- 1Department of Translational Medicine, The First Hospital of Jilin University, Changchun, China
- 2Department of Cardiovascular Diseases, The First Hospital of Jilin University, Changchun, China
- 3Department of Cardiology, Zhongshan Hospital, Shanghai, China
- 4Department of Nephrology, Linyi Traditional Chinese Medicine Hospital, Linyi, China
Background: Early revascularization of the culprit vessel is the most effective treatment for reducing the risk of mortality from acute STEMI with and without cardiogenic shock. However, the most recent trends and impact of multivessel percutaneous coronary intervention (PCI) during the index hospitalization on in-hospital outcomes are unknown.
Methods: The National Inpatient Sample was queried from October 2015 to 2019 for hospitalizations with STEMI. The impact of multivessel PCI on in-hospital outcomes of patients with and without cardiogenic shock was evaluated.
Results: Of 624,605 STEMI hospitalizations treated with PCI, 12.5% were complicated by cardiogenic shock. Among hospitalizations without cardiogenic shock, 15.7% were treated by multivessel PCI, which declined from 20.8% in 2015 to 13.9% in 2019 (Ptrend < 0.001). Multivessel and culprit-only PCI had similar rates of In-hospital mortality (2.4 vs. 2.3%, p = 0.027) and major adverse cardiac and cerebrovascular events (MACCE; 7.4 vs. 7.2%, p = 0.072). Among hospitalizations with cardiogenic shock, 22.1% were treated by multivessel PCI, which declined from 29.2% in 2015 to 19.4% in 2019 (Ptrend < 0.001). Multivessel PCI was associated with higher rates of in-hospital mortality (30.9 vs. 28.4%, p < 0.001) and MACCE (39.9 vs. 36.5%, p < 0.001) than culprit-only PCI.
Conclusion: The frequency of multivessel PCI for STEMI with and without cardiogenic shock is declining. Multivessel PCI is associated with worse in-hospital outcomes for STEMI with cardiogenic shock but not for STEMI without cardiogenic shock.
Introduction
In patients presenting with ST-segment elevation myocardial infarction (STEMI) with or without cardiogenic shock, early revascularization—mainly percutaneous coronary intervention (PCI) on the culprit vessel—is the most effective therapeutic strategy to reduce both short- and long-term mortality (1–3). However, over half of patients with hemodynamically stable STEMI have at least 1 other obstructive lesion in non-culprit vessels (4); in STEMI with cardiogenic shock, up to 80% of patients present with multivessel coronary artery disease (5). Optimal strategies for the treatment of non-culprit lesions have been widely studied (6). Several randomized controlled trials including the COMPLETE trials comparing multivessel vs. culprit-only PCI have reported improved clinical outcomes including decreased cardiac mortality, myocardial reinfarction, and revascularization (7–12); however, the optimal time to treat non-culprit lesions is not known. Additionally, data supporting multivessel PCI have been derived from hemodynamically stable myocardial infarction (MI) patients as cardiogenic shock patients were excluded from these studies. The results of the CULPRIT-SHOCK trial enrolling patients with acute MI complicated by cardiogenic shock suggested that immediate treatment of non-culprit lesions during primary PCI was harmful (13). Real-world data regarding impact of multivessel PCI for STEMI with and without cardiogenic shock on in-hospital outcomes are limited and inconsistent (14–16). The most recent practice trends of multiple PCI are unknown, To address these issues, in this study we analyzed data for patients hospitalized for STEMI with and without cardiogenic shock using the latest United States (US) National Inpatient Sample (NIS) database.
Materials and methods
Data source
The data were obtained from the NIS database developed for the Healthcare Cost and Utilization Project (HCUP) (17). It is the largest inpatient care database in the US and includes over 7 million unweighted hospital stays annually with more than 100 clinical and non-clinical data elements. It is accessible at https://www.ahrq.gov. In accordance with NIS recommendations, proper weighting was applied using the individual weight variable provided by the HCUP to establish national estimate statistics. Comorbidities were identified using Elixhauser Comorbidity Software Refined for International Classification of Disease, 10th Revision, Clinical Modification (ICD-10-CM), which assigns data elements provided by HCUP. The use of the NIS database to describe outcomes and trends in cardiovascular disease in different patient populations has been previously validated (18, 19). As data from the NIS are publicly available and de-identified, the study was exempt from Institutional Review Board approval. The authors vouch for the accuracy and completeness of the data. the date of last database interrogation was on 10 July 2022.
Study population and outcome measures
Starting from 1 October 2015, all hospitals in the US transitioned from ICD-9-CM (i.e., the 9th revision) to ICD-10-CM coding of diagnoses and procedures. Significant disruption of statistics has been reported, and it was suggested that analyses rely on a single coding system (20, 21). In the present study, we queried the NIS database from inception of the ICD-10-CM coding system to the latest available time (from October 2015 through 2019). STEMI hospitalizations were identified using the ICD-10-CM diagnosis codes I21.0x, I21.1x, I21.2x, and I21.3 (Supplementary Table 1), which have been previously validated (22, 23). We excluded records of patients who did not undergo PCI; with missing information on the number of treated vessels in procedure codes; with age at admission <18 years; and with missing data on in-hospital mortality (Supplementary Figure 1). The primary outcome was in-hospital all-cause mortality, and the secondary outcome was major adverse cardiac or cerebrovascular events (MACCE) including a composite of all-cause mortality, cardiac complications (hemopericardium and cardiac tamponade necessitating pericardiocentesis), and stroke. Hospital cost was obtained by merging the cost-to-charge ratio files with total charge.
Statistical analyses
Continuous variables are expressed as mean ± SD or median [interquartile range (IQR)] as appropriate. Categorical variables are expressed as numbers and percentages. Weights for each discharge were used to calculate national estimates as recommended by the HCUP for NIS data. Multivariable logistic regression models were generated to evaluate the association between in-hospital mortality, presented as odds ratios (OR) with 95% confidence interval (CI), and variables included in the model (multivessel PCI, age, sex, race, expected payer, hospital bed size, location and teaching status, atrial fibrillation, smoking status, history of MI, prior PCI, prior coronary artery bypass graft [CABG], family history of coronary artery disease, chronic lung disease, obesity, peripheral artery disease, hypothyroidism, hypertension, and diabetes mellitus). Differences between categorical variables were evaluated with the chi-squared test, and differences between continuous variables were assessed with the Student’s t-test or Mann–Whitney U test as appropriate; the corresponding ORs and 95% CIs are presented as forest plots. The Breslow–Day test was used to analyze the interaction between subgroups. Considering the large sample size, a 2-sided P-value <0.01 was considered statistically significant. SAS 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
Temporal trends in multivessel percutaneous coronary intervention
The flow chart of patient selection is shown in Supplementary Figure 1. We extracted 912,540 hospitalizations with a diagnosis of STEMI between October 2015 to October 2019 from the NIS database. After excluding age <18 years at admission (n = 440); patients with missing in-hospital mortality data (n = 3,300); hospitalizations did not undergo PCI (283,645); and hospitalizations with missing number of vessel treatments in procedure codes (n = 3,280), the final analysis included 624,605 STEMI hospitalizations, 546,305 (87.5%) without and 78,300 (12.5%) with cardiogenic shock. In the cohort without cardiogenic shock, there were 460,315 (84.3%) hospitalizations where the patient underwent culprit-only PCI and 85,990 (15.7%) where the patient underwent multivessel PCI. In the cohort with cardiogenic shock, there were 60,695 (77.9%) hospitalizations where the patient underwent culprit-only PCI and 17,335 (22.1%) where the patient underwent multivessel PCI. During the study period, the rate of multivessel PCI in overall STEMI hospitalizations declined from 21.8% in 2015 to 14.6% in 2019 (Ptrend < 0.001); the rate of multivessel PCI for STEMI without cardiogenic shock declined from 20.8 to 13.9% (Ptrend < 0.001); and the rate of multivessel PCI in hospitalizations with cardiogenic shock declined from 29.2 to 19.4% (Ptrend < 0.001) (Figure 1).

Figure 1. Trend of multivessel PCI performance during the study period. Percentage of overall STEMI hospitalizations, STEMI hospitalizations with cardiogenic shock, and STEMI hospitalizations without cardiogenic shock in which multivessel PCI was performed.
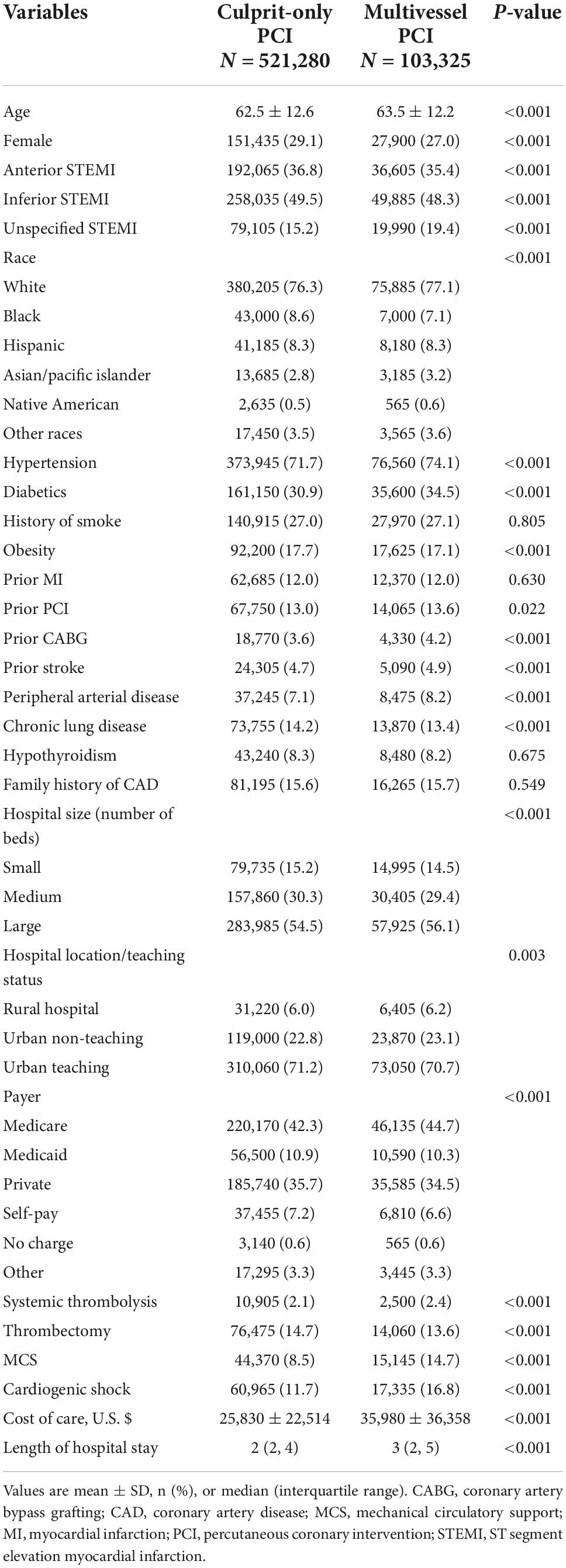
In the overall STEMI cohort, hospitalizations who underwent multivessel PCI were older (63.5 ± 12.2 vs. 62.5 ± 12.6) and mostly White (77.1 vs. 76.3%, P < 0.001); a higher proportion were males (73 vs. 70.9%, P < 0.001), and the patients had higher rates of hypertension (74.1 vs. 71.7%, P < 0.001), diabetes mellitus (34.5 vs. 30.9%, P < 0.001), and admission to a large hospital (56.1 vs. 54.5%, P < 0.001) compared to those who underwent culprit-only PCI. Multivessel PCI hospitalizations were associated higher rates of cardiogenic shock (16.8 vs. 11.7%, P < 0.001) and mechanical circulatory support (14.7 vs. 8.5%, P < 0.001), higher cost of care (35,980 ± 36,358 vs. 25,830 ± 22,514, P < 0.001), and longer hospital stay (median 3, IQR [2–5] vs. median = 3, IQR [2–4], P < 0.001) (Table 1); they also had higher in-hospital mortality compared to culprit-only PCI (7.2 vs. 5.4%, P < 0.001) and a higher rate of MACCE (12.8 vs. 10.6%, P < 0.001) (Figure 2). The increase in in-hospital mortality was observed in each calendar year during the study period (Supplementary Figures 2, 3). Logistic regression analysis in all STEMI cohorts showed that multivessel PCI was associated with increased risk for in-hospital mortality (OR = 1.33; 95% CI: 1.25–1.42, p < 0.001) (Figure 3). We categorized multivessel PCI into procedures involving 2 vessels and >2 vessels. The latter subgroup had higher rates of in-hospital mortality (9.6 vs. 6.8%, P < 0.001) and MACCE (15.8 vs. 12.3%, P < 0.001) compared to 2-vessel PCI (Supplementary Figure 4).

Figure 2. In-hospital mortality and MACCE in multivessel PCI vs. culprit-only PCI. (A,B) Shown are percentages of in-hospital mortality (A) and MACCE (B) comparing multivessel PCI vs. culprit-only PCI in the overall STEMI cohort, STEMI without cardiogenic shock cohort, and STEMI with cardiogenic shock cohort.

Figure 3. Forest plot of multivariable regression analysis to predict in-hospital mortality in overall STEMI cohort. AF, atrial fibrillation; CABG, coronary artery bypass graft; CAD, coronary artery disease; DM, diabetes mellitus; HT, hypertension; MI, myocardial infarction; PCI, percutaneous coronary intervention.
We next compared the impact of multivessel PCI with and without cardiogenic shock. Among hospitalizations without cardiogenic shock, hospitalizations underwent multivessel PCI are were older (63.0 ± 12.2 vs. 62.1 ± 12.5, P < 0.001) and mostly male (73.5 vs. 71.5%, P < 0.001) and White (77.6 vs. 76.5%, P < 0.001) (Table 2), with higher rates of hypertension (75.0 vs. 72.3%, P < 0.001), diabetes mellitus (33.3 vs. 30.5%, P < 0.001), and admission to a large hospital (55.3 vs. 54.3%, P < 0.001). Multivessel PCI hospitalizations were associated with a higher cost of care (30,691 ± 25,804 vs. 22,990 ± 15,863, P < 0.001) and longer hospital stay (median = 3, IQR [2–4] vs. median 2, IQR [2–3], P < 0.001), but had in-hospital mortality (2.4 vs. 2.3%, P = 0.027) and MACCE rate (7.4 vs. 7.2% P = 0.072) similar to culprit-only PCI hospitalizations (Figure 2). The rate of in-hospital mortality was comparable between the two groups in each calendar year during the study period (Supplementary Figures 2, 3). Logistic regression analysis showed that in the STEMI without cardiogenic shock cohort, multivessel PCI was not associated with an increased risk of in-hospital mortality (RR = 1.05; 95% CI:0.94–1.17) (Figure 4). When we stratified the procedure into 2-vessel and >2-vessel PCI (Supplementary Figure 4), the 2-vessel procedure had in-hospital mortality (2.3 vs. 2.3%) and MACCE rate (7.2 vs. 7.1%) similar to culprit-only PCI; however, PCI involving >2 vessels was associated with worse in-hospital outcomes (in-hospital mortality, 3.3% and MACCE, 8.8%). Additionally, although other subgroups showed comparable in-hospital mortality risk, >2-vessel PCI was associated with an increased risk of in-hospital death (OR = 1.45, 95% CI: 1.15–1.82) (Supplementary Figure 5).

Figure 4. Forest plot of multivariable regression analysis to predict in-hospital mortality in the STEMI without cardiogenic shock cohort. Abbreviations as in Figure 3.
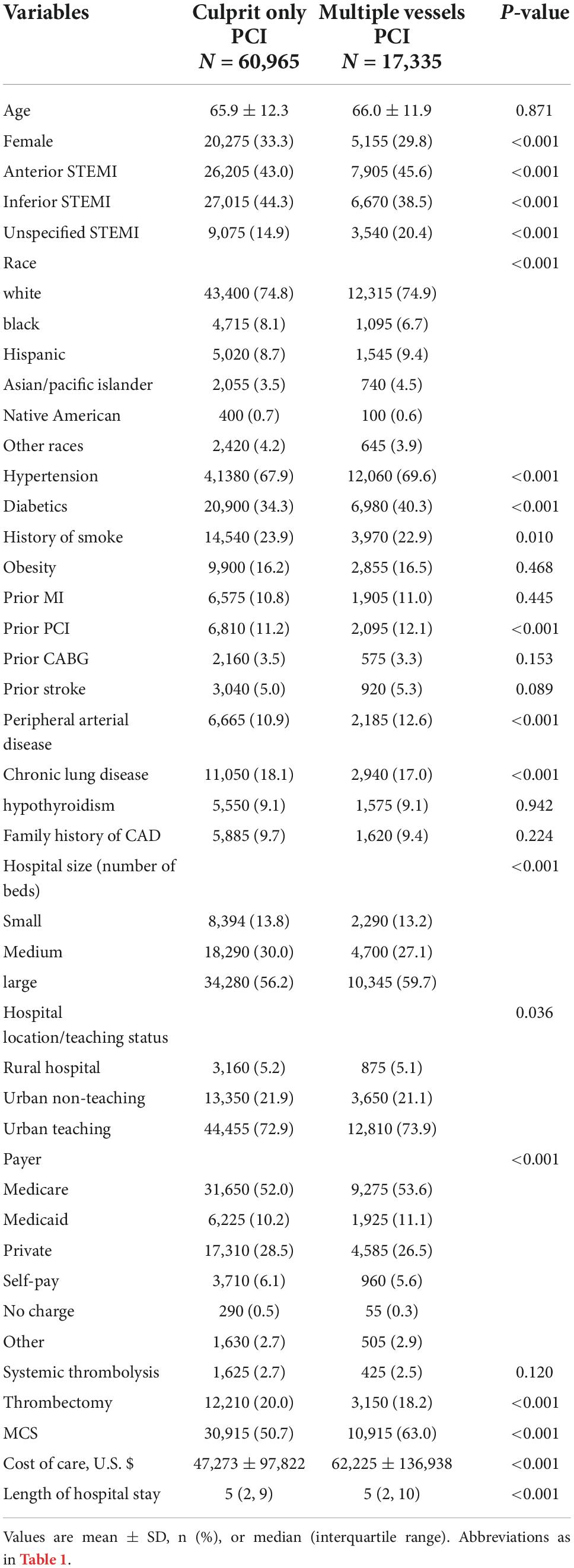
The mean ± SD age of patients hospitalized with cardiogenic shock was similar between those who underwent multivessel vs. culprit-only PCI (66.0 ± 11.9 vs. 65.9 ± 12.3, P = 0.871); however, the former cohort had more men (70.2 vs. 66.7%, P < 0.001) (Table 3) and higher rates of hypertension (69.6 vs. 67.9%, P < 0.001), diabetes mellitus (40.3 vs. 34.3%, P < 0.001), and admission to a large hospital (59.7 vs. 56.2%, P < 0.001). Multivessel PCI hospitalizations were associated with a higher cost of care (62,225 ± 136,938 vs. 47,273 ± 97,822, P < 0.001), longer hospital stay (median = 5, IQR [2–10] vs. median = 5, IQR [2–9], P < 0.001), and higher in-hospital mortality (30.9 vs. 28.4%, P < 0.001) and MACCE rate (39.9 vs. 36.5%, P < 0.001) than culprit-only PCI (Figure 2). The in-hospital mortality in each calendar year during the study period is shown in the Supplementary Figure 2. Logistic regression revealed that for STEMI with cardiogenic shock, multivessel PCI was associated with increased risk of in-hospital mortality (OR = 1.10; 95% CI: 1.06–1.14) (Figure 5). In the subgroup analysis, the rate of in-hospital mortality for 2-vessel and >2-vessel procedures were 30.7 and 31.6%, respectively, and the rate of MACCE was 39.8 and 40.3%, respectively, with similar results observed across all subgroups (Supplementary Figure 6).

Figure 5. Forest plot of multivariable regression analysis to predict in-hospital mortality in the STEMI with cardiogenic shock cohort. Abbreviations as in Figure 3.
Discussion
There were five main findings from this large-sample analysis of patients with STEMI with or without cardiogenic shock in the US. (1) The rate of multivessel PCI in the index hospitalization decreased during the study period, corresponding to the declining rates of STEMI with and without cardiogenic shock. (2) In the overall STEMI cohort, in-hospital mortality and rate of MACCE for multivessel PCI were significantly higher than the rate of culprit-only PCI. (3) In STEMI hospitalizations without cardiogenic shock, multivessel PCI was not associated with an elevated risk of in-hospital mortality and MACCE rate. (4) In STEMI hospitalizations with cardiogenic shock, multivessel PCI was associated with a significantly increased risk of in-hospital mortality and MACCE rate. (5) The elevated risk of multivessel PCI in the overall STEMI cohort was driven by the higher portion of cardiogenic shock hospitalizations in which patients underwent multivessel PCI, and higher risk associated with multivessel PCI in cardiogenic shock hospitalizations.
Multivessel disease is common in STEMI hospitalizations, and even more prevalent in the setting of cardiogenic shock (5). The presence of multivessel disease is associated with worse clinical outcomes compared with single-vessel disease (24). The optimal strategy for treatment of the non-culprit vessel is unclear, as reflected in the discrepancies in treatment guidelines. The current evidence indicates diverse effects of multivessel PCI on clinical outcomes depending on the presence of cardiogenic shock. Except for the CULPRIT-SHOCK trial, randomized clinical trials have excluded patients with cardiogenic shock and have reported favorable outcomes of multivessel PCI, with earlier trials showing that the benefit was mainly attributable to a reduction in repeated revascularizations (7–10, 25–29). The COMPLETE trial (11) showed that the benefit extended beyond repeated revascularizations, also reducing the rates of cardiac death and MI (12). However, the optimal timing of non-culprit vessel revascularization has not been adequately investigated. An analysis of 1,964 patients from 5 clinical trials that included multivessel PCI during the index hospitalization demonstrated a significant reduction in cardiovascular mortality in addition to repeated revascularizations (12). The present analysis of NIS data confirms the safety of non-culprit PCI during the index hospitalization for STEMI without cardiogenic shock.
During the study period, multivessel PCI was performed during the index hospitalization in only 15.7% of STEMI hospitalizations without cardiogenic shock; thus, most patients with multivessel disease admitted with STEMI did not have their non-culprit vessel treated before discharge. Although, the clinical benefit of non-culprit PCI has been established (11), several questions remain unanswered, like what is the optimal timing of non-culprit PCI (30), our data provide support for the treatment of non-culprit vessel coronary disease during the index hospitalization, considering the possible long-term benefit for complete revascularization (31). Thus, for STEMI without cardiogenic shock, multivessel PCI during the index hospitalization appears safe and should be considered, at least in selected hemodynamically stable myocardial infarction patients. However, it is worth noting that in this analysis, 85.7% of multivessel procedures were performed on two vessels. The 2-vessel procedure is safe and does not incur excessive risks of in-hospital mortality and MACCE compared with culprit-only PCI. Hospitalizations involving a >2-vessel procedure is still associated with a significant increase in in-hospital mortality and MACCE. These results indicate that there is a limit to how many vessels can be safely treated. In cases involving >2 vessels, it is important to consider whether staged PCI to treat the extra vessel(s) is more beneficial or whether CABG is a better option because of the complexity of the coronary artery disease.
The results of the CULPRIT-SHOCK trial showed the detrimental effect of immediate multivessel PCI on cardiogenic shock complicated by MI at 30 days (13). In line with this finding, our analysis showed that in contrast to STEMI hospitalizations without cardiogenic shock, multivessel PCI was associated with increased risk of in-hospital mortality and MACCE in STEMI hospitalizations with cardiogenic shock. An explanation for the differential impact of multivessel PCI in hospitalizations with vs. without cardiogenic shock is that the long procedure time may cause more stress and expose patients to more hemodynamic instability; additionally, injection of a large amount of contrast agent may further impair the function of an underperfused kidney in the setting of cardiogenic shock. To our knowledge, this analysis represents the largest-sample study of the impact of multivessel PCI on STEMI with cardiogenic shock. Our results provide real-world evidence of the harmful effects of immediate multivessel PCI as reported in the CULPRIT-SHOCK trial. The declining trend of multivessel PCI performance in the setting of cardiogenic shock during the study period may reflect the influence of the CULPRIT-SHOCK trial on clinical practice. It has been suggested that immediate multivessel PCI is associated with a higher short-term but lower long-term risk of death than culprit lesion-only PCI; however, this is not supported by the 1-year outcome from the CULPRIT-SHOCK trial that showed no reduction in the multivessel PCI group with a longer follow-up (between 30 days and 1 year) (32). This along with our findings suggest that immediate multivessel PCI should be avoided in STEMI with cardiogenic shock. In the CULPRIT-SHOCK trial, staged PCI of non-culprit lesions within 30 days was only performed on 17.4% patients. Whether performing more stage PCIs can improve outcomes and if so, the optimal time to treat the non-culprit lesion remain to be determined.
Study limitations
The present analysis had certain limitations. Large in-patient cohorts such as the NIS are subject to coding and documentation errors. The administrative database lacked clinical details for individual hospitalization including angiographic and procedural details, biochemistry data, echocardiography, and medications as well as long-term follow-up data; moreover, the retrospective observational study design made the analysis liable to selection bias. However, the NIS database has been widely validated internally and externally in studies with adequate sampling (33). Our analyses were robust and included subgroup analyses; moreover, they included the most current and largest sample of patients with STEMI with cardiogenic shock and provides insight into the practice patterns and impact of multivessel PCI in the real world, confirming the findings of the CULPRIT-SHOCK trial.
Conclusion
In this national analysis of STEMI hospitalizations with and without cardiogenic shock, we found a significant decrease in the performance of multivessel PCI for STEMI both with and without cardiogenic shock in the US from 2015 to 2019. In STEMI admissions without cardiogenic shock, PCI of no more than 1 non-culprit vessel can be safely performed during the index hospitalization. However, in STEMI with cardiogenic shock, multivessel PCI during the index hospitalization was associated with increased risks of in-hospital mortality and MACCE. Further study is needed to determine whether patients with STEMI with cardiogenic shock benefit from staged multivessel PCI, and the optimal procedure time thereof.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
MZ: full access to all the data in the study and responsible for the integrity of the data and the accuracy of the data analysis. MZ and QT: concept and design. JW, YW, MZ, and QT: acquisition, analysis, or interpretation of data. JW and YW: drafting of the manuscript and funding acquisition. JW and MZ: statistical analysis. HJ and WZ: administrative, technical, or material support. All authors: critical revision of the manuscript for important intellectual content.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81901591).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.992456/full#supplementary-material
Abbreviations
CABG, coronary artery bypass graft; HCUP, Healthcare Cost and Utilization Project; ICD-9/10-CM, International Classification of Disease, 9th/10th Revision, Clinical Modification; MACCE, major adverse cardiac and cerebrovascular event; MI, myocardial infarction; NIS, National Inpatient Sample; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
References
1. Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. (2006) 295:2511–5.
2. Terkelsen CJ, Christiansen EH, Sørensen JT, Kristensen SD, Lassen JF, Thuesen L, et al. Primary PCI as the preferred reperfusion therapy in STEMI: it is a matter of time. Heart. (2009) 95:362–9.
3. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. (1999) 341:625–34.
4. Park D-W, Clare RM, Schulte PJ, Pieper KS, Shaw LK, Califf RM, et al. Extent, location, and clinical significance of non–infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. (2014) 312:2019–27. doi: 10.1001/jama.2014.15095
5. Thiele H, Desch S, Piek JJ, Stepinska J, Oldroyd K, Serpytis P, et al. Multivessel versus culprit lesion only percutaneous revascularization plus potential staged revascularization in patients with acute myocardial infarction complicated by cardiogenic shock: design and rationale of CULPRIT-SHOCK trial. Am Heart J. (2016) 172:160–9. doi: 10.1016/j.ahj.2015.11.006
6. Bhatt DL. Do We Really Know the Cvlprit in Myocardial Infarction? Or Just Stent All Lesions? Washington, DC: American College of Cardiology Foundation (2015). p. 973–5.
7. Smits PC, Abdel-Wahab M, Neumann F-J, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional flow reserve–guided multivessel angioplasty in myocardial infarction. N Engl J Med. (2017) 376:1234–44.
8. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. (2015) 386:665–71. doi: 10.1016/s0140-6736(15)60648-1
9. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. (2015) 65:963–72.
10. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. (2013) 369:1115–23.
11. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. (2019) 381:1411–21.
12. Atti V, Gwon Y, Narayanan MA, Garcia S, Sandoval Y, Brilakis ES, et al. Multivessel versus culprit-only revascularization in STEMI and multivessel coronary artery disease: meta-analysis of randomized trials. JACC Cardiovasc Interv. (2020) 13:1571–82.
13. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. (2017) 377:2419–32.
14. Panaich SS, Arora S, Patel N, Schreiber T, Patel NJ, Pandya B, et al. Comparison of in-hospital mortality, length of stay, postprocedural complications, and cost of single-vessel versus multivessel percutaneous coronary intervention in hemodynamically stable patients with ST-segment elevation myocardial infarction (from nationwide inpatient sample [2006 to 2012]). Am J Cardiol. (2016) 118:950–8. doi: 10.1016/j.amjcard.2016.06.057
15. Arora S, Panaich SS, Patel NJ, Patel N, Solanki S, Deshmukh A, et al. Multivessel percutaneous coronary interventions in the United States: insights from the nationwide inpatient sample. Angiology. (2015) 67:326–35. doi: 10.1177/0003319715593853
16. Tummala R, Shah SD, Rawal E, Sandhu RK, Kavuri SP, Kaur G, et al. In-hospital mortality risk factor analysis in multivessel percutaneous coronary intervention inpatient recipients in the United States. Cureus. (2021) 13:e17520.
17. Elixhauser A. Clinical Classifications for Health Policy Research, Version 2: Hospital Inpatient Statistics. Washington, DC: US Department of Health and Human Services (1996).
18. Zhou LW, Allo M, Mlynash M, Field TS. Capturing intravenous thrombolysis for acute stroke at the ICD-9 to ICD-10 transition: case volume discontinuity in the United States national inpatient sample. J Am Heart Assoc. (2021) 10:e021614. doi: 10.1161/JAHA.121.021614
19. Hoffman GR, Stein DJ, Moore MB, Feuerstein JD. Safety of endoscopy for hospitalized patients with acute myocardial infarction: a national analysis. Am J Gastroenterol. (2020) 115:376–80.
20. Metcalfe A, Sheikh M, Hetherington E. Impact of the ICD-9-CM to ICD-10-CM transition on the incidence of severe maternal morbidity among delivery hospitalizations in the United States. Am J Obstet Gynecol. (2021) 225:422.e1–11. doi: 10.1016/j.ajog.2021.03.036
21. Hamedani AG, Blank L, Thibault DP, Willis AW. Impact of ICD-9 to ICD-10 coding transition on prevalence trends in neurology. Neurol Clin Pract. (2021) 11:e612–9. doi: 10.1212/CPJ.0000000000001046
22. Elbadawi A, Elzeneini M, Elgendy IY, Megaly M, Omer M, Jimenez E, et al. Coronary artery bypass grafting after acute ST-elevation myocardial infarction. J Thorac Cardiovasc Surg. (2021). [Epub ahead of print]. doi: 10.1016/j.jtcvs.2021.03.081
23. Wu J, Cong X, Lou Z, Zhang M. Trend and impact of concomitant CABG and multiple-valve procedure on In-hospital outcomes of SAVR patients. Front Cardiovasc Med. (2021) 8:740084. doi: 10.3389/fcvm.2021.740084
24. Lee JH, Park HS, Chae SC, Cho Y, Yang DH, Jeong MH, et al. Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea acute myocardial infarction registry). Am J Cardiol. (2009) 104:182–9. doi: 10.1016/j.amjcard.2009.03.010
25. Di Mario C, Mara S, Flavio A, Imad S, Antonio M, Anna P, et al. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for acute myocardial infarction (HELP AMI) study. Int J Cardiovasc Interv. (2004) 6:128–33.
26. Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Leuzzi C, et al. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart. (2010) 96:662–7. doi: 10.1136/hrt.2009.177162
27. Ghani A, Dambrink J, Van’t Hof A, Ottervanger J, Gosselink A, Hoorntje J. Treatment of non-culprit lesions detected during primary PCI: long-term follow-up of a randomised clinical trial. Netherlands Heart J. (2012) 20:347–53. doi: 10.1007/s12471-012-0281-y
28. Hlinomaz, O, Groch L, Polokova L, Lehar F, Vekov T, Griva M, et al. Multivessel disease diagnosed at the time of primary PCI for STEMI: complete revascularization versus conservative strategy. Eur Heart J. (2015) 17:214–20.
29. Hamza M, Mahmoud AN, Elgendy IY. A randomized trial of complete versus culprit−only revascularization during primary percutaneous coronary intervention in diabetic patients with acute ST elevation myocardial infarction and multi vessel disease. J Interv Cardiol. (2016) 29:241–7. doi: 10.1111/joic.12293
30. Montone RA, Niccoli G, Crea F, Jang IK. Management of non-culprit coronary plaques in patients with acute coronary syndrome. Eur Heart J. (2020) 41:3579–86.
31. Wood DA, Cairns JA, Wang J, Mehran R, Storey RF, Nguyen H, et al. Timing of staged nonculprit artery revascularization in patients with ST-segment elevation myocardial infarction: complete trial. J Am Coll Cardiol. (2019) 74:2713–23.
32. Thiele H, Akin I, Sandri M, de Waha-Thiele S, Meyer-Saraei R, Fuernau G, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. (2018) 379:1699–710.
Keywords: STEMI, cardiogenic shock, culprit-only PCI, multivessel PCI, National Inpatient Database (NIS)
Citation: Wu J, Wang Y, Li C, Ji H, Zhao W, Tong Q and Zhang M (2022) Multivessel vs. culprit vessel-only percutaneous coronary intervention in ST-segment elevation myocardial infarction with and without cardiogenic shock. Front. Cardiovasc. Med. 9:992456. doi: 10.3389/fcvm.2022.992456
Received: 12 July 2022; Accepted: 01 November 2022;
Published: 24 November 2022.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Rocco Antonio Montone, Agostino Gemelli University Polyclinic (IRCCS), ItalyRamesh Daggubati, West Virginia University, United States
Copyright © 2022 Wu, Wang, Li, Ji, Zhao, Tong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Tong, dG9uZ3FpYW5Aamx1LmVkdS5jbg==; Mingyou Zhang, em15QGpsdS5lZHUuY24=
†These authors have contributed equally to this work
 Jing Wu1†
Jing Wu1† Chenguang Li
Chenguang Li Qian Tong
Qian Tong Mingyou Zhang
Mingyou Zhang