- 1Department of Cardiology, Peking University People’s Hospital, Beijing, China
- 2Center for Cardiovascular Translational Research, Peking University People’s Hospital, Beijing, China
- 3Beijing Key Laboratory of Early Prediction and Intervention of Acute Myocardial Infarction, Peking University People’s Hospital, Beijing, China
Aim: The purpose of the study was to assess the incidence and predictors of left ventricular function change in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI.
Methods: 312 patients with STEMI who received primary percutaneous coronary intervention (PCI) between January 2015 and December 2016 were consecutively enrolled in this study. Multiple logistic regression analysis was used to evaluate independent predictors of left ventricular ejection fraction (LVEF) improvement after long-term follow-up.
Results: We finally analyzed the LVEF change in 186 patients from baseline to follow-up. The mean age was 61.3 ± 12.5 years, with 78.5% being male. The median duration of follow-up after STEMI was 1,021 (389–1,947) days. 54.3% had a decrease in LVEF and 45.7% experienced an improvement in LV function after primary PCI through long-term follow-up. Logistic regression analysis showed lower peak troponin I, non-anterior STEMI, lower baseline LVEF, and no previous myocardial infarction history were independently associated with LVEF improvement.
Conclusion: 54.3% of patients with STEMI undergoing primary PCI had a decrease in LVEF during long-term follow-up. LVEF recovery can be predicted by baseline characteristics.
Introduction
Heart failure (HF) is a major public health problem worldwide. China has experienced an epidemiological transition during the past several decades. The China Hypertension Survey demonstrated that the prevalence of HF is 1.3% in adults ≥35 years (1). The prevalence of HF will continue to increase as the aging of population and the growing incidence of cardiovascular risk factors. With the changes in diseases spectrum in China, the proportion of coronary heart disease and hypertension in the causes of HF is increasing. The China-HF study showed that the proportion of coronary heart disease in patients with HF is 49.6% (2). ST-segment elevation myocardial infarction (STEMI) is an important manifestation of coronary heart disease. Great improvement has been made in the management of STEMI, but HF after STEMI remains a problem. HF early after STEMI is related to adverse prognosis (3). Left ventricular ejection fraction (LVEF) can change dynamically during chronic LV remodeling after STEMI (4). The extent of cardiac chronic remodeling is associated with the development of HF. Primary percutaneous coronary intervention (PCI) is widely used in STEMI nowadays, and LV systolic dysfunction at the remote phase of STEMI remains poorly elucidated. We aim to identify the incidence and predictors of LVEF change several years after PCI in STEMI patients.
Methods
Study population
This retrospective study was performed at Peking University People's Hospital in China. Between January 2015 and December 2016, we consecutively enrolled 312 patients with STEMI who received primary PCI. We then screened patients who had both baseline and follow-up LVEF measurements. Exclusion criteria included: without receiving primary PCI; death in the hospital; without baseline and follow-up LVEF values. Diagnosis of STEMI was based on the universal definition of MI (5). The Ethics Review Board of Peking University People's Hospital reviewed and approved this study. All procedures were conducted in accordance with the guidelines of the Helsinki Declaration.
Data collection
We collected demographic information and clinical characteristics such as medical history, laboratory examinations, and coronary angiography images of the eligible patients. Transthoracic echocardiography was performed during initial hospitalization and at least 3 months after STEMI, and LVEF was estimated by the standard biplane Simpson method. Patients were divided into two groups according to the LVEF change from baseline to follow-up: improvement (ΔLVEF > 0), decline or no recovery (ΔLVEF ≤ 0).
Statistical analysis
For continuous variables, normally distributed data were reported as the mean ± SD and compared using the Student's t-test; non-parametric data are reported as the interquartile range (25%, 75%) and compared using the Mann-Whitney U-test. Categorical variables were presented as frequency (%) and compared using the χ2 test or Fisher's exact test. Logistic regression analysis was performed to evaluate multivariable predictors of LVEF improvement at follow-up. The variables with p < 0.10 in the univariate analysis and clinically significant variables entered in the multivariate analysis. Then the final predictive model was built with the independent predictors, which was developed by assigning weighted points according to the method of risk score establishment proposed in the Framingham Study (6). p values < 0.05 was considered statistically significant.
Results
Among 312 patients with STEMI who underwent primary PCI were eligible for this study, 126 patients were excluded. Thus, 186 patients were finally analyzed (Figure 1).
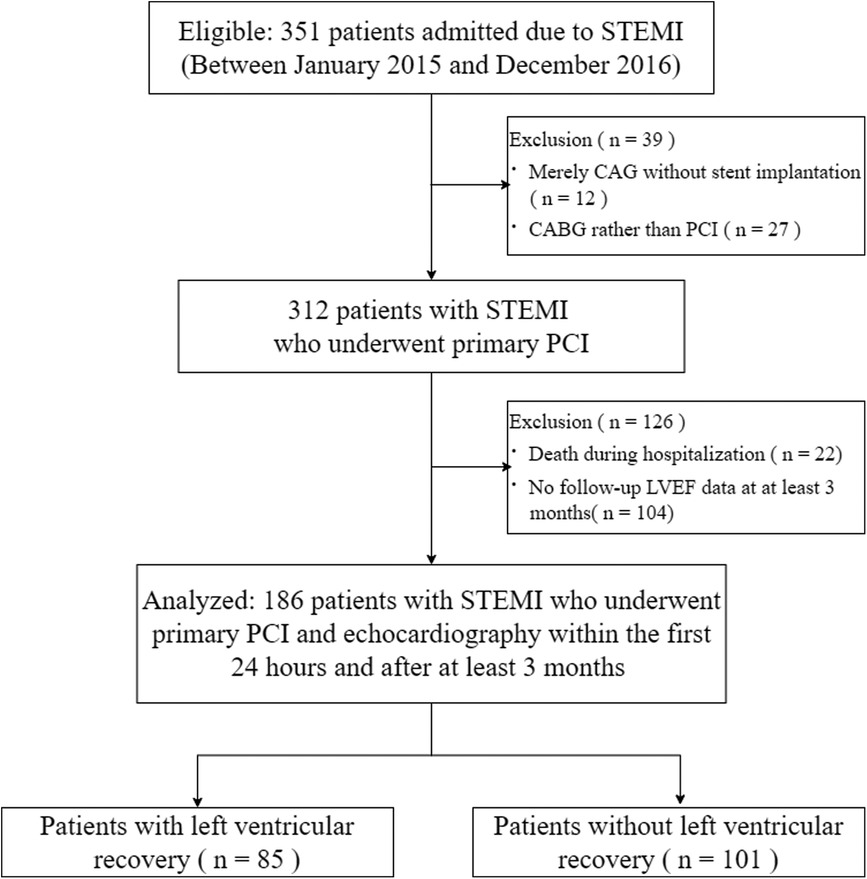
Figure 1. Study flowchart. STEMI, ST-segment elevation myocardial infarction; CAG, coronary angiography; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction.
Patient clinical characteristics during index hospitalization for STEMI
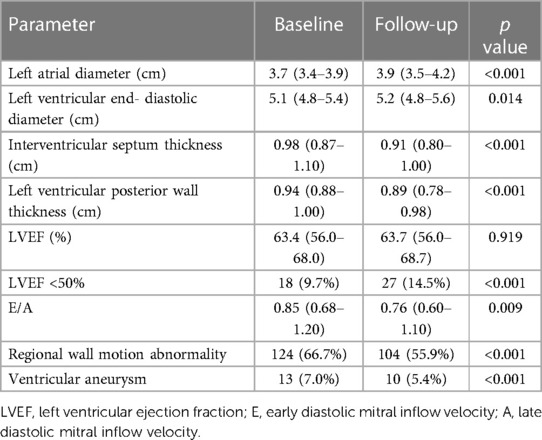
Table 1 summarized the baseline clinical characteristics of these patients. The mean age of the patients was 61.3 ± 12.5 years, with 78.5% being male. In terms of cardiovascular risk factors, more than half of the patients had hypertension. 32.3% had type 2 diabetes, and 21.5% had hyperlipemia. The patients whose infarction-related artery was left anterior descending (LAD) accounted for 51.1%. And the patients with left circumflex and right coronary as the culprit vessel were 16.2% and 34.4% respectively. The mean total ischemic time (time from onset-to-balloon) was 8.0 (4.0–24.0) h. And there were 135 (72.6%) patients with TIMI flow grade 3. Most patients received standard medications at discharge. 126 patients were excluded because of death during initial hospitalization (22) and without follow-up LVEF values (104). We compared patients with and without follow-up LVEF values in Supplementary Table S1.
Patient echocardiographic characteristics from baseline to follow-up
After a median follow-up of 1,021 (389–1,947) days, although 85 (45.7%) patients showed left ventricular function recovery (i.e., ΔLVEF > 0), there were no significant differences in LV function between baseline and follow-up echocardiography. However, there are more patients with LVEF < 50% at follow-up (p < 0.001). Left atrial diameter was greater at follow-up (p < 0.001). Another important echocardiographic measurement, the left ventricular end-diastolic diameter, also increased from baseline (p = 0.014) (Table 2). Left ventricular wall thickness including interventricular septum and left ventricular posterior wall became thinner (p < 0.001).
Predictors of left ventricular ejection fraction change
85 (45.7%) of all patients showed left ventricular function recovery. In patients with left ventricular function recovery, both the peak troponin I [13.75 (4.93–39.81) vs. 36.30 (8.37–80.0), p = 0.010] and peak CK-MB [41.1 (14.3–175.2) vs. 111.5 (28.9–294.0), p = 0.008] were significantly lower (Table 3). The symptom onset-to-balloon time was also shorter in these patients [5.33 (3.75–15.50) vs. 11.93 (5.00–24.00), p = 0.002].
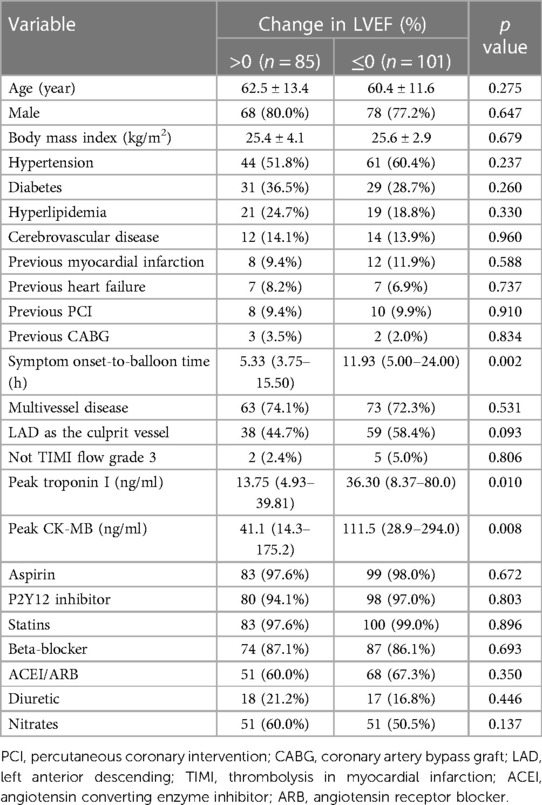
Table 3. Baseline clinical characteristics according to improvement in left ventricular ejection fraction (LVEF).
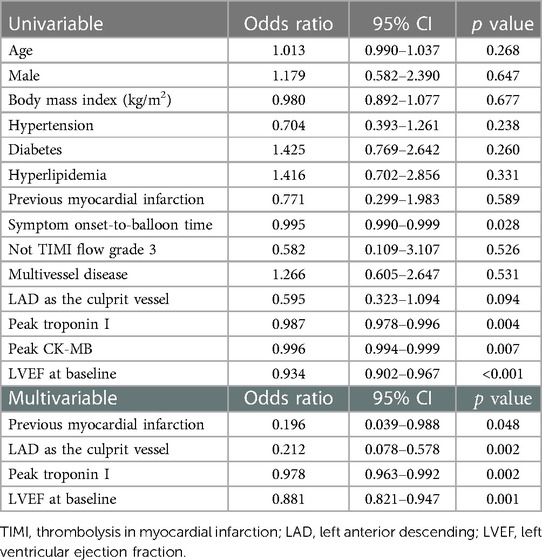
In univariate analysis, the symptom onset-to-balloon time, the peak troponin I, the peak CK-MB, and baseline LVEF were associated with left ventricular function recovery at follow-up. The variables entered into the multivariate analysis were age, sex, hypertension, diabetes, hyperlipidemia, previous myocardial infarction, symptom onset-to-balloon time, LAD as the culprit vessel, peak troponin I, peak CK-MB, and LVEF at baseline. The multivariate analysis demonstrated that previous history of myocardial infarction (OR: 0.196, 95% CI: 0.039–0.988, p = 0.048), LAD as the culprit vessel (OR: 0.212, 95% CI: 0.078–0.578, p = 0.002), peak troponin I (OR: 0.978, 95% CI: 0.963–0.992, p = 0.002) and LVEF at baseline (OR: 0.881, 95% CI: 0.821–0.947, p = 0.001) were independent predictors of LV function recovery (Table 4).
The predictive model for left ventricular function recovery
The points were assigned based on regression coefficients in the multivariate logistic regression model and we established a final predictive model as: 1.628 × previous myocardial infarction + 1.551 × LAD as the culprit vessel + 0.023 × peak troponin I + 0.126 × LVEF at baseline. Using ROC curve analysis, the optimal cut-off value of the predictive model in predicting left ventricular function recovery was 9.85 (sensitivity 0.800, specificity 0.710, positive predictive value 0.690, negative predictive value 0.815, AUC 0.768, 95% CI: 0.697–0.840, p < 0.001) (Figure 2).
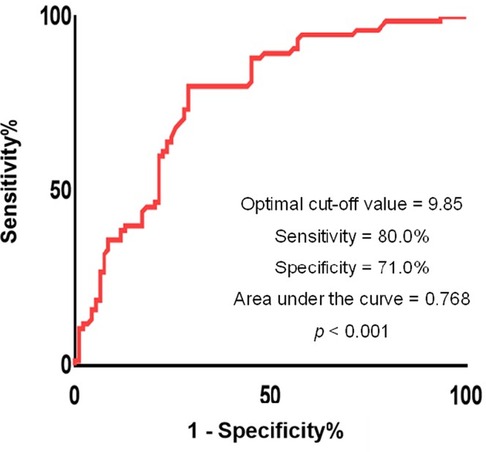
Figure 2. Receiver operating characteristics curves of the proposed predictive model. The optimal threshold of the model for predicting left ventricular recovery was −0.57 with sensitivity 0.800, specificity 0.710, and area under the curve 0.768, respectively (p < 0.001).
There were 87 patients with a predictive model score less than 9.85. Compared to the patients with a predictive model score more than 9.85, patients with a predictive model score less than 9.85 had shorter total ischemic time (p = 0.005), and lower peak CK-MB (p < 0.001). No significant difference was found in age, sex, cardiovascular risk factors, and most medications at discharge between the two groups (Table 5).
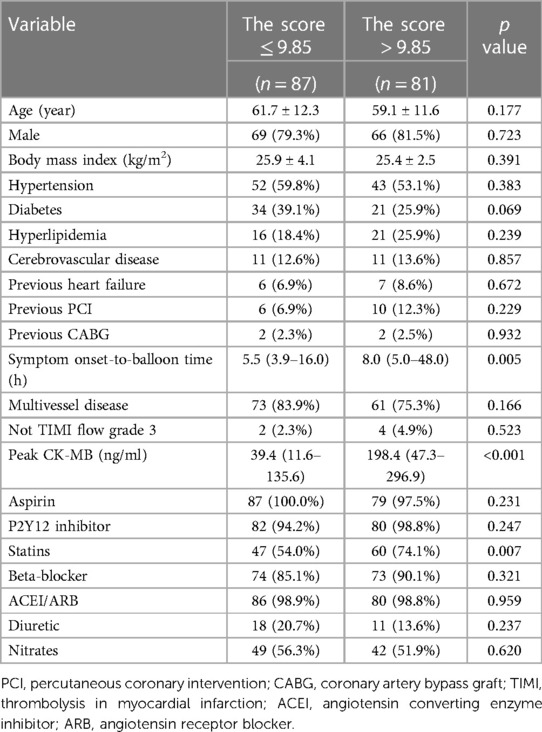
Table 5. Clinical characteristics of patients according to the optimal cut-off value for the predictive model.
Discussion
Our study showed in patients with STEMI, 54.3% had a decrease in LVEF and 45.7% experienced an improvement in LVEF after primary PCI through a nearly 4-year follow-up. Second, lower peak troponin I, non-anterior STEMI, lower baseline LVEF, and no previous myocardial infarction history were independently associated with LVEF improvement from baseline to long-term follow-up.
Rapid progress in primary coronary revascularization has reduced mortality in patients with STEMI, while the increased risk of HF after STEMI has become an emerging clinical problem. The change of LVEF after STEMI is a dynamic process. Myocardium stunning following acute coronary artery occlusions affects the recovery of LV function in the early stage of STEMI (7). However, LV remodeling is related to long-term LVEF recovery (8, 9). In our study, we found that after long-term follow-up, 54.3% of patients with STEMI had a decrease in LVEF, suggesting the effect of myocardial infarction on LV function is a long-term process.
A lower peak troponin I independently correlated with LVEF improvement in the present study, consistent with prior studies (10–15). Overwhelming evidences suggest that infarct size, which was quantified directly by Cardiac Magnetic Resonance Images or Single-Photon Emission Computed Tomography, can predict remodeling of the left ventricle (16, 17). The predictive value of peak troponin I is attributed to its ability to estimate infarct size, as previous studies proved peak troponin I correlated with infarct size (18, 19). Our study showed that non-anterior STEMI was an independent predictor of LV function recovery. A study found that an anterior MI location was associated with adverse remodeling (10). Another study also demonstrated that the worse LV function in anterior STEMI patients is due to its larger MI size (20). However, there is also some supporting evidence to suggest that those patients with the culprit vessel as the LAD were more likely to benefit from reperfusion therapy (21, 22). A lower baseline LVEF was associated with LVEF improvement in our study. The reason for this inverse relationship between baseline LVEF and LVEF improvement remains uncertain. This result may be explained by the greater recovery in LVEF after primary PCI in those patients with more ischemic damage (23). Those patients may have a greater potential for functional recuperation (10). At the same time, quite a few studies suggested the opposite results, that is, patients with a higher baseline LVEF were more likely with left ventricular recovery (15, 24). Studies that dynamically observe LVEF changes after primary PCI in STEMI patients may need further exploration.
In the present study, symptom onset-to-balloon time was not an independent predictor of LVEF improvement. Some studies also reported no correlation between time to reperfusion and LVEF improvement (25, 26). In contrast, some studies showed that the time to reperfusion is important for the recovery of left ventricular function (26). It is possible that total ischemic time is related to other measurements such as peak troponin I. It is also likely that early reperfusion is related to early LV function recovery, but in our study, we focused on the remote phase of STEMI.
Limitation
Several limitations should be noted in our study. First, in this single-center and retrospective study, the sample size is not big enough. Second, left ventricular function measures may have variability. Third, we didn't have time-scheduled LVEF measurements due to the observational design of the study. Finally, the prognostic impact of LVEF change on outcomes in patients with STEMI undergoing primary PCI was not assessed.
Conclusion
More than half of patients with STEMI had a decrease in LVEF during a long-term follow-up. Lower peak troponin I, non-anterior STEMI, lower baseline LVEF, and no previous myocardial infarction history were independent predictors of LVEF improvement from baseline to follow-up.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University People's Hospital. The Ethics Committee waived the requirement of written informed consent for participation.
Author contributions
CL and MG collected the data. CL and MG planned and wrote the manuscript. MG performed the statistical analysis. YC, MW, and HC reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81970301).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewers [LZ, LZ] declared a shared parent affiliation with the authors to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1079647/full#supplementary-material.
References
1. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China hypertension survey, 2012–2015. Eur J Heart Fail. (2019) 21(11):1329–37. doi: 10.1002/ejhf.1629
2. Zhang Y, Zhang J, Butler J, Yang X, Xie P, Guo D, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China heart failure (China-HF) registry. J Card Fail. (2017) 23(12):868–75. doi: 10.1016/j.cardfail.2017.09.014
3. Shimizu A. What are the most useful predictors of cardiac mortality in patients post myocardial infarction? Circ J. (2013) 77(2):319–20. doi: 10.1253/circj.CJ-12-1542
4. Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. (2011) 16(1):13–21. doi: 10.1007/s10741-010-9181-7
5. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138(20):e618–51. doi: 10.1161/CIR.0000000000000617
6. Sullivan LM, Massaro JM, D'Agostmo RB. Presentation of multivariate data for clinical use: the framingham study risk score functions. Stat Med. (2004) 23(10):1631–60. doi: 10.1002/sim.1742
7. Hoole SP, Heck PM, White PA, Read PA, Khan SN, West NEJ, et al. Stunning and cumulative left ventricular dysfunction occurs late after coronary balloon occlusion in humans: insights from simultaneous coronary and left ventricular hemodynamic assessment. JACC Cardiovasc Interv. (2010) 3(4):412–8. doi: 10.1016/j.jcin.2009.12.014
8. Rodriguez-Palomares JF, Gavara J, Ferreira-González I, Valente F, Rios C, Rodríguez-García J, et al. Prognostic value of initial left ventricular remodeling in patients with reperfused STEMI. JACC Cardiovasc Imaging. (2019) 12(12):2445–56. doi: 10.1016/j.jcmg.2019.02.025
9. van der Bijl P, Abou R, Goedemans L, Gersh BJ, Holmes DR Jr., Ajmone Marsan N, et al. Left ventricular post-infarct remodeling: implications for systolic function improvement and outcomes in the modern era. JACC Heart Fail. (2020) 8(2):131–40. doi: 10.1016/j.jchf.2019.08.014
10. Chew DS, Wilton SB, Kavanagh K, Southern DA, Tan-Mesiatowsky LE, Exner DV, et al. Left ventricular ejection fraction reassessment post-myocardial infarction: current clinical practice and determinants of adverse remodeling. Am Heart J. (2018) 198:91–6. doi: 10.1016/j.ahj.2017.11.014
11. Dauw J, Martens P, Deferm S, Bertrand P, Nijst P, Hermans L, et al. Left ventricular function recovery after ST-elevation myocardial infarction: correlates and outcomes. Clin Res Cardiol. (2021) 110(9):1504–15. doi: 10.1007/s00392-021-01887-y
12. Brooks GC, Lee BK, Rao R, Lin F, Morin DP, Zweibel SL, et al. Predicting persistent left ventricular dysfunction following myocardial infarction: the PREDICTS study. J Am Coll Cardiol. (2016) 67(10):1186–96. doi: 10.1016/j.jacc.2015.12.042
13. Parodi G, Memisha G, Carrabba N, Signorini U, Migliorini A, Cerisano G, et al. Prevalence, predictors, time course, and long-term clinical implications of left ventricular functional recovery after mechanical reperfusion for acute myocardial infarction. Am J Cardiol. (2007) 100(12):1718–22. doi: 10.1016/j.amjcard.2007.07.022
14. Bauters C, Fertin M, Delhaye C, Goeminne C, Le Tourneau T, Lamblin N, et al. Late recovery in left ventricular systolic function after discharge of patients with a first anterior myocardial infarction. Arch Cardiovasc Dis. (2010) 103(10):538–45. doi: 10.1016/j.acvd.2010.10.001
15. Lei Z, Li B, Li B, Peng W. Predictors and prognostic impact of left ventricular ejection fraction trajectories in patients with ST-segment elevation myocardial infarction. Aging Clin Exp Res. (2022) 34(6):1429–38. doi: 10.1007/s40520-022-02087-y
16. Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. (2008) 94(6):730–6. doi: 10.1136/hrt.2007.122622
17. Kosmidou I, Redfors B, Selker HP, Thiele H, Patel MR, Udelson JE, et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: results from an individual patient-level pooled analysis of 10 randomized trials. Eur Heart J. (2017) 38(21):1656–63. doi: 10.1093/eurheartj/ehx159
18. Arruda-Olson AM, Roger VL, Jaffe AS, Hodge DO, Gibbons RJ, Miller TD. Troponin T levels and infarct size by SPECT myocardial perfusion imaging. JACC Cardiovasc Imaging. (2011) 4(5):523–33. doi: 10.1016/j.jcmg.2011.03.010
19. Steen H, Ciannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 h after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol. (2006) 48(11):2192–4. doi: 10.1016/j.jacc.2006.06.002
20. Masci PG, Ganame J, Francone M, Desmet W, Lorenzoni V, Iacucci I, et al. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur Heart J. (2011) 32(13):1640–8. doi: 10.1093/eurheartj/ehr064
21. Heusch G, Gersh BJ. Is cardioprotection salvageable? Circulation. (2020) 141(6):415–7. doi: 10.1161/CIRCULATIONAHA.119.044176
22. Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities. Heart. (2016) 102(5):341–8. doi: 10.1136/heartjnl-2015-307855
23. Serrao GW, Lansky AJ, Mehran R, Stone GW. Predictors of left ventricular ejection fraction improvement after primary stenting in ST-segment elevation myocardial infarction (from the harmonizing outcomes with revascularization and stents in acute myocardial infarction trial). Am J Cardiol. (2018) 121(6):678–83. doi: 10.1016/j.amjcard.2017.12.004
24. Otero-Garcia O, Cid-Alvarez AB, Juskova M, Alvarez-Alvarez B, Tasende-Rey P, Gude-Sampedro F, et al. Prognostic impact of left ventricular ejection fraction recovery in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: analysis of an 11-year all-comers registry. Eur Heart J Acute Cardiovasc Care. (2021) 10(8):898–908. doi: 10.1093/ehjacc/zuab058
25. Halkin A, Stone GW, Dixon SR, Grines CL, Tcheng JE, Cox DA, et al. Impact and determinants of left ventricular function in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction. Am J Cardiol. (2005) 96(3):325–31. doi: 10.1016/j.amjcard.2005.03.069
26. Brodie BR, Stuckey TD, Wall TC, Kissling G, Hansen CJ, Muncy DB, et al. Importance of time to reperfusion for 30-day and late survival and recovery of left ventricular function after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. (1998) 32(5):1312–9. doi: 10.1016/S0735-1097(98)00395-7
Keywords: ST-segment elevation myocardial infarction, primary PCI, echocardiography, left ventricular ejection fraction, left ventricular recovery
Citation: Liu C, Guo M, Cui Y, Wu M and Chen H (2023) Incidence and predictors of left ventricular function change following ST-segment elevation myocardial infarction. Front. Cardiovasc. Med. 10:1079647. doi: 10.3389/fcvm.2023.1079647
Received: 25 October 2022; Accepted: 13 March 2023;
Published: 30 March 2023.
Edited by:
Jingyi Ren, China-Japan Friendship Hospital, ChinaReviewed by:
LiLi Zhang, Aerospace Clinical Medical College of Peking University, ChinaLingyun Zu, Peking University Third Hospital, China
Yuan-Lin Guo, Chinese Academy of Medical Sciences, China
Jinxing Liu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
© 2023 Liu, Guo, Cui, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Chen Y2hlbmhvbmdiakBtZWRtYWlsLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
 Chuanfen Liu
Chuanfen Liu Meng Guo
Meng Guo Yuxia Cui1,2,3
Yuxia Cui1,2,3 Hong Chen
Hong Chen