- 1Department of Cardiology, Xuzhou Third People’s Hospital, Xuzhou Medical University, Xuzhou, China
- 2Department of Cardiology, Barts Heart Center, Barts Health NHS Trust, London, United Kingdom
- 3Cardiovascular Devices Hub, Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London, London, United Kingdom
- 4Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: There is limited evidence about vessel wall healing response following implantation of next-generation drug-eluting stents (DES) in patients admitted with a non-ST elevation acute coronary syndrome (NSTE-ACS). Cumulative data indicate that optical coherence tomography (OCT) imaging can optimize percutaneous coronary intervention results and expedite stent endothelialization in the general population but there is lack of data in NSTE-ACS patients.
Methods: The EXPECT study is an investigator-initiated, prospective, randomized trial to assess early vascular healing response following next-generation DES implantation in patients admitted with NSTE-ACS based on OCT guidance and evaluation. Sixty patients are randomized at 1:1:1 ratio to OCT-guided percutaneous coronary intervention (PCI) with 3-month follow-up OCT imaging (O3 group, n = 20), to angiography-guided PCI with 3-month follow-up OCT imaging (A3 group, n = 20) and to angiography-guided PCI with 6-month follow-up OCT imaging (A6 group, n = 20). The primary endpoint of the study is stent strut coverage rate at 3- or 6- month follow-up in the studied groups. The secondary endpoints of the study include OCT imaging endpoints, clinical endpoints, and molecular biology endpoints at the different time points. The clinical endpoints comprised of major cardiovascular adverse events and individual components. The molecular biology endpoints comprised of lipid levels and the levels of inflammatory indicators.
Discussion: The findings of the EXPECT study are anticipated to provide novel insights into vessel wall healing in NSTE-ACS population following implantation of next-generation DES, underscore the value of OCT imaging in expediting strut coverage in this setting, and explore the potential of an early discontinuation of dual antiplatelet therapy (DAPT) in this population.
Clinical Trial Registration:: ClinicalTrials.gov, NCT04375319.
Introduction
Over the past few decades, percutaneous coronary intervention (PCI) has significantly improved clinical outcomes in acute coronary syndrome (ACS) patients. The improved prognosis in this high-risk population has been at least partially attributed to enhanced medical therapy, the broad use of PCI as well as to the introduction of the second-generation drug-eluting stents (DES) that have increased efficacy and high safety profile (1). Current guidelines recommend treatment with dual antiplatelet therapy (DAPT) for 1 year after PCI regardless of stent types (2, 3). However, long-term DAPT may increase the risk of bleeding in the elderly, frail patients and those prone to bleeding with hematological, gastro intestinal, and liver pathologies. DAPT is also associated with a risk of serious bleeding in vulnerable patients with history of falls, previous hemorrhagic cerebrovascular events and can be a limiting factor in patients awaiting elective surgery. In the era of next-generation DES, large-scale randomized studies and meta-analyses have demonstrated that the duration of DAPT can be shortened to 6 months or even to 3 months with no clinical consequences while recent studies currently examine the safety and efficacy of aspirin single antiplatelet therapy post stent implantation (4–7).
In patients admitted with non-ST elevation acute coronary syndrome (NSTE-ACS) that have complex and highly thrombotic lesions, shorter-term DAPT can be considered only in cases of early strut coverage which constitutes a marker of early endothelialization (8). Indeed, cumulative data have underscored the prognostic implications of incomplete strut coverage indicating that this is associated with a high incidence of stent thrombosis (ST) (9) and future major adverse cardiovascular events (MACE) (10–12).
The introduction of optical coherence tomography (OCT) enabled in vivo assessment of strut coverage and allow us to calculate vascular repair index (13) and to identify predictors associated with a delayed vessel wall healing. In particular, stent design (i.e., stent polymer, stent strut thickness, and drug elution), strut embedment and apposition, the composition of the underlying plaque, and the time interval between stent implantation and follow-up imaging seem to determine strut coverage (14). Moreover, several studies have shown that in patients with NSTE-ACS the incidence of incomplete strut apposition is greater than in patients with a chronic coronary syndrome while the recently published study (8) has shown that the use of OCT during next-generation stent implantation is associated with a higher incidence of stent embedment and faster endothelialization comparing to angiography-guided PCI. However, most of the recruited patients in this study were suffering from a stable angina. The present investigator-initiated, prospective, randomized trial was designed to investigate the vessel wall healing response following implantation of a next-generation DES with low dose sirolimus elution in NSTE-ACS population and to explore the value of OCT imaging in expediting strut endothelialization.
Methods and analysis
Study design
The EXPECT study is an investigator-initiated, prospective, multicenter, randomized clinical trial (www.clinicaltrials.gov, NCT04375319). The objective of this study is to evaluate vessel wall healing response over time in NSTE-ACS patients receiving a next-generation DES implantation, and to examine the value of OCT-guided PCI in expediting stent strut coverage, aiming to underscore the potential of a lower duration DAPT in high bleeding risk population.
Patient enrollment and randomization
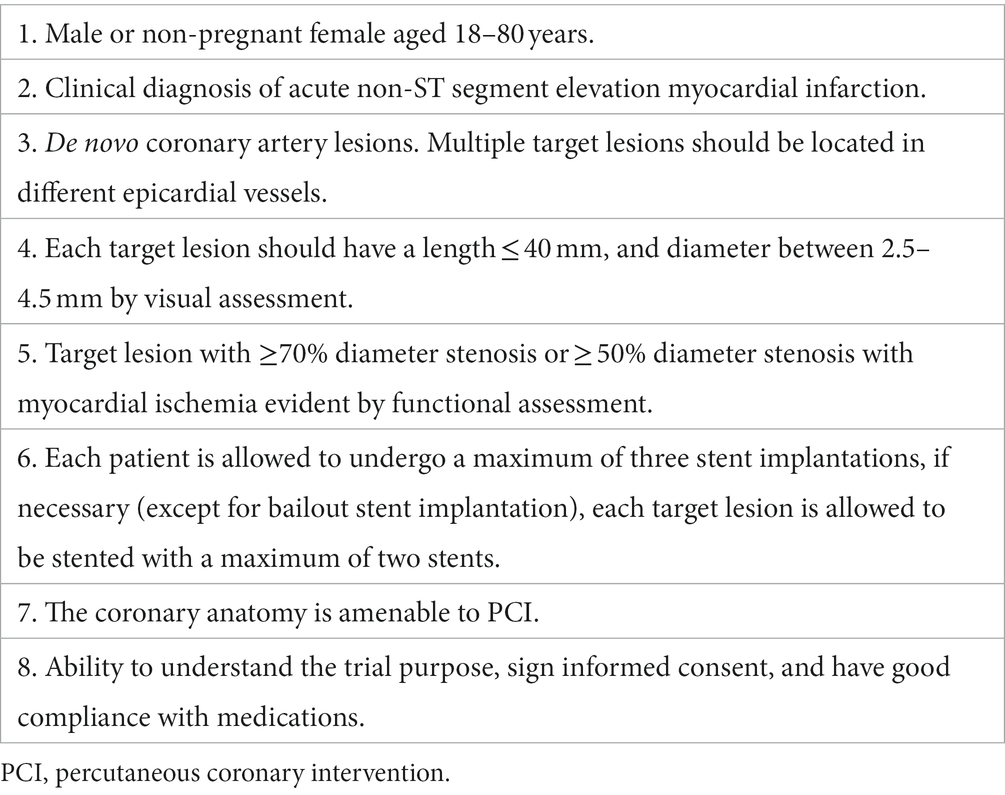
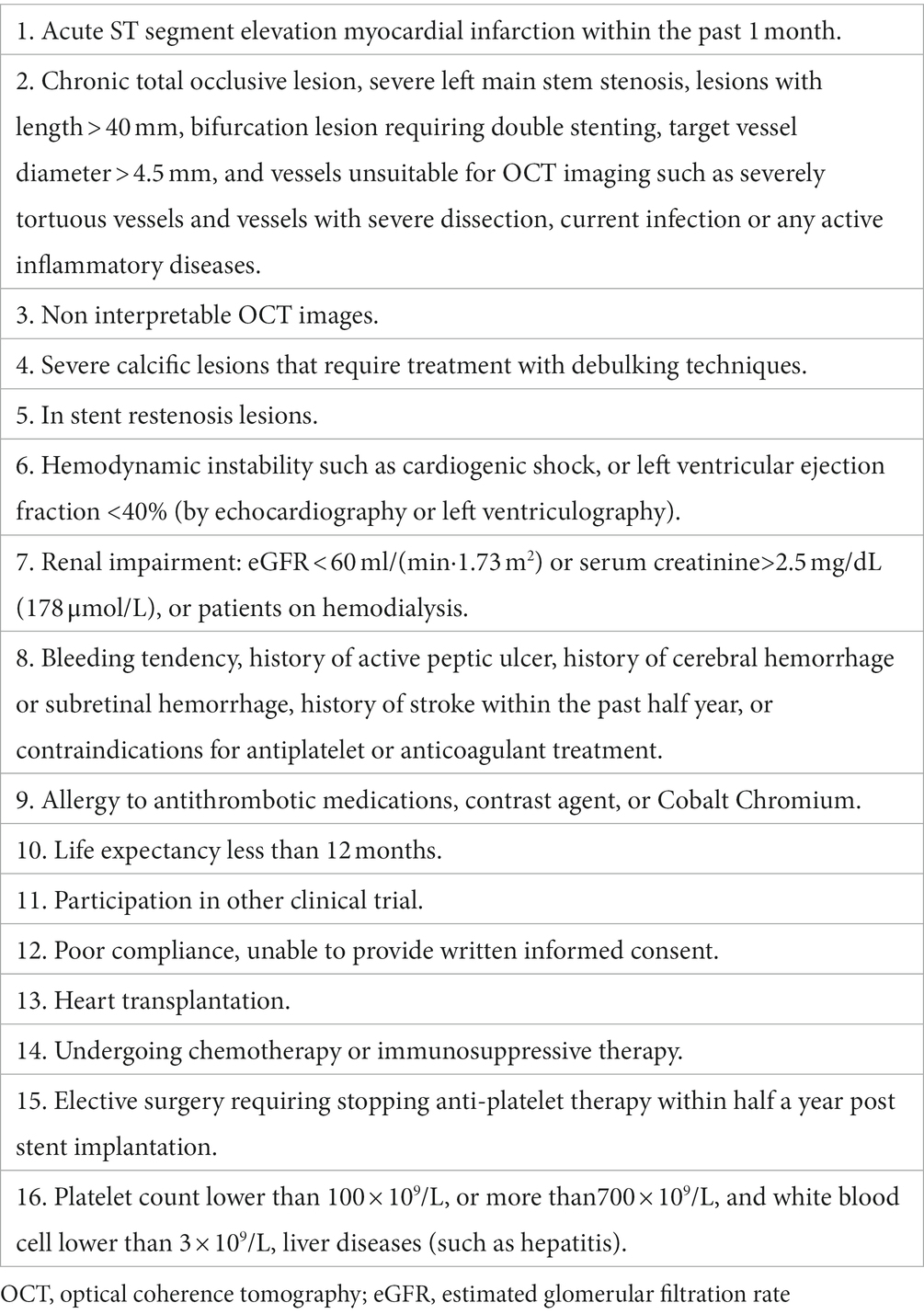
Sixty patients with NSTE-ACS (includes unstable angina pectoris and non-ST segment elevation myocardial infarction) from three centers in Huaihai economic zone (Supplementary Table S1) will be enrolled in this trial. The main inclusion and exclusion criteria are listed in Tables 1, 2, respectively.
The recruited patients will be randomized at 1:1:1 ratio to three groups using a computer-generated random sequence table. Patients enrolled in the first group will have OCT-guided revascularization and 3-month follow-up OCT imaging (O3 group, n = 20), those in the second group angiography-guided PCI and 3-month follow-up OCT imaging (A3 group, n = 20) while the patients recruited in the third group angiography-guided PCI and 6-month follow-up OCT imaging (A6 group, n = 20).
Treatment device
All the recruited patients will be implanted with Excrossal (JW Medical Systems, Shandong, China) stent, a novel next-generation sirolimus-eluting stent with a cobalt-chromium alloy and a biodegradable polymer. The dose of sirolimus in this device is reduced to 1/3 of the former product used while the polymer consists of biodegradable polylactic acid. Following stent implantation, the polymer coating degrades into lactic acid, which expedites vascular healing process while the low dose sirolimus elution provides the advantage of accelerating endothelialization without affecting the rate of restenosis (15, 16).
Treatment and follow-up procedures
In the OCT-guided group, OCT imaging will be performed before PCI to appropriately size stent implantation and following stenting to assess the final results and guide further optimization if needed. A final run will be performed at the end of the procedure to confirm optimal results and exclude common causes of stent failure reported in the literature (17). Patients will be given DAPT (oral aspirin 100 mg once daily, clopidogrel 75 mg once daily, or ticagrelor 90 mg twice daily) and will be put on secondary prevention medications according to the recommended ESC guidelines.
Post discharges all patients will have a follow-up appointment or will be contacted by phone at 1 month, 3 months, 6 months, and 1 year. Subjects assigned to O3/A3 arms will undergo coronary angiography and OCT imaging at 3 months, while those assigned to A6 arm will have invasive assessment and OCT imaging at 6 months after the index procedure (Figure 1). The schedule of enrolment, interventions, and assessments is outlined in Figure 2. The data of each center will be summarized and checked by an independent data monitoring committee.
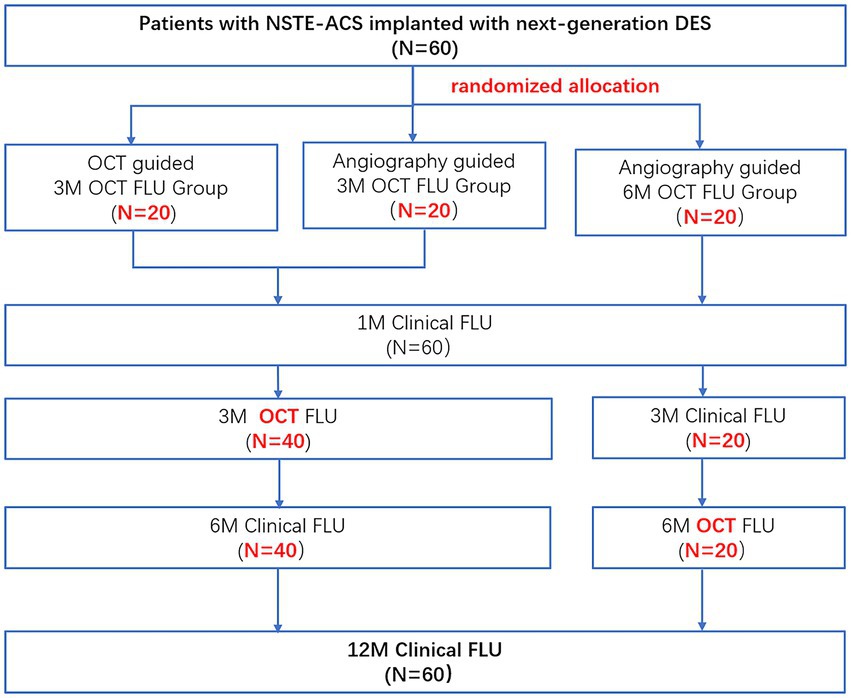
Figure 1. Study flow chart. NSET-ACS, non-ST elevation acute coronary syndrome; OCT, optical coherence tomography; FLU, follow up; 3 M, 3-month; 6 M, 6-month; and 12 M, 12-month.
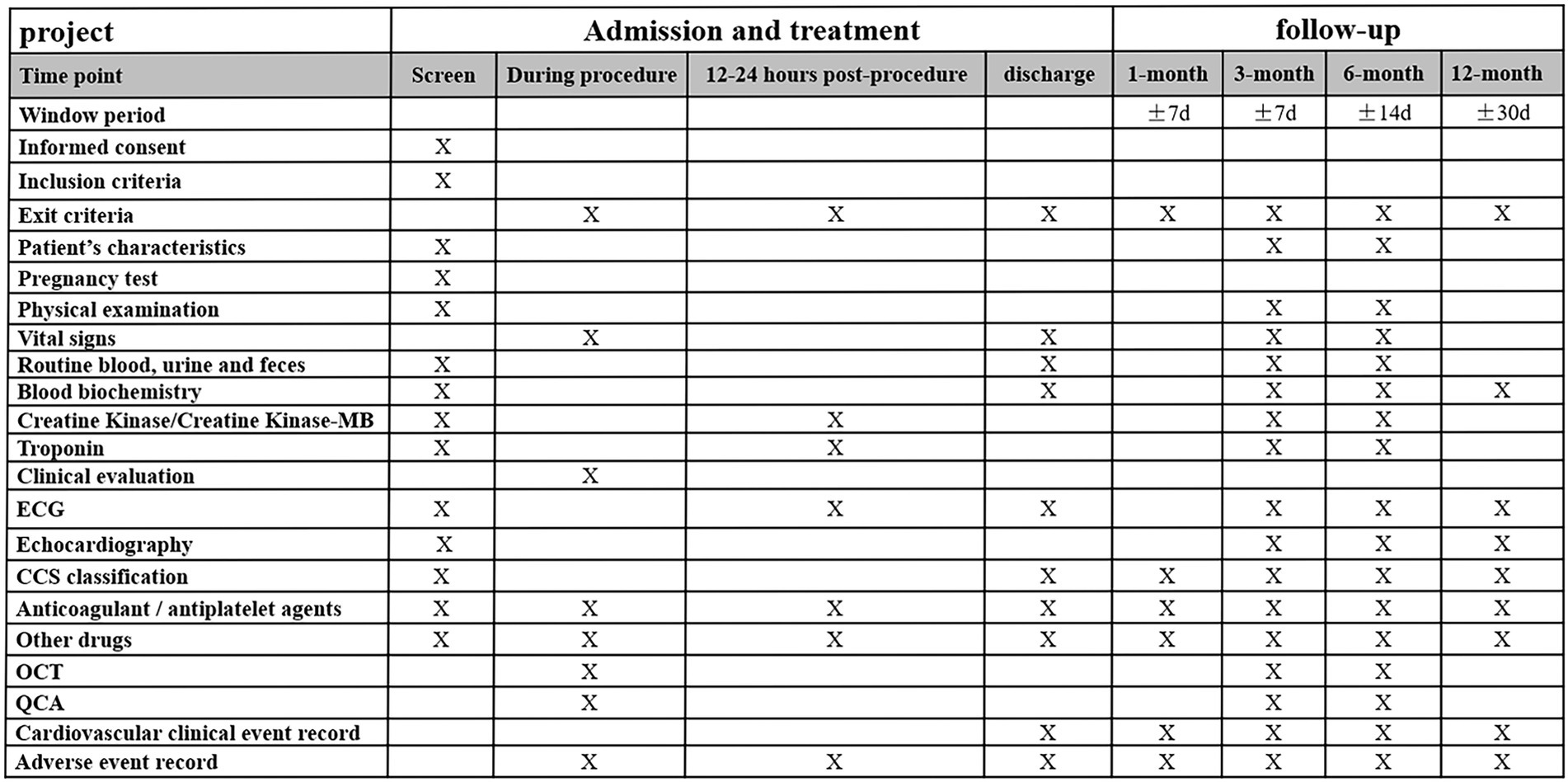
Figure 2. Time schedule of enrolment, interventions, and assessments of the study. ECG, electrocardiogram; CCS, Canadian cardiovascular society; OCT, optical coherence tomography; and QCA: quantitative coronary angiography.
Blood sample measurement
Blood samples (4–5 mL) will be collected from all subjects prior to the procedure and at 12–24 h after the procedure. Blood samples will be also collected at 3 and 6 months follow up. The samples will be centrifuged at 3,000 r/min for 10 min, and the serum obtained will be isolated and stored at −80°C. Serum levels of total cholesterol (TC), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), and triglyceride (TG) will be measured by automatic biochemical analyzer, the pentraxins 3 (PTX-3), vascular cell adhesion molecule 1 (VCAM-1), and matrix metalloproteinase 9 (MMP-9) by enzyme-linked immunosorbent assay, and the high-sensitivity C-reactive protein (hs-CRP) detected by the immune turbidity method.
QCA and OCT data acquisition and analysis
Intracoronary nitrate will be administered prior to diagnostic angiography in all the recruited patients unless contraindicated due to low blood pressure. Antithrombotic therapy will be administered according to the local protocol prior to insertion of a guidewire in the coronary arteries.
In the angiography-guided group, stent implantation will be performed and optimized with angiography guidance according to local standard practice based on operator’s visual assessment (stent to artery ratio, 1:1). At the end of the procedure, an OCT examination will be performed for research purposes. The collected images will not be available to the operators and these images will be stored and transferred to a dedicated core-lab for image analysis.
In the OCT-guided groups, PCI will be performed under OCT guidance according ILUMIEN III: OPTIMIZE PCI algorithm. The overall procedural approach for OCT-guided stent implantation and post-implant optimization also follows this algorithm (18). In short, the stent size is chosen based on OCT measurement, and post-dilation performed under OCT guidance to achieve fully stent struts apposition and satisfactory stent area (19).
Optical coherence tomography images will be acquired in the culprit vessel and non-culprit vessels using a commercially available C7 or OPTIS imaging system (Abbott, Santa Clara, United States). The imaging catheter will be advanced at least 10 mm distally to the culprit lesion and will be pulled-back using an automated pull-back device under contrast media injection for blood clearance. A second pull-back will be performed in case of long lesions so as the catheter to cover the entire segment of interest.
Repeat coronary angiography and OCT imaging will be also performed at 3- or 6-month follow-up according to the study protocol. The angiographic and OCT imaging data collected at baseline and follow-up will be anonymized and transferred to an independent core laboratory for further analysis.
Study endpoints
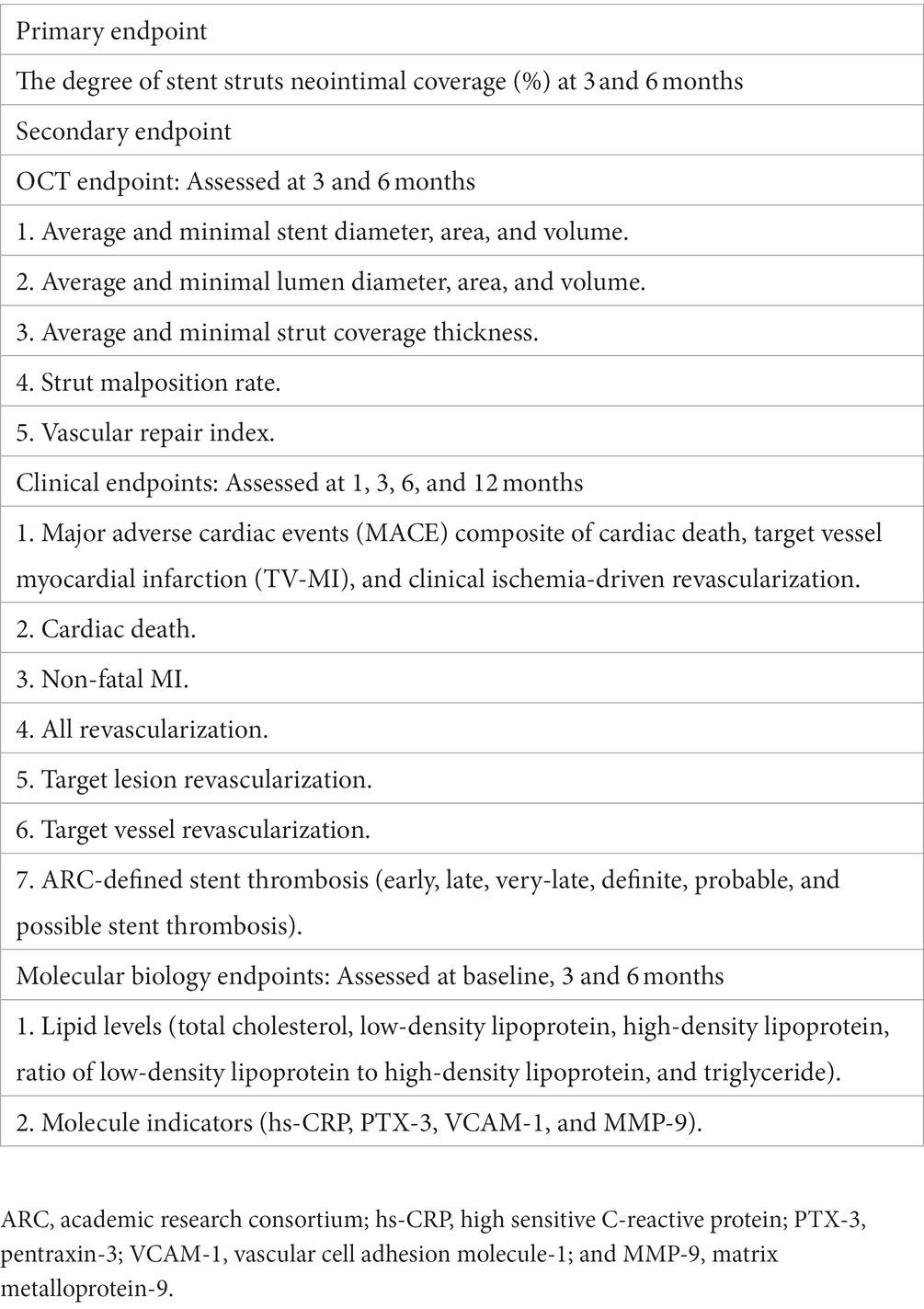
The primary endpoint of the study is the incidence of stent strut coverage (%) at 3- or 6-month follow-up.
The secondary endpoints of the study include angiographic, OCT, and clinical and biomarker outcomes that are listed in Table 3.
Statistical considerations
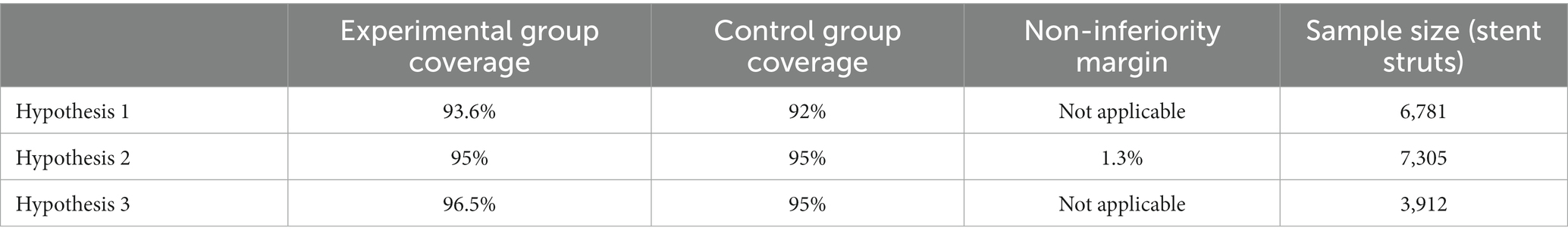
The sample size of this study is based on the previously published literature and the corresponding research hypothesis. This study intends to demonstrate that the incidence of strut coverage in the OCT-guided group is higher than that of angiography-guided group at 3-month follow-up (hypothesis 1). The second hypothesis of this study is that the incidence of stent strut coverage in the OCT-guided group at 3 months is similar to the incidence of strut coverage in the angiography-guided group at 6 months (non-inferiority analysis). If the above hypothesis is confirmed then an additional analysis will be performed that aims to demonstrate that the incidence of covered struts is higher at 3 months in the OCT-guided group compared to the rate of strut coverage noted at 6 months in the angiography-guided PCI (hypothesis 3). In order to control for the type I error, the test significance levels of hypothesis 1 and 2 are set at one-sided 0.025. Since hypothesis 3 is a sequential test carried out on the basis of hypothesis 2, the test significance level is set at one-sided 0.05.
The sample size calculation is based on the assumption that each patient will receive on average 1 stents, and that each stent will have a length of 24 mm. OCT analysis will be performed at 0.4/0.6 mm interval and that 7.9 struts in each cross section will be detected. Based on these assumptions, it is estimated that 474 strut results will be obtained from each patient. We estimate that we will need 7,305 strut estimations in each group to prove with 95% power the primary endpoint of the study. Based on the above assumptions, we estimate that 16 patients should be recruited in each group. Considering a 20% drop-off rate, we estimate that 20 patients should be included in each group (Table 4).
Continuous variables will be described as means and SDs if they are normally distributed or median and interquartile range in the case of asymmetric data distribution. Categorical variables will be presented as numbers and percentages. Comparisons of numerical variables are conducted with the Student’s t test or Mann–Whitney’ U test as appropriate, categorical variables and outcomes were compared by using the chi-squared test or Fisher exact test. A statistical package (SPSS 21.0) will be used for analysis. Two-tailed p < 0.05 will be considered statistically significant.
Discussion
Dual antiplatelet therapy is recommended following stent implantation, but the optimal duration remains controversial in ACS patients, the current ACC/AHA and ESC guidelines recommend DAPT for 12 months after PCI (2, 3). However, prolonged DAPT is associated with worse outcomes in high bleeding risk populations. A consensus from the Academic Research Consortium for High Bleeding Risk (ARC-HBR) defined 14 and six clinical criteria as major or minor criteria, respectively. If patients meet at least one major or two minor criteria, they are considered to be at high bleeding risk (20). According to the ARC-HBR criteria, 40% patients in real-world PCI registry are at high bleeding risk (21). These patients are often excluded from or underrepresented in clinical trials about stents and antiplatelet therapy. The development of advanced stent platforms and the optimal stent deployment—even with the use of intravascular imaging guidance—is expected to enable shorter duration of DAPT in high bleeding risk ACS patients undergoing PCI. Several studies have shown that shorter-term DAPT in ACS patients implanted with next-generation DES is non-inferior to the standard or longer duration of DAPT. More specifically the “all-comer” ITALIC randomized controlled trial demonstrated that after the implantation of next-generation DES, 6-month DAPT followed by aspirin monotherapy is non inferior to 24-month DAPT in terms of all-cause mortality, myocardial infraction (MI), target vessel revascularization (TVR), stroke, and major bleeding in a low risk profile for ischemic events population (22). Similarly, the randomized OPTIMA-C trial (NCT03056118) showed that the MACE rate at 12-month follow-up was not different in patients receiving 6-month DAPT and those treated for 12-month DAPT after implantation of next-generation DES (23). The multicenter REDUCE trial enrolled 1,496 patients admitted with an ACS treated with the next-generation COMBO stent who were randomized to 3-month (n = 751) or 12-month (n = 745) DAPT. The primary endpoint was a composite of all-cause mortality, MI, ST, stroke, TVR, and bleeding and its incidence was similar in the two groups (8.2 vs. 8.4%, p non-inferiority<0.001) at 1- or 2-year follow-up (11.6 vs. 12.1%, p = 0.76) (24).
There is compelling evidence that strut coverage is a predictor of ST (25). The next-generation DES with thinner struts, safer polymer profiles and improved drug kinetics appear to enable faster endothelialization and have known to reduce the incidence of ST compared to the first generation DES (25). The OCT sub-study of the OPTIMA-C trial revealed favorable strut coverage at 6 months after next-generation DES implantation (23), similarly the FUNCOMBO trial showed almost complete strut coverage of the next-generation DES in patients who presented with STEMI (26). Moreover, a small study reported that in the biodegradable polymer Synergy stent strut coverage was 94.5% at 3 months and 96.6% at 6 months (27) whereas the TARGET All Comers study showed a similar incidence of strut coverage in biodegradable polymer Firehawk and durable polymer XIENCE stent group (99.9 ± 0.3 vs. 100 ± 0.1%, p = 0.26) (28). Conversely, the biodegradable polymer BuMA Supreme stent was found to be superior to the XIENCE stent in terms of strut coverage at both 1 month and 2 months in the PIONEER-II OCT trial (29). However, stent implantation in these studies was mostly performed under angiography guidance. The DETECT-OCT (NCT01752894) trial was the first that highlighted the value of OCT imaging in optimizing stent apposition and in this way accelerating strut coverage. In this trial, the percentage of uncovered struts at 3 months was lower in the OCT-guided group (7.5%) than in the angiography-guided group (9.9%; p = 0.009) (8). However, the DETECT-OCT trial mainly included patients suffering from stable angina and did not include the group of patients undergoing OCT at a longer follow-up period. This study was designed to provide additional insights as it aims to include NSTE-ACS patients, and include the group of patients that will undergo OCT imaging at 6 months to examine late strut coverage in patients undergoing angiography guided PCI.
Additionally, clinical studies have found that MACE after PCI not only comes from in-stent restenosis or ST in ACS patients but also due to progression of mild to moderately graded disease in the non-culprit vessels as it is a well-known factor that patients presenting with ACS often have multivessel disease (30, 31). A study involving nearly 13,000 ACS patients showed that over 80% of ischemic events occurring after 30 days were unrelated to the culprit lesion that was stented, but were rather spontaneous, or de novo events in a non-culprit vessel (32). Intravascular imaging research revealed that non-culprit lesions in ACS patients are often vulnerable plaques, with characteristics such as thin-cap fibroatheroma and those with heavy lipid load. The progress and rupture of these vulnerable plaques in non-culprit vessels can also result in adverse events like thrombosis after PCI (33, 34). Therefore, understanding the evolution of plaque in non-culprit vessels is of great significance to prevent future adverse cardiac events and may potentially influence decision making in the use of DAPT duration. Thus, our study innovatively performs OCT examination in both culprit and non-culprit vessels at baseline and follow-up, to explore vulnerable plaque progression in non-target vessels, measure the changes in the thickness of the fibrous cap over lipid-rich plaques, and examine changes in macrophages accumulations and in the lipid content and in the vulnerable plaques, and their transformation at various time points. Furthermore, plaque progression is usually accompanied with lipid infiltration and inflammatory response. The positive correlation of cholesterol levels, proinflammatory cytokines, and atherosclerotic evolution is well established (35). Stent implantation is associated with vessel wall injury and endothelium disruption while it is well known that strut polymer and drug elution can trigger a pro-inflammatory and hypersensitivity reaction leading to the formation of neoatherosclerotic lesions (34). There are however limited data about the association between vulnerable plaque progression in native segments and neointima proliferation and neoatherosclerotic lesion formation in segments treated with stents. The present study has been designed to provide additional insights and explore the implications of the lipid profile and systemic inflammation assessed by circulatory biomarkers on neointima characteristics and atherosclerotic disease progression in native segments.
This study has several limitations. Firstly, only three centers in Huaihai economic zone are participated in this study. Therefore, the findings of this analysis may not be relevant to other populations. Secondly, “different operators” experience may impact the procedure outcomes, which is the source of uncontrolled bias. Finally, several studies revealed that the early strut coverage of biodegradable polymer DES is not similar with other next-generation DES (36, 37), which implies the results of our study may not be simply generalized to other DES with different design concept.
Ethics and dissemination
The study protocol has been approved by the ethics committee of Xuzhou Third People’s Hospital, Xuzhou Cancer Hospital (Xuzhou city, Jiangsu Province). The reference number is 2019-02-006-H02. A Chinese original document and an English translation of the ethical approval document are attached at Additional file 2. Written informed consent is provided by patients before enrollment.
The study results will be submitted for publication in peer-reviewed journals. Data will be disseminated and presented at scientific meetings.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this trial.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of Xuzhou Third People’s Hospital, Xuzhou Cancer Hospital (Xuzhou city, Jiangsu Province). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Y-XZ, LL, and Y-JZ co-designed the study protocol. Y-XZ, LL, and RP co-drafted the manuscript. ZL, QL, SC, and S-LF were involved with study conduct and data acquisition. W-RM and YW provided the statistical analysis. CB, BX, and Y-JZ further aided in assessment and revision of the protocol and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This investigator-initiated study is supported by JW Medical Systems (Shandong, China, grant number: N/A), 333 High-level Talent Training Program of Jiangsu Province (grant number: BAR2018275), and full-time introduction of special medical talents in 2018 of Xuzhou city (grant number: 2019-TPRC-1). These funding bodies are only providing financial support. The authors are solely responsible for the design and conduct of this study, analysis of the study data, drafting and editing of the paper, and the final content of the paper.
Acknowledgments
The authors wish to thank all participating centers, hospitals, researchers, and patients of the EXPECT study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor TG declared a past collaboration with the author CB.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1003546/full#supplementary-material
References
1. Gu, D, Qu, J, Zhang, H, and Zheng, Z. Revascularization for coronary artery disease: principle and challenges. Adv Exp Med Biol. (2020) 1177:75–100. doi: 10.1007/978-981-15-2517-9_3
2. Levine, GN, Bates, ER, Bittl, JA, Brindis, RG, Fihn, SD, Fleisher, LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and Management of Patients with Stable Ischemic Heart Disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the Management of Patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. (2016) 134:e123–55. doi: 10.1161/CIR.0000000000000404
3. Collet, JP, Thiele, H, Barbato, E, Barthélémy, O, Bauersachs, J, Bhatt, DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
4. Nakamura, M, Iijima, R, Ako, J, Shinke, T, Okada, H, Ito, Y, et al. Dual antiplatelet therapy for 6 versus 18 months after biodegradable polymer drug-eluting stent implantation. JACC Cardiovasc Interv. (2017) 10:1189–98. doi: 10.1016/j.jcin.2017.04.019
5. Yin, SH, Xu, P, Wang, B, Lu, Y, Wu, QY, Zhou, ML, et al. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent: systematic review and network meta-analysis. BMJ. (2019) 365:l2222. doi: 10.1136/bmj.l2222
6. Khan, SU, Singh, M, Valavoor, S, Khan, MU, Lone, AN, Khan, MZ, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation. (2020) 142:1425–36. doi: 10.1161/CIRCULATIONAHA.120.046308
7. Costa, F, Windecker, S, and Valgimigli, M. Dual antiplatelet therapy duration: reconciling the inconsistencies. Drugs. (2017) 77:1733–54. doi: 10.1007/s40265-017-0806-1
8. Lee, SY, Kim, JS, Yoon, HJ, Hur, SH, Lee, SG, Kim, JW, et al. Early strut coverage in patients receiving drug-eluting stents and its implications for dual antiplatelet therapy: a randomized trial. JACC Cardiovasc Imaging. (2018) 11:1810–9. doi: 10.1016/j.jcmg.2017.12.014
9. Cutlip, DE, Windecker, S, Mehran, R, Boam, A, Cohen, DJ, van Es, GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
10. Finn, AV, Joner, M, Nakazawa, G, Kolodgie, F, Newell, J, John, MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. (2007) 115:2435–41. doi: 10.1161/CIRCULATIONAHA.107.693739
11. Lee, R, Foin, N, Ng, J, Allen, J, Soh, N, Ang, I, et al. Early coverage of drug-eluting stents analysed by optical coherence tomography: evidence of the impact of stent apposition and strut characteristics on the neointimal healing process. EuroIntervention. (2016) 12:e605–14. doi: 10.4244/EIJV12I5A100
12. Gori, T, Polimeni, A, Indolfi, C, Räber, L, Adriaenssens, T, and Münzel, T. Predictors of stent thrombosis and their implications for clinical practice. Nat Rev Cardiol. (2019) 16:243–56. doi: 10.1038/s41569-018-0118-5
13. García-García, HM, Muramatsu, T, Nakatani, S, Lee, IS, Holm, NR, Thuesen, L, et al. Serial optical frequency domain imaging in STEMI patients: the follow-up report of TROFI study. Eur Heart J Cardiovasc Imaging. (2014) 15:987–95. doi: 10.1093/ehjci/jeu042
14. Kim, JS, Ha, J, Kim, BK, Shin, DH, Ko, YG, Choi, D, et al. The relationship between post-stent strut apposition and follow-up strut coverage assessed by a contour plot optical coherence tomography analysis. JACC Cardiovasc Interv. (2014) 7:641–51. doi: 10.1016/j.jcin.2013.12.205
15. Wang, G, Sun, Z, Jin, Q, Xu, K, Li, Y, Wang, X, et al. First-in-man study evaluating the safety and efficacy of a second generation biodegradable polymer sirolimus-eluting stent in the treatment of patients with de novo coronary lesions: clinical, angiographic, and OCT outcomes of CREDIT-1. Catheter Cardiovasc Interv. (2015) 85:744–51. doi: 10.1002/ccd.25862
16. Wang, G, Wang, H, Xu, B, Yang, Y, Yang, Z, Li, H, et al. Efficacy and safety of a biodegradable polymer cobalt-chromium sirolimus-eluting stent (EXCROSSAL) in treating de novo coronary artery disease: a pooled analysis of the CREDIT II and CREDIT III trials. Catheter Cardiovasc Interv. (2017) 89:512–9. doi: 10.1002/ccd.26887
17. Taniwaki, M, Windecker, S, Zaugg, S, Stefanini, GG, Baumgartner, S, Zanchin, T, et al. The association between in-stent neoatherosclerosis and native coronary artery disease progression: a long-term angiographic and optical coherence tomography cohort study. Eur Heart J. (2015) 36:2167–76. doi: 10.1093/eurheartj/ehv227
18. Ali, ZA, Maehara, A, Généreux, P, Shlofmitz, RA, Fabbiocchi, F, Nazif, TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. (2016) 388:2618–28. doi: 10.1016/S0140-6736(16)31922-5
19. Gupta, A, Shrivastava, A, Vijayvergiya, R, Chhikara, S, Datta, R, Aziz, A, et al. Optical coherence tomography: an eye into the coronary artery. Front Cardiovasc Med. (2022) 9:854554. doi: 10.3389/fcvm.2022.854554
20. Urban, P, Mehran, R, Colleran, R, Angiolillo, DJ, Byrne, RA, Capodanno, D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the academic research consortium for high bleeding risk. Eur Heart J. (2019) 40:2632–53. doi: 10.1093/eurheartj/ehz372
21. Ueki, Y, Bär, S, Losdat, S, Otsuka, T, Zanchin, C, Zanchin, T, et al. Validation of the academic research consortium for high bleeding risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. (2020) 16:371–9. doi: 10.4244/EIJ-D-20-00052
22. Didier, R, Morice, MC, Barragan, P, Noryani, AAL, Noor, HA, Majwal, T, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: final results of the ITALIC trial (is there a life for DES after discontinuation of Clopidogrel). JACC Cardiovasc Interv. (2017) 10:1202–10. doi: 10.1016/j.jcin.2017.03.049
23. Lee, BK, Kim, JS, Lee, OH, Min, PK, Yoon, YW, Hong, BK, et al. Safety of six-month dual antiplatelet therapy after second-generation drug-eluting stent implantation: OPTIMA-C randomised clinical trial and OCT substudy. EuroIntervention. (2018) 13:1923–30. doi: 10.4244/EIJ-D-17-00792
24. De Luca, G, Damen, SA, Camaro, C, Benit, E, Verdoia, M, Rasoul, S, et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention. (2019) 15:e990–8. doi: 10.4244/EIJ-D-19-00539
25. Torii, S, Jinnouchi, H, Sakamoto, A, Kutyna, M, Cornelissen, A, Kuntz, S, et al. Drug-eluting coronary stents: insights from preclinical and pathology studies. Nat Rev Cardiol. (2020) 17:37–51. doi: 10.1038/s41569-019-0234-x
26. Gómez-Lara, J, Oyarzabal, L, Brugaletta, S, Salvatella, N, Romaguera, R, Roura, G, et al. Coronary endothelial and microvascular function distal to polymer-free and endothelial cell-capturing drug-eluting stents. The randomized FUNCOMBO trial. Rev Esp Cardiol. (2021) 74:1013–22. doi: 10.1016/j.rec.2021.01.007
27. de la Torre Hernández, JM, Tejedor, P, Camarero, TG, Duran, JM, Lee, DH, Monedero, J, et al. Early healing assessment with optical coherence tomography of everolimus-eluting stents with bioabsorbable polymer (synergy™) at 3 and 6 months after implantation. Catheter Cardiovasc Interv. (2016) 88:E67–73. doi: 10.1002/ccd.26299
28. Baumbach, A, Lansky, AJ, Onuma, Y, Asano, T, Johnson, T, Anderson, R, et al. Optical coherence tomography substudy of a prospective multicentre randomised post-market trial to assess the safety and effectiveness of the Firehawk cobalt-chromium coronary stent (rapamycin target-eluting) system for the treatment of atherosclerotic lesions: TARGET all comers. EuroIntervention. (2018) 14:1121–8. doi: 10.4244/EIJ-D-18-00226
29. Asano, T, Jin, Q, Katagiri, Y, Kogame, N, Takahashi, K, Chang, CC, et al. A randomised comparison of healing response between the BuMA supreme stent and the XIENCE stent at one-month and two-month follow-up: PIONEER-II OCT randomised controlled trial. EuroIntervention. (2018) 14:e1306–15. doi: 10.4244/EIJ-D-18-00461
30. Taruya, A, Tanaka, A, Nishiguchi, T, Ozaki, Y, Kashiwagi, M, Yamano, T, et al. Lesion characteristics and prognosis of acute coronary syndrome without angiographically significant coronary artery stenosis. Eur Heart J Cardiovasc Imaging. (2020) 21:202–9. doi: 10.1093/ehjci/jez079
31. Montone, RA, Niccoli, G, Crea, F, and Jang, IK. Management of non-culprit coronary plaques in patients with acute coronary syndrome. Eur Heart J. (2020) 41:3579–86. doi: 10.1093/eurheartj/ehaa481
32. Scirica, BM, Bergmark, BA, Morrow, DA, Antman, EM, Bonaca, MP, Murphy, SA, et al. Nonculprit lesion myocardial infarction following percutaneous coronary intervention in patients with acute coronary syndrome. J Am Coll Cardiol. (2020) 75:1095–106. doi: 10.1016/j.jacc.2019.12.067
33. Russo, M, Kim, HO, Kurihara, O, Araki, M, Shinohara, H, Thondapu, V, et al. Characteristics of non-culprit plaques in acute coronary syndrome patients with layered culprit plaque. Eur Heart J Cardiovasc Imaging. (2020) 21:1421–30. doi: 10.1093/ehjci/jez308
34. Xing, L, Higuma, T, Wang, Z, Aguirre, AD, Mizuno, K, Takano, M, et al. Clinical significance of lipid-rich plaque detected by optical coherence tomography: a 4-year follow-up study. J Am Coll Cardiol. (2017) 69:2502–13. doi: 10.1016/j.jacc.2017.03.556
35. Zhu, Y, Xian, X, Wang, Z, Bi, Y, Chen, Q, Han, X, et al. Research Progress on the relationship between atherosclerosis and inflammation. Biomol Ther. (2018) 8:80. doi: 10.3390/biom8030080
36. Otaegui, I, Pérez de Prado, A, Massotti, M, López-Benito, M, Sabaté, M, Martí, G, et al. Intrapatient randomization to study strut coverage in polymer-free versus biodegradable-polymer Sirolimus-eluting stent implantations. JACC Cardiovasc Interv. (2020) 13:899–900. doi: 10.1016/j.jcin.2019.11.020
37. Suzuki, S, Sotomi, Y, Kobayashi, T, Hamanaka, Y, Nakatani, S, Shiojima, I, et al. Early vessel healing after implantation of biodegradable-polymer and durable-polymer drug-eluting stent: 3-month angioscopic evaluation of the RESTORE registry. Int J Card Imaging. (2019) 35:973–80. doi: 10.1007/s10554-019-01580-2
Keywords: non-ST elevation acute coronary syndrome, optical coherence tomography, percutaneous coronary intervention, early vascular healing, stent struts coverage
Citation: Zhu Y-X, Liang L, Parasa R, Li Z, Li Q, Chang S, Ma W-R, Feng S-L, Wang Y, Xu B, Bourantas CV and Zhang Y-J (2023) Early vascular healing after neXt-generation drug-eluting stent implantation in Patients with non-ST Elevation acute Coronary syndrome based on optical coherence Tomography guidance and evaluation (EXPECT): study protocol for a randomized controlled trial. Front. Cardiovasc. Med. 10:1003546. doi: 10.3389/fcvm.2023.1003546
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Peiren Shan, First Affiliated Hospital of Wenzhou Medical University, ChinaAnkush Gupta, Army Institute of Cardiothoracic Sciences (AICTS), India
Copyright © 2023 Zhu, Liang, Parasa, Li, Li, Chang, Ma, Feng, Wang, Xu, Bourantas and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Jun Zhang, ✉ MTM3NzA2Njg2NjdAMTM5LmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Yao-Jun Zhang, https://orcid.org/0000-0001-8357-5544
 Yong-Xiang Zhu
Yong-Xiang Zhu Li Liang
Li Liang Ramya Parasa
Ramya Parasa Zheng Li
Zheng Li Qian Li
Qian Li Shang Chang
Shang Chang Wen-Rui Ma
Wen-Rui Ma Si-Li Feng
Si-Li Feng Yang Wang
Yang Wang Bo Xu
Bo Xu Christos V. Bourantas
Christos V. Bourantas Yao-Jun Zhang
Yao-Jun Zhang