- 1Department of Cardiovascular Surgery, Fujian Medical University Union Hospital, Fuzhou, China
- 2Department of Clinical Medicine, Fujian Medical University, Fuzhou, China
- 3Department of Nursing, Fujian Medical University Union Hospital, Fuzhou, China
Background: Postoperative atrial fibrillation (POAF) is a common complication after cardiac surgery, which is associated with age and massive bleeding. However, whether thyroid hormone (TH) level can affect POAF remains controversial.
Aim: To investigate the occurrence and risk factors of POAF, in particular, the preoperative TH level of patients was introduced into this study as a variable for analysis, and a column graph prediction model of POAF was constructed.
Methods: Patients who underwent valve surgery in Fujian Cardiac Medical Center from January 2019 to May 2022 were retrospectively analyzed and divided into POAF group and NO-POAF group. Baseline characteristics and relevant clinical data were collected from the two groups of patients. Independent risk factors for POAF were screened using univariate analysis and binary logistic regression analysis, and a column line graph prediction model was established based on the regression analysis results, and the diagnostic efficacy and calibration of the model were evaluated using the Receiver Operating Characteristic Curve (ROC) and calibration curve.
Results: A total of 2,340 patients underwent valve surgery, excluding 1,751 patients, a total of 589 patients were included, including 89 patients in POAF group and 500 patients in NO-POAF group. The total incidence of POAF was 15.1%. The results of the Logistics regression analysis showed that gender, age, leukocytes and TSH were risk factors of POAF. The area under the ROC curve of the nomogram prediction model for POAF was 0.747 (95% CI: 0.688–0.806, P < 0.001), with a sensitivity of 74.2% and specificity of 68%. Hosmer-Lemeshow test showed χ2 = 11.141, P = 0.194 > 0.05, the calibration curve was well fitted.
Conclusion: The results of this study show that gender, age, leukocyte and TSH are risk factors of POAF, and the nomogram prediction model has a good prediction effect. Due to the limited sample size and included population, more studies are needed to validate this result.
1. Introduction
Postoperative atrial fibrillation (POAF) is a common complication after cardiac surgery, and 17.3%–76% of patients develop POAF after cardiac surgery (1–4). POAF usually occurs during hospitalization, has a short duration, recovers spontaneously without treatment, and rarely develops into chronic diseases (5, 6). However, POAF is significantly associated with increased mortality, length of hospital stay, and medical costs (7, 8). Therefore, the prevention of POAF after cardiac surgery is of great significance to promote disease recovery and reduce mortality (9, 10).
The relationship between thyroid hormone levels and disease has attracted more and more attention. Relevant datas show (11–13) that thyroid hormone changes within the normal range will also have an impact on the outcome of the disease, which may be related to impaired peripheral thyroxine deiodination and down-regulation of enzyme activity. Thyroid hormones play a wide range of roles in the cardiovascular system, causing arrhythmias, atherosclerotic vascular diseases, dyslipidemia and heart failure, which lead to an increased risk of death (14, 15).
Although previous studies have shown that the occurrence of POAF in patients undergoing heart valve surgery is correlated with age and hemorrhage (16, 17), however, no studies have investigated the effect of thyroid hormone levels on the occurrence of POAF in heart valves. Therefore, we introduced thyroid hormone levels into this study for analysis and constructed a nomogram prediction model in order to provide a more comprehensive understanding of the risk factors for the occurrence of POAF and to provide a reference for the prevention of POAF, so as to promote the life safety of patients.
2. Methods
2.1. Patients
This study collected patients who underwent valve surgery in Fujian Cardiac Medical Center from January 2019 to May 2022. All patients were ≥18 years old and had no preoperative atrial fibrillation, metabolic diseases, malignant tumors, history of autoimmune diseases, or severe hepatic or renal insufficiency. Exclusion criteria: patients with a history of heart disease, patients with thyroid disease.
2.2. Data collection
The baseline datas, preoperative, intraoperative and postoperative clinical datas of the patients were collected retrospectively. Baseline datas include gender, age, body mass index (BMI), smoking, alcohol consumption, hypertension and diabetes. Preoperative clinical datas include cardiac function classification (NYHA cardiac function classification divides cardiac function impairment into grades I to IV according to the activity of induceing heart failure symptoms), left ventricular ejection fraction, laboratory indicators. Intraoperative clinical datas include operation duration and cardiopulmonary bypass duration. Postoperative clinical datas include days of intensive care unit stay, duration of mechanical ventilation, and cardiac output (CO).
2.3. Assessment of atrial fibrillation
All patients after heart valve surgery were admitted to ICU for continuous vital signs monitoring, and whether the patient had atrial fibrillation was judged according to the data of the ECG monitoring instrument (18). POAF can be diagnosed if the patient has no AF before operation and is detected to have AF after operation. Atrial fibrillation that occurs from the day to 7 days after surgery is considered as early postoperative POAF.
2.4. Statistical analysis
SPSS24.0 software was used for datas analysis in this study. Continuous variables were expressed as mean ± standard deviation, and comparisons between groups were analyzed by two-independent sample t-test or Wilcoxon rank-sum test. Categorical variables were expressed as counts and percentages, and comparisons between groups were performed using χ2 test or Fisher's exact test. The independent risk factors of POAF were screened by binary Logistic regression analysis. Risk prediction models were developed, column line plots were drawn using R 4.2.2 software, and internal validation was performed. The area Under the Curve (AUC) of Receiver Operating Characteristic Curve (ROC) was used to evaluate the model differentiation, the Hosmer-Lemeshow goodness of fit test (H-L test) and calibration curve were used to evaluate the calibration degree of the model.
3. Results
3.1. Analysis of patients' inclusion
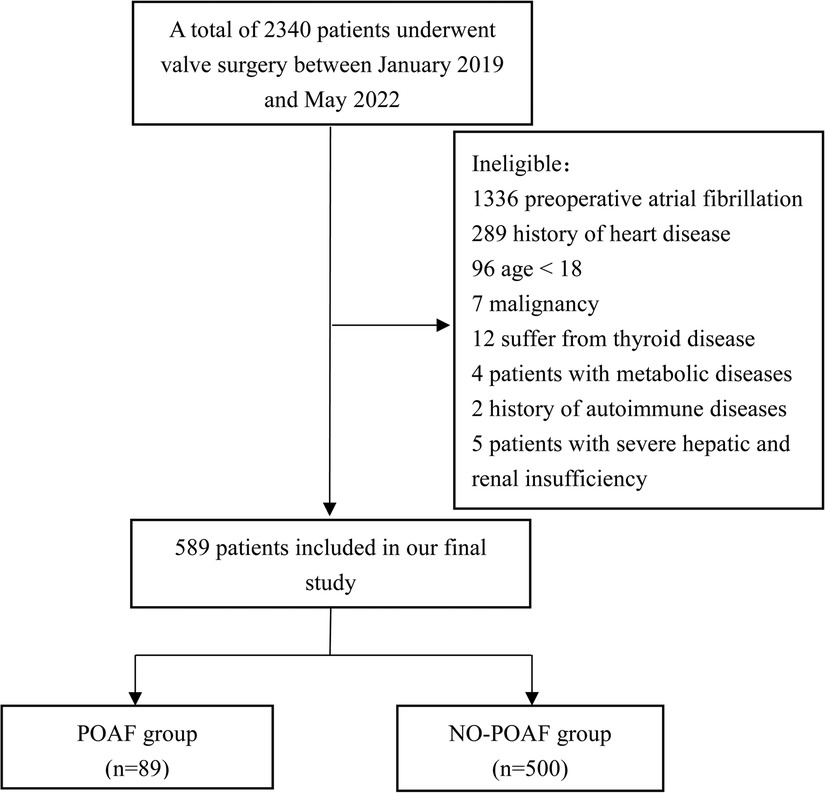
There are a total of 2,340 patients underwent valve surgery from January 2019 to May 2022. 1,751 patients were excluded (including 1,336 patients with preoperative atrial fibrillation, 289 patients with a history of heart diseases, 96 patients were younger than 18, 7 patients with malignant tumors, 12 patients with thyroid diseases, 4 patients with metabolic diseases, 2 patients with a history of autoimmune diseases, 5 patients with severe hepatic and renal insufficiency), a total of 589 patients were finally enrolled, there were 89 patients in POAF group and 500 patients in NO-POAF group. The flow chart of patients’ inclusion is shown in Figure 1.
3.2. Comparison of clinical datas between the two groups
3.2.1. Baseline patients' characteristics
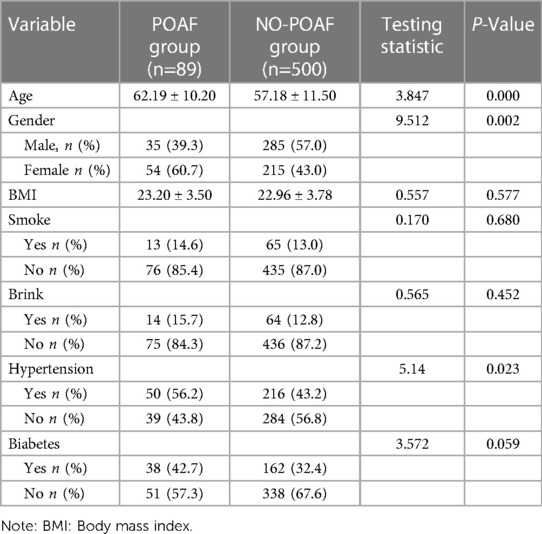
Table 1 shows the baseline characteristics of the two groups of patients. Among 589 patients, 89 (15.1%) were in the POAF group and 500 (84.9%) were in the NO-POAF group. Compared with the NO-POAF group, the age (61.067 ± 9.875 vs. 57.224 ± 11.517), the proportion of females (60.7% vs. 40%) and the proportion of hypertension patients (56.2% vs. 43.2%) in the POAF group were higher, and the differences were statistically significant (P < 0.05). There was no significant difference in BMI, smoking, drinking and diabetes between the two groups (P > 0.05). The difference is compared in Table 1.
3.2.2. Clinical datas of the patients
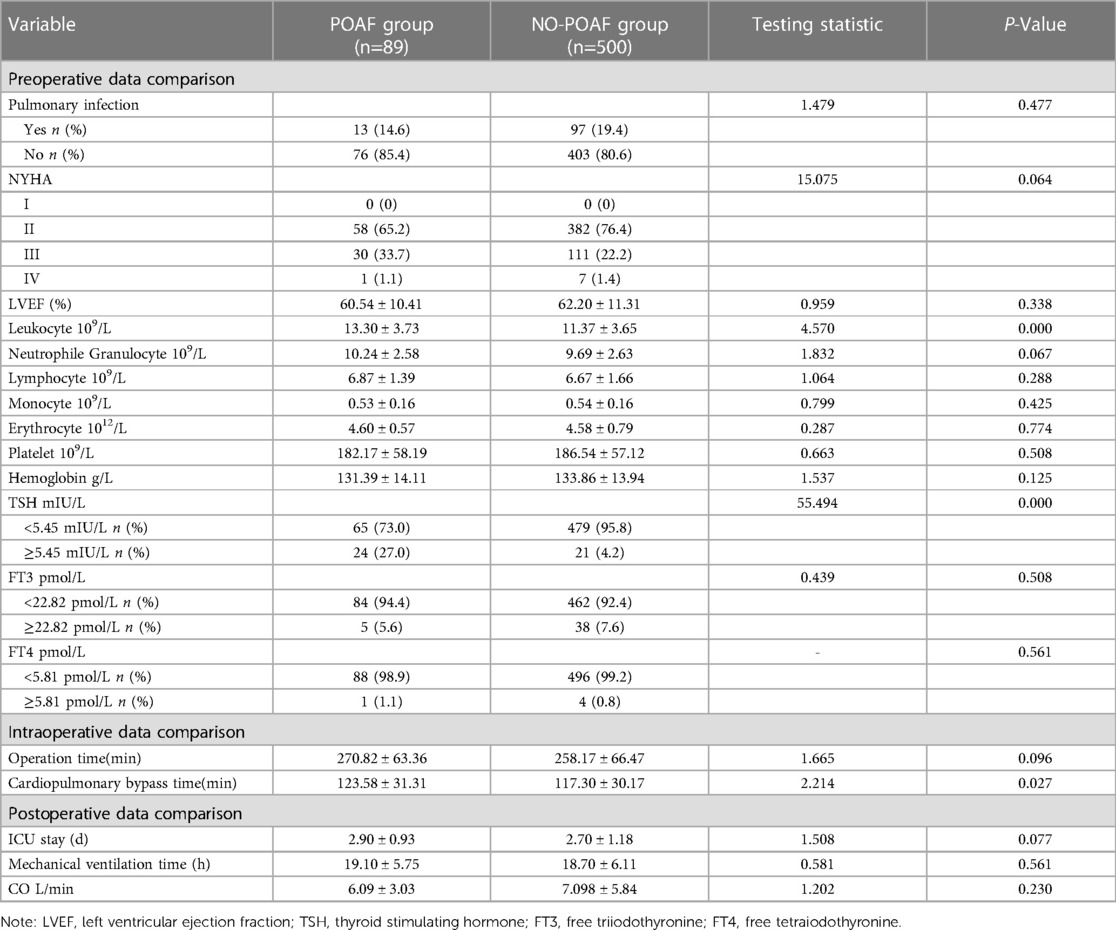
Table 2 Compares the clinical data of the two groups of patients. There were statistically significant differences in leukocyte, TSH and extracorporeal circulation time between the two groups (P < 0.05).
3.3. Binary logistic regression analysis
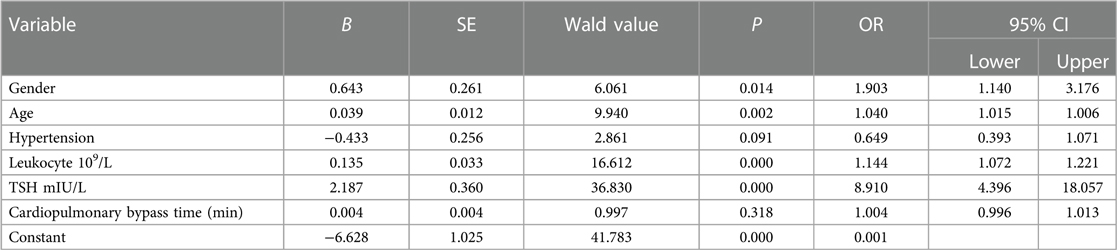
In Table 3, binary logistic regression analysis was performed with POAF as the dependent variable and gender, age, hypertension, leukocytes, TSH, and duration of extracorporeal circulation as independent variables. The results showed that gender (OR: 1.903, 95% CI: 1.140–3.176, P = 0.014), age (OR: 1.040, 95% CI: 1.015–1.006, P = 0.002), leukocytes (OR: 1.144, 95% CI: 1.072–1.221, P = 0.000), TSH (OR. 8.910, 95% CI: 4.396–18.057, P = 0.000) and other variables were risk factors for POAF in patients undergoing heart valve surgery.
3.4. POAF risk prediction line graph model
3.4.1. Constructing a line graph prediction model
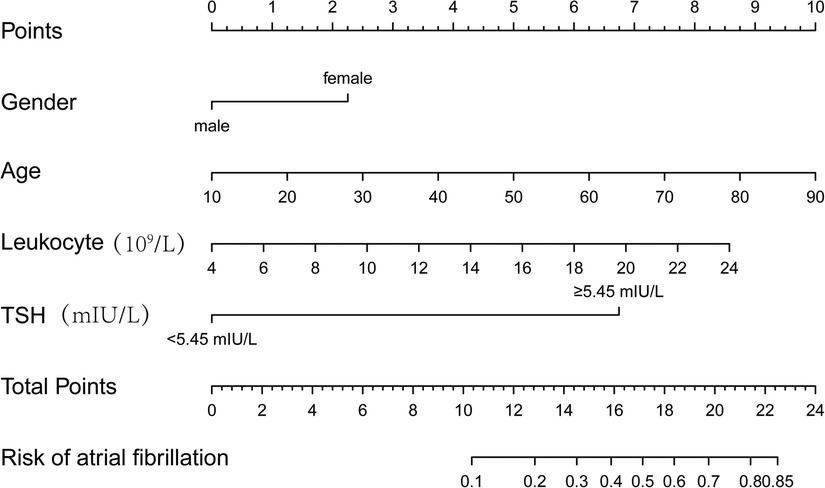
Columnar line graph of POAF risk prediction was drawn based on risk factors identified by binary logistic regression analysis. The column line plot prediction model showed that an increase of 2.25 points for women; 1.25 points for each 10-year increase in age; 0.75 points for each 2 units increase in leukocytes; and 6.75 points for TSH ≥ 5.45 mIU/L. The scores of the above risk factors were summed to calculate the total score, and the probability of occurrence of POAF corresponding to the column line graph was calculated based on the total score (Figure 2).
3.4.2. Distinguishability of the model
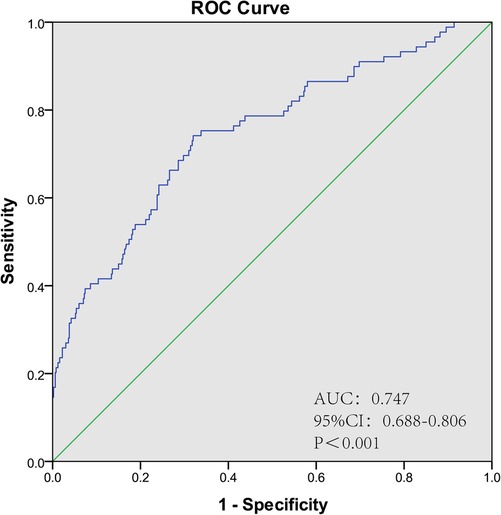
The ROC curve was plotted according to the prediction model, and the results showed an AUC of 0.747 (95% CI: 0.688–0.806, P < 0.001), a sensitivity of 74.2% and a specificity of 68%, suggesting that the model has a good discrimination. The ROC curve of POAF prediction model is shown in Figure 3.
3.4.3. Calibration degree of the model
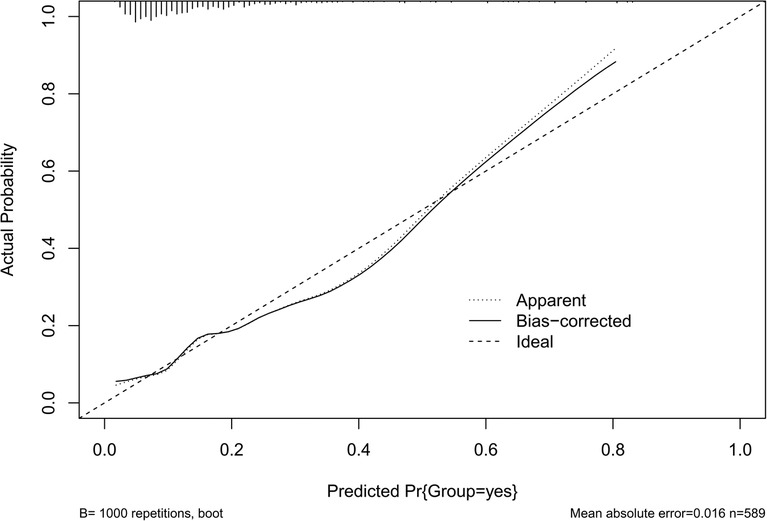
The results of the H-L test showed χ2 = 11.141, P = 0.194 > 0.05, and the calibration curve was well fitted as shown by bootstrap method with 1,000 repetitions (Figure 4), indicating that our predicted and observed values are close and in good agreement.
4. Discussion
4.1. Risk factors for POAF
There's a high incidence of POAF after cardiac surgery. Our study showed that the incidence of POAF in patients undergoing heart valve surgery was 15.1%, which was consistent with previous research results (19–21). Binary logistic regression analysis showed that gender, age, leukocyte and TSH were strongly correlated with the occurrence of POAF.
The results of this study showed that the age of patients in the POAF group was higher than that in the NO-POAF group, and the ROC curve showed that the optimal cut-off value of age was 64.5. Recent studies have also shown (22, 23) that advanced age is a risk factor for POAF in patients after cardiac surgery, which is consistent with the results of this study. It may be related to the frailty and loss of ventricular compliance in older patients (24, 25). Early identification of advanced age as a risk factor for POAF can enable medical staff to take targeted preventive measures before operation and carry out effective perioperative management.
Not only age is a risk factor for POAF, but gender can also have an important impact on the occurrence of POAF. In our study, women were a predictor of POAF in postoperative cardiac patients, and there was a strong correlation between women and the occurrence of POAF, which is consistent with the study of Li et al. (26). The study of Cheng et al. (27) also confirmed a higher recurrence rate of AF in women. This may be related to faster atrial remodeling and a higher rate of arrhythmia recurrence in women (28). Overall, it is not clear why gender difference has an impact on the recurrence of AF, and there are few relevant studies, which need to be further investigated.
Leukocyte is a risk factor for POAF. This may be related to increased local inflammation and pericardial effusion around the heart as a result of cardiac surgery. Postoperative pericardial effusion is highly oxidized, the myocardium itself produces pro-inflammatory cytokines, and local inflammation can directly affect cardiac function (29, 30). Inflammation in the pericardial cavity may alter myocardial cell electrical activity and apoptosis, causing elevated leukocyte counts, which can lead to action potential heterogeneity and arrhythmia formation and propagation (31, 32). Therefore, prevention of inflammation may be able to reduce the incidence of POAF (33).
In addition, our results showed a significant effect of TSH levels on the occurrence of POAF. The studies by Morishima et al., Sairaku et al. (34, 35) support our findings, while Baumgartner et al. (36) concluded that there was no significant correlation between TSH levels and AF. Considering the existence of different populations included in different studies differences, future studies with larger samples and multicenter studies are needed to fully analyze the effects of TSH levels on different populations.
4.2. POAF risk prediction line graph model
In this study, a POAF risk column line graph prediction model was established, especially introducing TH as a variable for analysis in this study. The risk prediction model developed in this study had good discrimination and calibration. Compared with previous studies that analyzed the influencing factors of AF (37, 38), the column line graph prediction model we developed was able to score patients more intuitively and quickly to predict the probability of POAF.
5. Limitations of the study
There are some limitations to our study. First of all, more large-sample, multicenter studies are needed to further evaluate the effect of TSH level on POAF due to the relatively small number of cases and single population included in the study. Secondly, due to the limitation of sample size, we only compared the difference of TSH level between the POAF group and the NO-POAF group, and did not stratify the TSH level. Third, this study was a retrospective study, which only evaluated early POAF and did not analyze the long-term occurrence of POAF.
6. Conclusion
We investigated the risk factors of POAF after heart valve surgery, and the prediction model constructed by the column graph has good predictive ability. However, due to the influence of sample size and included groups, more large-sample and multi-center studies are needed to verify the results of this study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted at the Fujian Cardiac Medical Center with institutional ethical approval (Ethics Committee, 2021KY135, Fujian, China). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SL and HZ were responsible for designing this research, collected clinical data and drafted the manuscript, XY and XL were responsible for thesis translation, YP and XL were responsible for data analysis, and LC and YL were responsible for research design and paper revision. All authors contributed to the article and approved the submitted version.
Funding
Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University (No. 2019-67). Special Finance Project of Fujian Province (2021XH019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abdelmoneim SS, Rosenberg E, Meykler M, Patel B, Reddy B, Ho J, et al. The incidence and natural progression of new-onset postoperative atrial fibrillation. JACC Clin Electrophysiol. (2021) 7(9):1134–44. doi: 10.1016/j.jacep.2021.02.005
2. Rezaei Y, Peighambari MM, Naghshbandi S, Samiei N, Ghavidel AA, Dehghani MR, et al. Postoperative atrial fibrillation following cardiac surgery: from pathogenesis to potential therapies. Am J Cardiovasc Drugs. (2020) 20:19–49. doi: 10.1007/s40256-019-00365-1
3. Seo EJ, Hong J, Lee HJ, Son YJ. Perioperative risk factors for new-onset postoperative atrial fibrillation after coronary artery bypass grafting: a systematic review. BMC Cardiovasc Disord. (2021) 21(1):418. doi: 10.1186/s12872-021-02224-x
4. Taha A, Nielsen SJ, Bergfeldt L, Ahlsson A, Friberg L, Björck S, et al. New-onset atrial fibrillation after coronary artery bypass grafting and long-term outcome: a population-based nationwide study from the SWEDEHEART registry. J Am Heart Assoc. (2021) 10(1):e017966. doi: 10.1161/JAHA.120.017966
5. Rostagno C, La Meir M, Gelsomino S, Ghilli L, Rossi A, Carone E, et al. Atrial fibrillation after cardiac surgery: incidence, risk factors, and economic burden. J Cardiothorac Vasc Anesth. (2010) 24(6):952–8. doi: 10.1053/j.jvca.2010.03.009
6. Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. J Am Med Assoc. (2004) 291(14):1720–9. doi: 10.1001/jama.291.14.1720
7. Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. (2019) 16(7):417–36. doi: 10.1038/s41569-019-0166-5
8. Echahidi N, Pibarot P, O'Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. (2008) 51(8):793–801. doi: 10.1016/j.jacc.2007.10.043
9. Dunham WC, Weinger MB, Slagle J, Pretorius M, Shah AS, Absi TS, et al. CYP2D6 genotype-guided metoprolol therapy in cardiac surgery patients: rationale and design of the pharmacogenetic-guided metoprolol management for postoperative atrial fibrillation in cardiac surgery (PREEMPTIVE) pilot study. J Cardiothorac Vasc Anesth. (2020) 34(1):20–8. doi: 10.1053/j.jvca.2019.09.003
10. Pal N, Kertai MD. Perioperative precision medicine: where are we in 2020? Curr Opin Anaesthesiol. (2020) 33(3):463–74. doi: 10.1097/ACO.0000000000000858
11. Pasqualetti G, Calsolaro V, Bernardini S, Linsalata G, Bigazzi R, Caraccio N, et al. Degree of peripheral thyroxin deiodination, frailty, and long-term survival in hospitalized older patients. J Clin Endocrinol Metab. (2018) 103(5):1867–76. doi: 10.1210/jc.2017-02149
12. Merke A, Merke J, Silbernagel G, März W. Free thyroid hormones and mortality in caucasians undergoing angiography: the ludwigshafen risk and cardiovascular health (luric) study. Endocr Pract. (2017) 23(3):288–98. doi: 10.4158/EP161217
13. Kozdag G, Ural D, Vural A, Agacdiken A, Kahraman G, Sahin T, et al. Relation between free triiodothyronine/free thyroxine ratio, echocardiographic parameters and mortality in dilated cardiomyopathy. Eur J Heart Fail. (2005) 7(1):113–8. doi: 10.1016/j.ejheart.2004.04.016
14. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41(1):111–88. doi: 10.1093/eurheartj/ehz455
15. Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, et al. Thyroid and cardiovascular disease: research agenda for enhancing knowledge, prevention, and treatment. Thyroid. (2019) 29(6):760–77. doi: 10.1089/thy.2018.0416
16. Ryan T, Grindal A, Jinah R, Um KJ, Vadakken ME, Pandey A, et al. New-onset atrial fibrillation after transcatheter aortic valve replacement: a systematic review and meta-analysis. JACC Cardiovasc Interv. (2022) 15(6):603–13. doi: 10.1016/j.jcin.2022.01.018
17. Shahim B, Malaisrie SC, George I, Thourani VH, Biviano AB, Russo M, et al. Postoperative atrial fibrillation or flutter following transcatheter or surgical aortic valve replacement: PARTNER 3 trial. JACC Cardiovasc Interv. (2021) 14(14):1565–74. doi: 10.1016/j.jcin.2021.05.026
18. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
19. van den Brink FS, Wijtsma I, Amrane H, Vossenberg TNE, Haenen J, Porta F, et al. Outcome of transcatheter aortic valve replacement in patients over 85 years of age versus patients aged 85 and younger. Neth Heart J. (2022) 30(10):473–8. doi: 10.1007/s12471-022-01693-9
20. Rezk M, Taha A, Nielsen SJ, Gudbjartsson T, Bergfeldt L, Ahlsson A, et al. Clinical course of postoperative atrial fibrillation after cardiac surgery and long-term outcome. Ann Thorac Surg. (2022) 114(6):2209–15. doi: 10.1016/j.athoracsur.2022.03.062
21. Feilberg Rasmussen L, Andreasen JJ, Riahi S, Lundbye-Christensen S, Johnsen SP, Andersen G, et al. Risk and subtypes of stroke following new-onset postoperative atrial fibrillation in coronary bypass surgery: a population-based cohort study. J Am Heart Assoc. (2022) 11(24):e8032. doi: 10.1161/JAHA.122.027010
22. Zhang Z, Hu C, Wang R, Lin J, Ruan Z. Predictive factors for new-onset atrial fibrillation in acute coronary syndrome patients undergoing percutaneous coronary intervention. Panminerva Med. (2020) 62(1):1–6. doi: 10.23736/S0031-0808.18.03556-5
23. Samaritaki E, Tsiligianni I, Basta M, Alegkakis A, Vlassiadis K, Lazopoulos G. Demographic and clinical predictors of post-operative atrial fibrillation in cardio-surgical patients. Eur J Cardiovasc Nurs. (2022) 22(1):98–106. doi: 10.1093/eurjcn/zvac024
24. Gudbjartsson T, Helgadottir S, Sigurdsson MI, Taha A, Jeppsson A, Christensen TD, et al. New-onset postoperative atrial fibrillation after heart surgery. Acta Anaesthesiol Scand. (2020) 64(2):145–55. doi: 10.1111/aas.13507
25. Coletta MJ, Lis G, Clark P, Dabir R, Daneshvar F. Reducing new-onset atrial fibrillation after coronary artery bypass graft surgery. Adv Crit Care. (2019) 30(3):249–58. doi: 10.4037/aacnacc2019470
26. Li H, Wang Z, Cheng Z, Zhu Y, Yuan Z, Gao J, et al. Sex differences involved in persistent atrial fibrillation recurrence after radiofrequency ablation. BMC Cardiovasc Disord. (2022) 22(1):549. doi: 10.1186/s12872-022-03002-z
27. Cheng X, Hu Q, Gao L, Liu J, Qin S, Zhang D. Sex-related differences in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. (2019) 21(10):1509–18. doi: 10.1093/europace/euz179
28. Wong GR, Nalliah CJ, Lee G, Voskoboinik A, Chieng D, Prabhu S, et al. Sex-related differences in atrial remodeling in patients with atrial fibrillation: relationship to ablation outcomes. Circ Arrhythm Electrophysiol. (2022) 15(1):e009925. doi: 10.1161/CIRCEP.121.009925
29. Lehto J, Kiviniemi T. Postpericardiotomy syndrome after cardiac surgery. Ann Med. (2020) 52(6):243–64. doi: 10.1080/07853890.2020.1758339
30. Erlich JF, Paz Z. Postpericardial injury syndrome: an autoimmune phenomenon. Clin Rev Allergy Immunol. (2010) 38(2–3):156–8. doi: 10.1007/s12016-009-8147-9
31. Imazio M, Brucato A, Ferrazzi P, Pullara A, Adler Y, Barosi A, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. J Am Med Assoc. (2014) 312(10):1016–23. doi: 10.1001/jama.2014.11026
32. Imazio M, Belli R, Brucato A, Ferrazzi P, Patrini D, Martinelli L, et al. Rationale and design of the COlchicine for prevention of the post-pericardiotomy syndrome and post-operative atrial fibrillation (COPPS-2 trial): a randomized, placebo-controlled, multicenter study on the use of colchicine for the primary prevention of the postpericardiotomy syndrome, postoperative effusions, and postoperative atrial fibrillation. Am Heart J. (2013) 166(1):13–9. doi: 10.1016/j.ahj.2013.03.025
33. Racca V, Torri A, Grati P, Panzarino C, Marventano I, Saresella M, et al. Inflammatory cytokines during cardiac rehabilitation after heart surgery and their association to postoperative atrial fibrillation. Sci Rep. (2020) 10(1):8618. doi: 10.1038/s41598-020-65581-1
34. Morishima I, Okumura K, Morita Y, Kanzaki Y, Takagi K, Yoshida R, et al. High-normal thyroid-stimulating hormone shows a potential causal association with arrhythmia recurrence after catheter ablation of atrial fibrillation. J Am Heart Assoc. (2018) 7(14):e009158. doi: 10.1161/JAHA.118.009158
35. Sairaku A, Nakano Y, Uchimura Y, Tokuyama T, Kawazoe H, Watanabe Y, et al. Increased left atrial pressure in non-heart failure patients with subclinical hypothyroidism and atrial fibrillation. Endocr Connect. (2016) 5(3):101–6. doi: 10.1530/EC-16-0012
36. Baumgartner C, da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. (2017) 136(22):2100–16. doi: 10.1161/CIRCULATIONAHA.117.028753
37. Dal Zotto B, Barbieri L, Tumminello G, Saviano M, Gentile D, Lucreziotti S, et al. New onset atrial fibrillation in STEMI patients: main prognostic factors and clinical outcome. Diagnostics. (2023) 13(4):613. doi: 10.3390/diagnostics13040613
Keywords: postoperative atrial fibrillation, cardiac valve surgery, thyrotropin, nomogram model, risk factors
Citation: Li S, Zhang H, Liao X, Yan X, Chen L, Lin Y and Peng Y (2023) The occurrence of early atrial fibrillation after cardiac valve operation and the establishment of a nomogram model. Front. Cardiovasc. Med. 10:1036888. doi: 10.3389/fcvm.2023.1036888
Received: 5 September 2022; Accepted: 27 March 2023;
Published: 17 April 2023.
Edited by:
Young Keun On, Sungkyunkwan University, Republic of KoreaReviewed by:
Changsheng Ma, Capital Medical University, ChinaRégis Guieu, Aix Marseille Université, France
© 2023 Li, Zhang, Liao, Yan, Chen, Lin and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjuan Lin Zmp4aHlqbEAxNjMuY29t Liangwan Chen Y2hlbmxpYW5nd2FuQHRvbS5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID Yanjuan Lin orcid.org/0000-0002-6890-7552
Specialty Section: This article was submitted to Heart Valve Disease, a section of the journal Frontiers in Cardiovascular Medicine
 Sailan Li1,†
Sailan Li1,† Yanjuan Lin
Yanjuan Lin Yanchun Peng
Yanchun Peng