- 1Charité Universitätsmedizin Berlin, Department of Cardiology, Berlin, Germany
- 2DZHK (German Centre for Cardiovascular Research), Partner Site Berlin, Berlin, Germany
Heart failure (HF) is one of the most common causes of death in industrialized countries and increases steadily with age. Patients with HF present many comorbidities that affect their clinical management, quality of life, and prognosis. Iron deficiency is a relevant comorbidity of all patients with heart failure. It remains the most prevalent nutritional deficiency worldwide, affecting an estimated 2 billion people and has a negative prognostic impact on hospitalization and mortality rate. To date, none of the previous studies, have provided evidence of reduced mortality or decrease in hospitalization with intravenous iron supplementation. This review describes the prevalence, clinical implications, and current trials on the treatment of iron deficiency in heart failure and discusses the Improvement of exercise and functional capacity and quality of life in patients with heart failure by iron therapy. Despite compelling evidence of the significant prevalence of ID in HF patients and current guidelines, ID is often not properly managed in clinical practice. Therefore, ID should be given greater consideration in HF health care practice to improve patient quality of life and outcome.
Introduction
Currently, many definitions of heart failure (HF) are ambiguous and focus on different characteristics. Some approaches focus primarily on characterizing the hemodynamic and physiologic components, whereas others focus on the diagnostic elements of the clinical syndrome. A revised definition was proposed by the Japanese Heart Failure Society (JHFS), the Heart Failure Association of the European Society of Cardiology (HFA/ESC), and the Heart Failure Society of America (HFSA) in 2021. HF, like many noncategorical diseases, is regarded as a clinical syndrome since it lacks a specific pathognomonic histological or biochemical sign and could emerge from a variety of clinical disease states that are quite different from one another (1). HF is one of the most common causes of death in industrialized countries with a prevalence of 1%–2% in the adult population and increasing steadily with age (2). Numerous comorbidities are prevalent in HF patients and have an impact on their clinical care, quality of life, and prognosis. Iron deficiency (ID) represents one frequent comorbidity in patients with HF. It remains the most prevalent nutritional deficiency worldwide, affecting an estimated 2 billion people.
This review describes the prevalence, clinical implications, and current trials on the treatment of ID in HF and discusses the improvement of exercise and functional capacity, and quality of life in patients with HF by iron therapy.
Prevalence and prognostic impact of ID in HF
ID is a relevant comorbidity in approximately 35%–50% of all patients with HF. ID is defined by current HF guidelines as a serum ferritin level of less than 100 ng/ml or when between 100 and 299 ng/ml with a transferrin saturation level of less than 20% (3). Inflammation may make it difficult to interpret blood ferritin levels. Proinflammatory cytokines can cause “iron entrapment” in macrophages, hepatocytes, and enterocytes by degrading ferroportin, the transmembrane protein responsible for iron transfer outside cells. This process often guards against pathogens that rely on iron availability for life, and it promotes a functional state of ID, rendering iron ineffective despite adequate iron storage (4).
Iron is essential for aerobic metabolism in addition to its impact on hemoglobin and myoglobin the primary proteins for O2 transport and accumulation. The citric acid cycle and the respiratory chain are two major pathways for oxidoreductive processes involved in the production of cellular energy. Iron sufficiency is necessary for the synthesis of mitochondrial proteins. These elements undoubtedly play a part in HF's decreased ability to exercise. Melenovsky et al. studied left ventricular samples from 91 HF patients undergoing transplantation and compared them with samples from 38 HF-free organ donors (controls). HF patients showed significantly reduced myocardial oxygen respiration and decreased activity of all tested mitochondrial enzymes as compared to controls (5). This supports the hypothesis that ID may be associated with the exacerbation of mitochondrial dysfunction already present in HF. Being highly active cells, cardiomyocytes require effective energy generation, hence, appropriate mitochondrial activity is essential. According to Bakogiannis et al. iron deficiency also hampers the electrochemical stability of cardiomyocytes due to impaired mitochondrial enzyme activity. This results in dysfunctional contractility of the cardiomyocytes and may trigger lethal arrhythmias (6). Since their production depends on the availability of iron, ferritin, and transferrin saturation blood levels in clinical settings might reveal information about iron homeostasis (4). In addition to its important role in oxygen transport and metabolism, iron also plays a critical role in microRNA biogenesis, thyroid, central nervous system, and immune system function (7).
Anemia in HF has a complex etiology, it is often brought on by a variety of conditions, such as hypoplastic bone marrow, insufficient erythropoiesis, gastrointestinal bleeding, drug interactions, or food-reducing absorption (8). ID persists (>30%) in individuals without anemia or abnormalities in hematologic indexes regardless of the presence of anemia (9). It can be observed that the prevalence of ID rises with the increasing NYHA class. Other risk factors such as high plasma N-terminal natriuretic peptide type B (NT-pro-BNP), high serum high-sensitivity C-reactive protein (hsCRP), and female gender also negatively affect disease progression in HF patients with ID (10). As illustrated in Figure 1, the prevalence is higher in patients with acutely decompensated HF, varying between 72% and 83% (11).
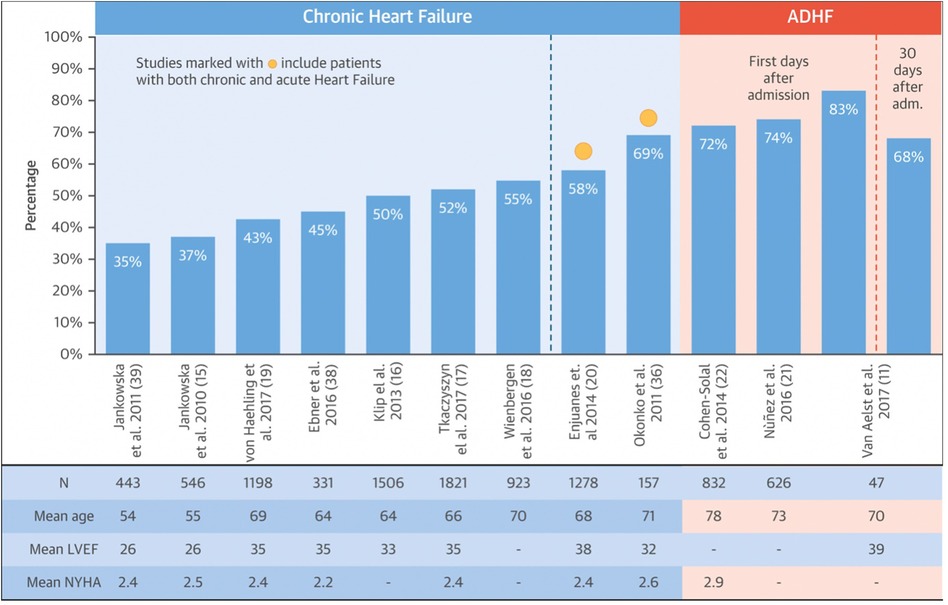
Figure 1. Prevalence of ID in HF. Modified after Rocha et al. (11).
To date, none of the previous studies, nor the randomized AFFIRM-AHF trial presented at the 2020 AHA Congress, have provided evidence of reduced mortality or decrease in hospitalization with intravenous iron supplementation. However, nearly all studies were not powered for this endpoint.
Between 2016 and 2017, there were two meta-analyses devoted to IV iron therapy in patients with HF. With a total of 851 patients across 5 clinical trials, Jankowska et al. found that IV iron therapy decreased the risk of both the combined endpoint of all-cause death or cardiovascular hospitalization (OR: 0.44; 95% CI: 0.30 to 0.64; p < 0.0001) and the combined endpoint of cardiovascular death or hospitalization for worsening HF (OR: 0.39; 95% CI: 0.24 to 0.63; p = 0.0001) (12).
Using data from 4 randomized controlled trials (FER-CARS-01, FAIR-HF, EFFICACY-HF, and CONFIRM-HF) that included 839 patients with chronic HF a second metanalysis was carried out in 2017. The results showed that, compared with placebo, FCM reduced recurrent HF hospitalizations (relative risk RR: 0.41; 95% CI: 0.23 to 0.73; p 1⁄4 0.003) and recurrent cardiovascular hospitalizations (RR: 0.54; 95% CI: 0.36 to 0.83; p 1⁄4 0.004) (13).
These findings support the use of IV iron therapy in HF, which led to the creation of the prospective FAIR-HF 2 trial [NCT03036462 (14)] with a primary endpoint of the combined rate of recurrent hospitalizations for HF and cardiovascular death.
Impact of ID on exercise and functional capacity and quality of life
Iron is an essential micronutrient involved in a wide range of biological processes, such as oxygen delivery, energy production in the mitochondria, and metabolic homeostasis.
Exercise and functional capacity
In the published data exercise and functional capacity are frequently mentioned and used as equivalent terms but it is important to distinguish these. Contrary to functional capacity, which is described as “the ability to perform activities of daily living that require sustained, submaximal aerobic metabolism”, exercise capacity can be characterized as “the maximum amount of physical exertion that a subject can sustain” (15). Objective quantitative methods for the assessment are the 6-min walk test (6MWT—functional capacity), the graded exercise testing with electrocardiography (ECG), and cardiopulmonary exercise testing with the evaluation of peak oxygen consumption (CPET—exercise capacity).
Quality of life—PRO
Patient-reported outcomes (PROs), as defined by the World Health Organization and the U.S. Food and Drug Administration, are patient-reported evaluations of their health status that are not interpreted by anyone else, such as physicians (16). Consequently, a patient-reported outcome measure (PROM) is an instrument, such as a questionnaire, used to quantify and gather data on a PRO, which is often associated with health-related quality of life. PROMs can be classified as general and disease-specific measures. The purpose of generic PROMs is to assess a patient's overall condition, such as her physical, functional, and emotional state, and is not limited to a specific condition. Disease-group-specific PROMs pertain to a particular set of diseases. These PROMs are often more sensitive than generic PROMs by measuring one specific disease, e.g., sleep apnea in HF patients (17). PROs are becoming more significant as predictors of mortality and hospitalization in patients with HF and can be used as primary or secondary endpoints in clinical studies, as both clinicians and patients are increasingly realizing (18). Several commonly used questionnaires have been validated in HF for assessing the quality of life, including the Kansas City Cardiomyopathy Questionnaire (KCCQ), the Minnesota Living with Heart Failure Questionnaire (MLHFQ), the European Quality of Life 5 Dimensions (EQ-5D) or the Short Form 36 (SF-36) (19, 20). In a recently published cohort study of 2021, 2,872 patients with chronic HF with reduced ejection fraction have been enrolled with an evaluation of NYHA class and KCCQ-OS data at baseline and 12 months. Examined were the longitudinal changes and correlations between the two measures, NYHA and KCCQ-OS, from baseline to 12 months, with clinical outcomes occurring between months 12 and 24. The cohort analysis demonstrated that the patient-reported KCCQ-OS was more likely than the clinician-assigned NYHA class to detect a substantial change in health status over time (21).
Evaluating PROs for sex and gender differences reveals consistent evidence that women and men perceive their physical symptoms and psychological load differently. Most investigations discovered gender discrepancies in PRO, with female patients faring worse than male patients (22, 23). In the process of developing and validating PROMs, it is critical to have a gender-balanced population, examine discrepancies between male and female reports, and consider gender identities beyond the binary idea. However, despite the importance of these PROs, the chance to incorporate them into standard clinical practice is seldom taken advantage of. The practicality of integrating PROMs into clinical workflow, linking it to medical records, interpretability of the data, and the expense of collecting and analyzing the data are just some of the hurdles to the regular use of PRO (24). The most prevalent formal HF-specific PROMs were designed primarily for outpatient usage and were not originally intended for hospitalizations (25). Nonetheless, these PROMs may have therapeutic applications in the inpatient context and, if utilized effectively, may enhance risk classification and patient-centered treatment.
Clinical trials—oral iron therapy
Two clinical studies the IRON-5 trial (Short Term Oral Iron Supplementation in Systolic Heart Failure Patients Suffering From Iron Deficiency Anemia) and the IRONOUT HF trial (Oral Iron Repletion Effects On Oxygen Uptake in Heart Failure) assessed the effects of oral iron supplementation in comparison to intravenous iron (IV) supplementation in patients with HF (summarized in Table 1).
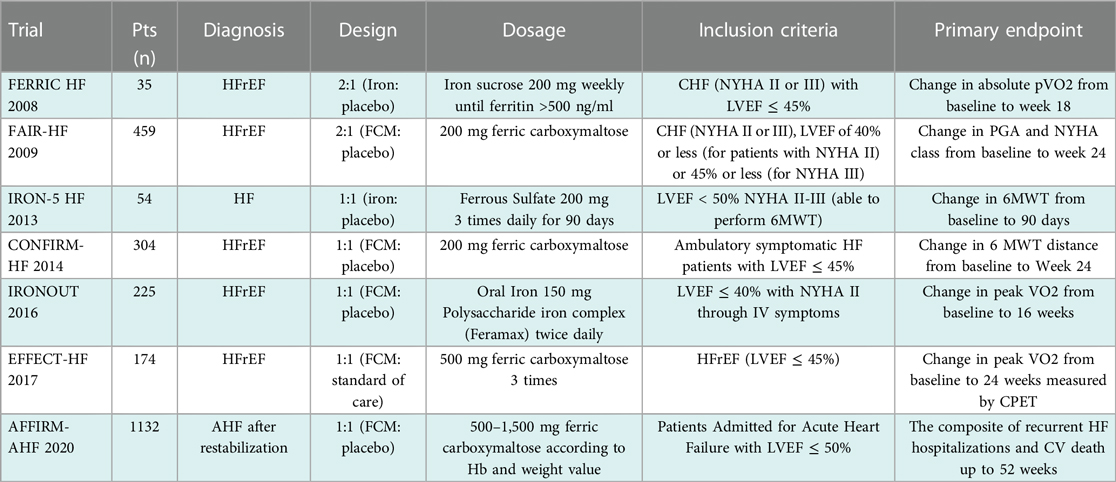
Table 1. Main trials of iron supplementation in heart failure. Modified after Rizzo et al. (4).
The IRON 5 trial, published in 2013, randomized patients with a LVEF of <50% and NYHA class II to IV, a hemoglobin concentration between 9 and 12 g/dl, a transferrin saturation (TSAT) level of >20%, and a ferritin concentration of <500 µg/dl. Patients were randomly allocated to three groups with three distinct treatments using a double-blind method: 1. oral ferrous sulfate treatment, 200 mg three times a day, plus IV placebo, 2. oral placebo plus IV iron sucrose, 200 mg weekly, and 3. oral and IV placebo. Patients received oral iron for 8 weeks, whereas IV iron was administered over 5 weeks. The primary endpoint was the change in peak oxygen consumption (peak VO2) measured by ergospirometry throughout a 3-month follow-up period. The trial was stopped early with only 23 randomized subjects due to financing issues. After 3 months, the IV iron group's peak oxygen consumption significantly increased, whereas the other groups did not (26).
A phase II double-blind, randomized, placebo-controlled trial called IRONOUT was also published in 2017. It included 225 patients with HF, an LVEF of <40%, and ID, which was defined as a ferritin concentration of 15–100 μg/L or ferritin 101–299 μg/L with TSAT of <20%. Patients were given 150 mg of iron polysaccharide twice a day for 16 weeks. The primary endpoint was the difference in peak oxygen uptake from baseline to 16 weeks, which did not vary significantly between the two groups at the end of the study (27).
Several clinical trials, such as those mentioned above, have had inconclusive outcomes for oral administration, therefore oral iron supplementation remains contentious. In addition, it is important to note that up to 40% of patients report side effects, most often gastrointestinal discomfort such as nausea, flatulence, stomach pain, diarrhea, and constipation (10). The ESC guidelines thus suggest IV iron supplementation for HF patients with ID as oral treatment does not adequately replenish iron reserves (28).
Clinical trials—intravenous iron therapy
As summarized in Table 1, several major studies have investigated intravenous iron supplementation and shown significant benefits in improved exercise and functional capacity. The first large-scale trial, FAIR-HF (Ferinject Assessment in patients with IRon deficiency and chronic Heart Failure) enrolled 459 patients with NYHA class II (LVEF ≤ 40%) or NYHA class III (LVEF ≤ 45%) and a hemoglobin concentration ranging between 9.5 and 13.5 g/dl. ID was defined as a serum ferritin concentration of <100 µg/L or a ferritin concentration range from 100 to 300 µg/L with a TSAT of <20%. Patients were randomly assigned to receive either IV ferric carboxymaltose (FCM) or a placebo 2:1. FCM was administered weekly during the correction phase and then every 4 weeks during the maintenance phase until iron repletion was accomplished. The trial demonstrated that IV FCM improved the main outcome, the self-reported patient global assessment (PGA), which compares the patient's present well-being to their prior assessment. Secondary endpoints included NYHA functional class, 6-min walk distance, and quality of life as measured by the Kansas City Cardiomyopathy Questionnaire, all of which showed a statistically significant improvement regardless of the presence of anemia at baseline (47% had an NYHA functional class I or II at week 24 after IV FCM, as compared with 30% of patients assigned to placebo; 6MWT increased from 274 ± 6 to 313 ± 7 m after 24 weeks; mean KCCQ increased from 52 ± 1 to 66 ± 1 points) (29). The CONFIRM HF trial validated these findings (A Study to Compare the Use of Ferric Carboxymaltose With Placebo in Patients With Chronic Heart Failure and Iron Deficiency). This mentioned trial involved 304 patients with symptomatic HF, NYHA class II or III, a LVEF of ≤45%, and an elevated level of either NT-proBNP or B-type natriuretic peptide. Patients were given a 1:1 ratio of either a placebo or an iron dose ranging from 500 to 2,000 mg during the first 6 weeks of the trial. If ferritin and/or TSAT remained to exhibit ID beyond this period, participants received 500 mg of FCM at each visit in weeks 12, 24, and 36. The main outcome was the change in the 6MWT from baseline to 24 weeks, which was significantly improved in the IV FCM group compared to the placebo group (difference: 33 ± 11 m; p = 0.002). Quality-of-life scores were significantly improved in FCM participants compared with placebo-treated patients on the KCCQ and the EQ-5D questionnaire (30).
In the 2017 EFFECT-HF study (Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Chronic Heart Failure and Iron Deficiency), 174 patients with LVEF ≤ 45% and predominantly NYHA class II at baseline were randomized, in a nonblinded fashion, to IV FCM therapy or standard of care for a 24-week follow-up. The main outcome was the change in peak VO2 between baseline and 24 weeks, as measured by CPET. After 24 weeks, peak oxygen decreased in the control group (−0.16 ± 0.387 ml/min/kg; p = 0.02) (31).
Positive effects for HF hospitalization rates and reduction in cardiovascular mortality rates can be inferred from the results of data from meta-analyses but have yet to be confirmed in a prospective study (12).
The randomized, double-blind, placebo-controlled AFFIRM-AHF study assesses the influence of intravenous FCM on hospitalizations and mortality in iron-deficient patients treated for acute HF. Patients hospitalized for acute HF with an LVEF of <50% and NYHA class II or III were recruited for the trial. Patients were randomly assigned to receive either IV FCM as an active treatment or a placebo. To qualify, patients' blood ferritin levels are required to be <100 or 100–299 µg/L with a TSAT of <20%, according to the ID criteria used in the previous trial. The trial's main outcome was a composite of HF hospitalizations and cardiovascular deaths over a 1-year follow-up period. At 52 weeks of follow-up, the incidence of cardiovascular death did not vary between the treatment and placebo groups. For the combined primary endpoint of total hospitalizations and cardiovascular (CV) death, the total number of events was numerically reduced in those treated with IV FCM compared to placebo by 21%, although this difference was not statistically significant (RR 0.79; 95% CI 0.62–1.01) (32).
As shown in Table 1 the most researched IV formulations for HF are IS and FCM. According to the labeling for the individual products, the dosage regimens for FCM and IS are different. While IS dosage is limited to 200/300 mg iron per infusion and 600 mg once weekly, FCM can be provided in quantities of up to 1,000 mg iron in a single infusion. From an economic perspective, the main disadvantage of IS is the limited dosage per session that requires multiple sessions, even if IS is cheaper than FCM. Nevertheless, economic analyses support the cost-effectiveness of FCM, which is driven by improved symptomatic status (NYHA class) and a drop in hospitalization rates (33). Because a complete iron dose may be delivered in fewer infusions with FCM compared to other formulations, the former may have cheaper expenses per therapy (34).
Ongoing trials
There are now several clinical trials being undertaken, the majority of which concentrate on the use of IV iron therapy in patients with chronic HF employing strict primary endpoints such as CV death or HF hospitalization.
◾ FAIR-HFpEF (Effect of IV Iron in Patients With Heart Failure With Preserved Ejection Fraction; NCT03074591)
The FAIR-HFpEF trial examines whether treatment with IV FCM for patients with heart failure with preserved ejection fraction (HFpEF) and ID, both with or without anemia, can increase exercise capacity as determined by a 6-min walking test (primary endpoint after 52 weeks). Secondary endpoints are the PGA quality of life questionnaire, the difference in NYHA class, and the impact on mortality and HF-related hospitalization rates from baseline to the conclusion of the trial (35).
◾ HEART-FID (Randomized Placebo-controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency; NCT03037931)
The HEART-FID trial is a double-blind, multicenter, prospective, randomized, placebo-controlled trial aimed to evaluate the efficacy of intravenous FCM over that of a placebo. The combined primary endpoint is a composite of death and HF hospitalization at 12 months and changes from baseline to 6 months in the 6MWT distance (36).
◾ FAIR-HF2 (Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity & Mortality; NCT03036462)
The clinical trial is designed as an international, prospective, multicenter, double-blind, parallel-group, randomized, controlled, interventional trial to determine whether long-term therapy with IV FCM compared to placebo can reduce the rate of recurrent HF hospitalizations and CV death in patients with heart failure with reduced ejection fraction (HFrEF). The major outcome measure is the combined rate of recurrent hospitalizations for HF and cardiovascular death (number of events) (14).
◾ IRONMAN (Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency; NCT02642562)
Compared to other trials investigating iron therapy, IRONMAN will use a different type of iron therapy, IV iron isomaltose (IIM), compared to FCM used in the above-mentioned trial.
IIM is compared to FCM dextran-based. They differ in type, structure, half-life, stability of polymerization, pharmacokinetics, dosage, and tolerability (37). The European Medicines Agency (EMA) issued a positive risk-benefit assessment for both iron preparations. However, the current evidence for the clinical benefit of i.v. iron supplementation primarily relates to FCM, such that FCM is also the only i.v. iron supplementation is recommended in the ESC guidelines for the treatment of HF patients with ID.
The IRONMAN trial is a randomized, open-label multicentre trial compared to the other trials. The purpose of the trial was to determine whether the treatment of IV IIM in addition to standard treatment would enhance the prognosis for patients with HF and ID. The primary endpoint is CV mortality or hospitalization for worsening HF (14).
◾ RESAFE-HF trial (Iron Intravenous Therapy in Reducing the burden of Severe Arrhythmias in HFrEF; NCT04974021)
The RESAFE-HF trial is a prospective, single-center, and open-label registry trial, to evaluate the effect of IV FCM on HFrEF patients' iron stores, arrhythmic burden, hospitalizations, functional capacity, quality of life, ultrasonographic parameters, and disease biomarkers. Patients with HFrEF and CIEDs scheduled to receive IV FCM as a treatment for ID were eligible to participate. The primary endpoint is a composite endpoint of haemoglobin ≥12 g/dl, ferritin ≥50 ng/L, and transferrin saturation >20%. Secondary endpoints include unplanned HF-related hospitalizations, ventricular tachyarrhythmias detected by CIEDs and Holter monitors, echocardiographic markers, functional status (VO2 max and 6 min walk test), blood biomarkers, and quality of life (38).
ESC guidelines
The updated 2021 European Society of Cardiology (ESC) guidelines for the diagnosis and management of acute and chronic HF emphasize the importance of ID diagnostics. It is now a class Ic recommendation that all patients with HF be screened periodically for anemia and ID, with a full blood count, serum ferritin concentration, and transferrin saturation. Regarding discharge of patients with HFrEF, recently hospitalized for worsening HF, intravenous FCM supplementation should also be considered to reduce HF rehospitalizations and symptom burden (28).
There is no update to the 2016 published guidelines regarding intravenous iron supplementation. It remains a class IIa recommendation that patients with ID and symptomatic HFrEF receive intravenous FCM to improve exercise and functional capacity and quality of life. Iron therapy is propagated for symptomatic patients with HF when ferritin is <100 μg/L or when ferritin is between 100 and 299 μg/L and transferrin saturation is <20% (39) (Figure 2).
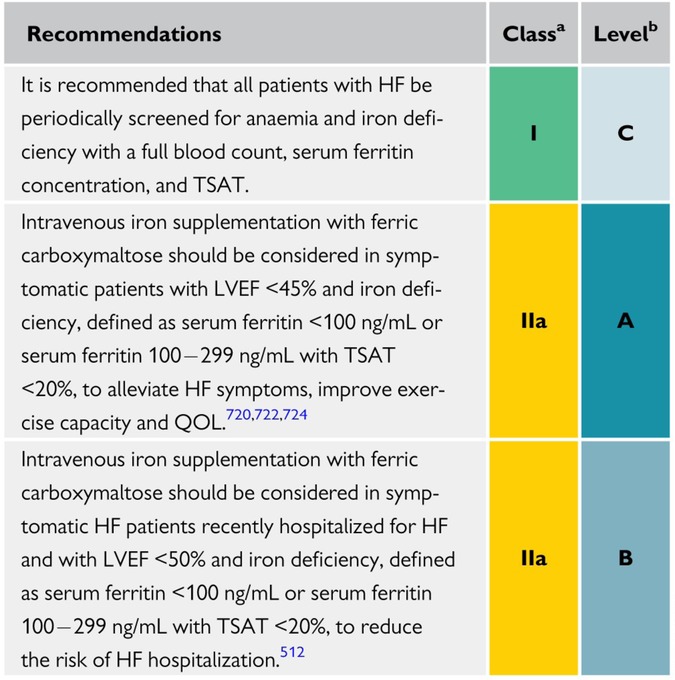
Figure 2. Recommendations for the management of anemia and iron deficiency in patients with heart failure (28).
Conclusion
Iron deficiency is a relevant co-morbidity in HF patients, which has a poor prognostic impact on hospitalization and mortality rates while impairing physical and functional performance and quality of life. Despite compelling evidence of the significant prevalence of ID in HF patients and current guidelines, ID is often not properly managed in clinical practice. Therefore, ID should be given greater consideration in HF health care practice to improve patient quality of life and outcome. Furthermore, numerous issues are still unsolved despite increased research efforts to understand the function of ID in HF. The mechanistic research now being done to explain the advantages of IV iron is cardio-centric in character, even though iron's functions are not only related to the cardiovascular system. Understanding the pathophysiology of ID will facilitate research into the effects of IV iron on the entire circulation.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This paper was supported by financial assistance from Vifor Pharma. The funder was not involved in the analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
FE reports personal fees and non-financial support from Novartis, grants and personal fees from Boehringer Ingelheim, personal fees from CVRx, Pfizer, Medtronic, grants and personal fees from Servier, personal fees from MSD/Bayer, personal fees from Bayer, personal fees from Resmed, personal fees from Berlin Chemie, grants from Thermo Fischer, personal fees from Vifor Pharma, personal fees from PharmaCosmos, personal fees from Merck, outside the submitted work.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Biykem Bozkurt, Andrew JS Coats, Hiroyuki Tsutsui, Magdy Abdelhamid, Stamatis Adamopoulos, Nancy Albert. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure. J Card Fail. (2021) 27(4):387–413. doi: 10.1002/ejhf.2115.
2. Emelia J Benjamin, Paul Muntner, Alvaro Alonso, Marcio S Bittencourt, Clifton W Callaway, April P Carson. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. (2019) 139(10):e56–e528. doi: 10.1161/CIR.0000000000000659
3. Theresa A McDonagh, Marco Metra, Marianna Adamo, Roy S Gardner, Andreas Baumbach, Michael Böhm. Corrigendum to: 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42(48):4901. doi: 10.1093/eurheartj/ehab670
4. Caterina Rizzo, Rosa Carbonara, Roberta Ruggieri, Andrea Passantino, Domenico Scrutinio. Iron deficiency: a new target for patients with heart failure. Front Cardiovasc Med. (2021) 8:709872. doi: 10.3389/fcvm.2021.709872
5. Vojtech Melenovsky, Jiri Petrak, Tomas Mracek, Jan Benes, Barry A Borlau, Hana Nuskova. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail. (2017) 19(4):522–30. doi: 10.1002/ejhf.640
6. Constantinos Bakogiannis, Alexandros Briasoulis, Dimitrios Mouselimis, Anastasios Tsarouchas, Nikolaos Papageorgiou, Christodoulos Papadopoulos. Iron deficiency as therapeutic target in heart failure: a translational approach. Heart Fail Rev. (2020) 25(2):173–82. doi: 10.1007/s10741-019-09815-z
7. Martina U Muckenthaler, Stefano Rivella, Matthias W Hentze, Bruno Galy. A red carpet for iron metabolism. Cell. (2017) 168(3):344–61. doi: 10.1016/j.cell.2016.12.034
8. Goran Loncar, Danilo Obradovic, Holger Thiele, Stephan von Haehling, Mitja Lainsca. Iron deficiency in heart failure. ESC Heart Fail. (2021) 8(4):2368–79. doi: 10.1002/ehf2.13265
9. Tkaczyszyn M, Jankowska EA. Iron deficiency and red cell indices in patients with heart failure: reply. Eur J Heart Fail. (2018) 20(4):828–9. doi: 10.1002/ejhf.1092
10. Stephan von Haehling, Waldemar Banasiak, Piotr Ponikowski, Ewa A. Jankowska, Wolfram Doehner, Andrew J. S. Coats. Iron deficiency in heart failure: an overview. JACC Heart Fail. (2019) 7(1):36–46. doi: 10.1016/j.jchf.2018.07.015
11. Rocha BML, Cunha GJL, Menezes Falcao LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. (2018) 71(7):782–93. doi: 10.1016/j.jacc.2017.12.027
12. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail. (2016) 18(7):786–95. doi: 10.1002/ejhf.473
13. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. (2018) 20(1):125–33. doi: 10.1002/ejhf.823
14. Intravenous iron in patients with systolic heart failure and iron deficiency to improve morbidity & mortality. Available at: https://ClinicalTrials.gov/show/NCT03036462.
15. Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ. Assessment of functional capacity in clinical and research settings: a scientific statement from the American heart association committee on exercise, rehabilitation, and prevention of the council on clinical cardiology and the council on cardiovascular nursing. Circulation. (2007) 116(3):329–43. doi: 10.1161/CIRCULATIONAHA.106.184461
16. U.S. Department of Health, et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. (2006) 4:79. doi: 10.1186/1477-7525-4-79
17. Benjamin K, Vernon MK, Patrick DL, Perfetto EM, Nestler-Parr S, Burke L. Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an ISPOR COA emerging good practices task force report. Value Health. (2017) 20(7):838–55. doi: 10.1016/j.jval.2017.05.015
18. Mastenbroek MH, Versteeg H, Zijlstra WP, Jaarsma T, Lenzen MJ, van der Wal MH. Disease-specific health status as a predictor of mortality in patients with heart failure: a systematic literature review and meta-analysis of prospective cohort studies. Eur J Heart Fail. (2014) 16(4):384–93. doi: 10.1002/ejhf.55
19. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L. Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev. (2014) 19(3):359–67. doi: 10.1007/s10741-013-9394-7
20. von Haehling S, Kapelios CJ, Papadopoulos CE, Bekfani T, Anker MS, Anker SD. Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail. (2021) 23(1):92–113. doi: 10.1002/ejhf.1838
21. Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM. Comparison of New York heart association class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. (2021) 6(5):522–31. doi: 10.1001/jamacardio.2021.0372
22. Blinderman CD, Homel P, Billings JA, Tennstedt SL, Portenoy . Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. (2008) 35(6):594–603. doi: 10.1016/j.jpainsymman.2007.06.007
23. O'Meara E, et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circulation. (2007) 115(24):3111–20. doi: 10.1161/CIRCULATIONAHA.106.673442
24. Spertus J. Barriers to the use of patient-reported outcomes in clinical care. Circ Cardiovasc Qual Outcomes. (2014) 7(1):2–4. doi: 10.1161/CIRCOUTCOMES.113.000829
25. Psotka MA, von Maltzahn R, Anstrom KJ, Redfield MM, Bull DA, Desvigne-Nickens P. Patient-reported outcomes in chronic heart failure: applicability for regulatory approval. JACC Heart Fail. (2016) 4(10):791–804. doi: 10.1016/j.jchf.2016.04.010
26. Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque DC. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. (2013) 168(4):3439–42. doi: 10.1016/j.ijcard.2013.04.181
27. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. J Am Med Assoc. (2017) 317(19):1958–66. doi: 10.1001/jama.2017.5427
28. Authors/Task Force, Theresa A McDonagh, Marco Metra, Marianna Adamo, Roy S Gardner, Andreas Baumbach, Michael Böh. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2022) 24(1):4–131. doi: 10.1002/ejhf.2333
29. Anker SD, Comin-Colet J, Filippatos G, Ronnie Willenheimer, Kenneth Dickstein, Helmut Drexler. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. (2009) 361(25):2436–48. doi: 10.1056/NEJMoa0908355
30. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J. (2015) 36(11):657–68. doi: 10.1093/eurheartj/ehu385
31. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. (2017) 136(15):1374–83. doi: 10.1161/CIRCULATIONAHA.117.027497
32. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. (2020) 396(10266):1895–904. doi: 10.1016/S0140-6736(20)32339-4
33. Theidel U, Kuhlmann MK, Pfeil AM, Stang P, Cantré D, Neumann T. Budget impact of intravenous iron therapy with ferric carboxymaltose in patients with chronic heart failure and iron deficiency in Germany. ESC Heart Fail. (2017) 4(3):274–81. doi: 10.1002/ehf2.12179
34. Basha A, Hammad SA, Zaki A, Mousa M, Abdellatif AH, Radwan W. Efficacy and cost effectiveness of intravenous ferric carboxymaltose versus iron sucrose in adult patients with iron deficiency anaemia. PLoS One. (2021) 16(8):e0255104. doi: 10.1371/journal.pone.0255104
35. Effect of IV iron in patients with heart failure with preserved ejection fraction. Available at: https://ClinicalTrials.gov/show/NCT03074591.
36. Randomized placebo-controlled trial of FCM as treatment for heart failure with iron deficiency. Available at: https://ClinicalTrials.gov/show/NCT03037931.
37. Martin-Malo A, Aljama P, Canaud B, Rodriguez M, Sanz M, Ramos R. Differences between intravenous iron products: focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail. (2019) 6(2):241–53. doi: 10.1002/ehf2.12400
38. Bakogiannis C, Anker SD, Cleland JGF, Filippatos G, Gheorghiade M, Metra M. Iron therapy and severe arrhythmias in HFrEF: rationale, study design, and baseline results of the RESAFE-HF trial. ESC Heart Fail. (2023) 10(2):1184–92. doi: 10.1002/ehf2.14276.36647691
39. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2016) 18(8):891–975. doi: 10.1002/ejhf.592
Keywords: heart faillure, iron defciency, iron therapy, exercise capacity, quality of life
Citation: Deichl A and Edelmann F (2023) Improvement of exercise and functional capacity and quality of life in patients with heart failure by iron therapy. Front. Cardiovasc. Med. 10:1025957. doi: 10.3389/fcvm.2023.1025957
Received: 23 August 2022; Accepted: 10 April 2023;
Published: 22 May 2023.
Edited by:
Matteo Cameli, University of Siena, ItalyReviewed by:
Mohamed A. Yassin, Hamad Medical Corporation, QatarVassilios Vassilikos, Aristotle University of Thessaloniki, Greece
© 2023 Deichl and Edelmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank Edelmann ZnJhbmsuZWRlbG1hbm5AY2hhcml0ZS5kZQ==
Abbreviations 6MWT, 6-min walk test; CPET, cardiopulmonary exercise testing; CV, cardiovascular; ECG, electrocardiography; EMA, european medicines agency; EQ-5D, European quality of life 5 dimension; FCM, ferric carboxymaltose; LVEF, left ventricular ejection fraction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hsCRP, high-sensitivity C-reactive protein; ID, iron deficiency; IIM, iron isomaltose; KCCQ, kansas city cardiomyopathy questionnaire; MLHFQ, minnesota living with heart failure questionnaire; NT-pro-BNP, N-terminal natriuretic peptide type B; PGA, patient global assessment; PROs, patient-reported outcomes; PROM, patient-reported outcome measure; peak VO2, peak oxygen consumption; SF-36, short form 36; TSAT, transferrin saturation.
 Andrea Deichl
Andrea Deichl Frank Edelmann
Frank Edelmann