
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 30 September 2022
Sec. Lipids in Cardiovascular Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.996467
 Le Sun1†
Le Sun1† Jia-Lin Yuan1†
Jia-Lin Yuan1† Qiu-Cen Chen1
Qiu-Cen Chen1 Wen-Kang Xiao1
Wen-Kang Xiao1 Gui-Ping Ma1
Gui-Ping Ma1 Jia-Hua Liang2
Jia-Hua Liang2 Xiao-Kun Chen1
Xiao-Kun Chen1 Song Wang3
Song Wang3 Xiao-Xiong Zhou3
Xiao-Xiong Zhou3 Hui Wu3*
Hui Wu3* Chuang-Xiong Hong3*
Chuang-Xiong Hong3*Aim: The study (PROSPERO: CRD42021240905) aims to reveal the relationships among red meat, serum lipids and inflammatory biomarkers.
Methods and results: PubMed, EMBASE and the Cochrane databases were explored through December 2021 to identify 574 studies about red meat and serum lipids markers including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), C-reactive protein (CRP) or hypersensitive-CRP (hs-CRP). Finally, 20 randomized controlled trials (RCTs) involving 1001 people were included, red meat and serum lipid markers and their relevant information was extracted. The pooled standard mean difference (SMD) was obtained by applying a random-effects model, and subgroup analyses and meta-regression were employed to explain the heterogeneity. Compared with white meat or grain diets, the gross results showed that the consumption of red meat increased serum lipid concentrations like TG (0.29 mmol/L, 95% CI 0.14, 0.44,P<0.001), but did not significantly influence the TC (0.13 mmol/L, 95% CI −0.07, 0.33, P = 0.21), LDL-C (0.11 mmol/L, 95% CI −0.23, 0.45, P = 0.53), HDL-C (−0.07 mmol/L, 95% CI −0.31, 0.17, P = 0.57),CRP or hs-CRP (0.13 mmol/L, 95% CI −0.10, 0.37,P = 0.273).
Conclusion: Our study provided evidence to the fact that red meat consumption affected serum lipids levels like TG, but almost had no effect on TC, LDL-C, HDL-C and CRP or hs-CRP. Such diets with red meat should be taken seriously to avoid the problem of high lipid profiles.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPERO], identifier [CRD42021240905].
Red meat includes edible animal muscle from cows, pigs, and sheep, and it is a favorite food for most people worldwide (1, 2). In recent years, some groups have urged people to consume plant-derived foods rather than animal-derived foods (3). Red meat is considered as a kind of high-quality protein with many other beneficial nutrients, such as fatty acids, vitamins, minerals and molecules mediating various cellular responses (1, 4, 5). However, excessive intake of red meat also gives rise to abnormalities in lipid metabolism, inflammatory reactions and possibly chronic diseases (6). Serum total cholesterol levels change if there is excessive consumption of cholesterol and saturated fats, and high levels of serum cholesterol accumulates in macrophages and then activates the NLRP3 inflammasome through the NF-κB signaling pathway (6, 7).
On the other hand, dyslipidaemia is becoming a concern worldwide, and it has been proven to be a major risk factor for cardiovascular and metabolic diseases and the underlying cause of stroke and other life-threatening diseases (8–10). In recent years, chronic inflammation has been proven to be the trigger of abnormal lipid metabolism (11). Oxidative stress triggers inflammation, and a study on the consumption of red meat concluded that red meat could give rise to changes in oxidative stress and further induce inflammation and related diseases (12, 13). In addition, red meat is the major source of serum iron, especially for the meats with high myoglobin content (14). However, excessive intake of iron ions in human body may trigger oxidative stress and aggravate inflammatory reaction (2) (Figure 1).
Lipoproteins in the blood like low-density lipoprotein cholesterol (LDL-C) can enter the arterial intima from the circulation, and the accumulation of lipoproteins in the arterial intima can trigger inflammation and induce pathological changes that threaten people’s lives and health (15–17). In contrast to lipoproteins, oxidized lipids (ox-LDL) are considered to have a much stronger influence on inflammation; ox-LDL can not only be synthesized endogenously but can also be obtained through the diets (18). Therefore, inhibiting proinflammatory cytokines has emerged as a novel promising mode of therapy to improve and complement the current lipid-lowering approaches (7).
Some studies, especially those supporting the US Dietary Guidelines for Americans, demonstrated that daily consumption of red and processed meat might increase the risk of coronary heart disease (CHD) (19). A proposal in emphasized a transformation trend to a daily diet that consisted mainly of plant-derived foods (20). Similarly, a study from Boston conducted a follow-up with 1,023,872 people, comparing the effect of red meat with other dietary components, such as legumes and grain. The results showed that a greater intake of red meat was positively correlated with a relatively higher risk of CHD (21).
However, recent studies hold the opposite view: a large prospective study conducted by The Netherlands Cohort Study (NLCS) found that red meat intake does not increase the risk of cardiovascular and respiratory mortality (22). Another article published in the Annals of Internal Medicine found that there is not enough scientific evidence to establish a link between the intake of red meat and cardiometabolic diseases (23).
Therefore, our study aimed to provide relevant evidence about the effects of the consumption of red meat on serum lipid levels and inflammatory markers.
This systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42021240905).
We conducted the systematic review and meta-analysis through exploring studies on
databases and there were no additional patients or public involvements needed, all inclusion criteria were consistent with the original study.
Literature searches were conducted in three databases: PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (through 14 December 2021). Two authors (Y.J.L. and X.W.K.) independently searched the databases by using standardized terms without year and language restrictions, including: Group 1) “red meat,” “red meats,” “beef,” “pork,” “lamb”; Group 2) “randomized controlled trial,” “randomized,” “placebo”; Group 3) keywords for lipid-related markers: Adiponectin, Adipocyte Complement-Related Protein 30 kDa, Adipocyte Complement Related Protein 30 kDa, Adipose Most Abundant Gene Transcript 1, apM-1 Protein, apM 1 Protein, ACRP30 Protein, Adipokynes, Adipocyte, Cytokines, IL-1β, IL-6, TNF-α, CRP, c-Reactive protein, Interleukin, Triacylglycerol, Triacylglycerols, Triglyceride, Triglycerides, Dyslipidaemia, Dyslipoproteinemias, Dyslipoproteinemia, Blood lipid, HDL lipoproteins, High density lipoprotein, Lipoprotein, Lipoproteins, High density lipoproteins, Alpha-lipoproteins, Alpha-lipoprotein, Heavy lipoproteins, Alpha-1 lipoprotein, HDL, Low density lipoprotein cholesterol, Low density lipoprotein, Low density lipoproteins, Low-density lipoprotein, Beta-lipoprotein cholesterol, Cholesterol, Beta lipoprotein, Beta-lipoproteins, Beta lipoproteins, Beta lipoprotein cholesterol, LDL lipoproteins, LDL cholesterol, Cholesteryl linoleate, LDL, LDL cholesteryl linoleate, LDL. Each database was searched using keywords in Group 1 combined with the terms in Groups 2 and 3. Then, inappropriate articles were excluded by manual screening.
Articles were included if they met the following criteria: (1) Randomized controlled trial (RCT) including parallel or crossover designs; (2) people recruited met the age restriction ≥ 18 years; (3) the intervention in one group was red meat, including beef, pork, lamb and mutton, and the other group was given non-red meat, including chicken, fish, soy, etc.; (4) the outcomes included at least one of the lipid parameters (LDL-C, HDL-C, TC, and TG); (5) mean and standard deviation (SD) were provided. The exclusion criteria were as follows: (1) recruited subjects were children, or the pregnant women; (2) the intervention had other programs which may influence the serum lipids levels, like walking or exercise training, etc.; (3) unclear habitual diet; (4) all participants are postmenopausal women.
Our team included 7 investigators guided by H.C.X, and two authors (Y.J.L. and X.W.K.) first conducted the study inclusion process by independently reading the titles and abstracts. If there were any discrepancies, the other authors (S.L. and L.J.H) were consulted. We identified 574 relevant studies on this topic, and all of the included articles had their relative characteristics extracted, including the first author’s name, publication year, country, population size, gender ratio, health condition, mean BMI or body weight, mean age and study design, intervention meat, control alternatives, study duration, and change before and after the intervention of the serum lipids and inflammation index, such as total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), C-reactive protein (CRP) and hypersensitive-CRP (hs-CRP).
Risk of bias was assessed by two authors (L.J.H. and M.G.P.) with the Cochrane risk-of-bias tool (RoB2), which considers the statistical analyses including the randomization method, allocation scheme concealment, blinding method, outcome data integrity, selective research results, other bias sources and the overall bias.
For the parallel or crossover trial design studies, we included the preintervention data and the final overall data, including means and standard deviations. For the analysis, all of the studies generally could be considered parallel designs of the respective groups, and if there were more than one intervention group or control group, we tended to adopt the data from the red meat groups and non-red meat alternative groups to analyze the differences between them (24, 25). The pooled standard mean difference (SMD) was obtained by meta-analyses of binary and continuous meta functions with a random-effects model after checking the heterogeneity. In terms of the heterogeneity among the studies, we used the I2 and Q statistics (26, 27). For the Q statistics, P<0.10 showed significant heterogeneity, and I2 values of 25%, 25-50%, 50-70%, and ¿75% were classified as indicating no, small, moderate, and significant heterogeneity, respectively. Moreover, we performed subgroup analysis by using the publication year, country, population size, gender, health condition, mean BMI or body weight, mean age and study design, intervention meat, control alternatives, and study duration to explore any heterogeneity.
We also performed meta-regression to examine the effect of potential factors on the serum TC concentration, and to assess the potential publication bias, we used Egger’s linear regression test. Sensitivity analyses were carried out by excluding each study one by one and re-analyzing the data. All statistical analyses were performed with STATA 13.0 (Stata Corp.).
We searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials and initially found 574 studies on our research objective and first eliminated 210 duplicated studies. Then, by reading the abstracts and titles, we preliminarily excluded 244 articles. Next, we read the full text to obtain detailed information and excluded 100 articles. Finally, we included 20 studies involving 1001 people about the consumption of red meat on blood lipids (Figure 2).
The research characteristics of the 20 RCTs are presented in Tables 1–4. The studies contained relatively few participants apart from 3 studies with more than 100 participants each (29, 41, 44). The pooled data showed that all of the studies were randomized, and there were 3 studies conforming to the parallel group design (33, 41, 45). The others were crossover studies (n = 17). The publication years were from 1980 to 2019, with 8 articles conducted in North America, including Canada (n = 1) (28),USA (n = 3) (29, 42, 44), Houston (n = 1) (33), Texas (n = 1) (34), Quebec (n = 1) (40), Chicago (n = 1) (41), and the others were carried out in Germany (n = 1) (39), Iran (n = 2) (45, 46), Australia (n = 3) (31, 32, 47), and South Africa (n = 2) (30, 43) and Columbia (n = 2) (35, 36), Brazil (n = 1) (38). Most of the studies included both men and women (n = 15), except for 4 studies that included only men (28, 32–34) and 1 study only for women (37). The mean age of all participants was 22 to 59. The control group in 13 articles included white meat and in 7 articles it was legume or dairy products. The intervention duration was < 10 wk in 16 studies and ≥ 10 wk in 4 studies.
We conducted a quality evaluation (risk of bias) with the Cochrane risk-of-bias tool (RoB2) (Table 5). We found that all of the studies were randomized; however, only 4 studies specifically described the allocation sequence method and the allocation concealment plan. The others did not mention it. Most of the studies did not follow blinding principles, except 1 study that adopted a triple-blind design. Outcome assessors in 3 studies were not aware of the intervention assignment, and they were considered to have a low risk of bias for blinding. There were no articles with conditions such as incomplete outcomes or selective reporting, so all of the studies were considered to have a low risk of bias, and none of the studies were found to have a high risk of bias.
We ultimately included 17 articles on red meat consumption and serum TG levels (Figure 3), and the combined results showed that TG levels increased by approximately 0.29 mmol/L (SMD 0.29 mmol/L, 95% CI 0.14 to 0.44; P<0.001). The final results from 19 studies showed that red meat based diets might have no significant effects on the serum TC concentrations (SMD 0.13 mmol/L, 95% CI -0.07 to 0.33; P = 0.21) (Figure 4), HDL-C concentrations (SMD -0.07 mmol/L, 95% CI -0.31 to 0.17; P = 0.57) (Figure 5). Similarly, the overall data from 14 studies showed that red meat diets did not affect the serum LDL-C concentrations (SMD 0.11 mmol/L, 95% CI −0.23 to 0.45; P = 0.53) (Figure 6). The influence of red meat on the serum relative inflammatory index such as CRP or hs-CRP was reported by 4 studies, and it might be increased by approximately 0.13 mmol/L (95% CI −0.10 to 0.37; P = 0.273) (Figure 7), which was not statistically significant.
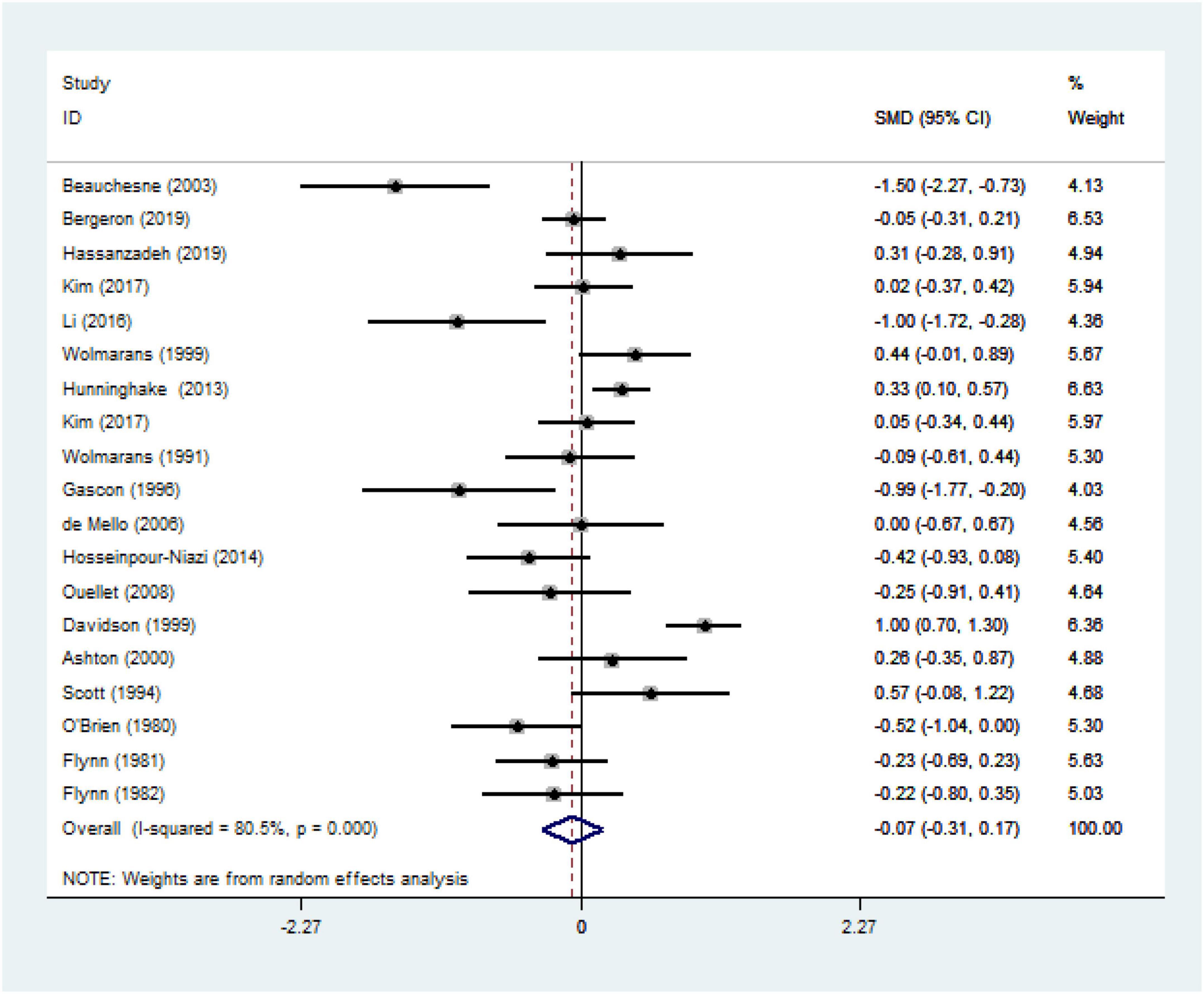
Figure 5. Effect of red meat consumption on HDL-C concentration. HDL-C, high-density lipoprotein cholesterol.
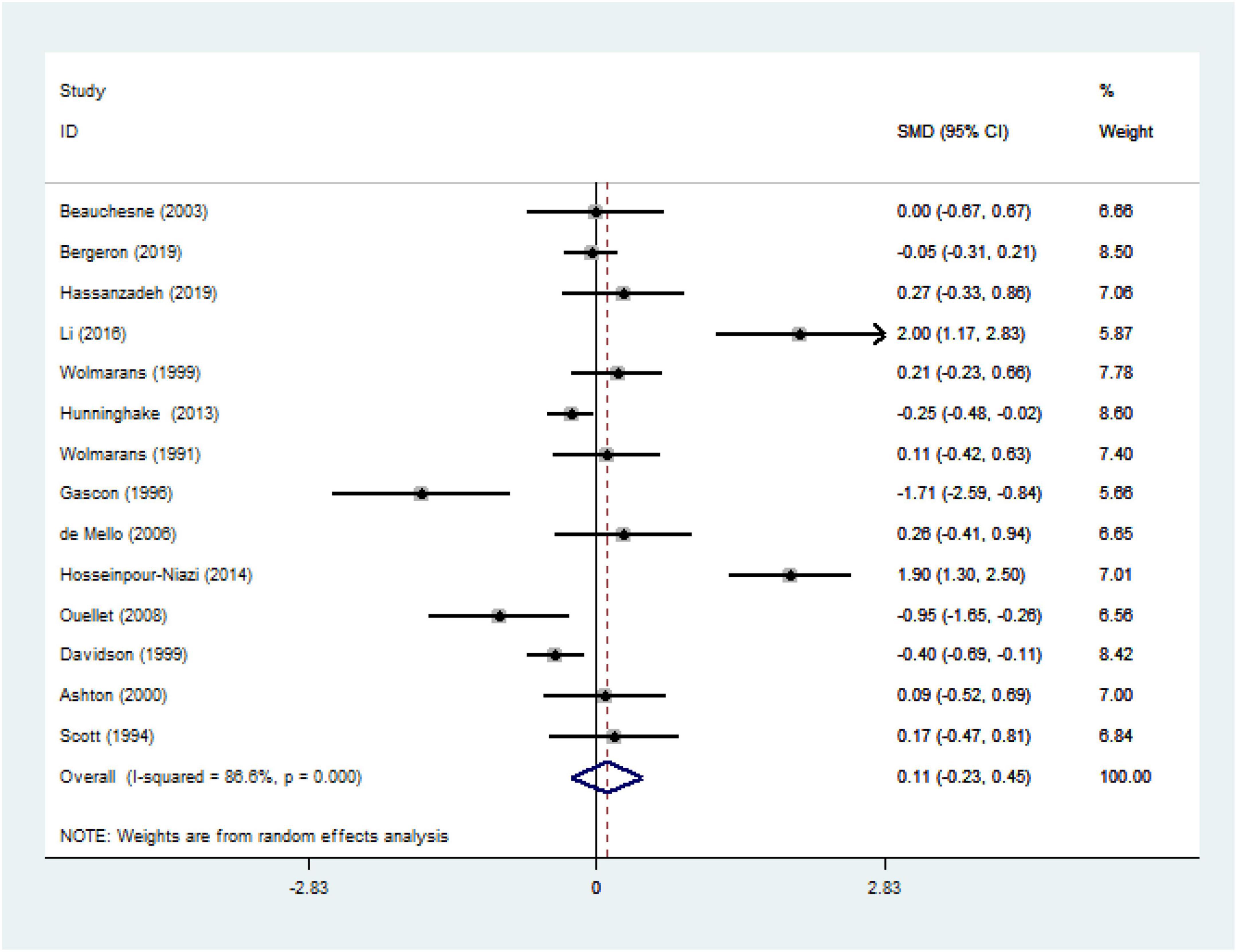
Figure 6. Effect of red meat consumption on LDL-C concentration. LDL-C, low-density lipoprotein cholesterol.
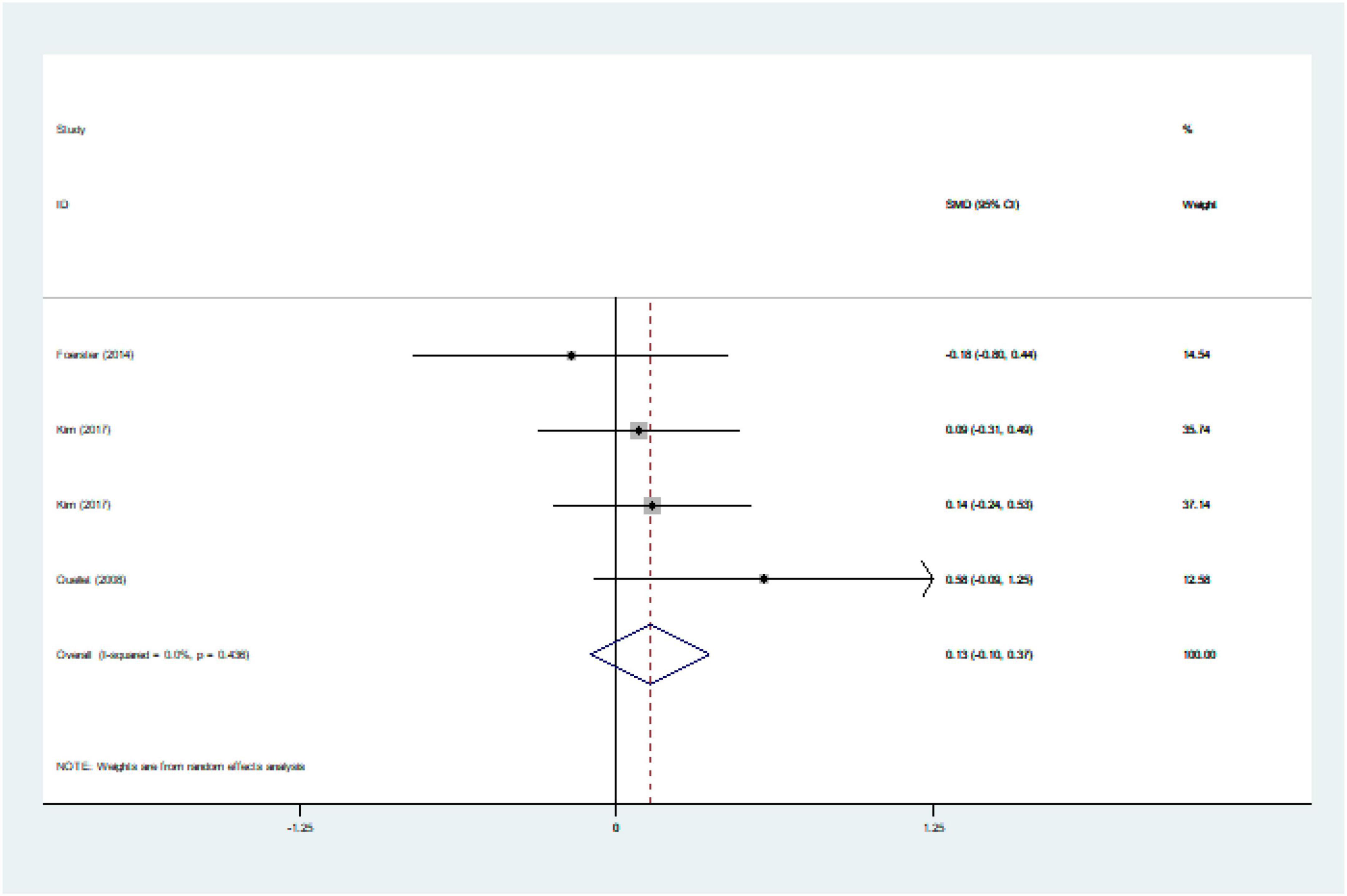
Figure 7. Effect of red meat consumption on CRP or hs-CRP concentration. CRP, C-reactive protein; hs-CRP, hypersensitive-CRP.
Regarding the effect of red meat on serum LDL-C, TC, TG, HDL-C, the subgroup analyses revealed that there were no reasonable subgroups to explain the moderate or high heterogeneity. We tried to explain the heterogeneity by analyzing the years, countries, number of participants, gender, BMI, age, study design, control group, and treatment period. Nevertheless, the outcome ultimately had unexplained moderate heterogeneity or relatively large differences (Tables 6, 7).
Meta-regression demonstrated that country might be a potential factor causing heterogeneity regarding to the TG levels (meta-regression P = 0.044). Unfortunately, meta-regression could not give a reasonable explanation of the results about the effect of red meat on the serum LDL-C, HDL-C, TC level when considering factors such as publication year, country, population size, gender, mean BMI or body weight, mean age and study design, intervention meat, control alternatives, and study duration.
Sensitivity analysis indicated that the gross results of the red meat on serum lipids (TC, TG, LDL-C, HDL-C) and inflammation index (CRP or hs-CRP) were not changed by the elimination of any one study: TC (SMD changed between −0.07 and 0.33), TG (SMD changed between 0.14 and 0.44), HDL-C (SMD changed between −0.31 and 0.17), and LDL-C (SMD changed between -0.23 and 0.45), CRP (SMD changed between −0.10 and 0.37).
We also evaluated publication bias through Egger’s linear regression test, and the results showed that there was no bias for TC (P = 0.443), LDL-C (P = 0.255),CRP (P = 0.772), but there was for TG (P = 0.045), or HDL-C (P = 0.015).
This meta-analysis explored the effects of red meat on serum lipid levels and inflammatory biomarkers. Our team included 20 RCTs published between 1980 and 2019. The analysis ultimately revealed that red meat consumption increased serum lipid concentrations like TG, and had no significant effects on TC, LDL-C, HDL-C, CRP, and hs-CRP.
Previous findings from a meta-analysis that included 1,803 participants in randomized controlled trials revealed that there were no significant differences among red meat, fish and low-quality carbohydrates in terms of their effects on blood lipids (48). However, it might have the potential impact on the final results because there were red meat in the comparison diets in several researches. In addition, another meta-analysis suggested that red meat, compared with non-red meat such as poultry or fish, was not necessarily correlated with increases in serum lipids; more precisely, ≥ 0.5 servings had no effect on serum lipid concentration (49). However, our research conducted subgroup analyses and the results showed that the blood lipids (TC, TG, LDL-C, HDL-C) had no direct relationship with the publication year, country, population size, gender, mean age, study design, intervention meat, control alternatives, or study duration. The only finding was that the consumption of red meat had a greater impact on the TG.
Disorders of lipid metabolism and obesity can induce higher secretion of interleukin-1β, and CRP or hs-CRP can reflect the upstream activity of inflammatory cytokines (50, 51). Meanwhile, studies have revealed that maintaining a low level of serum CRP is as important as maintaining a low serum LDL cholesterol, and statins have both anti-inflammatory and lipid-reducing functions (52–54). Elevated serum LDL cholesterol has been proven to promote the progression of coronary atherosclerotic plaques (55). They are easily oxidized under oxidative stress and turn into oxidized low-density lipoprotein (OX-LDL), which works as a damage signal in the progression of pathological conditions (56). Subsequently, macrophages release many inflammatory factors that interact with the human immune system (57–59). Overaccumulation of triglycerides in white adipose tissue will cause the release of inflammatory cytokines and has the risk of triggering systemic metabolic disease (60). In fact, medium-chain saturated fats in red meat are more likely to increase serum HDL cholesterol (16, 17). Excessive consumption of long-chain fatty acids in red meat can induce endoplasmic reticulum (ER) stress, and oxidative stress is upstream of vascular inflammation and relative dysfunction (16, 61–64).
Daily red meat consumption is often accompanied by an increased intake of NaCl, an essential nutrient for human health, which is crucial to cell homeostasis and body metabolism; however, excessive intake of NaCl can release reactive oxygen species (ROS) and have an impact on lipid metabolism, endothelial cell damage and atherosclerosis (65–67). Red meat contains more carnitine than other alternatives, and it is a metabolic precursor of trimethylamine N-oxide (TMAO), which inhibits the process of reversing cholesterol and triggers coronary artery inflammation (68–70). Carnitine is digested by the carnitine oxygenase enzyme derived from the gut microbiota into trimethylamine (TMA), which is transformed by the liver into TMAO (71). Researchers have shown that higher serum levels of TMAO after the consumption of red meat only decrease after several weeks (72).
It was proved that the nutraceuticals in daily diets could lower serum lipid levels with the help of the beneficial compounds (73). Carotenoids and resveratrol, which mainly exist in the fruits, vegetables diets and Mediterranean foods, are able to work as anti-inflammatory molecules in the management of lipid disorders to prevent cardiovascular diseases (74, 75). Proanthocyanidins are also proved to reduce the triacylglycerol concentration in the blood (76). Similarly, Water-insoluble fish proteins (IFP) is beneficial for dyslipidaemia treatment through lowering serum cholesterol (77). Fish oil are demonstrated to be rich in unsaturated fatty acids which are good for reducing triacylglycerol levels (78).
Our research not only extracted data on serum lipids but also paid attention to the relative inflammatory index. Inflammation is a potential risk factor for various chronic diseases and related basic causes (8–10). This review collected relevant inflammatory indicators to explore the potential impact of inflammation on blood lipids. In addition, all of the articles included in this study were RCTs with a high level of evidence. Moreover, our research performed subgroup analyses and meta-regression to verify the potential link between possible factors and blood lipids regarding the consumption of red meat. The outcome of the meta-regression indicated that country might be a potential factor to give rise to heterogeneity with regard to TG levels. Regarding the various diet habits in the different areas and differences among studies, we are supposed to further analyze the heterogeneity and be cautious about this outcome. Sensitivity analysis indicated that the gross results did not change with the elimination of any one study. Publication bias was assessed through Egger’s linear regression test. Considering that there were not enough relevant articles were included, we consider that the publication bias is related to the number of articles, and we advise caution about the results. This review could provide a useful reference for clinical treatment and disease prevention
However, our study had the following limitations. Notably, there was no deny that there was a higher heterogeneity involved in our study and we applied a random-effects model for statistical analyses, subgroup analyses and meta-regression were adopted to explain the heterogeneity. Meta-regression revealed that different countries might be the potential factors to induce the heterogeneity regarding to the TG levels. However, there were no reasonable subgroups to explain the moderate or high heterogeneity for serum lipids (TC, TG, LDL-C, HDL-C) and Egger’s linear regression test also showed the publication bias for TGs and HDL-C. Undeniably, the limited articles included might be the potential risk factors. Meanwhile, further large-scale researches should be explored in the future and we might be cautious about the results.
In addition, eating habits and lifestyle are crucial to health (4, 5, 79). We lacked data about the quantity of red meat and the proportion of energy obtained from protein and ignored daily habits. Moreover, due to different personal habits and hobbies, the studies could not be double-blinded, possibly causing bias. Different countries and regions had different ways of cooking food; these different ways and cooking oils might have potential effects on lipids, and we could not analyse these effects nor could we analyze different food additives (5, 80, 81). Therefore, future studies should include various processing methods and additives. A larger sample size is also necessary.
In conclusion, the pooled results of our meta-analysis showed that the consumption of red meat might increase the serum lipid concentrations, especially for TG concentration,. but had a little affect on TC, LDL-C, HDL-C and CRP or hs-CRP Therefore, considering the effect of red meat on blood lipids, we hold a negative opinion about eating red meat, especially for people with a higher TG concentration. In addition, future studies will advocate larger number of participants, clarify the quantities, cooking methods, in order to ensure the safety of red meat on lipid profiles.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
LS first proposed the suggestion under the guidance of C-XH. J-LY and W-KX were responsible for conducting the search, screening articles, and extracting the data. J-HL and G-PM assessed the quality of the articles. LS, X-KC, SW, and X-XZ performed the statistical analysis. LS wrote the article. Q-CC and HW were responsible for the final revision. C-XH was the guarantor of the entire content. All authors reviewed and agreed with the content of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wyness L. The role of red meat in the diet: nutrition and health benefits. Proc Nutr Soc. (2016) 75:227–32. doi: 10.1017/S0029665115004267
2. Wolk A. Potential health hazards of eating red meat. J Intern Med. (2017) 281:106–22. doi: 10.1111/joim.12543
3. Gonzalez N, Marques M, Nadal M, Domingo JL. Meat consumption: which are the current global risks? A review of recent (2010-2020) evidences. Food Res Int. (2020) 137:109341. doi: 10.1016/j.foodres.2020.109341
4. Suleman R, Wang Z, Aadil RM, Hui T, Hopkins DL, Zhang D. Effect of cooking on the nutritive quality, sensory properties and safety of lamb meat: current challenges and future prospects. Meat Sci. (2020) 167:108172. doi: 10.1016/j.meatsci.2020.108172
5. Delgado J, Ansorena D, Van Hecke T, Astiasaran I, De Smet S, Estevez M. Meat lipids, NaCl and carnitine: do they unveil the conundrum of the association between red and processed meat intake and cardiovascular diseases?_Invited review. Meat Sci. (2021) 171:108278. doi: 10.1016/j.meatsci.2020.108278
6. Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. (2015) 15:104–16. doi: 10.1038/nri3793
7. Rovio SP, Salo H, Niinikoski H, Lagstrom H, Salo P, Viikari JSA, et al. Dietary intervention in infancy and cognitive function in young adulthood: the special turku coronary risk factor intervention project. J Pediatr. (2022) 246:184–90.e1. doi: 10.1016/j.jpeds.2022.03.046
8. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. (2021) 18:689–700. doi: 10.1038/s41569-021-00541-4
9. Ronsein GE, Vaisar T. Inflammation, remodeling, and other factors affecting HDL cholesterol efflux. Curr Opin Lipidol. (2017) 28:52–9. doi: 10.1097/MOL.0000000000000382
10. Pirro M, Bianconi V, Paciullo F, Mannarino MR, Bagaglia F, Sahebkar A. Lipoprotein(a) and inflammation: a dangerous duet leading to endothelial loss of integrity. Pharmacol Res. (2017) 119:178–87. doi: 10.1016/j.phrs.2017.02.001
11. Back M, Yurdagul A Jr., Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. (2019) 16:389–406. doi: 10.1038/s41569-019-0169-2
12. Leroy F, Cofnas N. Should dietary guidelines recommend low red meat intake? Crit Rev Food Sci Nutr. (2020) 60:2763–72. doi: 10.1080/10408398.2019.1657063
13. Turner KM, Keogh JB, Meikle PJ, Clifton PM. Changes in lipids and inflammatory markers after consuming diets high in red meat or dairy for four weeks. Nutrients. (2017) 9:886. doi: 10.3390/nu9080886
14. Juarez M, Lam S, Bohrer BM, Dugan MER, Vahmani P, Aalhus J, et al. Enhancing the nutritional value of red meat through genetic and feeding strategies. Foods. (2021) 10:872. doi: 10.3390/foods10040872
15. Gidding SS, Allen NB. Cholesterol and atherosclerotic cardiovascular disease: a lifelong problem. J Am Heart Assoc. (2019) 8:e012924. doi: 10.1161/JAHA.119.012924
16. De Smet S, Vossen E. Meat: the balance between nutrition and health. A review. Meat Sci. (2016) 120:145–56. doi: 10.1016/j.meatsci.2016.04.008
17. Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. (2010) 91:46–63. doi: 10.3945/ajcn.2009.27661
18. Moss JW, Ramji DP. Cytokines: roles in atherosclerosis disease progression and potential therapeutic targets. Future Med Chem. (2016) 8:1317–30. doi: 10.4155/fmc-2016-0072
19. U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. Washington, DC: U.S. Department of Health and Human Services (2015).
20. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the anthropocene: the EAT–lancet commission on healthy diets from sustainable food systems. Lancet. (2019) 393:447–92. doi: 10.1016/s0140-6736(18)31788-4
21. Al-Shaar L, Satija A, Wang DD, Rimm EB, Smith-Warner SA, Stampfer MJ, et al. Red meat intake and risk of coronary heart disease among US men: prospective cohort study. BMJ. (2020) 371:m4141. doi: 10.1136/bmj.m4141
22. van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands cohort study. Eur J Epidemiol. (2019) 34:351–69. doi: 10.1007/s10654-019-00483-9
23. Johnston BC, Zeraatkar D, Han MA, Vernooij RWM, Valli C, El Dib R, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) consortium. Ann Intern Med. (2019) 171:756–64. doi: 10.7326/M19-1621
24. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
25. Katz DL, Gnanaraj J, Treu JA, Ma Y, Kavak Y, Njike VY. Effects of egg ingestion on endothelial function in adults with coronary artery disease: a randomized, controlled, crossover trial. Am Heart J. (2015) 169:162–9. doi: 10.1016/j.ahj.2014.10.001
26. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
27. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. (2011) 11:41. doi: 10.1186/1471-2288-11-41
28. Beauchesne-Rondeau E, Gascon A, Bergeron J, Jacques H. Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. Am J Clin Nutr. (2003) 2003:587–93. doi: 10.1093/ajcn/77.3.587
29. Bergeron N, Chiu S, Williams PT, M King S, Krauss RM. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: a randomized controlled trial. Am J Clin Nutr. (2019) 110:24–33. doi: 10.1093/ajcn/nqz035
30. Wolmarans P, Benadé AJ, Kotze TJ, Daubitzer AK, Marais MP, Laubscher R. Plasma lipoprotein response to substituting fish for red meat in the diet. Am J Clin Nutr. (1991) 53:1171–6. doi: 10.1093/ajcn/53.5.1171
31. Kim Y, Keogh JB, Clifton PM. Effects of two different dietary patterns on inflammatory markers, advanced glycation end products and lipids in subjects without type 2 diabetes: a randomised crossover study. Nutrients. (2017) 9:336. doi: 10.3390/nu9040336
32. Ashton E, Ball M. Effects of soy as tofu vs meat on lipoprotein concentrations. Eur J Clin Nutr. (2000) 54:14–9. doi: 10.1038/sj.ejcn.1600885
33. Scott LW, Dunn JK, Pownall HJ, Brauchi DJ, McMann MC, Herd JA, et al. Effects of beef and chicken consumption on plasma lipid levels in hypercholesterolemic men. Arch Intern Med. (1994) 154:1261–7.
34. O’Brien BC, Reiser R. Human plasma lipid responses to red meat, poultry, fish, and eggs. Am J Clin Nutr. (1980) 33:2573–80. doi: 10.1093/ajcn/33.12.2573
35. Flynn MA, Heine B, Nolph GB, Naumann HD, Parisi E, Ball D, et al. Serum lipids in humans fed diets containing beef or fish and poultry. Am J Clin Nutr. (1981) 34:2734–41. doi: 10.1093/ajcn/34.12.2734
36. Flynn MA, Naumann HD, Nolph GB, Krause G, Ellersieck M. Dietary “meats” and serum lipids. Am J Clin Nutr. (1982) 35:935–42. doi: 10.1093/ajcn/35.5.935
37. Gascon A, Jacques H, Moorjani S, Deshaies Y, Brun LD, Julien P. Plasma lipoprotein profile and lipolytic activities in response to the substitution of lean white fish for other animal protein sources in premenopausal women. Am J Clin Nutr. (1996) 63:315–21. doi: 10.1093/ajcn/63.3.315
38. de Mello VD, Zelmanovitz T, Perassolo MS, Azevedo MJ, Gross JL. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr. (2006) 83:1032–8. doi: 10.1093/ajcn/83.5.1032
39. Foerster J, Maskarinec G, Reichardt N, Tett A, Narbad A, Blaut M, et al. The influence of whole grain products and red meat on intestinal microbiota composition in normal weight adults: a randomized crossover intervention trial. PLoS One. (2014) 9:e109606. doi: 10.1371/journal.pone.0109606
40. Ouellet V, Weisnagel SJ, Marois J, Bergeron J, Julien P, Gougeon R, et al. Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J Nutr. (2008) 138:2386–91. doi: 10.3945/jn.108.092346
41. Davidson MH, Hunninghake D, Maki KC, Kwiterovich PO Jr., Kafonek S. Comparison of the effects of lean red meat vs lean white meat on serum lipid levels among free-living persons with hypercholesterolemia: a long-term, randomized clinical trial. Arch Intern Med. (1999) 159:1331–8. doi: 10.1001/archinte.159.12.1331
42. Li J, Armstrong CL, Campbell WW. Effects of dietary protein source and quantity during weight loss on appetite, energy expenditure, and cardio-metabolic responses. Nutrients. (2016) 8:63. doi: 10.3390/nu8020063
43. Wolmarans P, Laubscher JA, van der Merwe S, Kriek JA, Lombard CJ, Marais M, et al. Effects of a prudent diet containing either lean beef and mutton or fish and skinless chicken on the plasma lipoproteins and fatty acid composition of triacylglycerol and cholesteryl ester of hypercholesterolemic subjects. Vascul Pharmacol. (1999) 10:598–608. doi: 10.1016/s0955-2863(99)00048-0
44. Hunninghake DB, Maki KC, Kwiterovich PO Jr., Davidson MH, Dicklin MR, Kafonek SD. Incorporation of lean red meat into a national cholesterol education program step I diet: a long-term, randomized clinical trial in free-living persons with hypercholesterolemia. J Am Coll Nutr. (2000) 19:351–60. doi: 10.1080/07315724.2000.10718931
45. Hassanzadeh-Rostami Z, Hemmatdar Z, Pishdad GR, Faghih S. Moderate consumption of red meat, compared to soy or non-soy legume, has no adverse effect on cardio-metabolic factors in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. (2021) 129:429–37. doi: 10.1055/a-0929-6287
46. Hosseinpour-Niazi S, Mirmiran P, Hedayati M, Azizi F. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: a cross-over randomized clinical trial. Eur J Clin Nutr. (2015) 69:592–7. doi: 10.1038/ejcn.2014.228
47. Kim Y, Keogh JB, Clifton PM. Consumption of red and processed meat and refined grains for 4weeks decreases insulin sensitivity in insulin-resistant adults: a randomized crossover study. Metabolism. (2017) 68:173–83. doi: 10.1016/j.metabol.2016.12.011
48. Guasch-Ferre M, Satija A, Blondin SA, Janiszewski M, Emlen E, O’Connor LE, et al. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation. (2019) 139:1828–45. doi: 10.1161/CIRCULATIONAHA.118.035225
49. O’Connor LE, Kim JE, Campbell WW. Total red meat intake of >/=0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. Am J Clin Nutr. (2017) 105:57–69. doi: 10.3945/ajcn.116.142521
50. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. (2017) 127:1–4. doi: 10.1172/JCI92035
51. Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. (2016) 67:712–23. doi: 10.1016/j.jacc.2015.11.037
52. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. (2018) 391:319–28. doi: 10.1016/s0140-6736(17)32814-3
53. Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. (2015) 132:1224–33. doi: 10.1161/CIRCULATIONAHA.115.018381
54. Ridker PM. Anticytokine agents: targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res. (2019) 124:437–50. doi: 10.1161/CIRCRESAHA.118.313129
55. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. (2011) 473:317–25. doi: 10.1038/nature10146
56. Miller YI, Shyy JY. Context-dependent role of oxidized lipids and lipoproteins in inflammation. Trends Endocrinol Metab. (2017) 28:143–52. doi: 10.1016/j.tem.2016.11.002
57. Gistera A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. (2017) 13:368–80. doi: 10.1038/nrneph.2017.51
58. Testa G, Rossin D, Poli G, Biasi F, Leonarduzzi G. Implication of oxysterols in chronic inflammatory human diseases. Biochimie. (2018) 153:220–31. doi: 10.1016/j.biochi.2018.06.006
59. Zhong S, Li L, Shen X, Li Q, Xu W, Wang X, et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. (2019) 144:266–78. doi: 10.1016/j.freeradbiomed.2019.03.036
60. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. (2006) 116:1494–505. doi: 10.1172/JCI26498
61. Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol. (2015) 71:40–56. doi: 10.1016/j.vph.2015.03.005
62. Joseph LC, Avula UMR, Wan EY, Reyes MV, Lakkadi KR, Subramanyam P, et al. Dietary saturated fat promotes arrhythmia by activating NOX2 (NADPH oxidase 2). Circ Arrhythm Electrophysiol. (2019) 12:e007573. doi: 10.1161/CIRCEP.119.007573
63. Tian Z, Deng NH, Zhou ZX, Ren Z, Xiong WH, Jiang ZS. The role of adipose tissue-derived hydrogen sulfide in inhibiting atherosclerosis. Nitric Oxide. (2022) 127:18–25. doi: 10.1016/j.niox.2022.07.001
64. Ravaut G, Legiot A, Bergeron KF, Mounier C. Monounsaturated fatty acids in obesity-related inflammation. Int J Mol Sci. (2020) 22:330. doi: 10.3390/ijms22010330
65. Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the united states. JAMA. (2017) 317:912–24. doi: 10.1001/jama.2017.0947
66. Salvi P, Giannattasio C, Parati G. High sodium intake and arterial stiffness. J Hypertens. (2018) 36:754–8. doi: 10.1097/HJH.0000000000001658
67. de Medeiros G, Mesquita GXB, Lima S, Silva DFO, de Azevedo KPM, Pimenta I, et al. Associations of the consumption of unprocessed red meat and processed meat with the incidence of cardiovascular disease and mortality, and the dose-response relationship: a systematic review and meta-analysis of cohort studies. Crit Rev Food Sci Nutr. (2022) Epub ahead of print. doi: 10.1080/10408398.2022.2058461
68. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc. (2016) 5:e002767. doi: 10.1161/JAHA.115.002767
69. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
70. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. (2016) 165:111–24. doi: 10.1016/j.cell.2016.02.011
71. Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. (2017) 5:54. doi: 10.1186/s40168-017-0271-9
72. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. (2019) 40:583–94. doi: 10.1093/eurheartj/ehy799
73. Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, et al. Nutraceuticals and dyslipidaemia: beyond the common therapeutics. J Funct Foods. (2014) 6:11–32. doi: 10.1016/j.jff.2013.12.006
74. Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, et al. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. (2013) 2013:782137. doi: 10.1155/2013/782137
75. Chen X, Song X, Zhao X, Zhang Y, Wang Y, Jia R, et al. Insights into the anti-inflammatory and antiviral mechanisms of resveratrol. Mediators Inflamm. (2022) 2022:7138756. doi: 10.1155/2022/7138756
76. Wang TK, Xu S, Li S, Zhang Y. Proanthocyanidins should be a candidate in the treatment of cancer, cardiovascular diseases and lipid metabolic disorder. Molecules. (2020) 25:5971. doi: 10.3390/molecules25245971
77. Kato M, Ogawa H, Kishida T, Ebihara K. The mechanism of the cholesterol-lowering effect of water-insoluble fish protein in ovariectomised rats. Br J Nutr. (2009) 102:816–24. doi: 10.1017/S0007114509316153
78. Jiang H, Wang L, Wang D, Yan N, Li C, Wu M, et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin Nutr. (2022) 41:1798–807. doi: 10.1016/j.clnu.2022.06.034
79. Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. (2013) 159:543–51. doi: 10.7326/0003-4819-159-8-201310150-00007
80. Teng M, Zhao YJ, Khoo AL, Yeo TC, Yong QW, Lim BP. Impact of coconut oil consumption on cardiovascular health: a systematic review and meta-analysis. Nutr Rev. (2020) 78:249–59. doi: 10.1093/nutrit/nuz074
Keywords: red meat, lipids, dyslipidaemia, inflammation, meta-analysis
Citation: Sun L, Yuan J-L, Chen Q-C, Xiao W-K, Ma G-P, Liang J-H, Chen X-K, Wang S, Zhou X-X, Wu H and Hong C-X (2022) Red meat consumption and risk for dyslipidaemia and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:996467. doi: 10.3389/fcvm.2022.996467
Received: 17 July 2022; Accepted: 14 September 2022;
Published: 30 September 2022.
Edited by:
Nathalie Pamir, Oregon Health and Science University, United StatesReviewed by:
Marco Matteo Ciccone, University of Bari Aldo Moro, ItalyCopyright © 2022 Sun, Yuan, Chen, Xiao, Ma, Liang, Chen, Wang, Zhou, Wu and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wu, d3VodWkwMjZAMTYzLmNvbQ==; Chuang-Xiong Hong, Z3poY3gxOTY2QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.