
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 20 October 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.998842
 Jiri Parenica1,2
Jiri Parenica1,2 Klara Benesova3,4
Klara Benesova3,4 Martin Radvan1,2
Martin Radvan1,2 Ondrej Sanca3,4
Ondrej Sanca3,4 Jiri Hlasensky1,2
Jiri Hlasensky1,2 Petr Lokaj1,2
Petr Lokaj1,2 Tomas Ondrus1,2
Tomas Ondrus1,2 Katerina Helanova1,2
Katerina Helanova1,2 Petr Kala1,2
Petr Kala1,2 Ladislav Dusek3,4
Ladislav Dusek3,4 Jiri Jarkovsky3,4*
Jiri Jarkovsky3,4*Background: Heart failure (HF) patients are at higher risk of severe coronavirus disease 2019 (COVID-19). The Omicron variant has many novel mutations including those in the spike protein, leading to questions about vaccine effectiveness. The aim of this analysis was to evaluate the effectiveness of the COVID-19 vaccine with or without a booster (i.e., after the third dose) during the Omicron variant wave.
Methods: Chronic heart failure patients in the Czech Republic were included in the analysis. COVID-19 infection was monitored from January 1st 2022 to March 31st 2022. The analysis was conducted on data collected in the National Health Information System. Vaccine effectiveness of vaccinated (with or without booster) vs. unvaccinated patients was analyzed for incidence of COVID-19, COVID-19-related hospitalizations, COVID-19 related intensive care unit admissions, and COVID-19 related mechanical ventilation/extracorporeal membrane oxygenation treatment.
Findings: From a total 165,453 HF patients in the Czech Republic, 9,728 contracted COVID-19 (22.9% of them not vaccinated, 23.2% vaccinated and 53.8% vaccinated and boosted). Risk of intensive care unit (ICU) hospitalization was 7.6% in the unvaccinated group, 4.8% in the vaccinated group and 2.9% in the boosted group. The calculated effectiveness of the COVID-19 vaccine in prevention of ICU hospitalization in the vaccinated group was 41.9 and 76.6% in the boosted group.
Interpretation: The results demonstrated moderate vaccine effectiveness in the prevention of severe COVID-19 in vaccinated but not boosted HF patients. Much stronger effectiveness was found in those who were vaccinated and boosted.
Patients with heart failure (HF), particularly older adults, are at higher risk of severe coronavirus disease 2019 (COVID-19) (1–4). Bronchopulmonary infection can lead to decompensation of chronic heart failure and congestion in the respiratory tract leaves it more vulnerable to infection (5, 6). The original severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified at the end of 2019 (7) and has been found to continuously evolve new variants which evade host immune response, increase transmission, lead to a more severe course of the disease, or lower effectiveness of treatment and vaccination. The variants of concern of SARS-CoV2 include Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2), and each led to a new wave of the pandemic (8). Omicron (B.1.1.529) was described in November 2021 in South Africa and there was early information concerning lower virulency and increased transmission compared to earlier variants (9, 10). Omicron has many novel mutations including those in the spike protein (8, 11), leading to questions about vaccine efficacy (12, 13).
The primary aim of this analysis was to evaluate vaccine efficacy (with or without a booster dose) in preventing COVID-19 intensive care unit hospitalization in chronic heart failure patients during the Omicron variant wave.
SARS-CoV-2 infection was diagnosed by real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assays. The Omicron variant was identified on the basis of genome sequencing, which was performed in about 1.0% of SARS-CoV-2 virus infections in the Czech Republic (14). From January 1st 2022 to March 31st 2022, a total of 1,338,068 cases of COVID-19 were diagnosed in the Czech Republic (12,503 cases per 100,000 population), the mean age was 36.5 (±19.6) years. The proportion of Omicron infections had been growing significantly since Dec 6th 2021 and prevailed during January 2022. Variant BA.1 spread first, followed by variant BA.1.1. and since mid-January, the number of patients with the BA.2 variant has been gradually increasing (14).
Chronic heart failure patients in the Czech Republic were included in the analysis. COVID-19 infection was monitored from January 1st 2022 to March 31st 2022. Hospitalization or ICU admission was considered COVID-19-related if it occurred within 14 days of a positive RT-PCR test.
The methodology for obtaining and processing data from the health information system for COVID-19 has been described elsewhere (3), briefly: the analysis was conducted on data collected in the National Health Information System (NHIS) which is managed by the Institute of Health Information and Statistics of the Czech Republic. Patients with chronic heart failure were identified using data from The National Registry of Reimbursed Health Services. The registry contains data from health insurance companies for both outpatients and inpatients including diagnoses, procedures, and medications. Data are currently available for the period 01/2010–12/2021 which is also the timeframe for the detection of patients with HF. Data on COVID-19 were extracted from the Information System of Infectious Diseases (ISID) containing, among other things, records of tests, positive cases, related hospitalizations, and vaccination status. Data in the ISID are collected in compliance with Act No. 258/2000 Coll. on Protection of Public Health, whereas data in the NHIS are collected—and linked with data from ISID—in accordance with Act No. 372/2011 Coll., on Health Services and Conditions of Their Provision. Due to this legal mandate, the retrospective analyses did not require either approval by an ethics committee or informed consent from participants.
Standard descriptive statistics were applied in the analysis; absolute and relative frequencies for categorical variables and means supplemented with standard deviations for continuous variables. The life-table method was applied to estimate the probability of hospital and ICU admission. Patients were followed from the day of COVID-19 diagnosis. Those without events were censored on 14th April 2022. Vaccine effectiveness of vaccinated (with or without booster) vs. unvaccinated patients was analyzed for incidence of COVID-19, COVID-19-related hospitalizations, COVID-19 related intensive care unit admissions, and COVID-19 related mechanical ventilation/extracorporeal membrane oxygenation treatment. The computation used a days at risk approach with the following formulas: a) Vaccine effectiveness of completed vaccination without booster vs. unvaccinated.
b) Vaccine effectiveness of completed vaccination with booster vs. unvaccinated
Available patient characteristics (sex, age, hypertension, diabetes mellitus, malignancy in the past 5 years, history of stroke, history of renal failure, number of HF hospitalizations in the past 2 years and COVID-19 vaccine status) were used and significant predictors assessed using univariable Cox regression models. Significant factors (<0.05) were entered into a multivariable Cox regression model with backward stepwise algorithm for selection of independent predictors of COVID-19-related hospitalization and ICU admissions.
At the beginning of 2022, there were an estimated 165,453 chronic heart failure patients in the Czech Republic (point prevalence 1.546 cases per 100,000 population). Of these, 52.7% were men, with a mean age (±standard deviation) of 71.8 (±11.8) years, the mean of age of women was 78.1 (±10.8). Out of total of 165,453 patients, 45,777 (27.7%) were vaccinated with 2 doses (or 1 dose of Jansen COVID-19 vaccine) (vaccinated group), 89,127 (53.9%) patients received 3 doses (boosted group) and 30,549 (18.5%) were not vaccinated. Of a total 134,904 patients, 65.8% received the Pfizer-BioNTech vaccine, 18.4% received Oxford-AstraZeneca, 11.5% received Moderna vaccine, and 4.2% received Janssen COVID-19 vaccine. Booster doses were administered to 89,127 patients, of them 85.0% received the Pfizer-BioNTech and 15.0% received Moderna.
There were a total of 9,728 COVID-19 cases in patients with chronic heart failure, of which 54.9% were men. The unvaccinated group comprised 2,231 (22.9%) patients, vaccinated but not boosted were 2,260 (23.2%), and 5,237 (53.8%) were boosted. The baseline characteristics are shown in Table 1. All three groups were comparable in terms of age, gender, comorbidities, pharmacological treatment of chronic heart failure and number of hospitalizations for acute heart failure decompensation within the last 2 years.
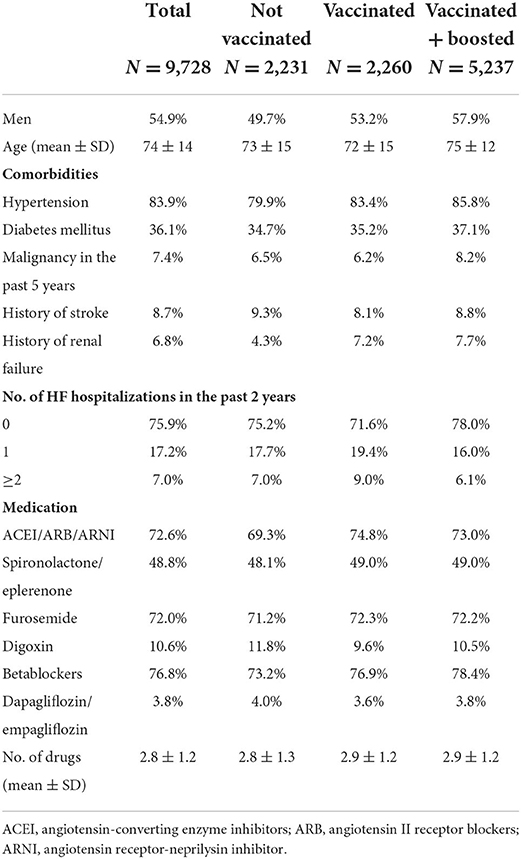
Table 1. Comorbidities and medication of patients with chronic heart failure diagnosed with COVID-19 from January to March 2022.
The risk of hospitalization within 14 days of a COVID-19 diagnosis was 52.1% with 95% confidence interval (CI) (50.0–54.2%) in the unvaccinated group, 39.4% (37.4–41.4%) in the vaccinated group, and 26.8% (25.6–28.0%) in the boosted group. Most hospitalizations occurred within 2 days of a
positive test. The risk of intensive care was 7.6% (6.5–8.7%) in the unvaccinated group, 4.8% (3.9–5.7%) in the
vaccinated group and 2.9% (2.4–3.3%) in the boosted group (Figure 1).
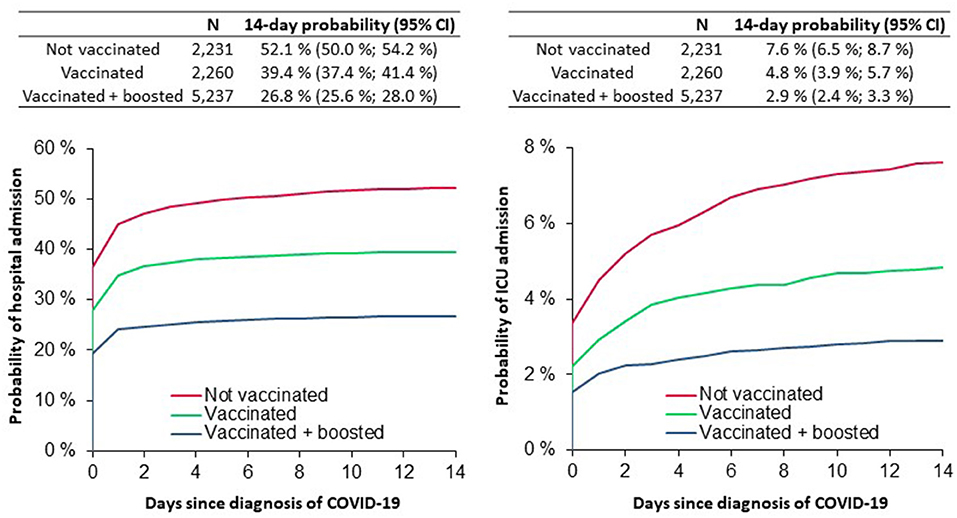
Figure 1. The probability of hospital/ICU admission in heart failure patients during the Omicron variant wave A.
The calculated vaccine effectiveness of 5.7% for COVID-19 positivity demonstrates the small benefit of completed vaccination compared with the unvaccinated group. Vaccination efficacy against intensive care unit admissions was 41.9%. The effectiveness in the boosted population was 76.6% for intensive care unit admissions and 79.2% for mechanical ventilation/extracorporeal membrane oxygenation treatment (Table 2).
Using univariable and multivariable Cox regression analysis, models for prediction of COVID-19 ICU admission in chronic heart failure patients diagnosed with COVID-19 were calculated (Supplementary Table 1). Vaccination and vaccination with booster dose were independent predictors of COVID-19 related ICU admissions. Hazard ratio for ICU admission was 0.61 (CI 0.48–0.77; p < 0.001) for vaccinated group and 0.36 (CI 0.29–0.44; p < 0.001) for boosted group.
The present analysis demonstrates the protective effect of vaccination against severe COVID-19 in a large, real-world population of 165,453 chronic heart failure patients. There was a clear reduction in-hospital intensive care for heart failure patients with history of vaccination, which was more pronounced in those with a booster dose. There was a demonstrated decreased rate of any hospitalization due to COVID-19 in the vaccinated group. These results confirm the efficacy of vaccination against Omicron variants.
The safety and efficacy of COVID-19 vaccines approved in the Czech Republic- Pfizer-BioNTech COVID-19 vaccine (15), Moderna (16), Janssen (17), Oxford-AstraZeneca (18), and Nuvaxovid (19) were confirmed in randomized, placebo-controlled trials. Vaccine effectiveness in preventing severe COVID-19 was reported to be in the range of 85.4–100%. It should be noted that the number of severe cases in the study ranged up to a maximum of ten patients and the trials were conducted before widespread circulation of the SARS-CoV-2 Delta variant and Omicron variant.
According to the presented results vaccine effectiveness was slightly lower compared to published results: vaccine effectiveness against the most severe outcome of hospitalizations, including invasive mechanical ventilation or death, was reported as 90%. Vaccine effectiveness was 94% after three doses during the Omicron wave (20).
It is well-documented that patients with chronic heart failure, primarily characterized by advanced age, advanced heart failure and the presence of comorbidities, have an increased risk of hospitalization and severe disease due to COVID-19 (1–4, 21). In our analysis, we expressed the severity of heart failure by the number of drugs for the treatment of heart failure (22) and by the number of heart failure hospitalizations in the last 2 years (23). It is known that infection is a common precipitating factor in acute decompensation of heart failure (24). Acute myocardial injury, myocardial infarction, myocarditis, respiratory failure, secondary bacterial infection, or renal injury could be other reasons leading to acute decompensation of chronic heart failure and worsening prognosis of these patients during COVID-19 (25).
The main limitations of the study are non-randomized design covering potential differences between the unvaccinated and vaccinated group and the fact that it does not distinguish between hospitalizations due to COVID-19 or those where COVID-19 was not the main reason for the hospital stay. Several studies have shown that vaccine effectiveness against hospitalization appears to be lower for Omicron variant compared to previous variant (26, 27). One reason could be because with Omicron incidences have become much higher than experienced ever before and there are more “with but not because of COVID-19” hospitalizations and consequently lower vaccine effectiveness. Vaccine effectiveness estimates improve when definitions of endpoints are chosen more specific to severe respiratory disease (28, 29).
Theis study had number of other limitations: the analysis did not consider previous COVID-19 infections and time from the vaccination. We cannot rule out a small number of patients who may have developed COVID-19 and not been tested using RT-PCR testing. We are not able to clearly distinguish patients with preserved, mid-range and reduced ejection fraction. Body mass index as important characteristic of heart failure population (30) and negative prognostic factor of COVID-19 (31) was not known and not included in the analysis. It was also hypothesized that physical activity and air pollution could play a role in the spread of the virus (32, 33), our data do not allow us identifying patients who live in geographic areas with increased air pollution.
Heart failure patients represent a group at high risk of severe COVID-19. Despite several study limitation our study demonstrates that approved COVID-19 vaccines remained effective against COVID-19- associated ICU hospitalization among a large population of patients with heart failure during the SARS-CoV-2 Omicron wave. Booster doses significantly increased the protection effect.
The results continue to highlight the urgent need to educate patients with chronic heart failure about the benefits of vaccination and the booster dose with existing approved vaccines, even during the relaxation of measures against coronavirus.
Heart failure patients are at higher risk of severe COVID-19. The Omicron variant has prevailed since January 2022.
Vaccine effectiveness in preventing severe COVID-19 with admission to the ICU was 41.9% in heart failure patients. The booster dose significantly increased the protective effect of vaccination and lead to vaccine effectiveness of 66.2% in preventing hospitalization and 76.6% in ICU admission.
It is important that heart failure patients be educated about the protective effect of vaccination and the booster dose which brings strong protection against severe COVID-19 with the Omicron variant.
The anonymized data is available upon reasonable request. The data are deidentified participant data, and available from the author JJ (amlyaS5qYXJrb3Zza3lAdXppcy5jeg==). The reuse of the data subset is permitted only for revalidation of the results.
The analysis was performed with data from the National Health Information System (NHIS), which was supplemented with data from the Information System of Infectious Diseases (ISID). Data in the ISID are collected in compliance with Act No. 258/2000 Coll. on Protection of Public Health, data in the NHIS are collected-and linked with data from ISID-in accordance with Act No. 372/2011 Coll., on Health Services and Conditions of Their Provision. The anonymized data is available upon reasonable request. The data are deidentified participant data, and available from the author JJ (amlyaS5qYXJrb3Zza3lAdXppcy5jeg==). The reuse of the data subset is permitted only for revalidation of the results.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JP: methodology, resources, validation, writing-original draft, visualization, formal analysis, project administration, and funding acquisition. KB: visualization and formal analysis. MR, JH, OS, and KH: visualization, formal analysis, and project administration. PL and TO: writing-original draft, visualization, and formal analysis. PK: project administration and funding acquisition. LD: project administration, funding acquisition, and formal analysis. JJ: project administration and supervision. All authors contributed to the article and approved the submitted version.
This research was supported by a grant from the Czech Republic Operational Program eHealth and Rare Disease CZ.03.4.74/0.0/0.0/15_025/ and by a grant from the Ministry of Health of the Czech Republic—conceptual development of research organization (FNBr, 65269705; funding was given to University Hospital Brno).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.998842/full#supplementary-material
1. Hergens M-P, Bell M, Haglund P, Sundström J, Lampa E, Nederby-Öhd J, et al. Risk factors for COVID-19-related death, hospitalization and intensive care: a population-wide study of all inhabitants in Stockholm. Eur J Epidemiol. (2022) 37:157–65. doi: 10.1007/s10654-021-00840-7
2. Semenzato L, Botton J, Drouin J, Cuenot F, Dray-Spira R, Weill A, et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. (2021) 8:100158. doi: 10.1016/j.lanepe.2021.100158
3. Jarkovsky J, Benesova K, Cerny V, Razova J, Kala P, Dolina J, et al. Covidogram as a simple tool for predicting severe course of COVID-19: population-based study. BMJ Open. (2021) 11:e045442. doi: 10.1136/bmjopen-2020-045442
4. Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. (2020) 76:2334–48. doi: 10.1016/j.jacc.2020.09.549
5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab670
6. Parenica J, Hanslianová M. Infectious complications in patients with acute heart failure –an overview. Kardiologicka Rev. (2016) 18:18–21.
7. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
8. He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 Omicron variant: characteristics and prevention. Med Comm. (2021) 2:838–45. doi: 10.1002/mco2.110
9. CDC. Omicron Variant: What You Need to Know. Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html (accessed March 31, 2022).
10. Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature. (2022) 603:679–86. doi: 10.1038/s41586-022-04411-y
11. Shah M, Woo HG. Omicron: a heavily mutated SARS-CoV-2 variant exhibits stronger binding to ACE2 and potently escapes approved COVID-19 therapeutic antibodies. Front Immunol. (2022) 12:2–3. doi: 10.3389/fimmu.2021.830527
12. Supasa P, Zhou D, Dejnirattisai W, Liu C, Mentzer AJ, Ginn HM, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. (2021) 184:2201–11. doi: 10.1016/j.cell.2021.02.033
13. Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. (2021) 19:409–24. doi: 10.1038/s41579-021-00573-0
14. Available online at: COG-CZhttps://virus.img.cas.cz/lineages (accessed March 31, 2022).
15. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
16. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
17. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26COV2S vaccine against Covid-19. N Engl J Med. (2021) 384:2187–201. doi: 10.1056/NEJMoa2101544
18. Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. (2021) 385:2348–60. doi: 10.1056/NEJMoa2105290
19. Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. (2022) 386:531–43. doi: 10.1056/NEJMoa2116185
20. Tenforde MW, Self WH, Gaglani M, Ginde AA, Douin DJ, Talbot HK, et al. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death - United States, March 2021-January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:459–65. doi: 10.15585/mmwr.mm7112e1
21. Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One. (2020) 15:e0238281. doi: 10.1371/journal.pone.0238281
22. Gilstrap L, Zipkin RJ, Barnes JA, King A, O'Malley AJ, Gaziano TA, et al. Sacubitril/valsartan vs ACEi/ARB at hospital discharge and 5-year survival in older patients with heart failure with reduced ejection fraction: a decision analysis approach. Am Heart J. (2022) 250:23–8. doi: 10.1016/j.ahj.2022.04.007
23. Canepa M, Leporatti L, Persico L, Ameri P, Porto I, Ansaldi F, et al. Frequency, characteristics and prognostic impact of hospital readmissions in elderly patients with heart failure: a population study from 2013 to 2017 in Liguria, Northern Italy. Int J Cardiol. (2022) 363:111–8. doi: 10.1016/j.ijcard.2022.06.052
24. Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi D-J, et al. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail. (2017) 19:201–8. doi: 10.1002/ejhf.682
25. Baigent C, Windecker S, Andreini D, Arbelo E, Barbato E, Bartorelli AL, et al. Task force for the management of COVID-19 of the European society of cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2-care pathways, treatment, and follow-up. Cardiovasc Res. (2021) 2021:cvab343. doi: 10.1093/eurheartj/ehab697
26. Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:139–45. doi: 10.15585/mmwr.mm7104e3
27. Gram MA, Emborg H-D, Schelde AB, Friis NU, Nielsen KF, Moustsen-Helms IR, et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or omicron SARS-CoV-2 variant: a nationwide danish cohort study. PLoS Med. (2022) 19:e1003992. doi: 10.1371/journal.pmed.1003992
28. Stowe J, Andrews N, Kirsebom F, Ramsay M, Bernal JL. Effectiveness of COVID-19 Vaccines Against Omicron and Delta Hospitalisation: Test Negative Case-Control Study. Available online at: https://www.medrxiv.org/content/10.1101/2022.04.01.22273281v1 (accessed July 18, 2022).
29. Kirsebom FCM, Andrews N, Stowe J, Toffa S, Sachdeva R, Gallagher E, et al. COVID-19 vaccine effectiveness against the omicron (BA2) variant in England. Lancet Infect Dis. (2022) 22:931–3. doi: 10.1016/S1473-3099(22)00309-7
30. Sciomer S, Moscucci F, Salvioni E, Marchese G, Bussotti M, Corrà U, et al. Role of gender, age and BMI in prognosis of heart failure. Eur J Prev Cardiol. (2020) 27:46–51. doi: 10.1177/2047487320961980
31. Keller K, Sagoschen I, Schmitt VH, Sivanathan V, Espinola-Klein C, Lavie CJ, et al. Obesity and its impact on adverse in-hospital outcomes in hospitalized patients with COVID-19. Front Endocrinol. (2022) 13:4–8. doi: 10.3389/fendo.2022.876028
32. Sciomer S, Moscucci F, Magrì D, Badagliacca R, Piccirillo G, Agostoni P. SARS-CoV-2 spread in Northern Italy: what about the pollution role? Environ Monit Assess. (2020) 192:325. doi: 10.1007/s10661-020-08317-y
Keywords: COVID-19, severe course, Omicron, vaccination, heart failure
Citation: Parenica J, Benesova K, Radvan M, Sanca O, Hlasensky J, Lokaj P, Ondrus T, Helanova K, Kala P, Dusek L and Jarkovsky J (2022) COVID-19 vaccine booster significantly decreases the risk of intensive care unit hospitalization in heart failure patients during the Omicron variant wave: A population-based study. Front. Cardiovasc. Med. 9:998842. doi: 10.3389/fcvm.2022.998842
Received: 20 July 2022; Accepted: 05 October 2022;
Published: 20 October 2022.
Edited by:
Elisabetta Salvioni, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Federica Moscucci, Sapienza University of Rome, ItalyCopyright © 2022 Parenica, Benesova, Radvan, Sanca, Hlasensky, Lokaj, Ondrus, Helanova, Kala, Dusek and Jarkovsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiri Jarkovsky, amlyaS5qYXJrb3Zza3lAdXppcy5jeg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.