
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 21 March 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1079332
Background: Whether guided antiplatelet therapy in patients with acute coronary syndrome (ACS) is effective in improving net clinical benefits compared with conventional antiplatelet therapy remains controversial. Therefore, we assessed the safety and efficacy of guided antiplatelet therapy in patients with ACS and undergoing percutaneous coronary intervention.
Method: We searched PubMed, EMBASE, and Cochrane Library databases to select the relevant randomized controlled trials comparing the guided and conventional antiplatelet therapy in patients with ACS. The primary and safety outcomes are major adverse cardiovascular events (MACE) and major bleeding, respectively. The efficacy outcomes included myocardial infarction, stent thrombosis, all-cause death, and cardiovascular death. We selected the relative risk (RR) and 95% confidence intervals (CIs) as effect size and calculated it using the Review Manager software. In addition, we evaluated the final results by trial sequential analysis (registered by PROSPERO, CRD 42020210912).
Results: We selected seven randomized controlled trials and included 8,451 patients in this meta-analysis. Guided antiplatelet therapy can significantly reduce the risk of MACE (RR 0.64, 95% CI 0.54–0.76, P < 0.00001), myocardial infarction (RR 0.62, 95% CI 0.49–0.79, P = 0.0001), all-cause death (RR 0.61, 95% CI 0.44–0.85, P = 0.003), and cardiovascular death (RR 0.66, 0.49–0.90, P = 0.009). In addition, there is no significant difference between the two groups in stent thrombosis (RR 0.67, 95% CI 0.44–1.03, P = 0.07) and major bleeding (RR 0.86, 95% CI 0.65–1.13, P = 0.27). The subgroup analysis showed that the guided group based on genotype tests could bring benefits in MACE and myocardial infarction.
Conclusions: The guided antiplatelet therapy is not only associated with a comparable risk of bleeding but also with a lower risk of MACE, myocardial infarction, all-cause death, cardiovascular death, and stent thrombosis than the conventional strategy in patients with ACS.
Dual antiplatelet therapy (DAPT) is a cornerstone for preventing ischemic complications in patients with acute coronary syndrome (ACS) and undergoing percutaneous coronary intervention (PCI) (1, 2). Clopidogrel is the most common type of P2Y12 receptor inhibitor, which needs to be converted to its active metabolite through cytochrome P450 (CYP2C19) enzymes in the liver (3, 4). However, there are individual differences in metabolic processes. In clinical practice, some patients could have high platelet reactivity (HPR) and/or CYP2C19 loss-of-function alleles, which are associated with higher thrombosis risk (4–6). On the contrary, the newer generation P2Y12 inhibitors, prasugrel and ticagrelor, have potent effects in inhibiting the aggregation of platelet and are not modulated by CYP2C19 genes (4, 7). Hence, the potent P2Y12 inhibitors could reduce the ischemic risk than clopidogrel in patients mentioned above, and clopidogrel could be fitter for patients without HPR or CYP2C19 loss-of-function alleles (8, 9). Meanwhile, there is higher risk of thrombosis in patients with ACS. Both the American Heart Association and European Society of Cardiology guidelines recommended that prasugrel or ticagrelor should be considered for patients with ACS (10, 11). However, the application of a potent P2Y12 inhibitor will increase the risk of bleeding and cause financial burden (8, 9). Therefore, genotype and platelet function testing have been two new potential approaches to choosing the optional P2Y12 inhibitor based on the individual difference.
A series of clinical trials have verified the efficacy and safety of shortening the duration of DAPT. Therefore, the 2017 European Society of Cardiology guideline recommended that patients with ACS should accept 12 months of DAPT, and the duration can be adjusted according to the risk of ischemia and bleeding (10, 11). However, the risk of ischemia and bleeding is hard to evaluate, and the individual antiplatelet strategy could be the best strategy for patients with ACS. Platelet function and genetic testing in patients after PCI represent two new schemes to guide antiplatelet therapy (4). More recent studies compared the guided and conventional strategies but have not provided an unequivocal result (12–14).
Therefore, we aim to explore the safety and efficacy of guided comparing conventional selection of antiplatelet therapy in patients with ACS. Related subgroup analysis was also performed to explore the impact of different strategies. In addition, trial sequential analysis (TSA) was used to assess the outcomes.
We implemented this meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guideline (15). We searched PubMed, EMBASE, and Cochrane Library databases for related randomized controlled trials, which compared guided antiplatelet therapy strategies (platelet function or genetic testing) with the conventional antiplatelet therapy. We also screened the abstracts of the scientific conferences in recent 3 years, such as the American Heart Association, American College of Cardiology, Transcatheter Cardiovascular Therapeutics, European Society of Cardiology, and Congress of the European Association of Percutaneous Cardiovascular Interventions. The major search terms in PubMed are as follows: “acute coronary syndrome” OR “percutaneous coronary intervention” AND “clopidogrel” OR “aspirin” OR “P2Y12 inhibitor” OR “prasugrel” OR “ticagrelor” AND “Randomized Controlled Trial”. The language was not limited in the process of literature retrieval and the detailed search strategy is shown (Supplementary Tables S1–S3).
The inclusion criteria of this meta-analysis met the following requirements: (a) patients with ACS and undergoing percutaneous coronary intervention; (b) compared guided and conventional antiplatelet therapy; (c) follow-up duration ≥6 months; (d) reported the efficacy and/or safety outcomes; and (e) randomized controlled trials. The exclusion criteria included: (a) reduplicate report and insufficient data from original studies and (b) nonrandomized controlled trial.
We conducted quality assessment using the Cochrane tool of Collaboration. Meanwhile, Grades of Recommendations Assessment, Development and Evaluation (GRADE) was applied to evaluate the quality of each outcome (16, 17). The study was registered in PROSPERO (CRD42020210912). The primary outcome is major adverse cardiovascular events (MACE), which are composed of death, myocardial infarction, stroke, stent thrombosis, or bleeding. The efficacy outcomes included all-cause death, cardiovascular death, myocardial infarction, and definite or probable stent thrombosis. We selected major bleeding as the safety outcome, but there are different definitions of major bleeding in different trials. As a result of Bleeding Academic Research Consortium (BARC) 3 or 5 is same as TIMI minor or major, the major bleeding of this study mainly include those two types of bleeding.
The data from randomized controlled trials based on intention-to-treat analysis were extracted independently by two investigators (P-YZ and J-PD). We selected the relevant trials by screening initially all the titles and abstracts. After that, eligible trials were included by reviewing the full-text articles of the relevant studies. Any disagreement was solved through a discussion with a third party. In addition, the baseline characteristics were also independently extracted by two researchers, and the discrepancy was resolved through negotiation with one of the authors (TL).
We performed the statistical analysis by Review Manager version 5.4 (Revman, The Cochrane Collaboration, Oxford, United Kingdom) and STATA 14.1 (StataCorp, College Station, TX, United States). The effect size is the relative risk (RR) with 95% confidence intervals (95% CI), which was calculated by the fixed-effects model based on the M-H statistical method. The P value of the chi-square test was used to evaluate heterogeneity, and the I2 index was employed to summarize the degree of heterogeneity. Significant heterogeneity was found, when the P value was <0.1 in comparison within groups or it was ≤0.05 in comparison among groups. The I2 values of 25%, 50%, and 75% correspond to low, moderate, and high heterogeneity, respectively (18). Subgroup analysis was performed according to (a) the different guided strategies and (b) de-escalation and escalation strategies. Publication bias was assessed by Egger's and Begg's tests, as well as the visual funnel plot. Trial sequential analysis version 0.9.5.10 software (Copenhagen Trial Unit, CTU) was applied to assess the results (available from https://www.ctu.dk/tsa).
The process of literature screening and study selection is shown in Figure 1. A total of 2,826 randomized controlled trials were retrieved from PubMed, EMBASE, and Cochrane library databases. Finally, seven randomized controlled trials met the inclusion criteria after reviewing 27 full-text articles (19–25).
The characteristics of the included trials are shown (Table 1). Five and two randomized controlled trials applied genetic testing and platelet function testing, respectively. Meanwhile, this study included escalation and de-escalation strategies. The baseline characteristics of the patient are shown in Table 2. There were no significant distinctions in clinical presentations between the guided and conventional groups.
All included studies reported the incidence of MACE between the guided and conventional groups (Figure 2 and Supplementary Tables S2, S3). Compared with conventional group, the guided group can significantly reduce the incidence of MACE (RR 0.64, 95% CI 0.54–0.76, P < 0.00001, I2 = 68%, Pheterogeneity = 0.004). There is statistical heterogeneity, and we conducted subgroup analyses to find the possible reason. This result is consistent in the escalation subgroup (RR 0.34, 95% CI 0.23–0.50, P < 0.00001, I2 = 0%, Pheterogeneity = 0.85), but the incidence of MACE was not reduced in the guided group in de-escalation (RR 0.89, 95% CI 0.69–1.14, P = 0.34, I2 = 0%, Pheterogeneity = 0.53). Meanwhile, there is significant statistical distinction between escalation and de-escalation subgroups (Pinteraction < 0.00001). In addition, guided group based on genotype testing also can reduce the MACE risk (RR 0.54, 95% CI 0.44–0.66, P < 0.00001, I2 = 61%, Pheterogeneity = 0.04). However, this risk not decreased in the guided group based on platelet function (RR 0.91, 95% CI 0.67–1.23, P = 0.55, I2 = 14%, Pheterogeneity = 0.28), and two subgroups have significant distinction (Pinteraction < 0.005). Similarly, there is a significant distinction in the genotype and platelet function testing subgroups (Pinteraction < 0.005).
All the trails reported myocardial infarction and stent thrombosis (Figure 3). Compared with the conventional antiplatelet therapy, the guided group had a significantly reduced risk of myocardial infarction (RR 0.62, 95% CI 0.49–0.79, P = 0.0001, I2 = 54%, Pheterogeneity = 0.04). On the contrary, there are no significant differences between the two groups in stent thrombosis (RR 0.65, 95% CI 0.43–1.00, P = 0.05, I2 = 0%, Pheterogeneity = 0.58). The all-cause death and cardiovascular death are reported in six and five trials, respectively (Figure 3). The guided group is associated with a lower risk of all-cause death than the conventional group (RR 0.61, 95% CI 0.44–0.85, P = 0.003, I2 = 53%, Pheterogeneity = 0.06). Similarly, the guided group also has a lower risk of cardiovascular death (RR 0.66, 95% CI 0.49–0.90, P = 0.009, I2 = 48%, Pheterogeneity = 0.09) than the conventional group.
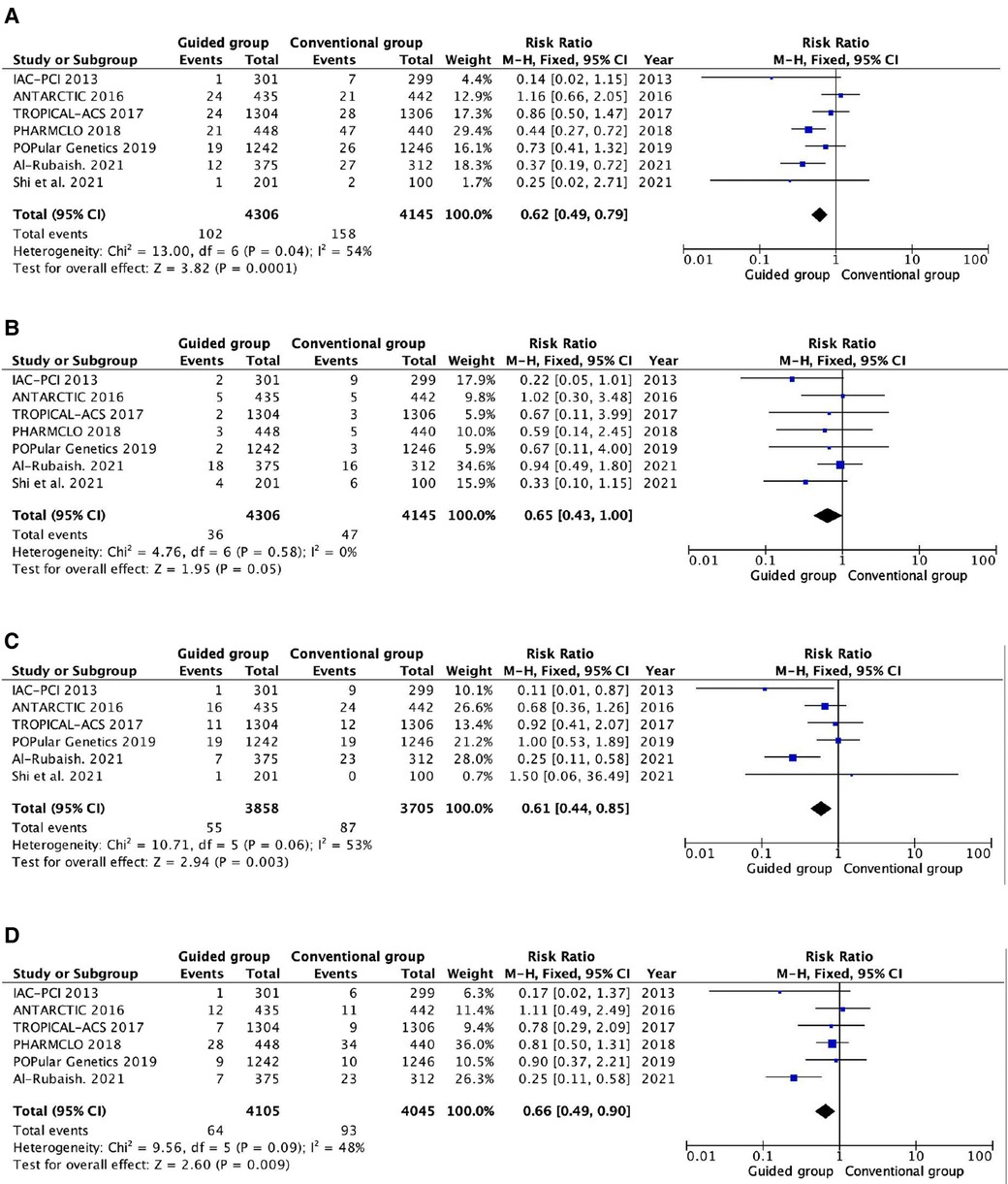
Figure 3. Pooled analyses of guided vs. standard antiplatelet therapy for the efficacy outcomes. (A) Myocardial infarction; (B) stent thrombosis; (C) all-cause death; (D) cardiovascular death.
High heterogeneity was found in myocardial infarction, all-cause death, and cardiovascular death. We conducted two subgroup analyses to find the possible reason, and the results are shown in Supplementary Tables S2, S3. The distinction of the guided groups based on escalation and de-escalation strategies could be the most reasonable cause for the heterogeneity. In the escalation subgroup, no heterogeneity was found and the guided group had a significantly reduced incidence of myocardial infarction (RR 0.32, 95% CI 0.17–0.59, P = 0.0002, I2 = 0%, Pheterogeneity = 0.67), all-cause death (RR 0.24, 95% CI 0.12–0.50, P = 0.0001, I2 = 0%, Pheterogeneity = 0.40), and cardiovascular death (RR 0.24, 95% CI 0.11–0.51, P = 0.0003, I2 = 0%, Pheterogeneity = 0.71). However, there is no significant distinction between guided and conventional groups for those outcomes in the de-escalation subgroup.
In addition, the difference between guided antiplatelet therapy based on genotype and platelet function testing could be also another reason for heterogeneity. In the genotype testing subgroup, heterogeneity could be significantly reduced. The guided group is associated with a lower risk of myocardial infarction (RR 0.47, 95% CI 0.34–0.64, P < 0.00001, I2 = 8%, Pheterogeneity = 0.36), cardiovascular death (RR 0.24, 95% CI 0.11–0.51, P = 0.0003, I2 = 0%, Pheterogeneity = 0.7), and stent thrombosis (RR 0.61, 95% CI 0.38–0.98, P = 0.04, I2 = 7%, Pheterogeneity = 0.37). On the contrary, the guided group has a similar risk of myocardial infarction, cardiovascular death, and stent thrombosis compared to the conventional group in the platelet function testing.
All the trials reported the incidence of major bleeding (Figure 4 and Supplementary Tables S2, S3). There were no significant differences and heterogeneity in major bleeding between the guided and conventional groups (RR 0.86, 0.65–1.13, P = 0.27, I2 = 23%, Pheterogeneity = 0.26). The subgroup analysis suggested that no difference was found between escalation and de-escalation subgroups. Meanwhile, the different types of testing also did not impact the risk of major bleeding.
The quality assessment of each trial and the quality assessment of GRADE evidence are shown in the supplementary materials (Supplementary Figure S1 and Table S4). There is a low risk of bias in selection, detection, and reporting, but there is a high risk of bias in performance in three out of seven trials. The quality assessments of GRADE evidence of major bleeding is moderate, and other outcomes are high. The trial sequential analysis of each outcome was performed, and the results are shown in Supplementary Figure S2. The curves of MACE and myocardial infarction are beyond the TSA boundary. All-cause death and cardiovascular death were beyond the conventional boundary but did not reach the TSA boundary and met the expected sample size. The curves of stent thrombosis and major bleeding did not reach both the conventional boundary and the expected sample size. The expected sample size of both stent thrombosis and major bleeding is 19,606. The publication bias evaluation of each outcome shows that the spots of the funnel plot were symmetrically distributed, and the P values of Begg's and Egger's tests are more than 0.05 (Supplementary Figure S3 and Table S5).
The results of this meta-analysis were based on seven randomized controlled trials and 8,451 patients. The results showed that the guided group is associated with similar efficacy and lower safety than the conventional group. In addition, the guided group based on escalation strategy has a significantly reduced incidence of ischemic events and primary outcomes. Guided groups based on genotype testing and escalation strategies are associated with a lower risk of MACE and myocardial infarction.
The DAPT is effective in inhibiting platelet reactivity and preventing ischemic events in patients with ACS after PCI (26). However, it is doubtful whether guided DAPT can improve efficacy while decreasing bleeding risk. Ticlopidine, the first-generation P2Y12 inhibitor, was launched in the 1990s, but it was linked with several severe side effects (27). Aspirin combined with clopidogrel can reduce the risk of thrombotic events while maintaining an acceptable safety profile compared to aspirin monotherapy, which has been widely used in clinical practice. However, unlike the potent P2Y12 inhibitors, there are large individual differences in the metabolism process of clopidogrel, which may result in severe HPR. Furthermore, HPR is linked to thrombosis, which can be caused by a variety of conditions including age, BMI, chronic renal disease, and diabetes. In addition, as potent P2Y12 inhibitors, ticagrelor and prasugrel can further reduce the risk of ischemia than clopidogrel, but they bring higher risk of bleeding simultaneously (9). Therefore, potent P2Y12 receptor inhibitors can bring more ischemic benefit than clopidogrel in patients with HPR or CYP2C19 loss-of-function alleles. Based on the above-mentioned rationale, platelet function and genetic testing were applied to select the best antiplatelet agent to achieve individual therapy (4, 7, 28).
Galli et al. (29) performed a meta-analysis to compare the efficacy and safety of the guided and conventional groups. The results showed that guided antiplatelet therapy was associated with a lower incidence of MACE (RR 0.78, 95% CI 0.63–0.95, P = 0.015), cardiovascular death (RR 0.77, 95% CI 0.59–1.00, P = 0.049), myocardial infarction (RR 0.76, 95% CI 0.60–0.96, P = 0·021), and stent thrombosis (RR 0.64, 95% CI 0.46–0.89, P = 0.011). Meanwhile, the guided strategy had a lower bleeding risk but was not statistically significant (RR 0.88, 95% CI 0.77–1.01, P = 0.069). Our study is consistent with this meta-analysis. The subgroup analysis of this study showed that the escalation approach was associated with a significant reduction in ischemic events without any trade-off in safety, and the de-escalation approach was associated with a significant reduction in bleeding events, without any trade-off in efficacy.
A series of factors will affect the conclusions in clinical practice. At first, East Asians could be associated with higher bleeding risk and lower ischemic risk than Caucasians (30). Therefore, short-term DAPT composed of aspirin and clopidogrel could be fitter for them. However, the incidence of clopidogrel hyporesponsiveness in East Asian patients was higher than that in Caucasians, which may be due to their unique cytochrome gene polymorphism (30, 31). In addition, East Asians have a high rate of CYP2C19 loss-of-function alleles, and guided antiplatelet therapy could be more suited for East Asian patients. Second, although potent P2Y12 inhibitors (prasugrel or ticagrelor) are recommended in patients with ACS, clopidogrel is still applied in clinical practice, especially for old patients. There are significant differences between escalation and de-escalation strategies. Compared with clopidogrel, the guided strategy of escalation aims to increase efficacy by switching from clopidogrel to prasugrel or ticagrelor. Our study suggested that the guided group is correlated with higher efficacy than clopidogrel. Furthermore, the guided strategy would result in de-escalation, which is associated with lower risk of bleeding and without compromising efficacy. Our result is correlated with the recommendations, which showed a guided approach with compromising efficacy but not reducing the risk of bleeding.
At last, both genetic and platelet tests have superiority and inferiority in clinical practice. By characterizing the platelet phenotype, platelet function tests could better relate to thrombosis (32). However, platelet function tests require patients to be treated with clopidogrel to determine responsiveness and escalate to a more potent P2Y12 inhibitor when patients have HPR (32). In addition, there are variabilities in the test results. Therefore, it is a challenge to implement platelet function test monitoring in nonspecialized centers (33). On the contrary, genetic testing for CYP2C19 loss-of-function alleles can now be achieved by rapid bedside testing, which can overcome some of the limitations mentioned above (32). However, multiple factors can influence the effectiveness of antiplatelet agents including age, body mass index, and chronic kidney disease. Therefore, assessing only genetic polymorphisms may have limited accuracy in identifying patients with HPR status. Combining multiple clinical variables with genotype to predict HPR status is considered to have greater accuracy than the individual components.
This meta-analysis of randomized control trials has several limitations. First, this meta-analysis is based on study-level data and not based on the individual data. The majority of included trials are open-label, which may result in the risk of bias. Second, there are many distinctions in the baseline characteristics of included randomized controlled trials. For example, there are different rates of patients with ST-elevation myocardial infarction and durations of follow-up. Third, only two included trials researched the guided strategy based on platelet function, which does not bring clinical benefits for patients with ACS. Hence, the efficacy of platelet function needs to be tested by more trials in the future. Finally, there is high heterogeneity in primary and efficacy outcomes, and we have found possible reasons by subgroup analysis. Therefore, the main conclusions are stated by the results of the subgroup analysis, which included the limited number of patients and the need for a larger sample of randomized control trials.
This systematic review and meta-analysis demonstrates that guided antiplatelet therapy after PCI was associated with a lower risk of MACE, myocardial infarction, all-cause death, and cardiovascular death in patients with ACS. Meanwhile, the guided strategy based on genotype testing could reduce the risk of MACE and myocardial infraction. At last, the guided group based on escalation strategy did not have increased risk of bleeding and improved the net clinical benefit, but the guided group based on the de-escalation strategy did not obtain net clinical benefits for patients with ACS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
P-YZ: study design, data collection, statistical analysis, and manuscript writing. J-PD: data collection, statistical analysis, and verification. J-HZ, LP, and H-YW: data collection and verification. TL: scientific revision of the manuscript. All authors contributed to the article and approved the submitted version.
First of all, I (P-Y Z) would like to express my deepest gratitude to TL, an honorable, responsible, and resourceful scholar, has provided me with valuable guidance at every stage of writing this thesis. I would also like to thank all my colleagues who helped me to complete this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1079332/full#supplementary-material.
1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 40(2):87–165. doi: 10.1093/eurheartj/ehy394
2. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
3. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC Guidelines on dual antiplatelet therapy: JACC Guideline comparison. J Am Coll Cardiol. (2018) 72(23 Pt A):2915–31. doi: 10.1016/j.jacc.2018.09.057
4. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. (2019) 12(16):1521–37. doi: 10.1016/j.jcin.2019.03.034
5. Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. (2015) 36(27):1762–71. doi: 10.1093/eurheartj/ehv104
6. Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. (2009) 302(8):849–57. doi: 10.1001/jama.2009.1232
7. Moon JY, Franchi F, Rollini F, Rivas Rios JR, Kureti M, Cavallari LH, et al. Role of genetic testing in patients undergoing percutaneous coronary intervention. Expert Rev Clin Pharmacol. (2018) 11(2):151–64. doi: 10.1080/17512433.2017.1353909
8. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2007) 357(20):2001–15. doi: 10.1056/NEJMoa0706482
9. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361(11):1045–57. doi: 10.1056/NEJMoa0904327
10. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. (2016) 134:e123–55. doi: 10.1016/j.jtcvs.2016.07.044
11. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
12. Tuteja S, Glick H, Matthai W, Nachamkin I, Nathan A, Monono K, et al. Prospective CYP2C19 genotyping to guide antiplatelet therapy following percutaneous coronary intervention: a pragmatic randomized clinical trial. Circ Genom Precis Med. (2020) 13(1):e002640. doi: 10.1161/CIRCGEN.119.002640
13. Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. (2020) 324(8):761–71. doi: 10.1001/jama.2020.12443
14. Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. (2012) 367(22):2100–9. doi: 10.1056/NEJMoa1209979
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2020) 372:n71. doi: 10.1136/bmj.n71
16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
17. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
19. Xie X, Ma YT, Yang YN, Li XM, Zheng YY, Ma X, et al. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: a randomized control trial. Int J Cardiol. (2013) 168(4):3736–40. doi: 10.1016/j.ijcard.2013.06.014
20. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. (2019) 381(17):1621–31. doi: 10.1056/NEJMoa1907096
21. Shi X, Zhang Y, Zhang Y, Zhang R, Lin B, Han J, et al. Personalized antiplatelet therapy based on CYP2C19 genotypes in Chinese ACS patients undergoing PCI: a randomized controlled trial. Front Cardiovasc Med. (2021) 8:676954. doi: 10.3389/fcvm.2021.676954
22. Al-Rubaish AM, Al-Muhanna FA, Alshehri AM, Al-Mansori MA, Alali RA, Khalil RM, et al. Bedside testing of CYP2C19 vs. conventional clopidogrel treatment to guide antiplatelet therapy in ST-segment elevation myocardial infarction patients. Int J Cardiol. (2021) 343:15–20. doi: 10.1016/j.ijcard.2021.08.051
23. Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. (2016) 388(10055):2015–22. doi: 10.1016/S0140-6736(16)31323-X
24. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. (2017) 390(10104):1747–57. doi: 10.1016/S0140-6736(17)32155-4
25. Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLO trial. J Am Coll Cardiol. (2018) 71(17):1869–77. doi: 10.1016/j.jacc.2018.02.029
26. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. (2012) 79(3):453–95. doi: 10.1002/ccd.23438
27. Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH, CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. (2000) 102(6):624–9. doi: 10.1161/01.cir.102.6.624
28. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. (2009) 360(4):354–62. doi: 10.1056/NEJMoa0809171
29. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D'Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. (2021) 397(10283):1470–83. doi: 10.1016/S0140-6736(21)00533-X
30. Park KW, Park JJ, Jeon KH, Kang SH, Oh IY, Yang HM, et al. Clinical predictors of high posttreatment platelet reactivity to clopidogrel in Koreans. Cardiovasc Ther. (2012) 30(1):5–11. doi: 10.1111/j.1755-5922.2010.00249.x
31. Park KW, Kim HS. Options to overcome clopidogrel response variability. Circ J. (2012) 76(2):287–92. doi: 10.1253/circj.cj-11-1494
32. Franchi F, Rollini F, Cho JR, Ferrante E, Angiolillo DJ. Platelet function testing in contemporary clinical and interventional practice. Curr Treat Options Cardiovasc Med. (2014) 16(5):300. doi: 10.1007/s11936-014-0300-y
Keywords: acute coronary syndrome, percutaneous coronary intervention, dual antiplatelet therapy, genotype testing, platelet function testing
Citation: Zhong P-Y, Deng J-P, Zhao J-H, Peng L, Liu T and Wang H-Y (2023) Guided vs. conventional anti-platelet therapy for patients with acute coronary syndrome: A meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 10:1079332. doi: 10.3389/fcvm.2023.1079332
Received: 25 October 2022; Accepted: 24 February 2023;
Published: 21 March 2023.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Wei Wang, The Affiliated Hospital of Southwest Medical University, China© 2023 Zhong, Deng, Zhao, Peng, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Liu enh5eWxpdXRAMTYzLmNvbQ== Hao-Yu Wang enh5eXdhbmdoYW95dUAxNjMuY29t
Specialty Section: This article was submitted to Coronary Artery Disease, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.