
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 19 January 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1067984
This article is part of the Research TopicManagement of Right Ventricular Failure: Pathophysiology, medical treatment and use of ventricular assist devicesView all 5 articles
Objectives: This study was performed to investigate the relationship between right ventricular free wall longitudinal strain (RVFWSL) and low cardiac output syndrome (LCOS) after surgical aortic valve replacement (SAVR) and to further explore its association with readmission within 2 years in patients who developed LCOS after SAVR.
Methods: This single-center retrospective observational study involved consecutive patients who underwent SAVR at our hospital from May 2018 to June 2020. Preoperative echocardiography was obtained within 3 days before SAVR. The longitudinal strain of the right ventricle was analyzed using the right ventricle as the main section, and the RVFWSL and right ventricular four-chamber longitudinal strain (RV4CSL) were obtained. The primary observation was the occurrence of LCOS. The secondary prognostic indicators were mainly the readmission rates within 2 years.
Results: In total, 146 patients were finally included in this study. The RVFWSL was significantly lower in the LCOS group than in the No-LCOS group (16.63 ± 2.10) vs. (23.95 ± 6.33), respectively; P < 0.001). The multivariate regression analysis showed that the RVFWSL was associated with LCOS (odds ratio, 1.676; 95% confidence interval, 1.258–2.232; P < 0.001). The receiver operating characteristic curve showed that the cut-off value for RVFWSL to predict LCOS was less than –18.3, with an area under the curve of 0.879, sensitivity of 100%, and specificity of 80.47%. The multivariate regression analysis showed that LCOS was an independent risk factor for readmission within 2 years in patients undergoing SAVR.
Conclusion: Patients with RVFWSL (<-18.3%) may be an increased risker for LCOS after SAVR. The occurrence of LCOS after SAVR is Yong-jian Zhang a risk factor for readmission within 2 years. Right ventricular function monitoring may have some predictive value for the postoperative prognosis in patients undergoing SAVR.
Aortic valve disease is the most common heart valve disease (1, 2). Surgical aortic valve replacement (SAVR) remains the predominant treatment for aortic valve disease. With advances in surgical techniques, mortality associated with cardiac surgery has substantially declined; however, the average perioperative mortality rate is currently 1–2%, and the incidence of major postoperative cardiovascular complications remains high (3, 4). Low cardiac output syndrome (LCOS) is the most serious complication after cardiac surgery, and the LCOS is strongly associated with short- and long-term postoperative mortality (5). Previous studies have revealed a mortality rate of more than 20% in patients who develop LCOS after cardiac surgery (6). It is important to rapidly and accurately identify LCOS after surgery. However, it is equally important to be able to accurately predict the occurrence of LCOS in patients before surgery to help the surgeon choose the most appropriate procedure and to reduce the time taken to determine LCOS postoperatively.
Studies have suggested that right ventricular (RV) function is strongly associated with a poor prognosis in patients with aortic valve disease after surgery (7–9). Recent studies have also concluded that RV function parameters such as the RV fractional area change (RVFAC) and tissue Doppler imaging-derived systolic velocity (TDI s’) are strongly associated with cardiovascular mortality 3 years after cardiac surgery (10). Strain imaging has been considered a very important risk assessment tool in recent years; it is highly reproducible and has better predictive power than traditional echocardiographic parameters (11, 12). It has also been suggested that RV longitudinal strain is associated with prognosis in patients with different cardiac diseases (13, 14). However, the predictive value of RV longitudinal strain in relation to postoperative LCOS in patients with aortic valve disease has been less thoroughly reported. Therefore, the aim of this study was to investigate the relationship between RV free wall longitudinal strain (RVFWSL) and LCOS after surgical treatment of aortic valve disease and to further explore its association with readmission within 2 years in patients who developed LCOS after SAVR.
This single-center retrospective observational study involved consecutive patients who underwent SAVR at our hospital from May 2018 to June 2020. Patients undergoing SAVR included those with aortic stenosis and aortic regurgitation; we referred to the latest guidelines for the diagnostic criteria (1, 2). The inclusion criteria were an age of >18 years and the presence of aortic valve disease. The exclusion criteria were perioperative death, poor-quality images of the right ventricle, and missing RV echo images. Poor quality was considered if the entire annulus of the tricuspid valve and the apex were not well visualized in the apical four-chamber view during the complete cardiac cycle or if foreshortening of the ventricle was present (15). The present study complied with the Declaration of Helsinki and was approved by the local ethics committee of the authors’ institution. All patients provided written informed consent. Figure 1 displays the patient selection and study design.
A color Doppler ultrasound diagnostic instrument (Philips CX50; Philips Medical Devices Group, Netherlands) equipped with a 1- to 5-MHz cardiac probe was used. Scans were performed and analyzed by two researchers with 5 years of experience in echocardiography. Preoperative echocardiography was obtained within 3 days before SAVR. Postoperative echocardiography was obtained before 10:00 on the first and second day after SAVR. Conventional views were obtained according to the American Society of Echocardiography guidelines, and measurements were performed as recommended by current guidelines (16). We measured overall left ventricular (LV) systolic function using the biplane Simpson method according to the American Society of Echocardiography guidelines (16). LV systolic function was divided into four grades for men (52–72%, normal range; 41–51%, mild abnormality; 30–40%, moderate abnormality; and <30%, severe abnormality) and women (54–74%, normal range; 41–53%, mild abnormality; 30–40%, moderate abnormality; and <30%, severe abnormality) (16).
Offline analysis was performed using QLAB 10.3 software (cardiac motion quantification; Phillips Medical Systems) to analyze the longitudinal myocardial strain in the right ventricle. The longitudinal strain of the right ventricle was analyzed using the right ventricle as the main section, and the RVFWSL and RV four-chamber longitudinal strain (RV4CSL) were obtained as shown in Figure 2.
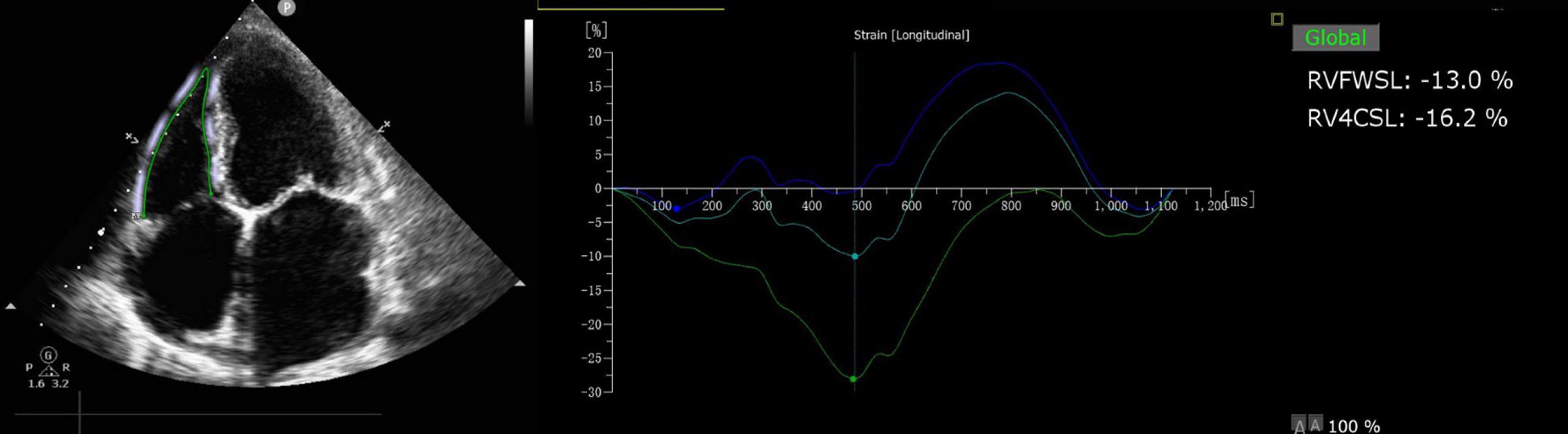
Figure 2. RVFWSL and RV4CSL analysis. Offline analysis was performed using QLAB 10.3 software (cardiac motion quantification; Phillips Medical Systems) to analyze the longitudinal myocardial strain in the right ventricle. The longitudinal strain of the right ventricle was analyzed using the right ventricle as the main section, and the RVFWSL and the RV4CSL were obtained.
The primary observation was the occurrence of LCOS. The criteria for the diagnosis of LCOS were the need for positive inotropic drugs (dobutamine, levosimendan, epinephrine, or milrinone) for at least 12 h after admission to the intensive care unit postoperatively and one of the following within 12 h of transfer to the intensive care unit: pulmonary capillary wedge pressure of >18 mmHg, central venous oxygen saturation of <60%, or urine output of <0.5 mL/kg/h (17).
The secondary prognostic indicators were mainly the readmission rates within 2 years, including readmission within 30 days of the patient’s initial admission. The primary cause of readmission was cardiac in origin.
Data analyses were performed with IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). Data are expressed as mean ± standard deviation, median and interquartile range (25th and 75th percentiles), or frequency (%). Categorical variables are expressed as absolute numbers with percentages. Parameters in the LCOS and No-LCOS groups were compared using the chi-squared test or Fisher’s exact test for categorical variables and the unpaired t-test or Mann–Whitney U test for continuous variables as appropriate. For variables with a statistically significant difference, a univariate logistic regression was performed to predict the event. After the univariate analysis, a multivariate logistic regression was performed to identify the independent predictors. To explore the optimal cutoff value of variable to predict the event, a receiver operating characteristic curve was constructed. Interobserver and intraobserver variability for RVFWSL were assessed in 20 patients using the intraclass correlation coefficient (ICC) (15). A P value of <0.05 was considered statistically significant.
After excluding patients with poor right heart images, 146 patients were finally included in this study. The mean age of all patients was 56 years (range, 49–79 years), and 106 (72.60%) patients were male and 40 (27.40%) were female. Of all 146 patients, LCOS occurred in 18 (incidence of approximately 13.33%). All 18 patients with LCOS needed positive inotropic drugs (dobutamine or epinephrine) at least 12 h after admission to the intensive care unit postoperatively and central venous oxygen saturation of <60% within 12 hours of transfer to the intensive care unit. All patients were divided into an LCOS group and No-LCOS group according to whether they had developed LCOS after surgery (Table 1).
The hospitalization days in the intensive care unit and total hospitalization days of patients with LCOS were significantly higher than those of patients with No-LCOS, which was statistically significant (Table 1).
RVFWSL showed good reproducibility with an intra-observer variability ICC of 0.906 (95% confidence interval, 0.780–0.962; P < 0.001) and an inter-observer variability ICC of 0.893 (95% confidence interval, 0.748–0.956; P < 0.001).
The comparison of LV-related echocardiographic parameters between the two groups showed no statistically significant differences in the LV end-diastolic diameter, LV end-systolic diameter, or LV ejection fraction (LVEF) between the two groups. The LVEF on the first and second postoperative day in the LCOS group were lower than those in the No-LCOS group, and the difference between the groups was statistically significant (Table 2).
The comparison of RV-related parameters between the two groups showed that the differences in diameter of right ventricular baseline (DRVB) and diameter of right ventricular mid-section (DRVM) between the two groups were not statistically significant (Table 2).
The RVFAC, TDI s’, and tricuspid annular plane systolic excursion (TAPSE), which are used to assess overall RV function, were significantly lower in the LCOS group than in the No-LCOS group (P < 0.05 for all) (Table 2).
The RV strain parameters RVFWSL (16.63 ± 2.10 vs. 23.29 ± 5.37) and RV4CSL (16.79 ± 4.43 vs. 19.86 ± 4.99) were significantly lower in the LCOS group than in the No-LCOS group (P < 0.001 and P = 0.015, respectively) (Table 2).
Regression analysis was performed to further analyze the correlation between echocardiographic parameters and LCOS. The results of the univariate regression analysis showed that the LVEF on the first and second postoperative day was associated with the development of LCOS and that the RVFAC, TDI s’, RVFWSL, and RV4CSL were associated with the development of LCOS. A multivariate regression analysis was performed to further verify the value of each parameter in predicting LCOS. The results of the analysis showed that only RVFWSL remained correlated with LCOS (Table 3).
Receiver operating characteristics curve analysis was performed to further determine the diagnostic cut-off value of RVFWSL for predicting LCOS. The results showed that the diagnostic cut-off value for RVFWSL to predict LCOS was less than –18.3, with an area under the curve of 0.879, sensitivity of 100%, and specificity of 80.47% (Figure 3).
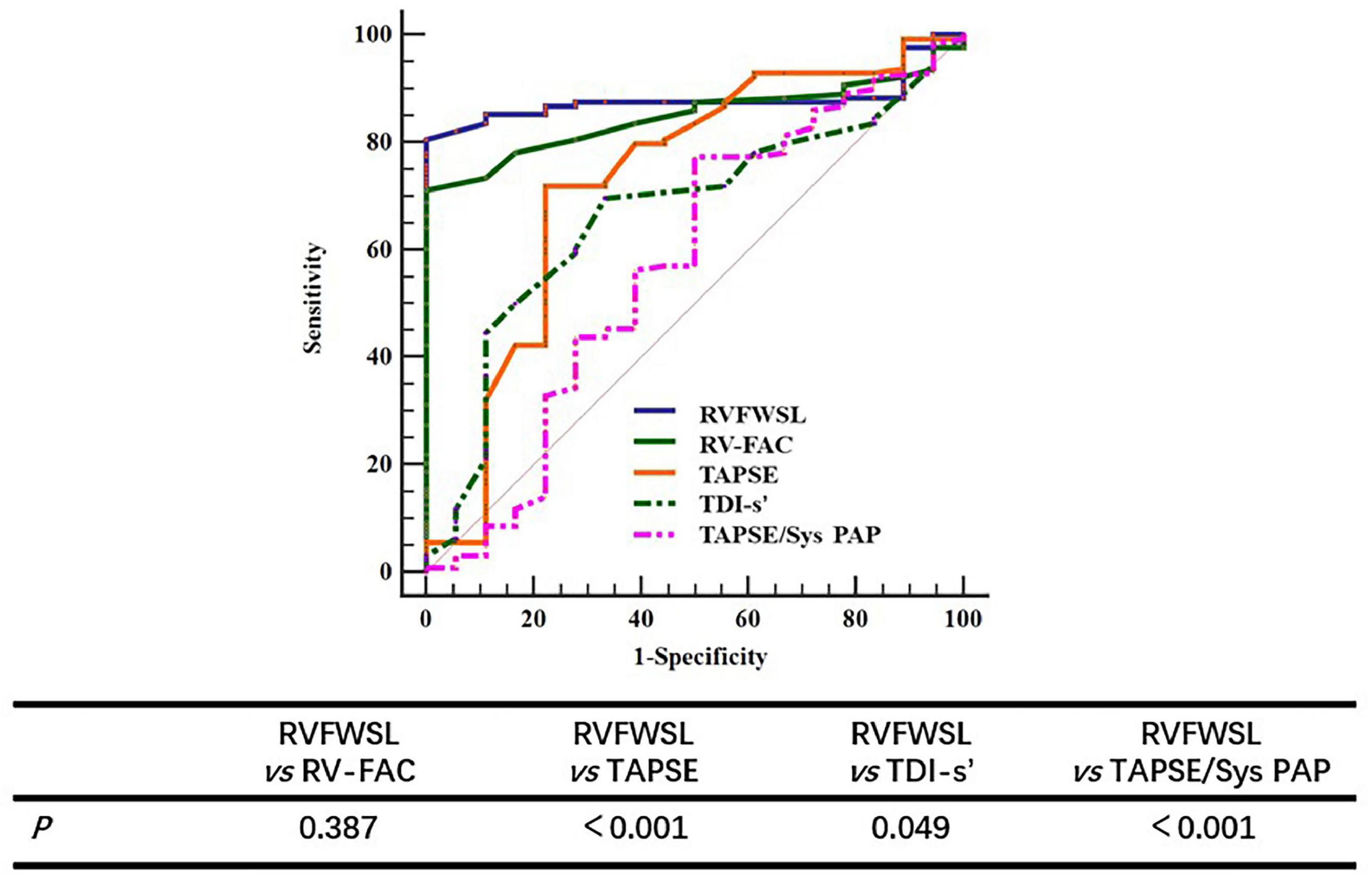
Figure 3. The ROC curve of RV parameters for predicting LCOS. Comparing the value of different parameters in predicting LCOS, the results showed that there was no significant difference between RVFWSL and RV-FAC in predicting LCOS, but there was significant difference between RV RVFWSL and other parameters (P < 0.05).
In order to further demonstrate that the value of RVFWSL in predicting LCOS is better than other parameters of evaluating right ventricular function, we further analyzed the results of RV-FAC, TDI’s, TAPSE and TAPSE/Sys PAP in predicting LCOS. The results showed that the AUC of FAC was 0.848 (95% CI: 0.780–0.902), the AUC of TDI s’ was 0.728 (95% CI: 0.648–0.798), and the AUC of TAPSE was 0.667 (95% CI: 0.585–0.743), the AUC of TAPSE/Sys PAP was 0.576 (95% CI: 0.491–0.657) (Table 4). The AUC of RV-FAC, TDI’s, TAPSE and TAPSE/Sys PAP in predicting LCOS is less than that of RVFWSL. Comparing the value of different parameters in predicting LCOS, the results showed that there was no significant difference between RVFWSL and RV-FAC in predicting LCOS (P = 0.387), but there was significant difference between RV RVFWSL and other parameters (P < 0.05) (Figure 3 and Table 4).
The secondary outcome during the follow-up period of this study was readmission of patients within 2 years, including patients readmitted 30 days after their initial admission. Among all patients, 14 were readmitted within 2 years, and all readmissions were for cardiac reasons. There were five readmissions in the LCOS group (incidence of 27.78%) and nine readmissions in the No-LCOS group (incidence of 7.03%), with a statistically significant difference in readmission rates between the two groups (P = 0.005) (Table 5).
Further analysis of risk factors for readmission within 2 years showed that LCOS was an independent risk factor for readmission, whereas RVFWSL was not significantly associated with readmission within 2 years (Table 6).
LCOS is the most common and serious complication after SAVR and is strongly associated with short- and long-term postoperative mortality (5). Therefore, it is important to be able to accurately predict the occurrence of LCOS before SAVR. The main finding of this study is that the RVFWSL may be associated with an increased risk for LCOS in patients who undergo SAVR. The occurrence of LCOS after SAVR is a risk factor for readmission within 2 years, whereas the RVFWSL is not.
Possible reasons for the development of LCOS after aortic stenosis and aortic insufficiency are as follows. First, perioperative RV ischemia may be one of the main causes of impaired RV systolic function postoperatively (18). Second, RV volume overload may occur due to tricuspid or pulmonary regurgitation. Third, aortic valve disease may lead to an increase in LV end-diastolic pressure, which leads to pulmonary hypertension and an excessive RV pressure load (9). Finally, a prolonged and sustained increase in LV afterload leads to thickening and fibrosis of the ventricular wall and a decrease in longitudinal function via the septum to the right ventricle (10). These causes directly lead to rapid RV dilatation and an increase in RV end-diastolic pressure (19), which in turn leads to a leftward shift of the septum (20), a reduction in LV preload, and ultimately the manifestation of LCOS. In our study, RV insufficiency parameters, including decreases in the RVFAC, TDI s’, were found to be closely associated with the development of LCOS. And we found that the AUC of RVFAC for predicting LCOS is higher than other parameters. Compared with RVFWSL, there is no significant difference in predicting LCOS, but the AUC of RVFAC is slightly lower than that of RVFWSL. RVFAC shows a better response to RV function, but it is dependent on image quality and requires a complete display of the entire RV structure. However, there was no significant correlation between TAPSE and LCOS in the present study, which is consistent with previous studies. One previous study suggested that TAPSE is a simple measure of longitudinal function but that it may not accurately reflect overall RV function because it is easily measured even with poor image quality, and therefore its reproducibility is poor (13). Additionally, previous studies have revealed contradictions between TAPSE and RVFAC as predictive parameters (21).
After excluding confounding factors, we found that RVFWSL was more closely related to LCOS than traditional parameters of RV insufficiency. Two-dimensional speckle tracking echocardiography is considered a better method for echocardiographic assessment of RV function and has the advantage of showing a more accurate response to RV function than RVFAC or TAPSE (22). Previous studies have found that RV strain parameters have important predictive value for the prognosis of patients with heart failure (23, 24). A recent study also suggested that both RVFWSL and RV4CSL are the most reliable echocardiographic indicators of RV function and that their value in predicting the risk of cardiovascular death exceeds that of RVFAC and TAPSE (13).
The present study showed that RVFWSL had better value than RV4CSL in predicting LCOS. The main fact on which this finding is based is that the septum is an integral part of the left ventricle, and RVFWSL is therefore closer to assessing RV function than RV4CSL. This is consistent with previous views. Cameli et al. (25) also concluded that the RVFWSL has the highest diagnostic accuracy and that it predicts the RV work-per-pulse index.
The secondary outcome in our study was the readmission rate within 2 years. We found that the readmission rate was 27.78% within 2 years for patients who developed postoperative LCOS. The multifactorial regression analysis showed that postoperative LCOS was a risk factor for readmission within 2 years, whereas RVFWSL was not significantly associated with readmission within 2 years. We consider that the main reason for this is related to the small number of patients included in this study.
The present study has some limitations. First, the sample size was small; a study with a larger sample size is needed to confirm the results of this study. Second, the present study did not include patients who died in the perioperative period, so there may be some bias in the population studied. Third, we excluded 13% of patients from the strain analysis because of poor image quality. Indeed, a very important limitation of RV strain remains the reliance on high-quality images and the frame rate.
Patients with RVFWSL (<18.3%) may be at increased risk for LCOS after SAVR. The occurrence of LCOS after SAVR is a risk factor for readmission within 2 years. RV function monitoring may have some predictive value for the postoperative prognosis in patients undergoing aortic valve surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Xi’an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
YS and YY conceived and designed the project. Y-JZ and YS interpreted the results and wrote the manuscript. HC, Y-LD, and J-NS collected the data and collated the echocardiographic data. All authors reviewed the manuscript.
We thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Baumgartner H, Falk V, Bax JJ, Bonis MD, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1016/j.rec.2017.12.013
2. Chair HB, Co-Chair JH, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European association of cardiovascular imaging and the American society of echocardiography. Eur Heart J Cardiovasc Imaging. (2017) 18:254–75. doi: 10.1093/ehjci/jew335
3. Chen J, Kaul P, Levy J, Haverich A, Menasché P, Smith P, et al. Myocardial infarction following coronary artery bypass graft surgery increases healthcare resource utilization. Crit Care Med. (2007) 35:1296–301. doi: 10.1097/01.CCM.0000262403.08546.A2
4. Lomivorotov VV, Efremov SM, Pokushalov EA, Romanov AB, Ponomarev DN, Cherniavsky AM, et al. Randomized trial of fish oil infusion to prevent atrial fibrillation after cardiac surgery: data from an implantable continuous cardiac monitor. J Cardiothorac Vasc Anesth. (2014) 28:1278–84. doi: 10.1053/j.jvca.2014.02.019
5. Algarni KD, Maganti M, Yau TM. Predictors of low cardiac output syndrome after isolated mitral valve surgery. J Thorac Cardiovasc Surg. (2010) 140:790–6. doi: 10.1016/j.jtcvs.2009.11.022
6. Algarni K, Maganti M, Yau T. redictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. (2011) 92:1678–84. doi: 10.1016/j.athoracsur.2011.06.017
7. Peyrou J, Chauvel C, Pathak A, Simon M, Dehant P, Abergel E. Preoperative right ventricular dysfunction is a strong predictor of 3 years survival after cardiac surgery. Clin Res Cardiol. (2017) 106:734–42. doi: 10.1007/s00392-017-1117-y
8. Furukawa K, Yano M, Nishimura M, Nakamura E, Watanabe N, Nishino S, et al. Significance of preoperative right ventricular function on mid-term outcomes after surgical ventricular restoration for ischemic cardiomyopathy. Gen Thorac Cardiovasc Surg. (2019) 67:925–33. doi: 10.1007/s11748-019-01123-5
9. Cavalcante JL, Rijal S, Althouse AD, Delgado-Montero A, Katz WE, Schindler JT, et al. Right ventricular function and prognosis in patients with low-flow, low-gradient severe aortic stenosis. J Am Soc Echocardiogr. (2016) 29:325–33. doi: 10.1016/j.echo.2015.12.001
10. Lomivorotov V, Efremov S, Kirov M, Fominskiy E, Karaskov A. Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth. (2017) 31:291–308. doi: 10.1053/j.jvca.2016.05.029
11. Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, et al. Variability and reproducibility of segmental longitudinal strain measurement: a report from the EACVIASE Strain Standardization Task Force. JACC Cardiovasc Imaging. (2018) 11:15–24. doi: 10.1016/j.jcmg.2017.01.027
12. Ng AC, Prihadi EA, Antoni ML, Bertini M, Ewe SH, Marsan NA, et al. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging. (2018) 19:859–67. doi: 10.1093/ehjci/jex189
13. Houard L, Benaets M, de Meester de Ravenstein C, Rousseau M, Ahn S, Amzulescu M, et al. Additional prognostic value of 2D right ventricular speckle-tracking strain for prediction of survival in heart failure and reduced ejection fraction: a comparative study with cardiac magnetic resonance. JACC Cardiovasc Imaging. (2019) 12:2373–85. doi: 10.1016/j.jcmg.2018.11.028
14. Ivey-Miranda JB, Almeida-Gutiérrez E, Borrayo-Sánchez G, Antezana-Castro J, Contreras-Rodríguez A, Posada-Martínez EL, et al. Right ventricular longitudinal strain predicts acute kidney injury and short-term prognosis in patients with right ventricular myocardial infarction. Int J Cardiovasc Imaging. (2019) 35:107–16. doi: 10.1007/s10554-018-1447-5
15. Posada-Martinez E, Fritche-Salazar J, Arias-Godinez J, Ortiz-Leon X, Balderas-Muñoz K, Ruiz-Esparza M, et al. Right ventricular longitudinal strain predicts low-cardiac- output syndrome after surgical aortic valve replacement in patients with preserved and mid-range ejection fraction. J Cardiothorac Vasc Anesth. (2021) 35:1638–45. doi: 10.1053/j.jvca.2020.12.008
16. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39. doi: 10.1016/j.echo.2014.10.003
17. Vela JL, Benítez JC, González MC, López MA, Pérez RH, Meneses VS, et al. Clinical practice guide for the management of low cardiac output syndrome in the postoperative period of heart surgery. Med Intensiva. (2012) 36:e1–44. doi: 10.1016/j.medine.2012.01.007
18. Galli E, Guirette Y, Feneon D, Daudin M, Fournet M, Leguerrier A, et al. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging. (2015) 16:531–8. doi: 10.1093/ehjci/jeu290
19. Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. (2010) 14:R169. doi: 10.1186/cc9264
20. Pfisterer M. Right ventricular involvement in myocardial infarction and cardiogenic shock. Lancet. (2003) 362:392–4. doi: 10.1016/S0140-6736(03)14028-7
21. Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. (2000) 85:837–42. doi: 10.1016/S0002-9149(99)00877-2
22. Longobardo L, Suma V, Jain R, Carerj S, Zito C, Zwicke DL, et al. Role of two-dimensional speckle-tracking echocardiography strain in the assessment of right ventricular systolic function and comparison with conventional parameters. J Am Soc Echocardiogr. (2017) 30:937–46. doi: 10.1016/j.echo.2017.06.016
23. Carluccio E, Biagioli P, Alunni G, Murrone A, Zuchi C, Coiro S, et al. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ Cardiovasc Imaging. (2018) 11:e006894. doi: 10.1161/CIRCIMAGING.117.006894
24. Iacoviello M, Citarelli G, Antoncecchi V, Romito R, Monitillo F, Leone M, et al. Right ventricular longitudinal strain measures independently predict chronic heart failure mortality. Echocardiography. (2016) 33:992–1000. doi: 10.1111/echo.13199
25. Cameli M, Bernazzali S, Lisi M, Tsioulpas C, Croccia MG, Lisi G, et al. Right ventricular longitudinal strain and right ventricular stroke work index in patients with severe heart failure: left ventricular assist device suitability for transplant candidates. Transplant Proc. (2012) 44:2013–5. doi: 10.1016/j.transproceed.2012.06.018
Keywords: low cardiac output syndrome, right ventricular free wall longitudinal strain, right ventricular function, aortic valve replacement, echocardiography
Citation: Zhang Y-j, Chen H, Dong Y-l, Shang J-n, Ruan L-t, Yan Y and Song Y (2023) The relationship between pre-operative right ventricular longitudinal strain and low-cardiac-output syndrome after surgical aortic valve replacement. Front. Cardiovasc. Med. 10:1067984. doi: 10.3389/fcvm.2023.1067984
Received: 12 October 2022; Accepted: 04 January 2023;
Published: 19 January 2023.
Edited by:
Edoardo Conte, University of Milan, ItalyReviewed by:
Ayse Baysal, Pendik Veterinary Control and Research Institute, TürkiyeCopyright © 2023 Zhang, Chen, Dong, Shang, Ruan, Yan and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Song,  c29uZ3lhbnhqdHVAMTI2LmNvbQ==,
c29uZ3lhbnhqdHVAMTI2LmNvbQ==,  c29uZ3lhbnhqdHVAeGp0dWZoLmVkdS5jbg==; Yang Yan,
c29uZ3lhbnhqdHVAeGp0dWZoLmVkdS5jbg==; Yang Yan,  eXlhbmczNzZAMTI2LmNvbQ==
eXlhbmczNzZAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.