- 1Department of Epidemiology and Biostatistics, Key Laboratory of Molecular Cancer Epidemiology, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin, China
- 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Beijing Office for Cancer Prevention and Control, Peking University Cancer Hospital and Institute, Beijing, China
- 3Department of Statistics, European Organization for Research and Treatment of Cancer, Brussels, Belgium
Background: Previous studies focused more on the short-term risk of cardiovascular (CV) death due to traumatic psychological stress after a cancer diagnosis and the acute cardiotoxicity of anticancer treatments than on the long-term risk of CV death.
Methods: Time trends in the proportions of CV death (PCV), cancer death (PCA), and other causes in deaths from all causes were used to show preliminary relationships among the three causes of death in 4,806,064 patients with cancer from the Surveillance, Epidemiology, and End Results (SEER) program. Competing mortality risk curves were used to investigate when the cumulative CV mortality rate (CMRCV) began to outweigh the cumulative cancer mortality rate (CMRCA) for patients with cancer who survived for more than 10 years. Multivariable competing risk models were further used to investigate the potential factors associated with CV death.
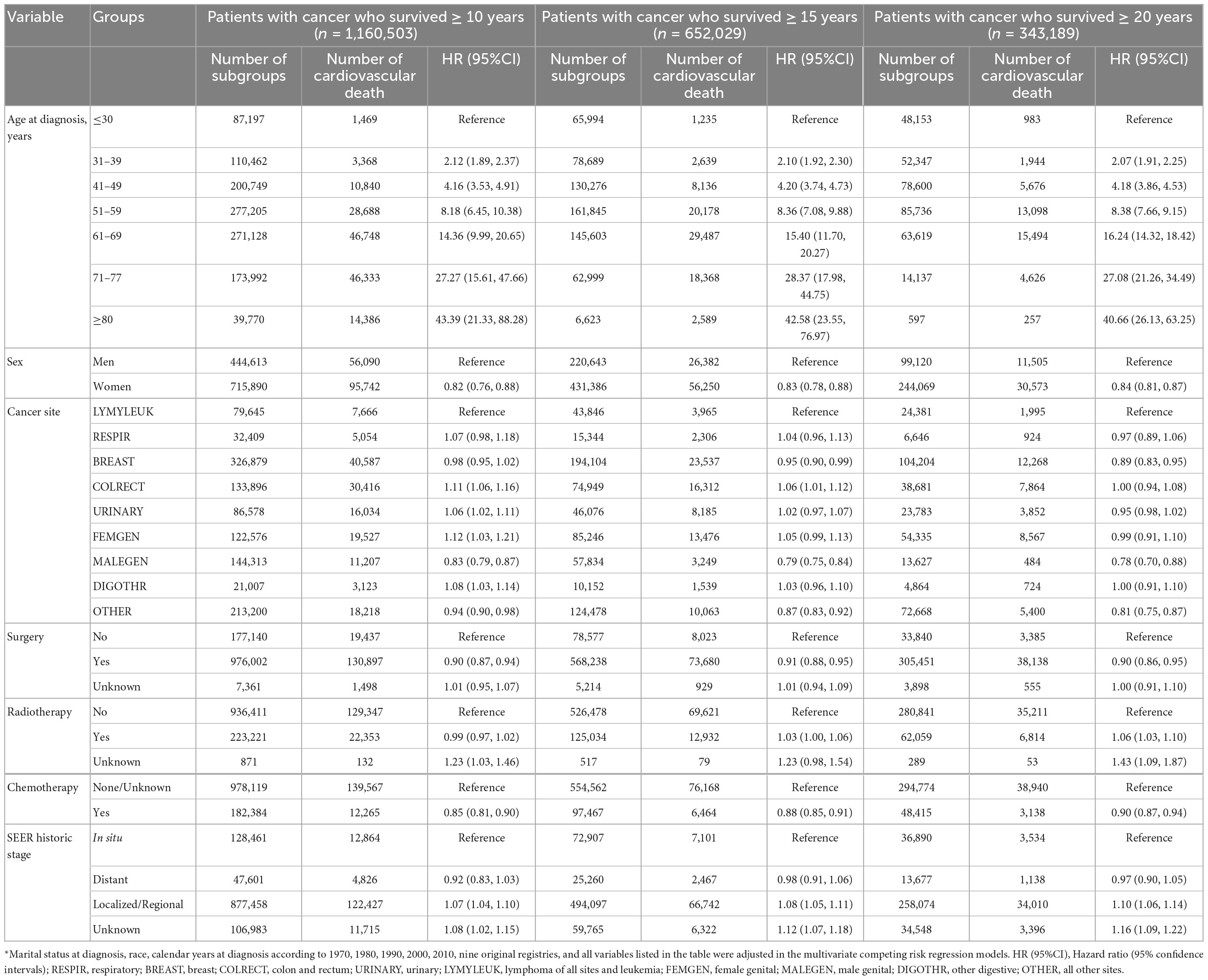
Results: For patients with cancer at all sites, the PCV increased from 22.8% in the 5th year after diagnosis to 31.0% in the 10th year and 35.7% in the 20th year, while the PCA decreased from 57.7% in the 5th year after diagnosis to 41.2 and 29.9% in the 10th year and 20th year, respectively. The PCV outweighed the PCA (34.6% vs. 34.1%) since the 15th year for patients with cancer at all sites, as early as the 9th year for patients with colorectal cancer (37.5% vs. 33.2%) and as late as the 22nd year for patients with breast cancer (33.5% vs. 30.6%). The CMRCV outweighed the CMRCA since the 25th year from diagnosis. Multivariate competing risk models showed that an increased risk of CV death was independently associated with older age at diagnosis [hazard ratio and 95% confidence intervals [HR (95%CI)] of 43.39 (21.33, 88.28) for ≥ 80 vs. ≤ 30 years] and local metastasis [1.07 (1.04, 1.10)] and a decreased risk among women [0.82 (0.76, 0.88)], surgery [0.90 (0.87, 0.94)], and chemotherapy [0.85 (0.81, 0.90)] among patients with cancer who survived for more than 10 years. Further analyses of patients with cancer who survived for more than 20 years and sensitivity analyses by cancer at all sites showed similar results.
Conclusion: CV death gradually outweighs cancer death as survival time increases for most patients with cancer. Both the cardio-oncologist and cardio-oncology care should be involved to reduce CV deaths in long-term cancer survivors.
Introduction
Cancer ranks as the leading cause of death and remains the primary barrier to increasing life expectancy in most global countries in the twenty-first century (1). According to recent estimates from the World Health Organization (WHO), cancer is the first or second leading cause of death below the age of 70 in 91 out of 172 countries and ranks third or fourth in another 22 countries (1). Moreover, according to a recent study on the Global Burden of Disease, cancer cases rose by 28% between 2006 and 2016 (2). This rise seems to continue due to the growing epidemic of cancer-associated risk factors, including aging, tobacco use, unhealthy diet, excess body weight, and physical inactivity. According to the most recent GLOBOCAN estimates, there would be 19.3 million new cases and almost 10 million cancer deaths worldwide in 2020 (3).
A cancer diagnosis is often associated with immediate and traumatic psychological stress, thereby increasing the risk of short-term cardiovascular (CV) death (4–6). As reported by Fang, the risk of CV death after a cancer diagnosis is 6–10 times higher than that in cancer-free people (5). However, the risk of CV death due to psychological stress began to decline rapidly 1 year after a cancer diagnosis (5, 7, 8). Therefore, several previous studies paid more attention to the immediate risks of CV death but less attention to the long-time risk of CV death. Due to the long-term cumulative cardiotoxicity of anticancer treatments, the potential long-time risk of CV death after anticancer treatments among patients with cancer has always been a great concern for both oncologists and cardiologists (9–12). Previous studies suggested the increased risk of developing CV disease (CVD) among long-term cancer survivors, including patients who were young (13) or patients older than 40 years (14), patients with colorectal cancer who survived for more than 10 years (15), and other patients with breast, prostate, or bladder cancer (16). A recent study suggested that the risk of fatal heart disease increased over time for almost cancer survivors at all sites (17). However, it is still unclear whether the long-term risk of CV mortality outweighs the cancer mortality risk for patients with cancer at all sites in a particular window of time. More importantly, as multimorbidity, that is, the simultaneous presence of multiple chronic conditions, including cancer and CVD due to shared risk factors, is becoming an increasing global health problem, and the integrated management of shared risk factors has become a global need to reduce the overall burden of multimorbidity (18–21). However, current treatment and management protocols that treat cancer as a separate disease entity are likely to ignore the long-term risk of CV death in cancer survivors.
Therefore, this study aimed to investigate the long-time risk of CV death among cancer survivors and investigate whether and when CV death outweighed cancer death for cancer survivors at different sites.
Materials and methods
Study cohort
A network of population-based incident tumor registries from geographically distinct regions in the USA was established as the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (22, 23). Data on patient demographics, the month and year of diagnosis, tumor characteristics, treatment utilization, and mortality from all incident cancer cases from the selected population-based cancer registries in the USA were reported uniformly and then collected by the SEER program.
The SEER 9 data, which covered 10% of the entire US population and were released in April 2018, were used (22, 24). All patients with cancer diagnosed between 1 January 1973 and 31 December 2015 in the nine original registries (San Francisco-Oakland, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta) were included in SEER 9 (25). In the most recent SEER data, all cancers were classified into nine sites: respiratory (RESPIR), breast (BREAST), colon and rectum (COLRECT), urinary (URINARY), lymphoma of all sites and leukemia (LYMYLEUK), female genital (FEMGEN), male genital (MALEGEN), other digestive except the colon and the rectum (DIGOTHR), and all other sites except the abovementioned sites (OTHER).
Radiation treatment variables were removed from the public research database since November 2016, and chemotherapy had never been released in the research data. Therefore, radiation and chemotherapy data were available through personalized data (26). Finally, a total of 5,094,238 patients with cancer diagnosed as the first primary malignancy were initially identified. We excluded 48,820 (1.0%) patients with cancer with no clear cause of death, 67,735 (1.3%) patients with cancer loss to a follow-up, and 171,619 (3.3%) patients with cancer diagnosed in 2015 for less than a 1-year follow-up. After diagnosis, a total of 4,806,064 patients were included in the final analyses.
Ascertainment of mortality and survival time
Data from the SEER were linked with data from the National Center for Health Statistics to determine death and its cause. Survival time, as recorded in months, started on the date of a cancer diagnosis and ended on the date of death, the last date known to be alive, or the end of a follow-up (31 December 2015), whichever happened first.
Definition of CV death and cancer-related death
According to a previous study (13), based on the SEER Causes of Death Register and International Classification of Diseases (10th Revision, ICD10), CV deaths included deaths from heart diseases (ICD10: I00-I09, I11, I13, I20-I51), hypertension without heart disease (ICD10: I10, I12), cerebrovascular diseases (ICD10: I60-I69), atherosclerosis (ICD10: I70), and diabetes mellitus (ICD10: E10-E14). Cancer-related deaths were defined as deaths from cancer at all sites and included deaths from both primary and additional cancer sites.
Statistical analysis
Time trends in the proportions of CV death (PCV), cancer death (PCA), and other causes in all-cause deaths were used to show preliminary relationships among the three aforementioned causes of death in patients with cancer. To investigate when the cumulative CV mortality rate (CMRCV) began to outweigh the cumulative cancer mortality rate (CMRCA) since diagnosis, cumulative incidence functions based on the Fine-Gray hypothesis were first used to calculate CMRCV and CMRCA, respectively. Fine-Gray tests were then used to select for potential factors associated with CV death in long-time cancer survivors. Multivariable competing risk models based on the Fine-Gray tests were further used to investigate the independent factors associated with CV death (27–29). Hazard ratios and 95% confidence intervals [HR (95%CI)] were calculated based on multivariate competing risk models to measure associations between risk factors and CV death. Given the potential higher risk of arterial thromboembolic events caused by tumor thrombi among patients with LYMYLEUK (30), patients with LYMYLEUK were used as the reference group in the multivariate competing risk models.
To reduce the “dilution” effect of a short-time follow-up on the long-time risk of CV death, we selected patients with cancer who survived for more than 15 and 20 years for subgroup analysis. Moreover, to determine whether some patients with cancer never have a higher risk of CV death than cancer death in their lifetime, we further selected patients with cancer who survived for more than 25 years for a sensitivity analysis.
All analyses were performed with R software (version 4.0.3). All statistics were tested on two sides, and p-values of < 0.05 were considered statistically significant.
Results
Overall mortality rates of CV death among patients with cancer
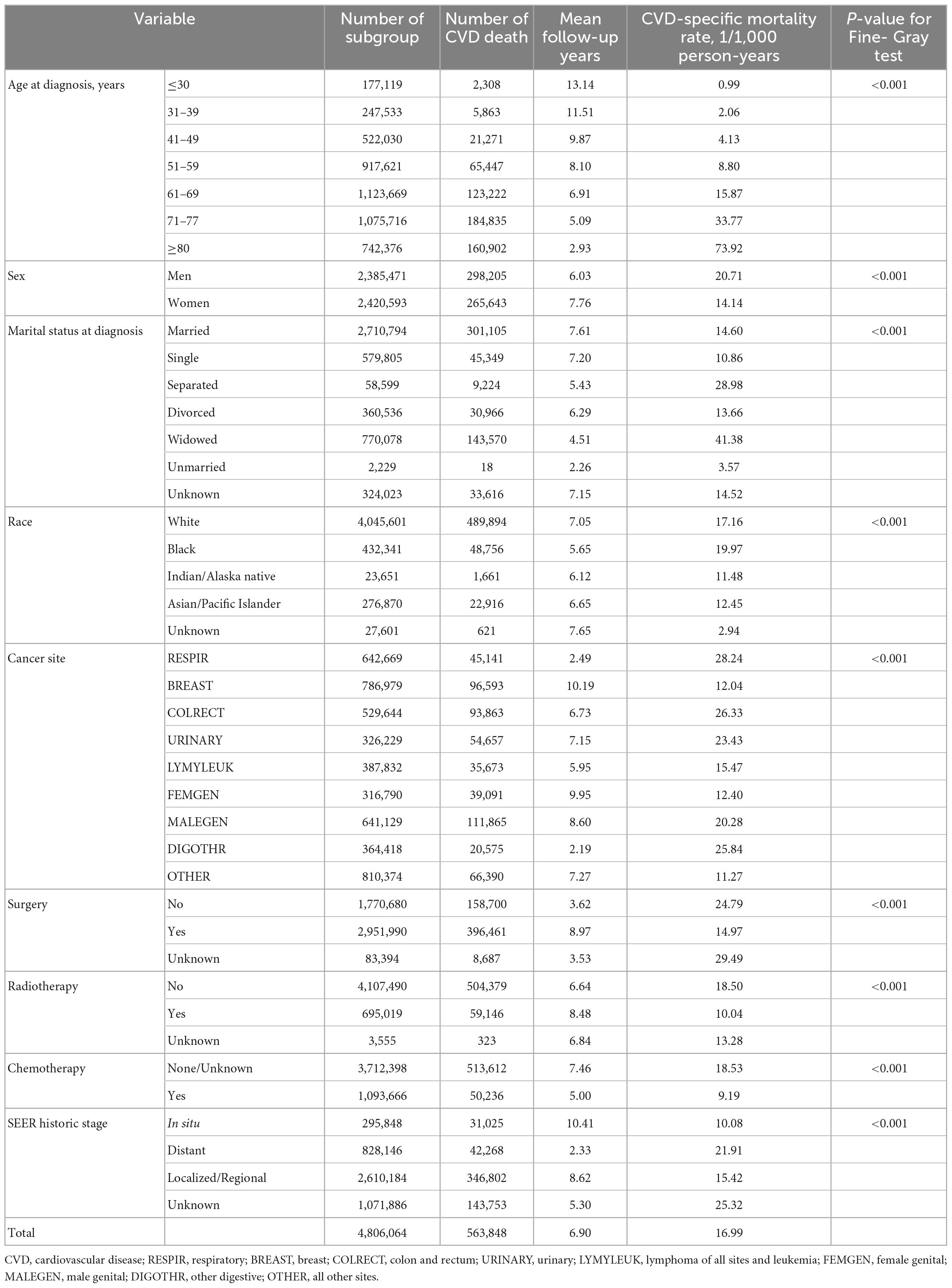
After an average follow-up of 6.90 years, 563,848 patients with cancer died from CVD (16.99/1,000 person-years), which accounted for 17.9% of all-cause deaths (3,149,601) and 51.4% of non-cancer deaths (1,095,209). The Fine-Gray tests showed that a significantly higher overall mortality rate of CVD was found among patients with cancer who were older at diagnosis, men, widowed, African-American, patients with RESPIR, without surgery, without radiation therapy, without chemotherapy, and distant metastasis (all p-values < 0.001, Table 1).
Temporal trends in the PCV and PCA
As shown in Figure 1, for all patients with cancer, the PCV increased from 22.8% in the 5th year after a cancer diagnosis, to 31.0% in 10th year, and to 35.7% in the 20th year, while the PCA decreased from 57.7% in the 5th year to 41.2 and 29.9% in 10th year and 20th year, respectively. The PCV outweighed the PCA (34.6% vs. 34.1%) since the 15th year for all patients with cancer, as early as the 9th year for patients with colorectal cancer (37.5% vs. 33.2%) and as late as the 22nd year for patients with breast cancer (33.5% vs. 30.6%). For patients with respiratory and hematological cancers, it was not observed that the PCV outweighed the PCA even at a follow-up of ≥ 30 years. The same was true for patients with digestive cancers than for those with colorectal cancer and cancer at all other sites.
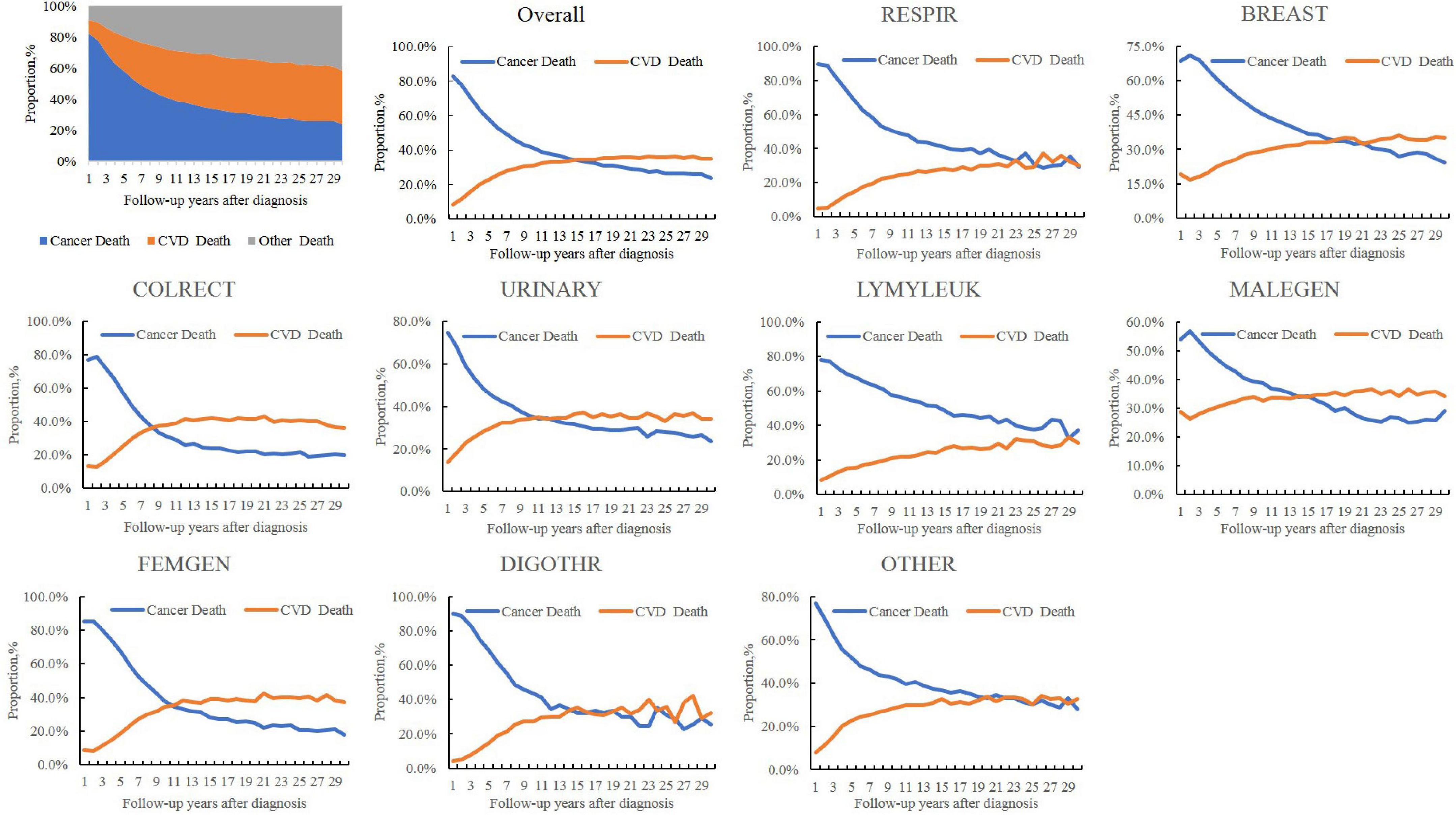
Figure 1. Temporal trends in the proportions of cardiovascular (CV) death (PCV), cancer death (PCA), and other causes in all-cause deaths in patients with cancer. CVD, cardiovascular disease; RESPIR, respiratory; BREAST, breast; COLRECT, colon and rectum; URINARY, urinary; LYMYLEUK, lymphoma of all sites and leukemia; FEMGEN, female genital; MALEGEN, male genital; DIGOTHR, other digestive; OTHER, all other sites.
Cumulative risk of cardiovascular death (CMRCV) and cancer death (CMRCA)
For all patients with cancer, the CMRCV outweighed the CMRCA since the 15th year for patients with cancer who survived for more than 10 years (Supplementary Figure 1), the 5th year for patients with cancer who survived for more than 15 years (Supplementary Figure 1), and the 1st year for patients with cancer who survived for more than 20 years (Figure 2). For cancer at different sites, it was observed that the CMRCV finally outweighed CMRCA in most patients with cancer survived for more than 10 years, except respiratory cancer, hematological cancer, digestive cancer except colorectal cancer, and cancer at all other sites (Supplementary Figure 1). For patients with cancer who survived for more than 20 years, it was only observed that CMRCV finally did not outweigh CMRCA in patients with respiratory and hematological cancers (Figure 2). For patients with cancer who survived for more than 15 years, it was observed that CMRCV finally did not outweigh CMRCA in patients with respiratory, hematological and all other types of cancers (Supplementary Figure 2). Sensitivity analyses showed that the CMRCV began to outweigh CMRCA in the 10th year for patients with respiratory cancer who survived for more than 25 years and in the 7th year for patients with respiratory cancer who survived for more than 30 years (Supplementary Figure 3).
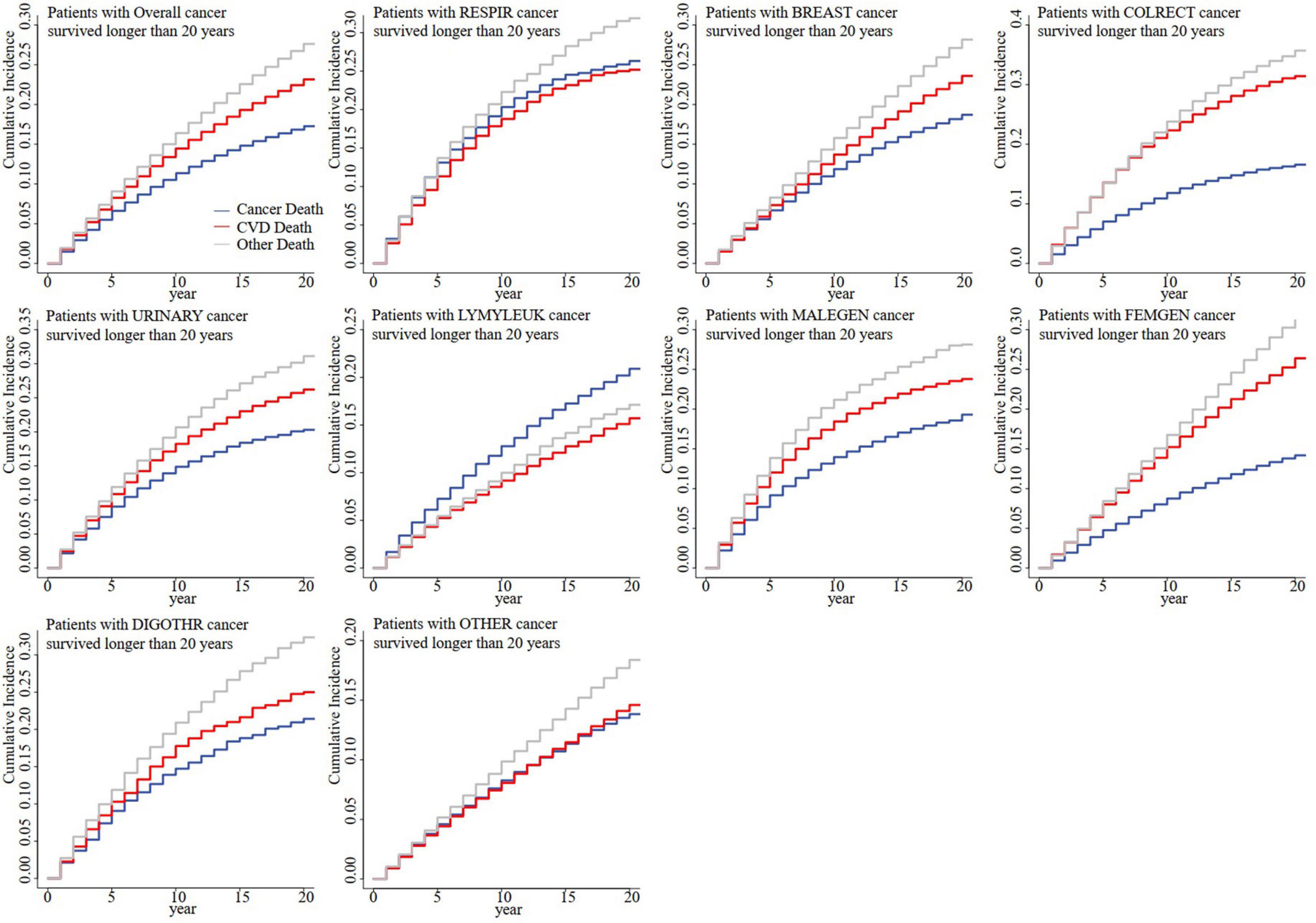
Figure 2. Competing mortality risk curves for patients with cancer who survived for more than 20 years by cancer sites. CVD, cardiovascular disease; RESPIR, respiratory; BREAST, breast; COLRECT, colon and rectum; URINARY, urinary; LYMYLEUK, lymphoma of all sites and leukemia; FEMGEN, female genital; MALEGEN, male genital; DIGOTHR, other digestive; OTHER, all other sites.
Factors associated with CV death for long-term cancer survivors
As presented in Table 2, the multivariate competing risk models showed that an increased risk of CV death was independently associated with older age at diagnosis [HR (95%CI) of 43.39 (21.33, 88.28) for ≥ 80 years vs. ≤ 30 years] and local metastasis [1.07 (1.04, 1.10)] and a decreased risk with women [0.82 (0.76, 0.88)], surgery [0.90 (0.87, 0.94)], and chemotherapy [0.85 (0.81, 0.90)] among patients with cancer who survived for more than 10 years. Further analyses of patients with cancer who survived for more than 20 years showed similar results, while radiotherapy significantly increased the risk of CV death in long-term cancer survivors. Of all the risk factors associated with CV death, elder age at diagnosis was the most important factor. Compared with patients with cancer who survived for more than 20 years and were diagnosed at ≤ 30 years, the HRs for the risk of CV death increased from 2.07 (95%CI: 1.91–2.25) for patients who were diagnosed at 31–39 years, to 27.08 (95%CI: 21.26–34.49) for patients who were diagnosed at 71–77 years, and 40.66 (95%CI: 26.13–63.25) for patients who were diagnosed at ≥ 80 years. Sensitivity analyses by cancer sites showed similar results (Figure 3 and Supplementary Table 1).
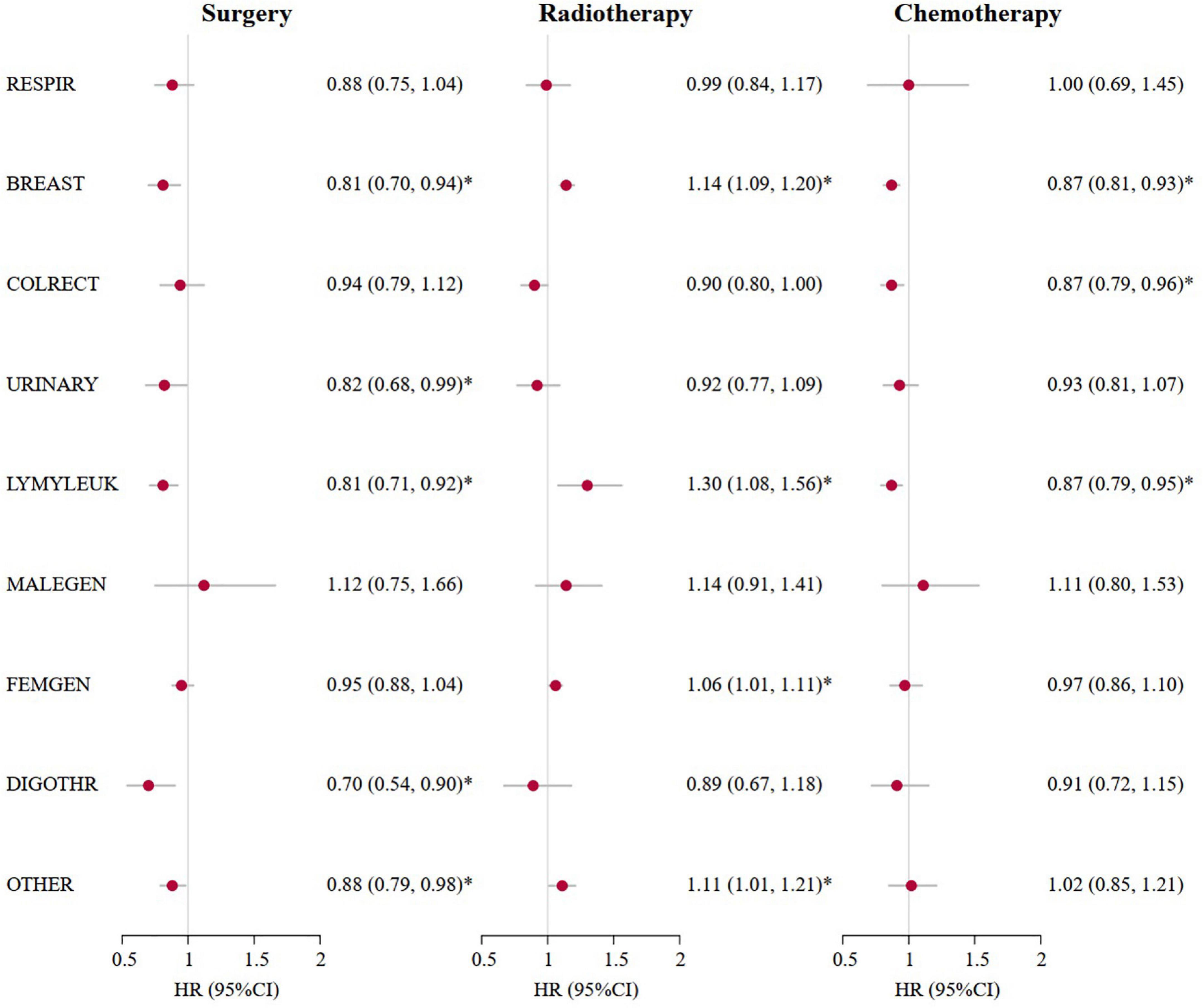
Figure 3. Cardiovascular mortality risk and anticancer treatments for patients with cancer who survived for more than 20 years by cancer sites. *A p-value of < 0.05. Marital status at diagnosis, race, calendar years at diagnosis according to 1970s, 1980s, 1990s, 2000s, 2010s, nine original registries, sex, SEER historic stage, surgery, radiotherapy and chemotherapy were adjusted in the multivariate competing risk regression models. HR (95%CI), Hazard ratio (95% confidence intervals); RESPIR, respiratory; BREAST, breast; COLRECT, colon and rectum; URINARY, urinary; LYMYLEUK, lymphoma of all sites and leukemia; FEMGEN, female genital; MALEGEN, male genital; DIGOTHR, other digestive; OTHER, all other sites.
Discussion
In this cohort of more than 4.8 million patients with cancer, the risk of CV death begins to climb after a cancer diagnosis and will eventually outweigh the risk of cancer death for most patients with cancer. Specifically, it took 15 years for PCV and 25 years for CMRCV in the aggregate of patients to outweigh PCA and CMRCA. We also observed that older age at diagnosis was the most important factor associated with the increased risk of CV death in patients with cancer. Moreover, as reported in recently published studies, the overall CV mortality rates for the general population in the USA were 252.7 deaths and 219.4 deaths per 100,000 person-years in 2014 (31) and 2017 (32), respectively. For the population older than 65 years in the USA, the CV mortality rate was 1,407.2 deaths per 100,000 person-years. In this study, the overall CV mortality rate was 16.99 deaths/1,000 person-years (namely, 1,699 deaths per 100,000 person-years), which was much higher in older patients with cancer. These results indirectly supported the higher CV mortality in patients with cancer than that in the general population.
Previous studies suggested that CV death in patients with cancer can be primarily attributable to CV toxicity associated with various anticancer treatments, including chemotherapy, radiotherapy, endocrine therapy (10, 33–35), and targeted therapy (i.e., trastuzumab) (36, 37). Patients with cancer are also potentially at high CV risk when undergoing surgery. This would be due to the traumatic stress on the CV system caused by surgery and chronic inflammation after surgery (4, 13). An increased risk of stress-induced CV death caused by surgery was usually an immediate risk but not a long-term risk (4). The difference between this study and previous studies is that chemotherapy and surgery do not significantly increase the risk of CV death but reduce the risk in 10-year survivors. Even though long-term cancer survivors were selected to minimize the dilution effect of a short-term follow-up, a reduced risk of CV death associated with chemotherapy was still observed, even further adjusting the main confounding factors, such as age at diagnosis, cancer stage, and competing risk of death. Due to the non-randomized design within this study, other unknown factors may also contribute to a reduced risk of CV death, such as potentially higher exposure to risk factors for CVD for patients who do not receive chemotherapy than for those who can receive chemotherapy. Therefore, further studies with a more sophisticated design are needed in the future to validate the results. These results also suggested that the combined effect of chemotherapy on CV death for patients with cancer should be re-evaluated in addition to well-established CV toxicity. A similar re-evaluation is also needed for a reduced risk of CV death associated with surgery for patients with cancer. Unlike a reduced risk of CV death associated with both chemotherapy and surgery, the combined effect of radiotherapy changed from no effect to a significantly increased risk of CV death (Table 2). The results suggest that cardio-oncologists should pay more attention to the CV toxicity of radiotherapy than to chemotherapy.
Compared to the associations between the risk of CV death and other factors, this study suggested that the most important risk factor for CV death was older age at diagnosis. The risk of CV death was even 40 times higher in patients with cancer diagnosed at age ≥ 80 years compared to those diagnosed at age ≤ 30 years. This huge difference is similar to the results of previous studies (13, 38, 39). This is probably due to the presence of shared risk factors and chronic inflammation between CVD and cancer (39). However, this huge difference has not received enough attention up to now. These results also suggested that integrated management of shared risk factors would be an urgent need to reduce the overall burden of multimorbidity among long-time cancer survivors.
Another important finding of this study is that the timing of CV death over cancer death varies among patients at different sites of cancer. It first appeared in patients with colorectal cancer and was last observed in patients with breast cancer. However, we also found that patients with cancer not at all sites would experience more CV deaths than cancer deaths. This is likely to be related to the different treatment regimens of patients with different sites of cancer. It may also be related to the potency of local/distant metastases for cancers at different sites. Distant metastasis is based on vascular invasion. Patients with cancer may be in a state where the CV system continuously faces the invasion process by cancer cells and inflammatory factors. Cancer patients who are more prone to distant metastasis are more prone to CV invasion (40–42). Therefore, prevention of distant metastasis in patients with cancer may be an effective way to reduce the risk of CV death.
Several limitations deserved attention in this study. First, preexisting illnesses, especially preexisting CVD, may modify the risk of CV death after a cancer diagnosis. Second, shared risk factors between cancer and CVD, such as residual confounding, may bias our findings. Third, as the data were collected from cancer registries rather than CV registries, underreporting or misclassification of CVD would also bias our results. Fourth, different treatment components may be associated with different CV mortality risks. However, the SEER data did not provide more detailed information to reclassify patients who received radiotherapy into subgroups with chest or non-chest radiotherapy, subgroups with right- or left-side chest radiotherapy, or other subgroups. Another potential limitation would be selection bias for all-cause mortality due to better health conditions of long-term survivors compared to short-term survivors. Due to the exclusion of patients who died from any cause within a specific time point (e.g., 10 years), including those who died from CV death within that time point, this selection bias would dilute the overall risk of CV death in patients with cancer. Finally, as there was no available information in SEER to analyze age-standardized CV mortality in the general population, we cannot directly calculate the relative risk of CV mortality in patients with cancer compared to those in the general population.
Despite the limitations of this study mentioned earlier, such a large real-world cohort study with relatively reasonable analyses would provide many insights into the current practice of cardio-oncology. All these limitations will be our next focus in the future. Additionally, although we observed higher CVD-specific mortality than the index cancer-specific mortality among long-term cancer survivors, we recommend focusing more on cardio-oncology care than cardiotoxic therapy for long-term cancer survivors, as they would not normally be exposed in long-term exposure to cardiotoxic therapy after receiving standard anticancer therapies.
In conclusion, for patients with cancer, the risk of CV death persists throughout life and is likely to exceed the risk of cancer death over time. The risk of CV death in elderly patients with cancer and radiotherapy, as well as the prevention of CV death by reducing local/distant metastases, will be the focus in the field of cardio-oncology.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements.
Author contributions
All authors involved with the conception and design, manuscript writing, and final approval of the manuscript.
Funding
This study was funded by the National Key R&D Program of China (Grant No. 2021YFC2500400), the Tianjin Science and Technology Committee Foundation (Grant No. 20JCZXJC00090), and the Tianjin Municipal Health Commission Foundation (Grant No. TJWJ2021MS008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1014400/full#supplementary-material
Supplementary Figure 1 | Competing mortality risk curves for patients with cancer who survived for more than 10 years by cancer sites. CVD, cardiovascular disease; RESPIR, respiratory; BREAST, breast; COLRECT, colon and rectum; URINARY, urinary; LYMYLEUK, lymphoma of all sites and leukemia; FEMGEN, female genital; MALEGEN, male genital; DIGOTHR, other digestive; OTHER, all other sites.
Supplementary Figure 2 | Competing mortality risk curves for patients with cancer who survived for more than 15 years by cancer sites. CVD, cardiovascular disease; RESPIR, respiratory; BREAST, breast; COLRECT, colon and rectum; URINARY, urinary; LYMYLEUK, lymphoma of all sites and leukemia; FEMGEN, female genital; MALEGEN, male genital; DIGOTHR, other digestive; OTHER, all other sites.
Supplementary Figure 3 | Competing mortality risk curves for selected patients with cancer who survived for more than 25 and 30 years. RESPIR, respiratory; LYMYLEUK, lymphoma of all sites and leukemia.
Abbreviations
PCV, proportion of cardiovascular death; PCA, proportion of cancer death; CMRCV, cumulative cardiovascular mortality rate; CMRCA, cumulative cancer mortality rate; SEER, Surveillance, Epidemiology, and End Results; RESPIR, respiratory; BREAST, breast; COLRECT, colon and rectum; URINARY, urinary; LYMYLEUK, lymphoma of all sites and leukemia; FEMGEN, female genital; MALEGEN, male genital; DIGOTHR, other digestive except colon and rectum; OTHER, all other sites except the abovementioned sites; CVD, cardiovascular disease.
References
2. Fitzmaurice C, Akinyemiju T, Al L, Alam T, AlizadehNavaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted Life-Years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. (2018) 4:1553–68. doi: 10.1001/jamaoncol.2018.2706
3. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Fang F, Keating N, Mucci L, Adami H, Stampfer M, Valdimarsdottir U, et al. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: Cohort study in the United States. J Natl Cancer Inst. (2010) 102:307–14. doi: 10.1093/jnci/djp537
5. Fang F, Fall K, Mittleman M, Sparen P, Ye W, Adami H, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. (2012) 366:1310–8. doi: 10.1056/NEJMoa1110307
6. Fall K, Fang F, Mucci L, Ye W, Andren O, Johansson J, et al. Immediate risk for cardiovascular events and suicide following a prostate cancer diagnosis: Prospective cohort study. PLoS Med. (2009) 6:e1000197. doi: 10.1371/journal.pmed.1000197
7. Zhou H, Xian W, Zhang Y, Chen G, Zhao S, Chen X, et al. Trends in incidence and associated risk factors of suicide mortality in patients with non-small cell lung cancer. Cancer Med. (2018) 7:4146–55. doi: 10.1002/cam4.1656
8. Wang S, Chang J, Weng S, Yeh M, Lee C. Risk of suicide within 1 year of cancer diagnosis. Int J Cancer. (2018) 142:1986–93. doi: 10.1002/ijc.31224
9. Khosrow-Khavar F, Filion K, Al-Qurashi S, Torabi N, Bouganim N, Suissa S, et al. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann Oncol. (2017) 28:487–96. doi: 10.1093/annonc/mdw673
10. Haque R, Shi J, Schottinger J, Chung J, Avila C, Amundsen B, et al. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. (2016) 2:1590–7. doi: 10.1001/jamaoncol.2016.0429
11. Van Hemelrijck M, Garmo H, Holmberg L, Ingelsson E, Bratt O, Bill-Axelson A, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: Results from the Population-Based PCBaSe Sweden. J Clin Oncol. (2010) 28:3448–56. doi: 10.1200/JCO.2010.29.1567
12. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan D. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. (2010) 102:14–25. doi: 10.1093/jnci/djp440
13. Zaorsky N, Churilla T, Egleston B, Fisher S, Ridge J, Horwitz E, et al. Causes of death among cancer patients. Ann Oncol. (2017) 28:400–7. doi: 10.1093/annonc/mdw604
14. Strongman H, Gadd S, Matthews A, Mansfield K, Stanway S, Lyon A, et al. Does cardiovascular mortality overtake cancer mortality during cancer survivorship?: An english retrospective cohort study. JACC CardioOncol. (2022) 4:113–23. doi: 10.1016/j.jaccao.2022.01.102
15. Feng Y, Jin H, Guo K, Wasan H, Ruan S, Chen C. Causes of death after colorectal cancer diagnosis: A Population-Based study. Front Oncol. (2021) 11:647179. doi: 10.3389/fonc.2021.647179
16. Sturgeon K, Deng L, Bluethmann S, Zhou S, Trifiletti D, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. (2019) 40:3889–97. doi: 10.1093/eurheartj/ehz766
17. Stoltzfus K, Zhang Y, Sturgeon K, Sinoway L, Trifiletti D, Chinchilli V, et al. Fatal heart disease among cancer patients. Nat Commun. (2020) 11:2011. doi: 10.1038/s41467-020-15639-5
18. Zullig L, Drake C, Shahsahebi M, Avecilla R, Whitney C, Mills C, et al. Adherence to cardiovascular disease risk factor medications among patients with cancer: A systematic review. J Cancer Surviv. (2022) 44:288. doi: 10.1007/s11764-022-01212-0
19. Koene R, Prizment A, Blaes A, Konety S. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133:1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
20. Pietzner M, Stewart I, Raffler J, Khaw K, Michelotti G, Kastenmuller G, et al. Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat Med. (2021) 27:471–9. doi: 10.1038/s41591-021-01266-0
21. Karlstaedt A, Moslehi J, de Boer R. Cardio-onco-metabolism: Metabolic remodelling in cardiovascular disease and cancer. Nat Rev Cardiol. (2022) 19:414–25. doi: 10.1038/s41569-022-00698-6
22. Merrill R, Dearden K. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States? Cancer Causes Control. (2004) 15:1027–34. doi: 10.1007/s10552-004-1324-5
23. Zippin C, Lum D, Hankey B. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer-Am Cancer Soc. (1995) 76:2343–50. doi: 10.1002/1097-0142(19951201)76:113.0.co;2-#
24. Warren J, Klabunde C, Schrag D, Bach P, Riley G. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. (2002) 40:3–18. doi: 10.1097/01.MLR.0000020942.47004.03
25. NIH. The Surveillance, Epidemiology, Research Data (1973-2015), National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2018, Based on the November 2017 Submission. (2018). Available online at: http://www.seer.cancer.gov (accessed 2018).
26. NIH. The Surveillance, Epidemiology, Radiation/Chemotherapy Databases, National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2018, Based on the November 2017 Submission. (2018). Available online at: http://www.seer.cancer.gov (accessed 2018).
27. Austin P, Fine J. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. (2017) 36:4391–400. doi: 10.1002/sim.7501
28. Moore D. Applied Survival Analysis Using R. Piscataway, NJ: Springer International Publishing (2016).
29. Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Publicat Am Stat Assoc. (1999) 94:496–509.
30. Navi B, Reiner A, Kamel H, Iadecola C, Okin P, Tagawa S, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. (2019) 133:781–9. doi: 10.1182/blood-2018-06-860874
31. Roth G, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs R, Morozoff C, Naghavi M, et al. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980-2014. JAMA. (2017) 317:1976–92. doi: 10.1001/jama.2017.4150
32. Cross S, Mehra M, Bhatt D, Nasir K, O’Donnell C, Califf R, et al. Rural-Urban Differences in Cardiovascular Mortality in the US, 1999-2017. JAMA. (2020) 323:1852–4. doi: 10.1001/jama.2020.2047
33. Matthews A, Stanway S, Farmer R, Strongman H, Thomas S, Lyon A, et al. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: Systematic review. BMJ. (2018) 363:k3845. doi: 10.1136/bmj.k3845
34. Mouridsen H, Keshaviah A, Coates A, Rabaglio M, Castiglione-Gertsch M, Sun Z, et al. Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: Safety analysis of BIG 1-98 trial. J Clin Oncol. (2007) 25:5715–22. doi: 10.1200/JCO.2007.12.1665
35. Lyon A, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. (2020) 22:1945–60. doi: 10.1002/ejhf.1920
36. He X, Dai X, Ji J, Liu H, Shi G, Yeung S. Nine-Year median follow-up of cardiotoxicity and efficacy of trastuzumab concurrently with Anthracycline-Based and Anthracycline-Free neoadjuvant chemotherapy in HER2-Positive breast cancer patients. Clin Breast Cancer. (2022) 22:e80–90. doi: 10.1016/j.clbc.2021.05.008
37. Gonciar D, Mocan L, Zlibut A, Mocan T, Agoston-Coldea L. Cardiotoxicity in HER2-positive breast cancer patients. Heart Fail Rev. (2021) 26:919–35. doi: 10.1007/s10741-020-10072-8
38. Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. (2021) 385:1067–77. doi: 10.1056/NEJMoa2105675
39. Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. (2018) 379:623–32. doi: 10.1056/NEJMoa1803626
40. Sejben I, Bori R, Cserni G. Venous invasion demonstrated by orcein staining of colorectal carcinoma specimens is associated with the development of distant metastasis. J Clin Pathol. (2010) 63:575–8. doi: 10.1136/jcp.2010.075846
41. Xu X, Zhang H, Liu Q, Sun S, Zhang J, Zhu F, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. (2019) 70:1133–44. doi: 10.1016/j.jhep.2019.02.023
Keywords: cancer, cardiovascular deaths and mortality, cardio-oncology, long-term cancer survivors, anticancer treatment
Citation: Wang Z, Fan Z, Yang L, Liu L, Sheng C, Song F, Huang Y and Chen K (2023) Higher risk of cardiovascular mortality than cancer mortality among long-term cancer survivors. Front. Cardiovasc. Med. 10:1014400. doi: 10.3389/fcvm.2023.1014400
Received: 08 August 2022; Accepted: 02 January 2023;
Published: 25 January 2023.
Edited by:
Wouter Kok, Amsterdam University Medical Center, NetherlandsReviewed by:
Bogda Koczwara, Flinders University, AustraliaDaniel Chen, University College London, United Kingdom
Copyright © 2023 Wang, Fan, Yang, Liu, Sheng, Song, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yubei Huang,  eXViZWlfaHVhbmdAMTYzLmNvbQ==; Fengju Song,
eXViZWlfaHVhbmdAMTYzLmNvbQ==; Fengju Song,  c29uZ2ZlbmdqdUAxNjMuY29t; Kexin Chen,
c29uZ2ZlbmdqdUAxNjMuY29t; Kexin Chen,  Y2hlbmtleGluQHRqbXVjaC5jb20=
Y2hlbmtleGluQHRqbXVjaC5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Zhipeng Wang
Zhipeng Wang Zeyu Fan1†
Zeyu Fan1† Chao Sheng
Chao Sheng Yubei Huang
Yubei Huang