other studies which show that frail patients with cardiovascular disease have 2.5–3.5-fold higher mortality than non-frail patients (53). The FRAILTY-AVR (Frailty Assessment Before Cardiac Surgery & Transcatheter Interventions) showed that frailty was as high as 68% in patients undergoing aortic valve replacement, which in turn, increased mortality (13). Across the cardiovascular cohort, frail patients with HF are at greatest risk, with mortality rates as high as 52% (53).
Overlapping frailty and HF syndromes
It is estimated that over 50% of HF have concomitant frailty (45), which is associated with higher rates of hospitalisation and death (54). The true prevalence of frailty in a HF population is unclear due to the lack of universal definition or frailty tool. The literature is clear however, that HF and frailty are overlapping syndromes. Wang et al., found that in 10 studies involving 3,033 patients, frailty was highly prevalent in patients with HF, with rates of 25.4% to 76% in patients aged >65 years and 70% increased mortality (55). This was replicated in a systematic review of 8 studies and 2,645 patient by Yang et al., where frailty resulted in an 50% increase in hospitalisations and death for HF patients (56).
In the advanced HF spectrum, Jha et al., (54) studied 156 HF patients referred for heart transplantation using the PFP and PFP plus cognition. They found that using both tools, non-frail patients had a higher 12-month survival than frail patients. A further study showed that frailty in an advanced HF population was independent of age, however correlated with NYHA class, body mass index, lower cardiac index, cognitive impairment, and depression. Frailty in this population was an independent predictor of mortality.
There are several studies that show the bi-directional effects of HF and frailty. The mechanisms are several-fold. HF leads to an up regulated neurohormonal state, and chronic inflammation (41). There are higher circulating levels of inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), C-reactive protein (CRP), and tumour necrosis factor-α (TNF-α), which lead to both tissue depletion and sarcopenia (57). In the HF syndrome, this manifests as dyspnoea, fatigue, and exercise intolerance, which are all contributory to the overall frailty phenotype. Sarcopenia and cachexia are also both present in frailty and HF, and can lead to worse outcomes (58).
There are many common mechanistic pathways that explain the relationship between frailty and HF including effects on the coagulation system, platelets, and endothelium. In in HF, a heightened immune state is present. Risk factors for ischaemic cardiomyopathy, a leading cause of HF including obesity, hypertension, and diabetes and all pro-inflammatory. Moreover, from an early age the process of vascular aging begins leading to a reduction in nitric oxide availably and progressively increasing arterial stiffness (41, 59). This process of “cardiac inflammaging” leads to endothelial dysfunction and myocardial damage. Another key process common to both frailty and HF is mitochondrial dysfunction, which leads to changes in the balance of reactive oxygen species and higher levels of oxidative stress (60). This in turn can lead to platelet and endothelial dysfunction (41).
There are sex differences in the process of cardiac inflammaging. Interestingly, in the same way that frailty is more prevalent in women, there is also a higher prevalence of immune dysfunction, particularly in the form of cardiac inflammaging. The loss of oestrogen that occurs with menopause leads to an up-regulation of pro-inflammatory cytokines, and a decline in anti-inflammatory mechanisms (61). This may explain why women with HF, particularly HF with preserved ejection fraction have worse outcomes than men, although this needs further research.
Frailty in mechanical circulatory support and transplantation
Frailty is a modifiable risk factor for poor outcomes with advanced HF therapy such as mechanical circulatory support and transplantation. It is an additional diagnostic tool, not currently in the guidelines to help guide the decision making of multi-disciplinary teams. Importantly, it should be measured serially as a patient's journey may change with therapy.
Frailty is a risk factor for post-operative mortality after insertion of left ventricular assist device (LVAD) in both a bridge to transplant and destination therapy cohorts (62).. However, with durable support and treatment of their HF, some patients can recover, and even reverse their frailty. For example, Jha et al., demonstrated that PFP was partly or completely reversible in 12 of 13 patients after LVAD insertion (63).
In contrast, Muthiah et al., (64), showed that patients deemed frail by the PFP had prohibitively poor outcomes post Biventricular assist device (BiVAD) insertion, with mortality rates of n = 4/5 in frail patients compared with n = 0/6 in non-frail BiVAD supported patients, suggesting that frailty should be a red flag if patients are thought to require BiVAD support during the transplant work-up.
Pre-transplant frailty predicts worse post-transplant outcomes across the range of solid-organ transplants. In a study of 140 heart transplant patients, frailty assessed by the PFP within the 6 months prior to transplant surgery predicted poorer outcomes post-transplant (65). McAdams-DeMarco showed that in renal transplant recipients, PFP score on admission for transplant predicted outcomes (66).
Given the key role that inflammation and immune activation plays in frailty, mapping long term frailty in solid organ recipients would be interesting. Heart transplant recipients have a unique inflammatory predisposition. Regardless of the accuracy of donor-recipient matching, they essentially have a long-term foreign body, and therefore a heightened immune response. Despite improvements in immunosuppression, rejection still occurs in 1/3 recipients and is most prevalence initially post-transplant. The interactions between immunosuppression and frailty are complex, and this is a potential exciting area for research in the future.
It is important to note that frailty is a dynamic process in these complex patients. For example, non-frail patients prior to transplant who have major complications requiring a long hospital admission post-transplant are likely to become frail post-operatively (33).
Reversal of frailty and future areas of research
One emerging area of future research is the differentiation between fixed vs. dynamic components of frailty. Once this is known, targeted intervention can be applied to optimize reversible elements of the frailty syndrome and increase levels of robustness.
The immune system is central to the process of aging and may play a key role in the dynamic nature of frailty. It may also explain why chronological age may not reflect physiological age. The thymus begins to involute around∼age 15, leading to gradual decline in immune reserve, referred to as immunosenescence (67, 68). A secondary process, inflammaging, may be a potential target for anti-inflammatory therapy to reduce both cardiovascular disease and frailty (41). The more we understand about these dual processes, the more likely this is as a therapeutic target.
Inflammation has become an important therapeutic target in cardiovascular disease and may be a way of also addressing frailty. For example, the CANTOS (69) study showed that monoclonal antibody canakinumab which targets interleukin-1β reduces atherosclerosis. It would be interesting to see what impact such medications could have on frailty in the long-term.
It is important to address and, where possible, reverse cognitive and psychological frailty. Approximately 40 per cent of the population attributable risk of dementia is now considered preventable based on 12 modifiable risk factors including hearing loss, alcohol and smoking, hypertension, physical inactivity and social isolation (70). Strict control of cardiovascular risk factors and prevention of stroke is particularly relevant to people with cardiovascular disease, particularly those with end-stage disease awaiting transplant or supported by an LVAD. For those patients who are hospitalized, prevention of delirium is especially important as this is an independent risk factor for the subsequent development of dementia (71). Other measures recommended in the treatment of mild cognitive impairment such as identification and cessation of medications that may contribute to cognitive impairment, treatment of depression and regular exercise (72) would also be appropriate. Patients with conditions such as depression or dementia treated with anti-depressants can demonstrate neuroplasticity (73). The same may be true for cognitive deficits (independent of reduced cerebral perfusion due to a low output state) in HF patients (74). “Social prescription” as a way of linking older adults with sources of support in the community may combat social frailty although evidence for this intervention is thus far limited (75).
Finally, exercise has been shown to be better than any drug for preventing cardiac disease and maintaining robustness (76). Exercise reduces obesity, insulin resistance, blood pressure and overall cardiovascular disease risk (77). Prehabilitation, defined as the screening for and identification of pre-existing disorder followed by medical optimisation (78), is emerging as a useful tool for assessing patients prior to major cardiac surgery (33). In addition, exercise such as that incorporated in cardiac rehabilitation has been shown to improve prognosis and be excellent secondary prevention once a cardiac event has occurred (79). Determining the appropriate level of exercise intensity for HF patients to maintain safety and maximise benefits is important. The gold standard for such a maximum aerobic exercise intensity prescription is the cardiopulmonary exercise test (CPET) which demonstrates metabolic, respiratory and cardiac responses at anaerobic threshold and respiratory compensation point (80). Where CPET is not available, heart rate during the six minute walk test and step test can guide such prescriptions (81).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, et al. Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the council for cardiology practice (CCP), association for acute cardio vascular care (ACVC), association of cardiovascular nursing and allied professions (ACNAP), European association of preventive cardiology (EAPC), European heart rhythm association (EHRA), council on valvular heart diseases (VHD), council on hypertension (CHT), council of cardio-oncology (CCO), working group (WG) aorta and peripheral vascular diseases, WG e-cardiology, WG thrombosis, of the European society of cardiology, European primary care cardiology society (EPCCS). Eur J Prev Cardiol. (2022) 29:216–27. doi: 10.1093/eurjpc/zwaa167
2. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6
3. Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. (2015) 70:1427–34. doi: 10.1093/gerona/glv133
4. Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan: a systematic review and meta-analysis. J Epidemiol. (2017) 27:347–53. doi: 10.1016/j.je.2016.09.008
5. Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. (2016) 70:716–21. doi: 10.1136/jech-2015-206717
6. Ensrud KE, Kats AM, Schousboe JT, Taylor BC, Cawthon PM, Hillier TA, et al. Frailty phenotype and healthcare costs and utilization in older women. J Am Geriatr Soc. (2018) 66:1276–83. doi: 10.1111/jgs.15381
7. Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci. (2019) 74:1271–6. doi: 10.1093/gerona/gly197
8. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. (2012) 60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x
9. Gobbens RJ, van Assen MA. Frailty and its prediction of disability and health care utilization: the added value of interviews and physical measures following a self-report questionnaire. Arch Gerontol Geriatr. (2012) 55:369–79. doi: 10.1016/j.archger.2012.04.008
10. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.M146
11. Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. (2001) 56:M158–66. doi: 10.1093/gerona/56.3.M158
12. Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: emergence and consequences in women aged 65 and older in the women's health initiative observational study. J Am Geriatr Soc. (2005) 53:1321–30. doi: 10.1111/j.1532-5415.2005.53405.x
13. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. (2017) 70:689–700. doi: 10.1016/j.jacc.2017.06.024
14. Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R, et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. (2019) 74:659–66. doi: 10.1093/gerona/gly110
15. Hansen M, Kennedy BK. Does longer lifespan mean longer healthspan? Trends Cell Biol. (2016) 26:565–8. doi: 10.1016/j.tcb.2016.05.002
16. Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. (2012) 147:190–3. doi: 10.1001/archsurg.2011.1229
17. Angioni D, Macaron T, Takeda C, Sourdet S, Cesari M, Virecoulon Giudici K, et al. Can we distinguish age-related frailty from frailty related to diseases? Data from the MAPT study. J Nutr Health Aging. (2020) 24:1144–51. doi: 10.1007/s12603-020-1518-x
19. Beard JR, Jotheeswaran AT, Cesari M, Araujo de Carvalho I. The structure and predictive value of intrinsic capacity in a longitudinal study of ageing. BMJ Open. (2019) 9:e026119. doi: 10.1136/bmjopen-2018-026119
20. McDonagh J, Martin L, Ferguson C, Jha SR, Macdonald PS, Davidson PM, et al. Frailty assessment instruments in HF: a systematic review. Eur J Cardiovasc Nurs. (2018) 17:23–35. doi: 10.1177/1474515117708888
21. Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook. (2010) 58:76–86. doi: 10.1016/j.outlook.2009.09.005
22. Sager MA, Rudberg MA, Jalaluddin M, Franke T, Inouye SK, Landefeld CS, et al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc. (1996) 44:251–7. doi: 10.1111/j.1532-5415.1996.tb00910.x
23. Sobhani A, Fadayevatan R, Sharifi F, Kamrani AA, Ejtahed HS, Hosseini RS, et al. The conceptual and practical definitions of frailty in older adults: a systematic review. J Diabetes Metab Disord. (2021) 20:1975–2013. doi: 10.1007/s40200-021-00897-x
24. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. (2001) 1:323–36. doi: 10.1100/tsw.2001.58
25. Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. (2013) 17:726–34. doi: 10.1007/s12603-013-0367-2
26. Gobbens RJ, van Assen MA, Luijkx KG, Schols JM. Testing an integral conceptual model of frailty. J Adv Nurs. (2012) 68:2047–60. doi: 10.1111/j.1365-2648.2011.05896.x
27. Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. (2002) 22:309–23. doi: 10.1146/annurev.nutr.22.010402.102715
28. Louise Finlay S. Frailty: an overview of concepts, risk factors, assessment tools and interventions. Nurs Older People. (2022) 34:35–42. doi: 10.7748/nop.2022.e1394
29. van de Pol MHJ, Fluit C, Lagro J, Slaats Y, Olde Rikkert MGM, Lagro-Janssen ALM. Shared decision making with frail older patients: proposed teaching framework and practice recommendations. Gerontol Geriatr Educ. (2017) 38:482–95. doi: 10.1080/02701960.2016.1276014
30. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. (2005) 173:489–95. doi: 10.1503/cmaj.050051
31. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. (2016) 31:3–10. doi: 10.1016/j.ejim.2016.03.007
32. Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The accumulation of deficits with age and possible invariants of aging. ScientificWorldJournal. (2002) 2:1816–22. doi: 10.1100/tsw.2002.861
33. Macdonald P. Frailty of the heart recipient. Transplantation. (2021) 105:2352–61. doi: 10.1097/TP.0000000000003692
34. Oviedo-Briones M, Rodriguez-Laso A, Carnicero JA, Gryglewska B, Sinclair AJ, Landi F, et al. The ability of eight frailty instruments to identify adverse outcomes across different settings: the FRAILTOOLS project. J Cachexia Sarcopenia Muscle. (2022) 13:1487–501. doi: 10.1002/jcsm.12990
35. Kobashigawa J, Dadhania D, Bhorade S, Adey D, Berger J, Bhat G, et al. Report from the American society of transplantation on frailty in solid organ transplantation. Am J Transplant. (2019) 19:984–94. doi: 10.1111/ajt.15198
36. Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. (2011) 93:385–404. doi: 10.1016/j.pneurobio.2011.01.002
37. Iqbal J, Denvir M, Gunn J. Frailty assessment in elderly people. Lancet. (2013) 381:1985–6. doi: 10.1016/S0140-6736(13)61203-9
38. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. (2016) 31:1–8. doi: 10.1016/j.arr.2016.08.006
39. Ridker PM, Lüscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. (2014) 35:1782–91. doi: 10.1093/eurheartj/ehu203
40. Lorenzatti A, Servato ML. Role of anti-inflammatory interventions in coronary artery disease: understanding the canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Eur Cardiol. (2018) 13:38–41. doi: 10.15420/ecr.2018.11.1
41. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
42. Salaffi F, Di Matteo A, Farah S, Di Carlo M. Inflammaging and frailty in immune-mediated rheumatic diseases: how to address and score the issue. Clin Rev Allergy Immunol. (2023) 64:206–21. doi: 10.1007/s12016-022-08943-z
43. Motta F, Sica A, Selmi C. Frailty in rheumatic diseases. Front Immunol. (2020) 11:576134. doi: 10.3389/fimmu.2020.576134
44. Mensah E, Ali K, Banya W, Kirkham FA, Mengozzi M, Ghezzi P, et al. FRailty and arterial stiffness—the role of oXidative stress and inflammation (FRAXI study). Biomark Insights. (2022) 17:11772719221130719. doi: 10.1177/11772719221130719
45. Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in HF: a systematic review and meta-analysis. Int J Cardiol. (2017) 236:283–9. doi: 10.1016/j.ijcard.2017.01.153
46. Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, et al. Comparison of sarcopenia and cachexia in men with chronic HF: results from the studies investigating co-morbidities aggravating HF (SICA-HF). Eur J Heart Fail. (2018) 20:1580–7. doi: 10.1002/ejhf.1304
47. Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, et al. Elevated circulating level of ghrelin in cachexia associated with chronic HF: relationships between ghrelin and anabolic/catabolic factors. Circulation. (2001) 104:2034–8. doi: 10.1161/hc4201.097836
48. Doehner W, Pflaum CD, Rauchhaus M, Godsland IF, Egerer K, Cicoira M, et al. Leptin, insulin sensitivity and growth hormone binding protein in chronic HF with and without cardiac cachexia. Eur J Endocrinol. (2001) 145:727–35. doi: 10.1530/eje.0.1450727
49. Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol. (2014) 63:185–95. doi: 10.1097/FJC.0000000000000003
50. Daskalopoulou M, George J, Walters K, Osborn DP, Batty GD, Stogiannis D, et al. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PLoS One. (2016) 11:e0153838. doi: 10.1371/journal.pone.0153838
51. Sbolli M, Fiuzat M, Cani D, O'Connor CM. Depression and HF: the lonely comorbidity. Eur J Heart Fail. (2020) 22:2007–17. doi: 10.1002/ejhf.1865
52. Veronese N, Sigeirsdottir K, Eiriksdottir G, Marques EA, Chalhoub D, Phillips CL, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment susceptibility-Reykjavik study. Rejuvenation Res. (2017) 20:517–24. doi: 10.1089/rej.2016.1905
53. Marinus N, Vigorito C, Giallauria F, Haenen L, Jansegers T, Dendale P, et al. Frailty is highly prevalent in specific cardiovascular diseases and females, but significantly worsens prognosis in all affected patients: a systematic review. Ageing Res Rev. (2021) 66:101233. doi: 10.1016/j.arr.2020.101233
54. Jha SR, Hannu MK, Gore K, Chang S, Newton P, Wilhelm K, et al. Cognitive impairment improves the predictive validity of physical frailty for mortality in patients with advanced HF referred for heart transplantation. J Heart Lung Transplant. (2016) 35:1092–100. doi: 10.1016/j.healun.2016.04.008
55. Wang X, Zhou C, Li Y, Li H, Cao Q, Li F. Prognostic value of frailty for older patients with HF: a systematic review and meta-analysis of prospective studies. Biomed Res Int. (2018) 2018:8739058. doi: 10.1155/2018/8739058
56. Yang X, Lupon J, Vidan MT, Ferguson C, Gastelurrutia P, Newton PJ, et al. Impact of frailty on mortality and hospitalization in chronic HF: a systematic review and meta-analysis. J Am Heart Assoc. (2018) 7:e008251. doi: 10.1161/JAHA.117.008251
57. Joseph SM, Rich MW. Targeting frailty in HF. Curr Treat Opt Cardiovasc Med. (2017) 19:31. doi: 10.1007/s11936-017-0527-5
58. Beltrami M, Fumagalli C, Milli M. Frailty, sarcopenia and cachexia in HF patients: different clinical entities of the same painting. World J Cardiol. (2021) 13:1–10. doi: 10.4330/wjc.v13.i1.1
59. Barcena ML, Aslam M, Pozdniakova S, Norman K, Ladilov Y. Cardiovascular inflammaging: mechanisms and translational aspects. Cells. (2022) 11(6):1010. doi: 10.3390/cells11061010
60. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. (2018) 13:757–72. doi: 10.2147/CIA.S158513
61. Milan-Mattos JC, Anibal FF, Perseguini NM, Minatel V, Rehder-Santos P, Castro CA, et al. Effects of natural aging and gender on pro-inflammatory markers. Braz J Med Biol Res. (2019) 52:e8392. doi: 10.1590/1414-431x20198392
62. Chung CJ, Wu C, Jones M, Kato TS, Dam TT, Givens RC, et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with HF undergoing ventricular assist device placement. J Card Fail. (2014) 20:310–5. doi: 10.1016/j.cardfail.2014.02.008
63. Jha SR, Hannu MK, Newton PJ, Wilhelm K, Hayward CS, Jabbour A, et al. Reversibility of frailty after bridge-to-transplant ventricular assist device implantation or heart transplantation. Transplant Direct. (2017) 3:e167. doi: 10.1097/TXD.0000000000000690
64. Muthiah K, Wilhelm K, Robson D, Raju H, Aili SR, Jha SR, et al. Impact of frailty on mortality and morbidity in bridge to transplant recipients of contemporary durable mechanical circulatory support. J Heart Lung Transplant. (2022) 41:829–39. doi: 10.1016/j.healun.2022.02.008
65. Macdonald PS, Gorrie N, Brennan X, Aili SR, De Silva R, Jha SR, et al. The impact of frailty on mortality after heart transplantation. J Heart Lung Transplant. (2021) 40:87–94. doi: 10.1016/j.healun.2020.11.007
66. McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. (2015) 15:149–54. doi: 10.1111/ajt.12992
67. Rodrigues LP, Teixeira VR, Alencar-Silva T, Simonassi-Paiva B, Pereira RW, Pogue R, et al. Hallmarks of aging and immunosenescence: connecting the dots. Cytokine Growth Factor Rev. (2021) 59:9–21. doi: 10.1016/j.cytogfr.2021.01.006
68. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. (2019) 10:2247. doi: 10.3389/fimmu.2019.02247
69. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
70. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
71. Fong TG, Inouye SK. The inter-relationship between delirium and dementia: the importance of delirium prevention. Nat Rev Neurol. (2022) 18:579–96. doi: 10.1038/s41582-022-00698-7
72. Petersen RC. Mild cognitive impairment. Continuum (Minneap Minn). (2016) 22:404–18. doi: 10.1212/con.0000000000000313
73. Dafsari FS, Jessen F. Depression-an underrecognized target for prevention of dementia in Alzheimer's disease. Transl Psychiatry. (2020) 10:160. doi: 10.1038/s41398-020-0839-1
74. Zheng Z, Zeng Y, Wu J. Increased neuroplasticity may protect against cardiovascular disease. Int J Neurosci. (2013) 123:599–608. doi: 10.3109/00207454.2013.785949
75. Smith TO, Jimoh OF, Cross J, Allan L, Corbett A, Sadler E, et al. Social prescribing programmes to prevent or delay frailty in community-dwelling older adults. Geriatrics (Basel). (2019) 4(4):65. doi: 10.3390/geriatrics4040065.31783654
76. Bray NW, Smart RR, Jakobi JM, Jones GR. Exercise prescription to reverse frailty. Appl Physiol Nutr Metab. (2016) 41:1112–6. doi: 10.1139/apnm-2016-0226
77. Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. (2018) 5:135. doi: 10.3389/fcvm.2018.00135
78. Knight T, Atkin C, Martin FC, Subbe C, Holland M, Cooksley T, et al. Frailty assessment and acute frailty service provision in the UK: results of a national “day of care” survey. BMC Geriatr. (2022) 22:19. doi: 10.1186/s12877-021-02679-9
79. Taylor RS, Dalal HM, McDonagh STJ. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol. (2022) 19:180–94. doi: 10.1038/s41569-021-00611-7
80. Binder RK, Wonisch M, Corra U, Cohen-Solal A, Vanhees L, Saner H, et al. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur J Cardiovasc Prev Rehabil. (2008) 15:726–34. doi: 10.1097/HJR.0b013e328304fed4
Keywords: frailty, heart failure, cardiac disease, ageing, superAging, transplantation, robustness
Citation: Bart NK, Powell A and Macdonald PS (2023) The role of frailty in advanced HF and cardiac transplantation. Front. Cardiovasc. Med. 10:1082371. doi: 10.3389/fcvm.2023.1082371
Received: 28 October 2022; Accepted: 14 March 2023;
Published: 3 April 2023.
Edited by:
Yow Keat Tham, Baker Heart and Diabetes Institute, AustraliaReviewed by:
Diego Arauna, University of Talca, ChileMarta Wleklik, Wroclaw Medical University, Poland
© 2023 Bart, Powell and Macdonald. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole K. Bart bmljb2xlLmJhcnRAc3ZoYS5vcmcuYXU=
Specialty Section: This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
 Nicole K. Bart
Nicole K. Bart Alice Powell5,6
Alice Powell5,6 Peter S. Macdonald
Peter S. Macdonald