- 1Department of Endocrinology and Metabolism, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 2Department of Functional Examination, Gansu Provincial Maternal and Child Health Hospital, Lanzhou, China
- 3Clinical Experimental Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
Radiation therapy (RT) is one of the common and widely used treatment method for thyroid tumors. Considering that the thyroid is located close to the heart, the radiation generated during the treatment of thyroid tumors may have an adverse greater impact on the heart. This study is to explore the influencing factors, especially additional effects of RT, on cardiac-specific death among patients with malignant thyroid tumors. Collecting information from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database using SEER*Stat. Patients with malignant thyroid tumors were searched, whether receiving RT or not. Ultimately, 201, 346 eligible patients were included. Propensity Score Matching (PSM) was used to minimize bias of baseline characteristics by adjusting for confounding factors. COX (proportional hazards) and fine-gray (competing risk) model regression analysis were used to explore the effects of various influencing factors on cardiac-specific death. The present analysis showed that, compared with non-RT, RT based upon radioactive implants and beam radiation were associated with lower risk of cardiac-specific death in patients with thyroid malignancy, beam radiation therapy may had a similar effect. Besides, the remaining RT methods did not significantly increase the risk of cardiac-specific death. In addition, Asian or Pacific Islander ethnicity, female sex, marital status, combined summary stage (localized, regional, and distant), high-income, and later year of diagnosis were associated with lower risk of cardiac-specific death. While older age of diagnosis, African ethnicity, non-Hispanic ancestry, and derived AJCC stage (IV) were risk factors for cardiac-specific death. These results help to identify the factors influencing cardiac-specific death among patients with thyroid malignancies. Furthermore, it may helps to improve the clinical application of RT without too much concern about adverse cardiac effects.
Introduction
While radiation therapy (RT) can improve the condition of patients with cancer, it can also cause harmful off-target side, such as radiation-induced heart diseases, which presents a major concern in cancer therapy. Under the course of RT treatment – particularly for malignant tumors – the accumulation of high doses of radiation can lead to delayed-onset cardiac damage and, in some cases, cardiac death, which can occur years or even decades after radiation exposure (1). Current studies have demonstrated that RT significantly increases the risk of cardiac death in some tumors (2–4), as RT is widely used in the treatment of thyroid tumors (5), and considering that the thyroid is located near the heart, the present study sought to explore whether RT in the context of thyroid cancer treatment may be associated with increased rates of cardiac-specific death.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database is a population-based coordinated state cancer registry that collates the demographic and clinical information of cancer patients from multiple regions of the United States. Therefore, the SEER database is a potentially useful resource for exploring the cardiac complications of RT among patients with malignant thyroid tumors (6). In this study, we explored the incidence of post-radiation cardiovascular complications in patients with malignant thyroid tumors and identify the influencing factors of cardiac-specific death by collecting data from SEER databases (7, 8).
Patients and methods
Patient selection
The “Incidence” package of SEER database was accessed using SEER*Stat (version 8.4.0.1), and the data of patients with malignant thyroid tumors, whether receiving RT or not, from 2000 to 2019 was retrieved. Cardiac-specific death was defined as all deaths due to heart disease. Those who were unsure whether to receive RT were excluded. Ethical approval and patient consent were not required since the SEER data is publicly available (9).
Statistical analysis
Patients were divided into RT and non-RT groups, according to whether or not they received radiation therapy. Firstly, we calculated the differences in baseline characteristics of the two groups, and then used regression analysis to explore the factors influencing cardiac-specific death. The missing data were filled with multiple imputation. Categorical variables were expressed as frequencies and proportions, which were compared by chi-square or Fisher’s exact test. Continuous variables were expressed as mean ± SD and compared by t-test if also homoscedastic. Otherwise, these variables were expressed as median and compared by Mann-Whitney U test. After verifying that the variables meet the equal-proportional hazards assumption, COX (proportional hazards) and Fine-Gray (competing risk) model regression analysis were used to explore the factors influencing cardiac-specific death. All statistical tests were two-sided, and P < 0.05 was considered statistically significant. SPSS (version, 27.0; SPSS, Chicago, IL, USA) and STATA (version, 17.0; STATA, TX, USA) were used for all statistical analyses.
Propensity score matching
Propensity score matching (PSM) has been widely used to improve comparability between groups in observational studies by adjusting for confounding factors (10). A propensity 1:1 matching analysis was performed using SPSS to minimize bias, followed by the statistical analyses as described above.
Results
Patient characteristics
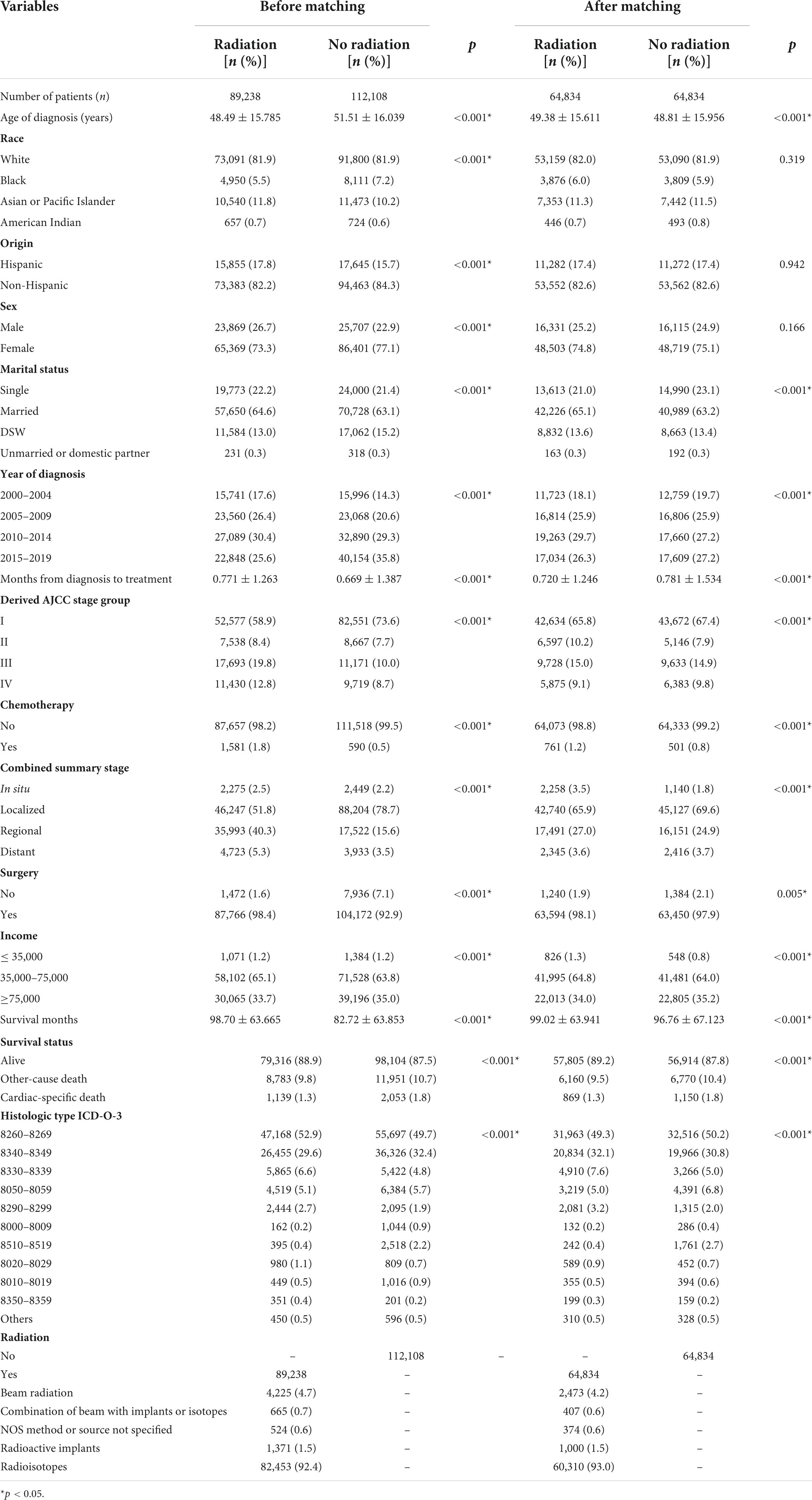
Ultimately, 201, 346 eligible patients were included in this study. The average age of patients at diagnosis was around 50 years old, and the patients were predominantly White ethnicity, non-Hispanic ancestry, female sex, marital status, and middle-income. About 44% of patients received various types of RT, the vast majority of patients received surgery, and very few patients were treated by chemotherapy. There were significant differences in all characteristics (Table 1).
After PSM (1:1), 129, 668 patients still retaining, and the distribution of baseline characteristics of the population were similar to before matching. The two groups became statistically indistinguishable in variables of race, origin, and sex, and the differences in other variables also decreased to a certain extent compared to pre-PSM (Table 1).
Effects of radiation therapy on cardiac-specific death before propensity score matching
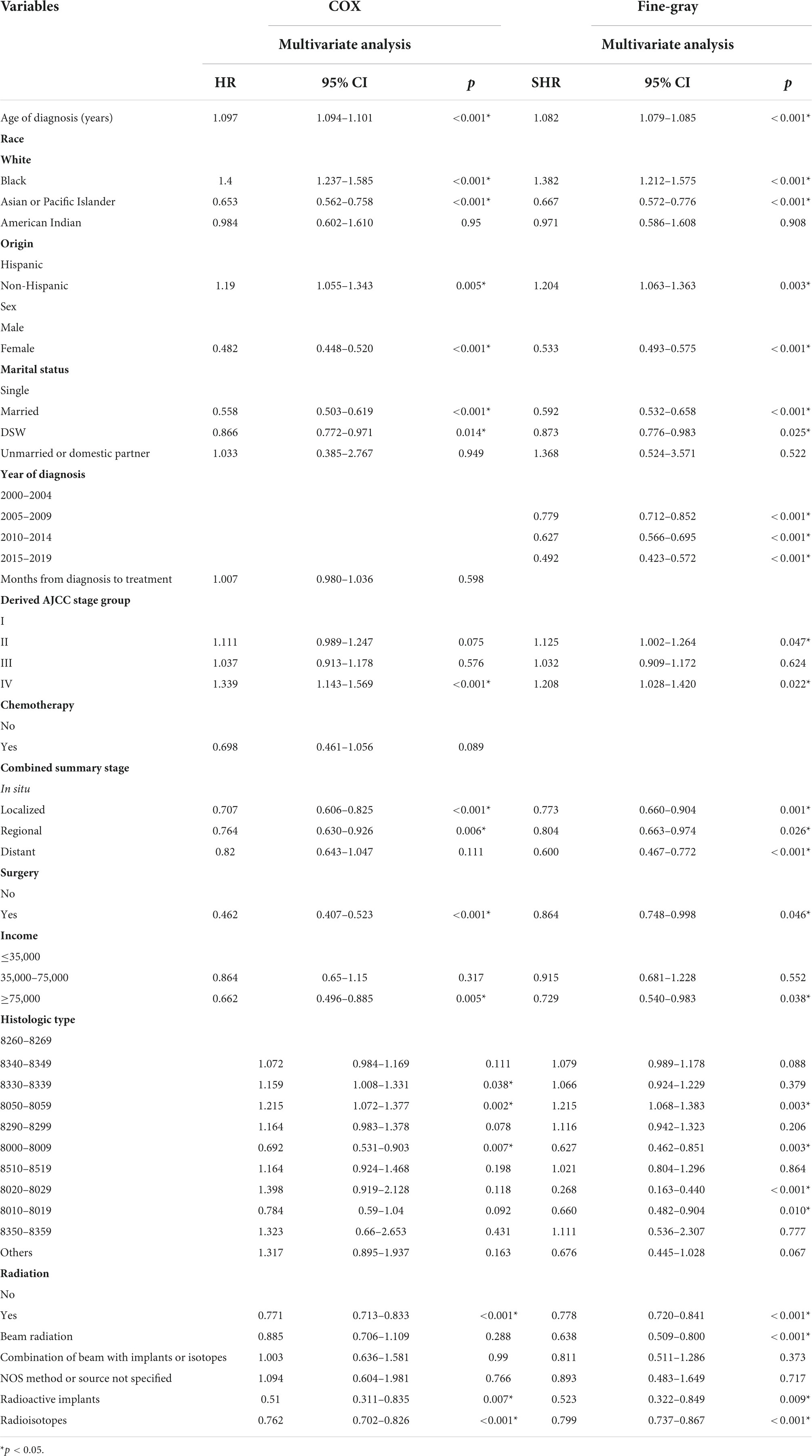
Multivariate COX regression analysis showed that RT {yes (hazard ratio [HR] = 0.771, 95% confidence interval [CI] = 0.713–0.833, p < 0.001) vs. no} was associated with a lower risk of cardiac-specific death among patients with malignant thyroid tumors (Figure 1A). Results of group analysis by type of RT received showed that RT based upon radioisotopes (HR = 0.762, CI = 0.702–0.826, p < 0.001) and radioactive implants (HR = 0.51, CI = 0.311–0.835, p = 0.007) could reduce the risk of cardiac-specific death with statistical significance. Conversely, RT based upon combination of beam with implants or isotopes (HR = 1.003, CI = 0.636–1.581, p = 0.99), as well as RT cases where the type was NOS (not otherwise specified) method (HR = 1.094, CI = 0.604–1.981, p = 0.766), could increase the risk of cardiac-specific death – albeit not with statistical significance, while beam radiation (HR = 0.885, CI = 0.706–1.109, p = 0.288) therapy alone could reduce the risk of cardiac-specific death, although again not with statistical significance (Figure 1B). The effects of RT on cardiac-specific death were consistent between the Fine-Gray and COX models of regression analysis, with the exception of beam radiation ([HR = 0.638, CI = 0.509–0.800, p < 0.001] vs. [HR = 0.885, CI = 0.706–1.109, p = 0.288]) alone (Figure 2 and Table 2).
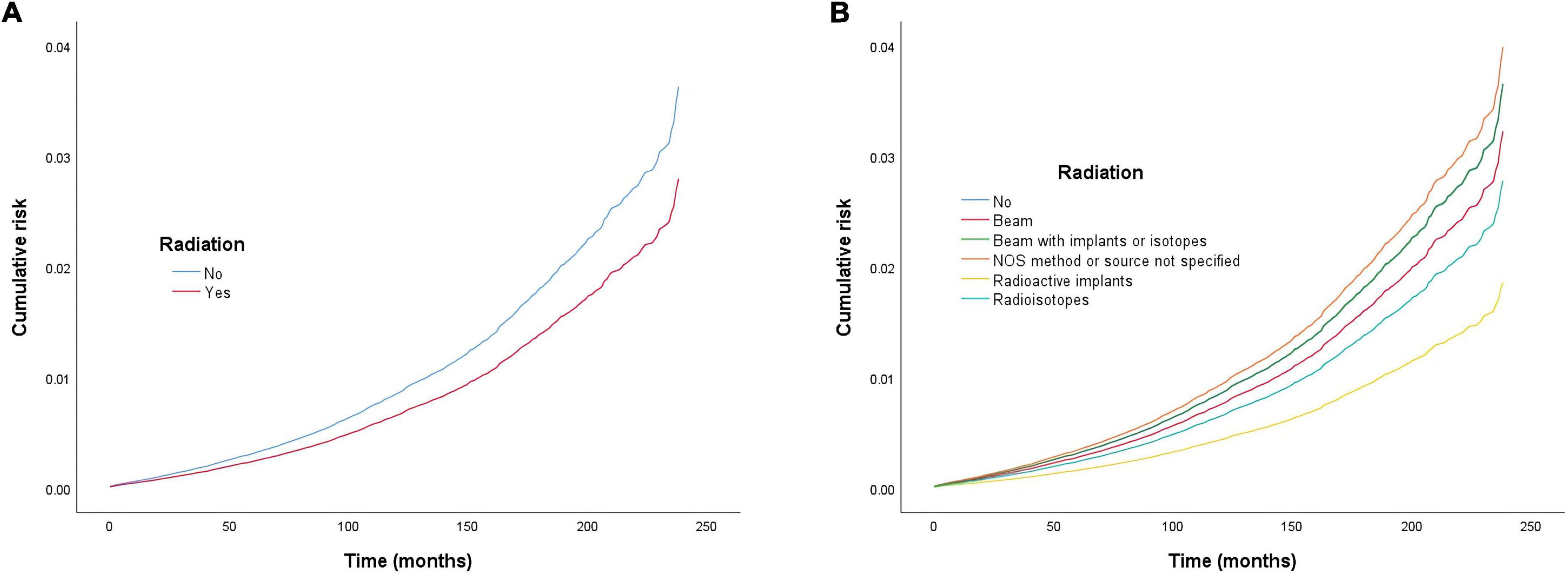
Figure 1. Cumulative risk of cardiac-specific death related to radiation therapy (RT) before propensity score matching (PSM) (the curves of “no radiation” and “beam with implants or radioisotopes” are overlapping and may not be drawn separately). The Y-axis of each panel shows the cumulative risk of cardiac-specific death and the X-axis shows the time since diagnosis in months. Each line represents the cumulative risk of cardiac-specific death in patients after receiving a treatment. (A) Cumulative risk of cardiac-specific death in patients with receiving and not receiving radiotherapy. (B) Cumulative risk of cardiacspecific death in patients with not receiving radiotherapy and receiving different modalities of radiotherapy.
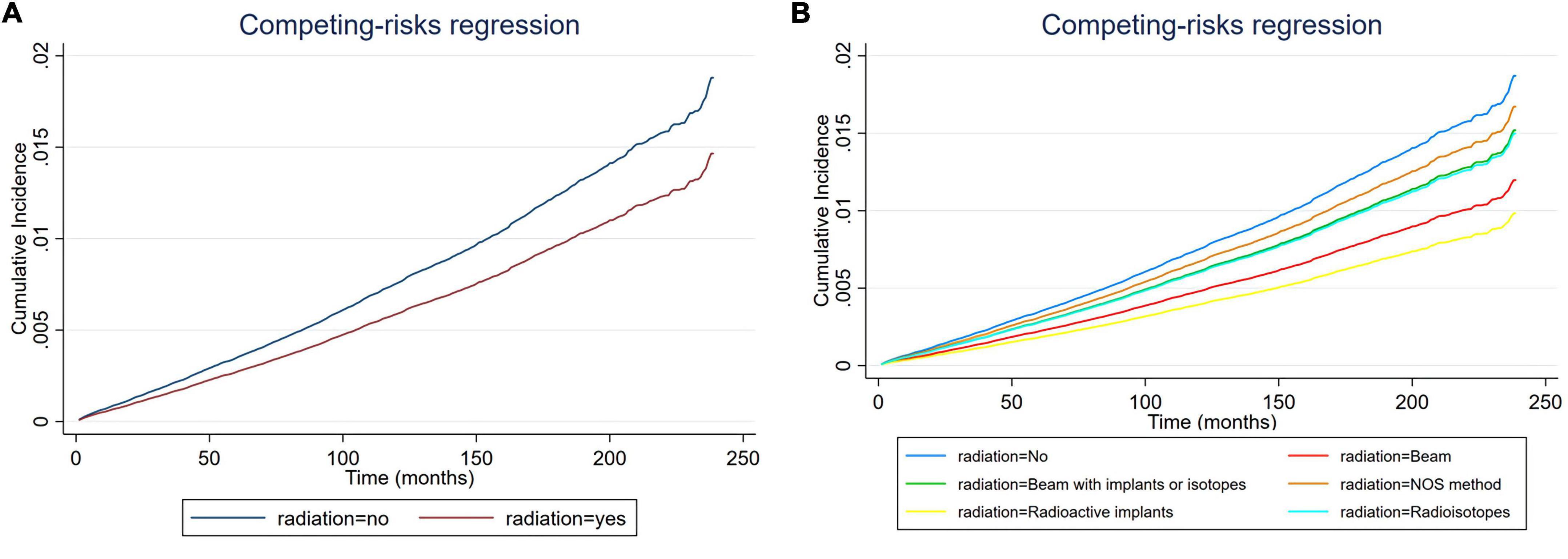
Figure 2. Cumulative incidence rate of cardiac-specific death related to radiation therapy (RT) before propensity score matching (PSM) (the curves of “radioactive isotopes” and “beam with implants or isotopes” are overlapping and may not be drawn separately). The Y-axis of each panel shows the cumulative risk of cardiac-specific death and the X-axis shows the time since diagnosis in months. Each line represents the cumulative risk of cardiac-specific death in patients after receiving a treatment. (A) Cumulative risk of cardiac-specific death in patients with receiving and not receiving radiotherapy. (B) Cumulative risk of cardiacspecific death in patients with not receiving radiotherapy and receiving different modalities of radiotherapy.
Other influencing factors for cardiac-specific death before propensity score matching
Multivariate COX model regression analysis showed that race (Asian or Pacific Islander ethnicity [HR = 0.653, CI = 0.562–0.758, p < 0.001] vs. White ethnicity), sex (female [HR = 0.482, CI = 0.448–0.520, p < 0.001] vs. male), marital status (married [HR = 0.558, CI = 0.503–0.619, p < 0.001], DSW (divorced, separated, and widowed) [HR = 0.866, CI = 0.772–0.971, p = 0.014] vs. single), combined summary stage (localized [HR = 0.707, CI = 0.606–0.825, p < 0.001], regional [HR = 0.764, CI = 0.630–0.926, p = 0.006] vs. in situ), surgery (yes [HR = 0.462, CI = 0.407–0.523, p < 0.001] vs. no), income (≥75,000 [HR = 0.662, CI = 0.496–0.885, p = 0.005] vs. ≤35,000), and histologic type (8000–8009 [HR = 0.692, CI = 0.5316–0.903, p = 0.007] vs. 8260–8269) were associated with lower risk of cardiac-specific death, while older age of diagnosis (HR = 1.097, CI = 1.094–1.101, p < 0.001), race (African ethnicity [HR = 1.4, CI = 1.237–1.585, p < 0.001] vs. White ethnicity), Origin (non-Hispanic ancestry [HR = 1.19, CI = 1.055–1.343, p = 0.005] vs. Hispanic ancestry), derived AJCC stage (IV [HR = 1.339, CI = 1.143–1.569, p < 0.001] vs. I), and histologic type (8330–8339 [HR = 1.159, CI = 1.008–1.331, p = 0.038] and 8050–8059 [HR = 1.215, CI = 1.072–1.377, p = 0.002] vs. 8260–8269) were risk factors for cardiac-specific death.
All variables tested for their impact on cardiac-specific death–with the exception of the year of diagnosis, derived AJCC stage group (II), combined summary stage (distant), histologic type (8330–8339, 8020–8029, 8010–8019)–were in agreement between the Fine-Gray and COX models of regression analysis (Table 2).
Effects of radiation therapy on cardiac-specific death after propensity score matching
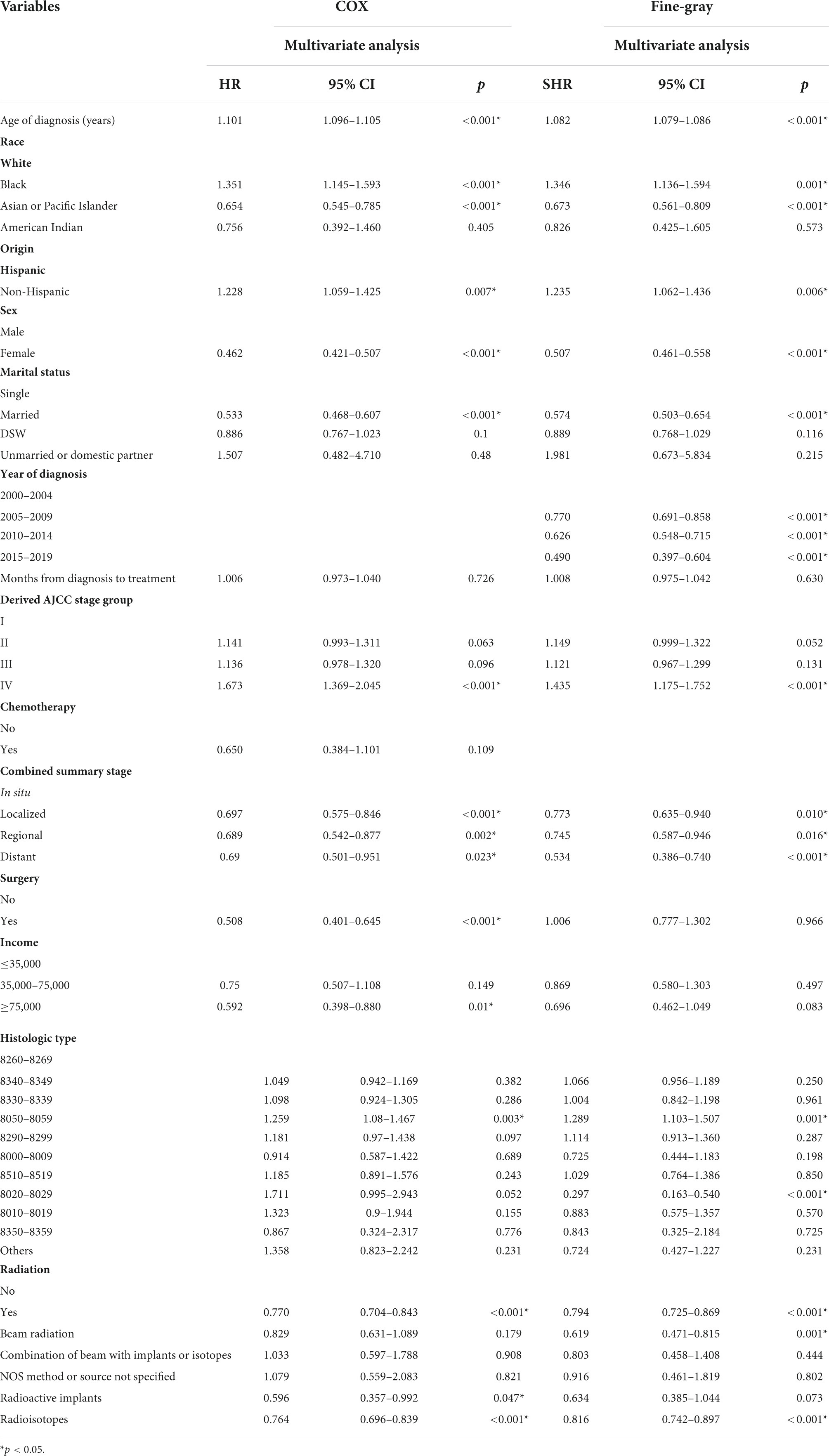
Multivariate COX model regression analysis showed that RT {yes (HR = 0.770, 95%, CI = 0.704–0.843, p < 0.001) vs. no} was associated with a lower risk of cardiac-specific death among patients with malignant thyroid tumors (Figure 3A). Results of group analysis by the types of RT received showed that RT based upon radioisotopes (HR = 0.764, CI = 0.696–0.839, p < 0.001) and radioactive implants (HR = 0.596, CI = 0.357–0.992, p = 0.047) could reduce the risk of cardiac-specific death with statistical significance. Conversely, RT based upon combination of beam with implants or isotopes (HR = 1.033, CI = 0.597–1.788, p = 0.908) therapy, as well as RT cases where the type was NOS (not otherwise specified) method (HR = 1.079, CI = 0.559–2.083, p = 0.821), could increase the risk of cardiac-specific death – albeit not with statistical significance, while beam radiation (HR = 0.829, CI = 0.631–1.089, p = 0.179) therapy alone could reduce the risk of cardiac-specific death, although again not with statistical significance (Figure 3B). The effects of RT on cardiac-specific death were consistent between the Fine-Gray and COX model of regression analysis, with the exception of beam radiation ([HR = 0.619, CI = 0.471–0.815, p = 0.001] vs. [HR = 0.829, CI = 0.631–1.089, p = 0.179]) and radioactive implants ([HR = 0.634, CI = 0.385–1.044, p = 0.073] vs. [HR = 0.596, CI = 0.357–0.992, p = 0.047]; Figure 4 and Table 3).
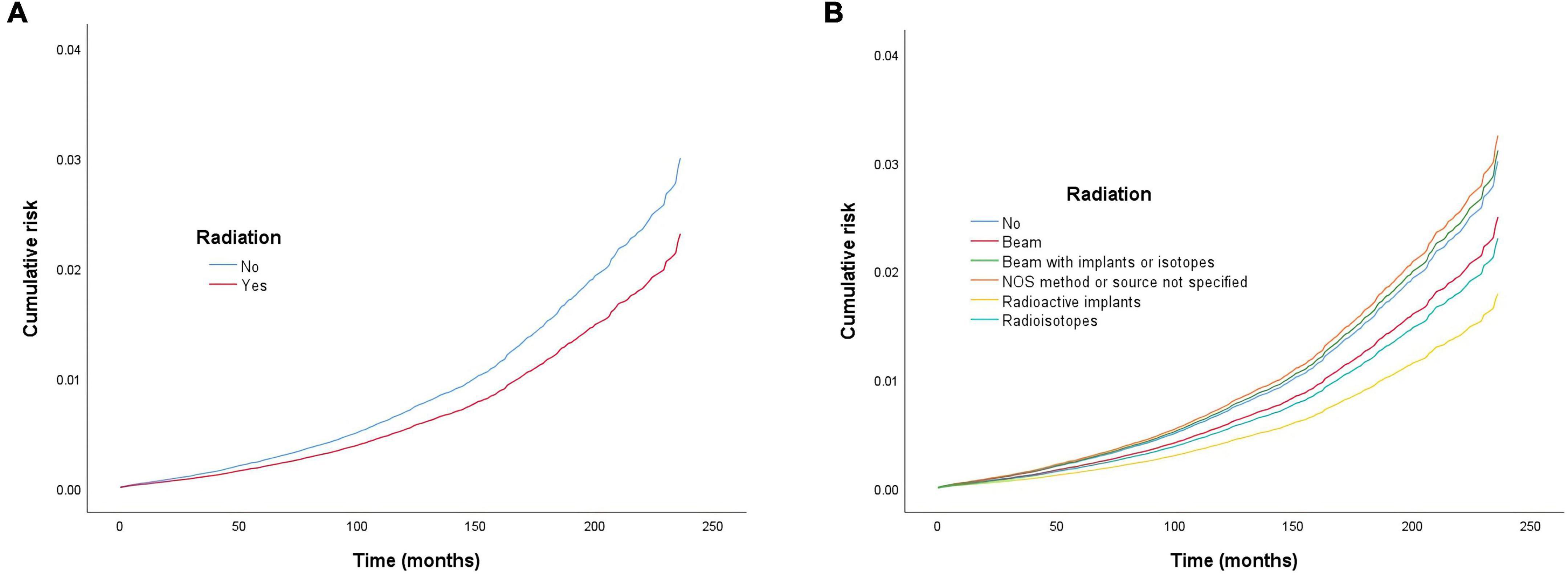
Figure 3. Cumulative risk of cardiac-specific death related to radiation therapy (RT) after propensity score matching (PSM). The Y-axis of each panel shows the cumulative risk of cardiac-specific death and the X-axis shows the time since diagnosis in months. Each line represents the cumulative risk of cardiac-specific death in patients after receiving a treatment. (A) Cumulative risk of cardiac-specific death in patients with receiving and not receiving radiotherapy. (B) Cumulative risk of cardiacspecific death in patients with not receiving radiotherapy and receiving different modalities of radiotherapy.
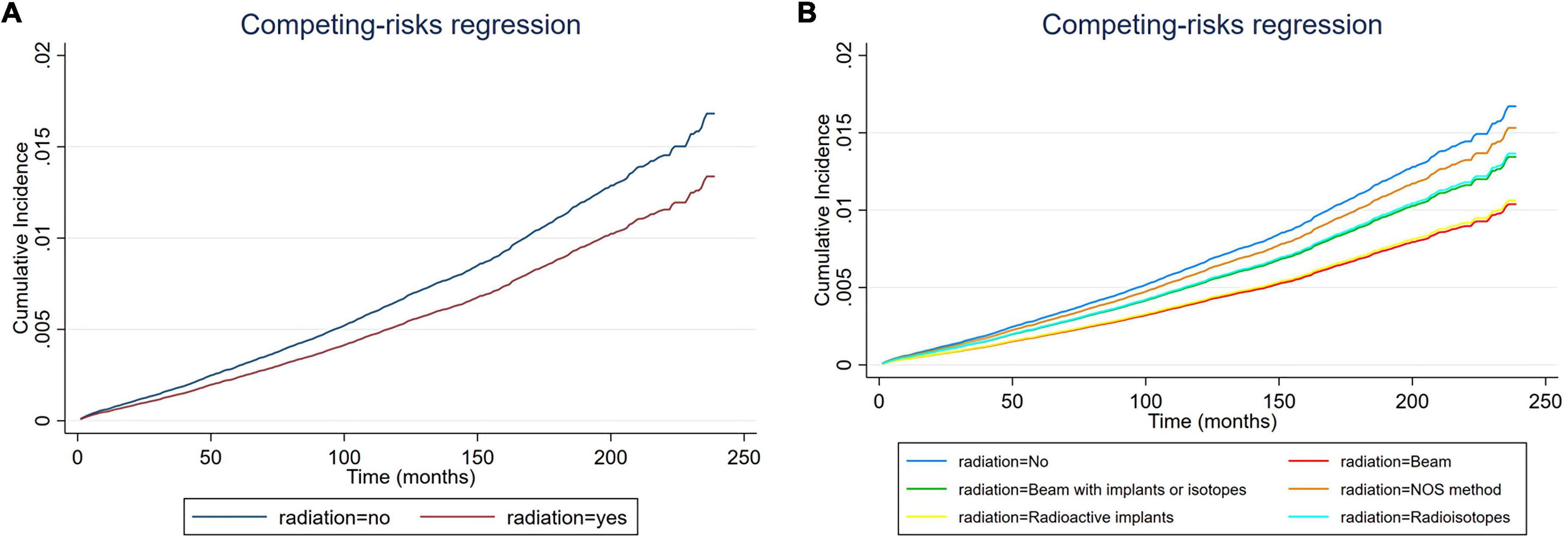
Figure 4. Cumulative incidence rate of cardiac-specific death related to radiation therapy (RT) after propensity score matching (PSM) (the curves of “radioactive isotopes” and “beam with implants or isotopes” are overlapping and may not be drawn separately, as was the curves of “beam” and “radioactive implants”). The Y-axis of each panel shows the cumulative risk of cardiac-specific death and the X-axis shows the time since diagnosis in months. Each line represents the cumulative risk of cardiac-specific death in patients after receiving a treatment. (A) Cumulative risk of cardiac-specific death in patients with receiving and not receiving radiotherapy. (B) Cumulative risk of cardiacspecific death in patients with not receiving radiotherapy and receiving different modalities of radiotherapy.
Other influencing factors for cardiac-specific death after propensity score matching
Multivariate COX model regression analysis showed that race (Asian or Pacific Islander ethnicity [HR = 0.654, CI = 0.545–0.785, p < 0.001] vs. White ethnicity), sex (female [HR = 0.462, CI = 0.421–0.507, p < 0.001] vs. male), marital status (married [HR = 0.533, CI = 0.468–0.607, p < 0.001] vs. single), combined summary stage (localized [HR = 0.697, CI = 0.575–0.846, p < 0.001], regional [HR = 0.689, CI = 0.542–0.877, p = 0.002], distant [HR = 0.690, CI = 0.501–0.951, p = 0.023] vs. in situ), surgery (yes [HR = 0.508, CI = 0.401–0.645, p < 0.001] vs. no), and income (≥75,000 [HR = 0.592, CI = 0.398–0.880, p = 0.01] vs. ≤35,000) were associated with lower risk of cardiac-specific death, while older age of diagnosis (HR = 1.101, CI = 1.096–1.105, p < 0.001), race (African ethnicity [HR = 1.351, CI = 1.145–1.593, p < 0.001] vs. White ethnicity), Origin (non-Hispanic ancestry [HR = 1.228, CI = 1.059–1.425, p = 0.007] vs. Hispanic ancestry), derived AJCC stage (IV [HR = 1.673, CI = 1.369–2.045, p < 0.001] vs. I), and histologic type (8050–8059 [HR = 1.259, CI = 1.08–1.467, p = 0.003] vs. 8260–8269) were risk factors for cardiac-specific death.
All variables tested for their impact on cardiac-specific death – with the exception of the year of diagnosis, surgery, income, histologic type (8020–8029) – were in agreement between the Fine-Gray and COX models of regression analysis (Table 3).
Discussion
Previous studies have reported that patients with thyroid cancer have no increased risk of dying from cardiovascular disease relative to the general population, but differences have been shown depending on the year, and the highest rates of heart disease-specific survival across various cancer types is observed in patients with thyroid cancer (11–13). Of course, there are also studies with opposite results showing a significant increase in cardiovascular as well as all-cause mortality in patients with thyroid cancer (14). These discrepancies may in part be due to variable patient baseline characteristics as some studies did not fully consider the effect of use or type of RT. Additionally, histological variations can also lead to different survival outcomes as the results of our study showed. Although previous studies have shown that patients with thyroid cancer may not have an increased risk of cardiovascular death as same as other cancers, the exact cause of this has not been clarified. Our study unexpectedly found that RT based upon beam radiation, radioisotopes and radioactive implant may be associated with lower risk of cardiac-specific death in patients with malignant thyroid tumors. Considering that 43.7% of patients received above three types of RT, we speculate that perhaps the low risk of cardiac-specific death in patients with thyroid cancer was associated with RT. Additionally, before PSM, the incidence of cardiac-specific death was lower in the RT group than in the non-RT group (1.2 vs. 1.8%, p < 0.001), with the incidence of cardiac death being lower in the groups of beam radiation (2.1%) and combination of beam with implants or isotopes (2.8%), NOS method or source not specified (2.0%) had higher incidences of cardiac death than the non-RT group, and the incidences of cardiac-specific death in groups receiving radioactive implants (1.1%) and radioisotopes (1.2%) were lower than that in the non-RT group (1.8%), but only the group receiving radioisotopes therapy reached statistical difference. The situation after PSM was similar to that before PSM, except for groups receiving NOS method or source not specified or combination of beam with implants or isotopes, RT based upon beam radiation, radioactive implants and radioisotopes reduced the risk of cardiac-specific death compared to non-RT. These results reflect the role of RT in decreasing the cardiac-specific deaths of patients with malignant thyroid tumors in a side-by-side manner, contradicting previous concept that RT generally increases cardiac-specific death. We know that everything has two sides, the overall effect is reflected in the offset of the positive and negative effects, and RT is no exception. RT can lead to direct toxic and negative effects on the heart, of course, it can also bring positive effects on the heart. The effect of radiation on the heart may be caused in a variety of ways, of course, the exact mechanism still needs further research. In addition, our study found some differences in the effects of different modalities of RT on the heart, after adjusting for multivariate confounding variables, i.e., RT based upon radioisotopes and radioactive implants were associated with lower risk of cardiac-specific death, beam radiation may had a similar effect, and the remaining RT methods did not significantly increase the risk of cardiac-specific death after comprehensive consideration. We think the result of radioisotopes is more credible as far more people (92.4%) receive this form of treatment than any others. In addition to effective treatment, internal RT can produce a better killing effect on cancer cells while minimizing non-targeted irradiation to surrounding healthy tissue (15).
Cancer patients have a consistently higher risk of death due to cardiovascular diseases compared to the general United States population, and this risk is inversely related to the age at diagnosis (11). Our study found that this trend also applies to patients with thyroid cancer. Previous studies have also shown that cardiovascular mortality is usually higher among patients with African ancestry, and lower among patients with Caucasian, Asian and Hispanic ancestry (16). Consistent with this, we found that Caucasian, Asian or Pacific Islander, and Hispanic ancestry were protective factors in reducing post-radiation cardiac-specific death in patients with malignant thyroid tumors, while African ancestry was a risk factor. Previous studies have shown that women have lower risk factors for most causes of death (17), a self-reported “good marital status” is a protective factor for reducing cardiovascular events, and being single, experiencing marital stress, or experiencing a divorce all increase risk of cardiovascular death (18, 19). Our results also showed that female sex and marital status were protective factors of radiation-induced cardiac-specific death in our cohort. Lower socioeconomic status may also continue to contribute to increased cancer rates and increased risk of death from cardiovascular disease in cancer survivors (20). Our study showed that higher income could reduce the risk of cardiac-specific death in patients with malignant thyroid tumors, but without statistical significance after PSM. It is possible that this may be due to bias before the PSM, as PSM could correct for some deviations in baseline characteristics of the two groups. In addition, hypofractionated or dedifferentiated cancer (histological type 8020–8029) was associated with a lower risk of cardiac-specific death in our study. Except for higher age (average age about 50 years old), patients included were predominantly Caucasian, female sex, non-Hispanic ancestry, marital status, Derived AJCC Stage Group I, non-chemotherapy, and moderate to high income, all of which were associated with a lower risk of cardiovascular mortality. Thus, the population included in this study was considered to have a lower risk of cardiovascular mortality, and the influence of confounding factors on the effect of RT action was small.
The primary differences between the results of the COX and Fine-Gray model analyses were that the statistical difference between the effect of surgery and radioactive implant treatments on cardiac-specific death were lost, Additionally, later year of diagnosis and receipt of beam radiation therapy became significant protective factors in reducing cardiac-specific death in the Fine-Gray model analysis, unlike in the COX model analysis. This may be caused by the different effects of the models, as the Fine-Gray model has the advantages of analysis and the results are more reliable in the presence of competitive death.
Limitation
Although this is the first study analyzing the effect of different types of RT on the risks of cardiac-specific death in patients with malignant thyroid tumors, it has a very severe limitation that ought to be considered. The SEER database is not allowing to evaluate any kind of clinical characteristics, like cardiovascular risk factors, type of cardiac deaths, comorbidities and so on. This limitation prevent us from analysing the results according to cardiovascular risk factors and clarifying the causes of death. Hence, our results only suggest (not demonstrating) that RT may not be dangerous or may be protective for the heart.
Conclusion
This study showed the factors influencing cardiac-specific death in patients with thyroid malignancies, and found a phenomenon whereby radioisotopes and radioactive implant therapies were associated with reduced risks of cardiac-specific death in patients with thyroid malignancy. Thus, our results may suggest additional effects of radiation on the heart that differ across different types of RT. These results lay the foundation for identification of the risk factors of cardiac-specific death among patients with thyroid malignancies after undergoing RT, and may allowing improvement of the clinical application of RT in thyroid malignancies without as much concern regarding adverse cardiac effects. Importantly, however, these findings should be confirmed by special randomized controlled trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval and patient consent were not required for this study in accordance with the local legislation and institutional requirements.
Author contributions
RW and HY contributed to the conception, design of the study, analyzed, and interpreted the data. YW and JW did the literature search and applied the inclusion and exclusion criteria. RW and LM contributed to the collection and assembly of data. RW, LW, YW, and XZ contributed to the writing of the report. All authors approved the final version of the report.
Funding
This study was supported by the Talent Introduction Funding Project of The First Affiliated Hospital of Jinan University (No. 808026) and Basic Scientific Research Project of Central University of Jinan University (No. 2162301).
Acknowledgments
Thanks to Lyu Jun from the Clinical Research Department of The First Affiliated Hospital, Jinan University, Guangzhou, China, and thanks to the Guangzhou Key Laboratory of Basic and Translational Research on Chronic Diseases, The First Affiliated Hospital, Jinan University, Guangzhou, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.996732/full#supplementary-material
References
1. Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. (2003) 13:346–56. doi: 10.1016/S1053-4296(03)00026-2
2. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ’t Veer MB, Baaijens MH, de Boer JP. Late cardiotoxicity after treatment for hodgkin lymphoma. Blood. (2007) 109:1878–86. doi: 10.1182/blood-2006-07-034405
3. de Vries S, Schaapveld M, Janus CPM, Daniëls LA, Petersen EJ, van der Maazen RWM, et al. Long-term cause-specific mortality in hodgkin lymphoma patients. J Natl Cancer Inst. (2021) 113:760–9. doi: 10.1093/jnci/djaa194
4. Senkus-Konefka E, Jassem J. Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. (2007) 33:578–93. doi: 10.1016/j.ctrv.2007.07.011
6. Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol. (2016) 40:e94–102. doi: 10.1097/pas.0000000000000749
7. Wu WT, Li YJ, Feng AZ, Li L, Huang T, Xu AD, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. (2021) 8:44. doi: 10.1186/s40779-021-00338-z
8. Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. (2020) 13:57–69. doi: 10.1111/jebm.12373
9. Guan X, Hu H, Chen W, Jiang Z, Liu Z, Zhao Z, et al. Comparison of long-term outcome between hemicolectomy and partial colectomy in the elderly: a large population-based study. Oncotarget. (2017) 8:51076–85. doi: 10.18632/oncotarget.16993
10. Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. (2015) 16:286–96. doi: 10.3348/kjr.2015.16.2.286
11. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. (2019) 40:3889–97. doi: 10.1093/eurheartj/ehz766
12. Abdel-Rahman O. Risk of cardiac death among cancer survivors in the United States: a SEER database analysis. Expert Rev Anticancer Ther. (2017) 17:873–8. doi: 10.1080/14737140.2017.1344099
13. Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. (2006) 91:313–9. doi: 10.1210/jc.2005-1322
14. Klein Hesselink EN, Klein Hesselink MS, de Bock GH, Gansevoort RT, Bakker SJ, Vredeveld EJ, et al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. J Clin Oncol. (2013) 31:4046–53. doi: 10.1200/jco.2013.49.1043
15. Pei P, Liu T, Shen W, Liu Z, Yang K. Biomaterial-mediated internal radioisotope therapy. Mater Horiz. (2021) 8:1348–66. doi: 10.1039/d0mh01761b
16. Savitz ST, Leong T, Sung SH, Lee K, Rana JS, Tabada G, et al. Contemporary reevaluation of race and ethnicity with outcomes in heart failure. J Am Heart Assoc. (2021) 10:e016601. doi: 10.1161/jaha.120.016601
17. Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health. (1984) 5:433–58. doi: 10.1146/annurev.pu.05.050184.002245
18. Orth-Gomér K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: the Stockholm female coronary risk study. JAMA. (2000) 284:3008–14. doi: 10.1001/jama.284.23.3008
19. Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB Sr., Benjamin EJ. Marital status, marital strain, and risk of coronary heart disease or total mortality: the Framingham offspring study. Psychosom Med. (2007) 69:509–13. doi: 10.1097/PSY.0b013e3180f62357
Keywords: radiation therapy, heart, thyroid malignant tumors, death of heart disease, cardiac death
Citation: Wang R, Ye H, Wang Y, Ma L, Wei J, Zhang X and Wang L (2022) Effects of different types of radiation therapy on cardiac-specific death in patients with thyroid malignancy. Front. Cardiovasc. Med. 9:996732. doi: 10.3389/fcvm.2022.996732
Received: 18 July 2022; Accepted: 27 October 2022;
Published: 10 November 2022.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Pei-Wei Shueng, Far Eastern Memorial Hospital (FEMH), TaiwanRiccardo Asteggiano, University of Insubria, Italy
Copyright © 2022 Wang, Ye, Wang, Ma, Wei, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Wang, bmQ2Njg4NjY4OEBnbWFpbC5jb20=; Xiaofang Zhang, emhhbmd4ZnR0QDE2My5jb20=
†These authors have contributed equally to this work
 Ruxin Wang
Ruxin Wang Haowen Ye
Haowen Ye Ying Wang1†
Ying Wang1† Li Ma
Li Ma Jinjing Wei
Jinjing Wei