
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 03 November 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.989574
 Menglu Liu1†
Menglu Liu1† Panpan Xia2†
Panpan Xia2† Ziqi Tan2
Ziqi Tan2 Tiangang Song2
Tiangang Song2 Kaibo Mei3
Kaibo Mei3 Jingfeng Wang4
Jingfeng Wang4 Jianyong Ma5
Jianyong Ma5 Yuan Jiang4
Yuan Jiang4 Jing Zhang6
Jing Zhang6 Yujie Zhao1*
Yujie Zhao1* Peng Yu2*
Peng Yu2* Xiao Liu4*
Xiao Liu4*Background: In the past decade, fibroblast growth factor 23 (FGF23) has been recognized as an important biomarker of cardiovascular diseases. This study aimed to assess the relationship between FGF23 and the risk of cardiovascular diseases (CVDs) in general populations.
Methods: The protocol was registered prospectively in PROSPERO (CRD42021281837) and two authors independently searched for relevant studies in the PubMed, EMBASE, and Cochrane Library databases. The random effects model was applied.
Results: In total, 29 prospective studies involving 135,576 participants were included. In the general population, the category analysis revealed that elevated FGF23 levels were related to increased risks of myocardial infarction (MI) (RR: 1.40, 95%CI: 1.03−1.89), stroke (RR: 1.20, 95%CI: 1.02−1.43), heart failure (HF) (RR: 1.37, 95%CI: 1.23−1.52), CVD events (RR: 1.22, 95%CI: 0.99−1.51), cardiovascular mortality (RR: 1.46, 95%CI: 1.29−1.65), and all-cause mortality (RR: 1.50, 95%CI: 1.29−1.74). In the continuous analysis, per doubling of FGF23 was associated with increased risks of MI (RR: 1.08, 95%CI: 0.94−1.25), stroke (RR: 1.21, 95%CI: 0.99−1.48), HF (RR: 1.24, 95%CI: 1.14−1.35), CVD events (RR: 1.12, 95%CI: 0.99−1.27), cardiovascular mortality (RR: 1.43, 95%CI: 1.09−1.88), all-cause mortality (RR: 1.37, 95%CI: 1.15−1.62). Furthermore, the dose-response analysis demonstrated a potentially non-linear relationship between FGF23 and stroke, HF, and all-cause mortality. In contrast, a potentially linear relationship between FGF23 and cardiovascular mortality was observed (p for non-linearity = 0.73).
Conclusion: The present study suggests that increased serum FGF23 levels are positively related to CVD events and mortality in the general population. The clinical application of FGF23 levels to predict CVD risk requires further research.
Cardiovascular diseases (CVDs) remain the leading cause of mortality worldwide, resulting in 17.3 million deaths each year. The number of annual deaths is expected to exceed 23.6 million by 2030. Meanwhile, cardiovascular diseases are responsible for approximately 40% of deaths in the Chinese population (1, 2). Therefore, it is essential to explore the prevention and treatment of CVDs and develop effective solutions.
Fibroblast growth factor 23 (FGF23) is a phosphaturic hormone primarily secreted by osteocytes and osteoblasts. It participates in adjusting systemic phosphate homeostasis, vitamin D metabolism, and a-Klotho expression through the bone-kidney axis (3, 4). FGF23 mainly exerts physiological effects in the kidneys and the parathyroid gland by binding to the FGF receptor (FGFR) and its co-receptor klotho (5). The main physiological role of FGF23 is to enhance urinary phosphate excretion, decrease the 1,25-dihydroxy Vitamin D levels in vivo, and suppress the secretion of parathyroid hormone (PTH) (6, 7). In the past decade, FGF23 has been recognized as an important biomarker of cardiovascular diseases. Furthermore, CVD is also the leading cause of death in patients with chronic kidney disease (CKD). The serum FGF23 levels in CKD patients were significantly higher than in healthy populations and demonstrated an increase with decreasing glomerular filtration rate (GFR) (8, 9). Previous meta-analyses have investigated the relationship between FGF23 and CVDs in CKD patients (10–13), but the association and dose-response in the general population remain unclear. Therefore, this systematic review and dose-response meta-analysis explored of the association between FGF23 levels and cardiovascular diseases and mortality risk in the general population.
This review strictly followed the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) and G-Dose checklists guidelines (Supplementary Tables 1, 2). The study was registered in PROSPERO (CRD42021281837).
Two authors (PX and ML) performed a literature search using the PubMed, EMBASE, and the Cochrane Library databases, including articles published before 10 September 2022. Medical subject headings were combined with free-text terms for retrieval without language restrictions. The search conditions were as follows: “fibroblast growth-factor 23 OR FGF23 protein OR fibroblast growth factor 23 OR FGF23 protein OR phosphatonin OR tumor-derived hypophosphatemia inducing factor” And “cardiovascular diseases OR cardiovascular disease OR disease, cardiovascular OR diseases, cardiovascular OR myocardial infarction OR stroke OR heart failure OR atrial fibrillation OR coronary heart disease OR left ventricular hypertrophy OR hypertension.” The details of the search strategy are described in Supplementary Table 3.
According to the PICOS (population, intervention, comparison, outcome, and study design) strategy, the inclusion criteria for this review were as follows:
(1) The participants were adults from the general population (age > 18 years).
(2) The studies compared high vs. low FGF23 levels.
(3) The outcomes included all kinds of cardiovascular diseases (including myocardial infarction, stroke, heart failure, atrial fibrillation, coronary heart disease, left ventricular hypertrophy, hypertension, composite of cardiovascular events, cardiovascular mortality, and all-cause mortality).
(4) Prospective cohort studies were included.
Adjusted relative risk (RR) or hazard ratio (HR), and the corresponding 95% confidence interval (CI) were required. Prospective case-cohorts were regarded as prospective cohort studies (14). Case-control or cross-sectional study, reviews, case reports, abstracts, letters or comments, and animal research were excluded. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the included studies (15), and studies with moderate-to-high risk of bias were included (score > 6). The reasons for study exclusion are detailed in Supplementary Table 4.
Two authors (PX and ML) independently recorded the following information for each related study: first author, country, publication year, study design, sample size, sex, mean or median age, follow-up duration, FGF23 categories, outcomes reported, FGF23 measurement, RR or HR with the 95%CI, and adjusted co-founders.
In prospective studies, HR was considered to be equivalent to RR. The adjusted RR was transformed to the natural logarithms (logRR) to fit a normal distribution, and the standard errors (SElog [RR]) were calculated according to the corresponding 95%CIs. The random effects model was applied to pool the risk estimates considering the heterogeneity of different cohort studies. When an included study compared the lowest level or the reference category of FGF23 with the higher categories (≥2 categories), the highest category was regarded as the high level, while the reference level or the lowest level was regarded as the low level. For the category analysis, the summary RRs and 95%CIs were calculated by comparing the highest level of FGF23 to the lowest level of FGF23. For the dose-response analysis, the method described by Greenland and Longnecker (16) was used, with linear trends per 20 RU/mL increment of FGF23. The study-specific slopes and 95%CIs for FGF23 were calculated from the natural logs of the RRs and CIs. The FGF23 results were converted into RU/mL for all the included studies (1 RU/mL is approximately equivalent to 2 pg/mL) (17). FGF23 was also unified as a continuous variable into log base 2 transformations, interpreted as “per doubling” to calculate the corresponding summary RRs and 95%CIs. For the non-linear analysis, the robust error meta-regression method (REMR) developed by Xu and Doi (18, 19) was applied. The method requires data on the levels of FGF23 doses and RRs with variance estimates for at least two quantitative dose categories. If the levels of FGF23 doses was not directly reported, the mean or median of each FGF23 level between the upper and lower boundaries in each category was used to estimate the corresponding dose for each study. For open terminal categories, the open interval was set to the same length as that of the adjacent interval (20, 21).
The presence of heterogeneity between studies was estimated using the Cochrane Q test and the I2 statistic. For the Q statistic, P < 0.1 indicated significant heterogeneity. For the I2 statistic, <25% indicated low or no heterogeneity; 25%−50% suggested moderate heterogeneity; >50% was considered high heterogeneity (22). For those outcomes which a number of included studies over 6, pre-defined subgroups were stratified by age (≤60 years vs. >60 years), follow-up duration (≤10 years vs. >10 years), FGF23 measurement (iFGF23 vs. cFGF23). All statistical analyses were performed using Review Manager (RevMan) version 5.3 (The Cochrane Collaboration 2014; Nordic Cochrane Center Copenhagen, Denmark) and STATA (Version 16.0, Stata Corp., LP, College Station, TX, United States) software. All P-values were two-sided, and P-value < 0.05 was considered statistically significant.
We initially identified 3900 (PubMed = 798, EMBASE = 2958, Cochrane Library = 144) articles from the electronic literature search. After removing irrelevant and duplicate articles, a full-text review was performed for the remaining 87 potentially relevant studies. Ultimately, 29 articles were identified for the meta-analysis. The reasons for exclusion (n = 58) are detailed in Supplementary Table 3 and the details of the study selection are listed in Figure 1.
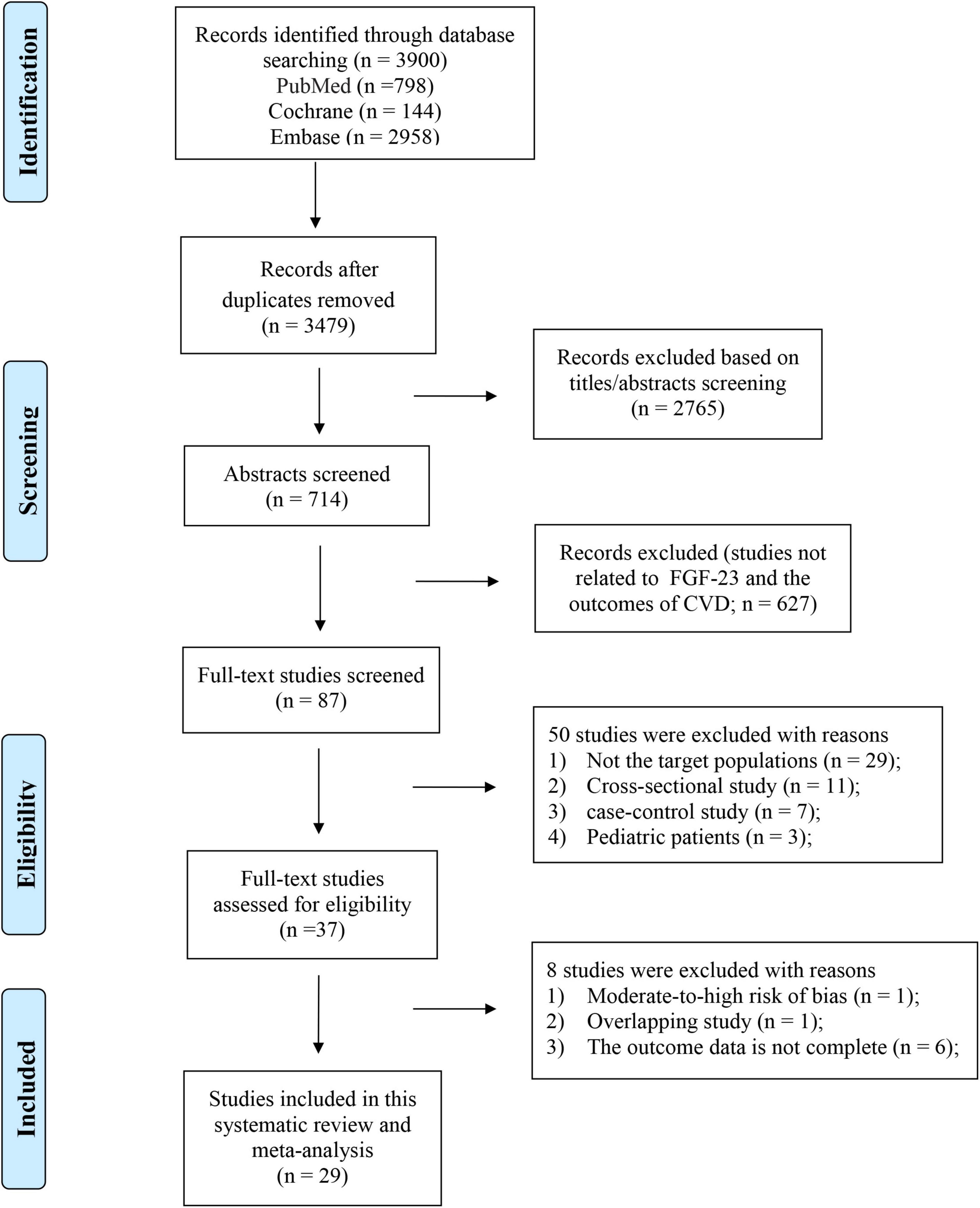
Figure 1. Flowchart of the study selection investigating the association between levels of FGF23 and risk of CVDs in the general population.
The baseline characteristics of the included studies are summarized in Table 1. Among the included studies, 23 studies reported associations between FGF23 and CVD risk in the general population: [myocardial infarction (MI) = 4, stroke = 6, heart failure (HF) = 10, CVD events = 6, sudden cardiac death (SCD) and Non-SCD = 1, atrial fibrillation (AF) = 2, hypertension = 3, coronary heart disease (CHD) = 3, left ventricular hypertrophy (LVH) = 1]. Furthermore, 11 studies investigated the association between FGF23 and all-cause mortality, and 8 studies assessed the association between FGF23 and cardiovascular mortality. In total, 18 studies presented the C-terminal FGF23 levels, and 14 studies reported intact-FGF23 levels. The sample sizes ranged from 727 to 22,127, with a total of 135,576 participants included. The duration of follow-up ranged from 1.75 years to 18.6 years. The included studies were of moderate-high quality, with NOS scores of 6 or higher (Supplementary Table 5).
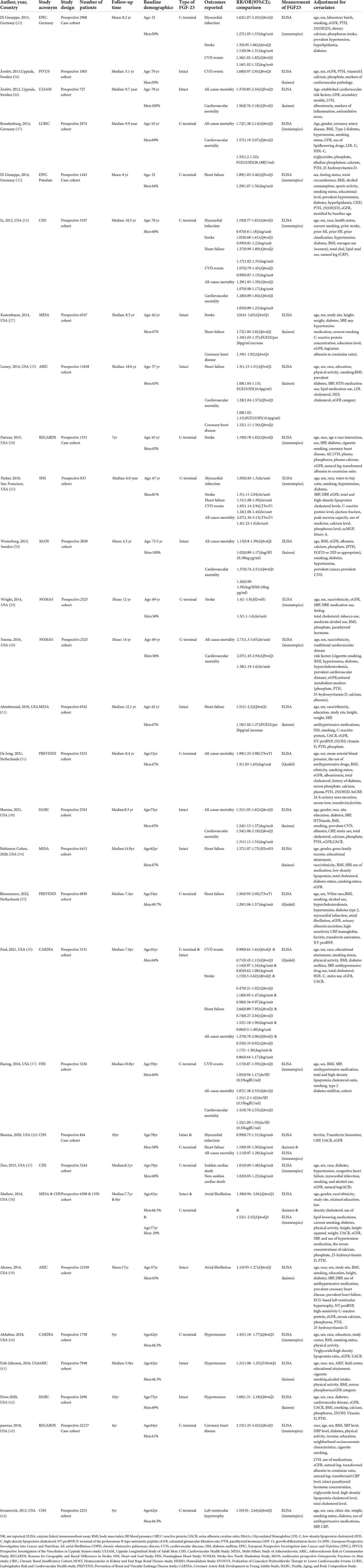
Table 1. Basic characteristics of the articles included in this systematic review and meta-analysis of FGF23 and the risk of cardiovascular diseases and mortality in the general population.
Four studies (23–26) reported the relationship between FGF23 and MI in the general population. The categorical analysis revealed that high FGF23 levels were related to increased risk of MI (RR: 1.40, 95%CI:1.03−1.89, p = 0.03; Figure 2A), with low heterogeneity (p = 0.31, I2 = 2%). Four studies reported continuous analysis (23–26); the RR of MI per doubling of FGF23 was 1.08 (95%CI: 0.94−1.25, p = 0.28; Figure 2B), with moderate heterogeneity (p = 0.22, I2 = 33%).
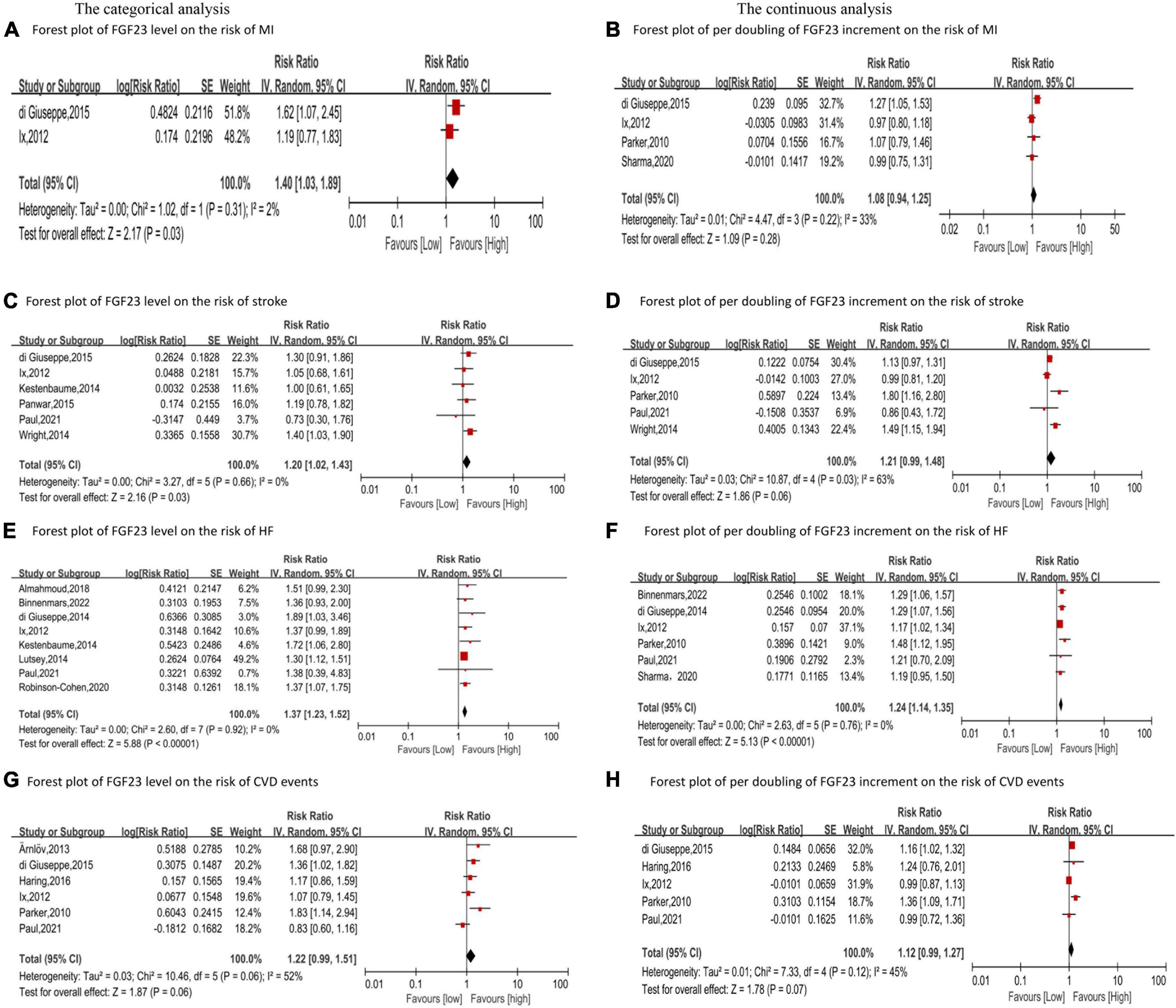
Figure 2. Forest plot for the association between FGF23 level and the risk of MI (A), stroke (C), HF (E), and CVD events (G) in the general population, analyzed as category variable, highest vs. lowest; the association between per doubling of FGF23 increment and the risk of MI (B), stroke (D), HF (F), and CVD events (H) in the general population, analyzed as a continuous variable.
Six studies analyzed the relationship between FGF23 levels and stroke (23, 24, 27–30). High FGF23 levels were related to increased risk of stroke in the categorical analysis (RR: 1.20, 95%CI: 1.02−1.43, p = 0.03; Figure 2C), without heterogeneity (p = 0.66, I2 = 0%). In the continuous analysis, the RR of stroke per doubling of FGF23 was 1.21 (95%CI: 0.99−1.48, p = 0.06; Figure 2D), with high heterogeneity (p = 0.02, I2 = 63%). Moreover, six studies performed a dose-response analysis (23, 24, 27–30), revealing a non-linear association between FGF23 and stroke (p for non-linearity = 0.10; Figure 3A).
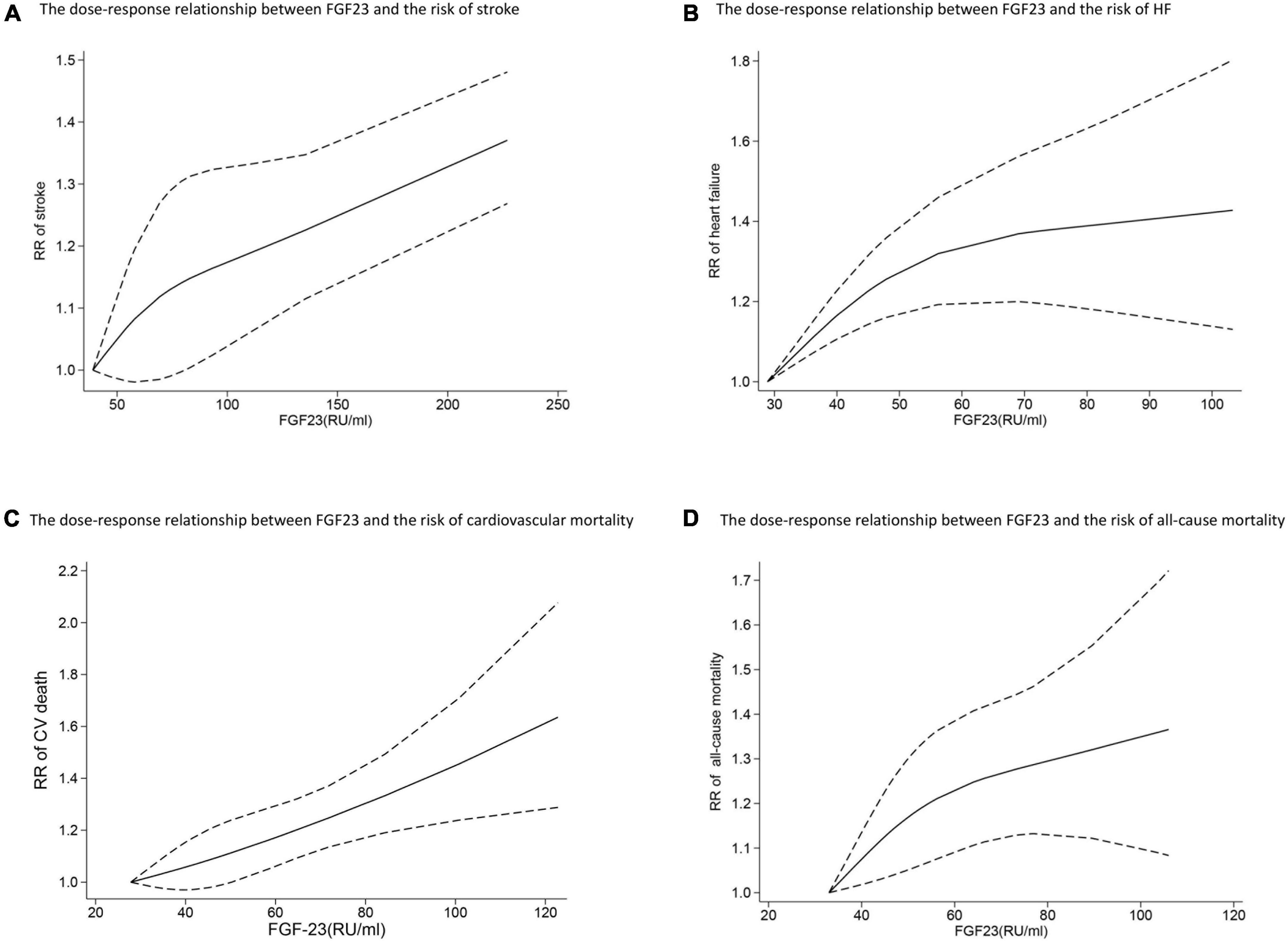
Figure 3. The dose-response relationship between FGF23 levels and the risk of stroke (A), HF (B), cardiovascular mortality (C), and all-cause mortality (D) in the general population. FGF23 levels were converted to RU/ml and the results were pooled in a one-stage random-effects model. The bold lines indicate the pooled restricted cubic spline model and the black dashed line indicates the 95% CIs of the pooled curve.
Ten studies (24–27, 30–35) reported the association between FGF23 levels and HF in general populations. A significant increase in HF risk was associated with high FGF23 levels (RR: 1.37, 95%CI: 1.23−1.52, p < 0.00001; Figure 2E), without heterogeneity (p = 0.92, I2 = 0%). In the continuous analysis, the summary RR for a 20 RU/ml increment of FGF23 was 1.25 (95%CI: 1.14−1.37, p < 0.00001; Supplementary Figure 1A), without heterogeneity (p = 0.47, I2 = 0%); and the RR of HF per doubling of FGF23 was 1.24 (95%CI: 1.14−1.35, p < 0.00001; Figure 2F), without heterogeneity (p = 0.76, I2 = 0%). In addition, eight studies carried out a dose-response analysis (24, 27, 30–35), and a potentially non-linear association of FGF23 was observed with HF (p for non-linearity = 0.001; Figure 3B).
Additionally, six studies (23–25, 30, 36, 37) considered the composite of MI, stroke, heart failure, and so on as CVD events, assessing FGF23 levels and CVD events in general populations. High FGF23 levels were related to an increased risk of CVD events (RR: 1.22, 95%CI: 0.99−1.51, p = 0.06; Figure 2G), with high heterogeneity (p = 0.06, I2 = 52%). In the continuous analysis, the RR of CVD per doubling of FGF23 was 1.12 (95%CI: 0.99−1.27, p = 0.07; Figure 2H), with moderate heterogeneity (p = 0.12, I2 = 45%).
A few studies examined the relationship between FGF23 and other cardiovascular diseases, but the results were not pooled due to the scarcity of data. As shown in Table 1, two studies (38, 39) reported the relationship between FGF23 levels and atrial fibrillation, including 2,092 cases out of 20,097 participants. Mathew et al. (38) described the association between FGF23 and AF incidence in The Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS) including 291 MESA patients (HR [quartile 4 vs. quartile 1]: 1.38) and 229 CHS patients (HR [quartile 4 vs. quartile 1]: 1.52) adjusted for potential confounding characteristics. However, Alonso et al. (39) revealed that baseline FGF23 levels were not associated with AF risk, regardless of kidney function. This study summarized data from 1,572 patients (HR [quartile 4 vs. quartile 1]:1.1) adjusted for potential confounding factors. Moreover, three studies (40–42) investigated the relationship between FGF23 levels and hypertension. The study from Akhabue et al. (40) included 618 patients and showed that elevated FGF23 levels were related to an increased risk of hypertension in fully adjusted models (RR [quartile 4 vs. quartile 1]: 1.45). In another cohort study, Fyfe-Johnson et al. (41) demonstrated that the HR for hypertension was 1.21 for decile 10 compared to quintile 1 after adjusting for demographics, behaviors, and adiposity. Drew et al. (42) reported that FGF23 was related to an increased hypertension risk after adjustments, including 576 patients (RR [quartile 4 vs. quartile 1]: 1.69).
Three studies (27, 33, 43) reported the relationship between FGF23 levels and the risk of CHD, including 2,317 cases. Panwar et al. (28) evaluated 829 patients adjusted for established CHD risk factors and kidney function, suggesting that elevated FGF23 concentrations were related to an increased CHD risk (HR [quartile 4 vs. quartile 1]: 2.15). In addition, Kestenbaum et al. (27) revealed that elevated FGF23 concentrations were related to an increased CHD risk, including 363 patients (RR [quartile 4 vs. quartile 1]: 1.39) after adjustments. Another cohort study involving 1125 patients with CHD reported similar results after adjustments (RR: 1.32).
Jovanovich et al. (44) found that FGF23 was associated with greater risk of LVH, including 310 patients (OR [quartile 4 vs. quartile 1]: 1.5) in adjusted analyses. Deo et al. (45) investigated 570 cases among the elderly population and observed that FGF23 elevations were independently associated with non-SCD (HR [quartile 4 vs. quartile 1]: 1.02) after adjustments.
Eight studies (24, 33, 37, 46–50) reported the association between FGF23 and cardiovascular mortality. The categorical analysis indicated that high FGF23 concentrations were related to increased risk of cardiovascular mortality (RR: 1.46, 95%CI: 1.29−1.65, p < 0.00001; Figure 4A), without heterogeneity (p = 0.50, I2 = 0%). In the continuous analysis, the summarized RR for a 20 RU/ml increment of FGF23 was 1.23 (95%CI: 1.15−1.32, p < 0.00001; Supplementary Figure 1B), without heterogeneity (p = 0.83, I2 = 0%); and the RR of cardiovascular mortality per doubling of FGF23 was 1.43 (95%CI: 1.09−1.88, p = 0.009; Figure 4B), with high heterogeneity (p = 0.001, I2 = 78%). Moreover, eight studies (24, 33, 37, 46–50) were included in the dose-response analysis, revealing a significant linear dose-response relationship between FGF23 levels and cardiovascular mortality (p for non-linearity = 0.73; Figure 3C).
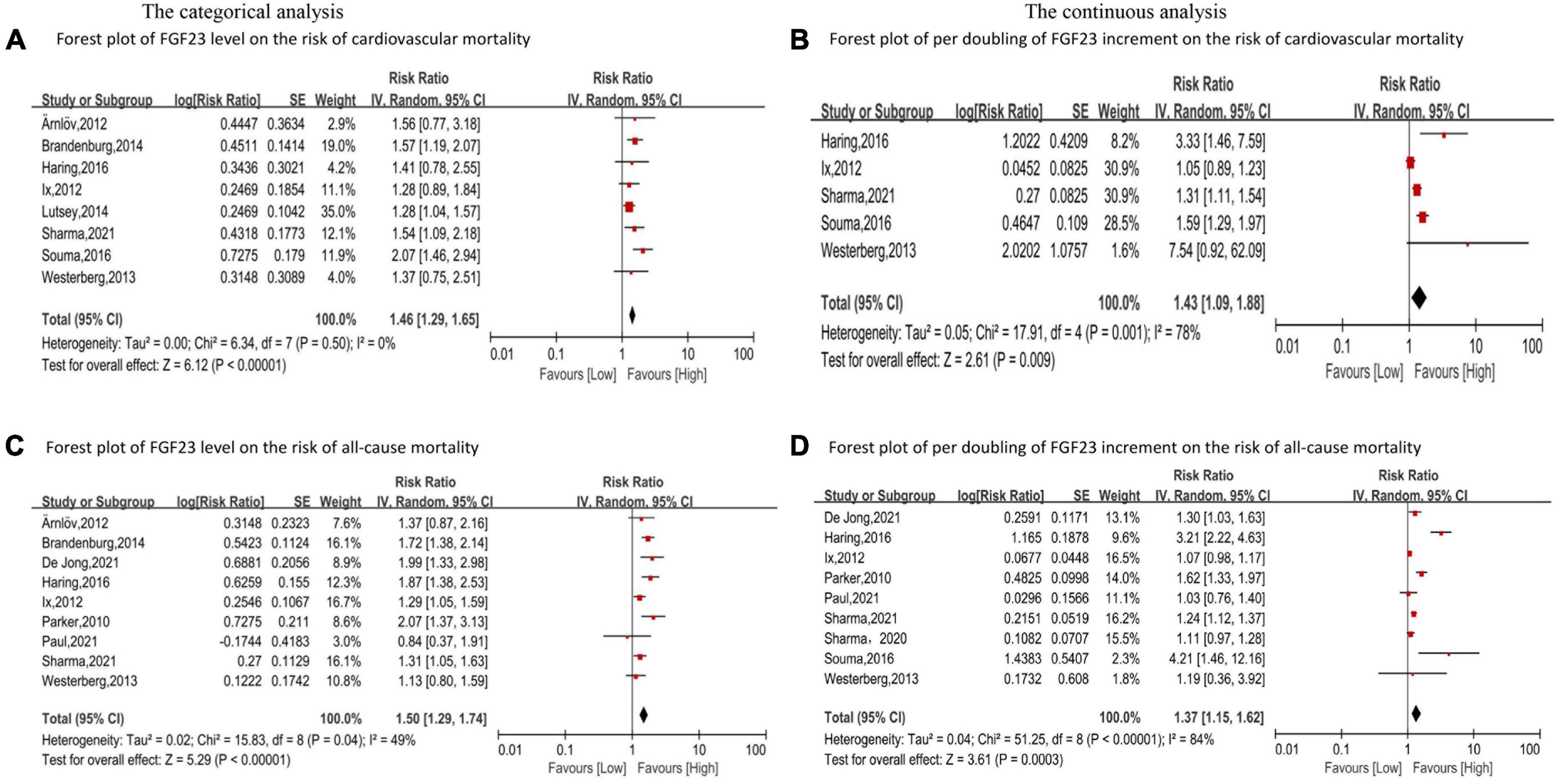
Figure 4. Forest plot for the association between FGF23 levels and the risk of cardiovascular mortality (A) and all-cause mortality (C) in the general population, analyzed as category variable, highest vs. lowest; the association between per doubling of FGF23 increment and the risk of cardiovascular mortality (B), and all-cause mortality (D) in the general population, analyzed as a continuous variable.
In addition, eleven studies (24–26, 30, 37, 46–51) evaluated the association between FGF23 and all-cause mortality in general populations. A significant increase in risk of death was associated with high FGF23 levels (RR: 1.50, 95%CI: 1.29−1.74, p < 0.00001; Figure 4C), with moderate heterogeneity (p = 0.04, I2 = 49%). The RR of all-cause mortality per doubling of FGF23 was 1.42 (95%CI: 1.37−1.15, p = 0.0003; Figure 4D), with high heterogeneity (p < 0.00001, I2 = 84%). Furthermore, nine studies were included in the dose-response analysis (24, 25, 30, 37, 46–48, 50, 51), and a non-linear association of FGF23 with all-cause mortality was observed (p for non-linearity = 0.10; Figure 3D).
Moreover, the above results were confirmed by the subgroup analysis based on age (≤60 years vs. > 60 years), follow-up duration (≤10 years vs. > 10 years), and FGF23 measurement (iFGF23 vs. cGFG23) (Supplementary Figures 2, 3).
Deleting individual studies in the sensitivity analysis did not significantly alter the pooled effect size (Supplementary Figure 4). The absence of publication bias was presented by using Egger’s test (p = 0.394 and 0.530) and a Funnel plot (Supplementary Figure 5).
The present study showed a significant relationship between FGF23 levels and the risk and mortality of CVDs in the general population. The dose-response analysis suggested a potentially non-linear relationship between FGF23 and stroke, and HF and all-cause mortality. In contrast, FGF23 levels and cardiovascular mortality exhibited a potentially linear relationship. To our knowledge, this is the first dose-response meta-analysis focusing on the association between FGF23 levels and cardiovascular diseases in general populations.
The findings were confirmed with a further analysis stratified by age (≤60 years vs. >60 years), follow-up duration (≤10 years vs. >10 years) and FGF23 types (iFGF23 vs. cGFG23). The results revealed a similar relationship between FGF23 and cardiovascular and all-cause mortality. Notably, most subgroups in this study did not exhibit heterogeneity, and no substantial changes were observed in the pooled RR when individual studies were deleted. However, moderate heterogeneity was found in all-cause mortality (I2 = 49%), and a stratified analysis was performed to explore the sources of heterogeneity. No substantial heterogeneity was detected in the intact FGF23 subgroup. The differences in variable adjustment across studies might lead to an inaccurate estimation of the effect size. Although our analysis showed moderate heterogeneity, a robust association was observed between FGF23 levels and all-cause mortality.
Gao and co-workers (10) reported that elevated FGF23 levels were related to all-cause mortality (RR: 1.25, 95%CI: 1.14−1.37) and CVDs (RR: 1.21, 95%CI: 1.13−1.39) in hemodialysis patients. These significant associations support the predictive role of FGF23 in CKD patients. Remarkably, most of the included studies had adjusted for potential confounders, including age, estimated glomerular filtration rate and other risk factors. Consequently, a significant association was observed between FGF23 levels and CVD risk and mortality. Three cohorts (24, 37, 44) did not adjust for CKD or kidney function, which were considered crucial variables in mediating the impact of FGF23 on cardiovascular disease. Nevertheless, higher FGF23 levels were still associated with CVDs and mortality after deleting the eGFR-unadjusted studies (MI RR: 1.62, 95%CI: 1.07−2.45; stroke RR: 1.24, 95%CI: 1.03−1.49; HF RR: 1.37, 95%CI: 1.23−1.53; CVD events RR: 1.32, 95%CI: 0.93−1.88; cardiovascular mortality RR: 1.50, 95%CI: 1.30−1.74; all-cause mortality RR: 1.50, 95%CI: 1.24−1.80). Collectively, our results provided compelling evidence for the close relationship of FGF23 levels with CVDs and mortality in the general population, independent of CKD status. These findings suggest that FGF23 may potentially be applied to predict the risk and mortality of CVDs, irrespective of kidney function.
Age is another vital confounding factor, and its effects on the study results should be explored as it is a well-known traditional risk factor. In most populations, the incidence of CVD increases with age. It is believed that the association between age and CVD reflects metabolic risk factors, such as elevated blood pressure, cholesterol, and diabetes (52). Moreover, two major changes with advancing age are large elastic artery stiffening and endothelial dysfunction, contributing to the development of CVD in the elderly (53). Despite adjusting for those factors, the presence of residual confounding factors such as Klotho cannot be excluded. A close association between FGF23 and klotho levels has been established. FGF23 exerts its biological effects by activating FGFRs, which are dependent on the αKlotho co-receptor. Although klotho is absent in the heart, in vivo experiments by Hu et al. (54) showed that high FGF23 concentrations induced direct cardiac toxicity in a klotho-deficient state. Serum and urinary Klotho levels are dramatically decreased during early CKD, while FGF23 levels are increased. Klotho deficiency is a pathogenic factor of CKD progression and CVD. Marcais et al. (55) suggested that evaluating FGF23 in the absence of Klotho data may overemphasize its adverse effects. Unfortunately, most studies did not consider the effect of klotho and did not conduct a separate classification analysis of klotho.
Based on the current evidence, high FGF23 levels are associated with increased risks of CVDs and mortality in the general population. Experimental data in CKD and general populations showed that FGF23 exerts direct cardiac and vascular toxicity, mediating cardiac hypertrophy, cardiac fibrosis, cardiac dysfunction, and diffuse vascular calcification by activating specific myocardial FGF receptors (FGFR) (56). Faul et al. (57) reported that injecting recombinant FGF23 into the myocardium of mice resulted in LVH, inducing a significant increase in heart weight, left ventricular wall thickness, and cross-sectional surface area of individual cardiomyocytes. Previous studies have suggested that FGF23 is associated with vascular endothelial dysfunction, arterial stiffness, and diffuse vascular calcification. In addition, the ERK1/2 signaling pathway may play an essential role in vascular calcification (58). Furthermore, FGF23 exerts indirect adverse cardiac effects, such as regulating sodium retention and excretion in the distal renal tubules, increasing the activation of the renin-angiotensin system, and the production of inflammation and oxidative stress markers (59, 60). However, the predictive effects of FGF23 remains to be demonstrated.
Up to now, several meta-analyses have explored the relationship between FGF23 and CVDs (10–13). The predictive value of FGF23 in CKD patients has been extensively summarized. Cheng et al. (13) concluded that high FGF23 levels were related to all-cause mortality (RR 1.46, 95% CI 1.38−1.55, p < 0.001), CVD (RR 1.37, 95% CI 1.15−1.63, p < 0.001) and renal events (RR 1.31, 95% CI 1.07−1.59, p = 0.008) in pre-dialysis CKD patients. Marthi et al. (11) described the association between FGF23 levels and CVDs in the general population, but did not perform a dose-response analysis. Our study extends previous findings and further clarifies the potential dose-response association between FGF23 and CVD risk and mortality in the general population.
Theoretically, FGF23 may be applied in the identification of high-risk individuals and could be a novel target to reduce the incidence of cardiovascular events. Phosphate binders, FGF23 antibodies, and FGFR blockers are currently the key therapeutic options. Studies have proposed that circulating FGF23 levels are related to dietary phosphate (Pi) intake levels in healthy people. Consequently, reducing the absorption of dietary phosphate can hypothetically decrease the circulating FGF23 concentrations. Commonly used phosphorus binders include Ca2+-containing binders, aluminum-containing binders and non-Ca2+ or Ca2+-free phosphate binders. However, reducing the absorption of dietary phosphate by phosphate binders or combination therapy only results in modest decreases in FGF23 levels and yields short-lived effects. Whether this is caused by increased intestinal total phosphorus absorption or medication resistance is unclear (61).
The mechanisms regulating FGF23 synthesis are poorly understood. Blocking the main FGFR isoform FGFR4 may reduce the cardiotoxic effects of FGF23, but does not affect its physiological functions. The safety of this method in cardiovascular diseases has already been demonstrated in clinical trials. Conversely, FGF23 antibodies might cause greater side effects than clinical benefits in patients with renal dysfunction (56). Optimally, low FGF23 levels should be maintained while blocking the non-target effects, as opposed to completely depleting it (62).
Traditional biomarkers such as troponin I and T have been widely used in clinical practice for the diagnosis of MI. FGF23, as a novel candidate biomarker of cardiovascular risk, is positively correlated with classical biomarkers of cardiac damage but does not directly depend on them (56). In addition, the combination of these biomarkers has been shown to have a significantly higher predictive value for cardiovascular risk assessment than individually (56). The ankle–brachial index (ABI) can be used to predict the risk of CVD and CHD events and is inexpensive, easily accessible, and non-invasive. However, its sensitivity and specificity still need to be explored (63). The coronary artery calcium score reflects the load of coronary calcification and the degree of coronary atherosclerosis. It is measured by cardiac computer tomography and requires patients to be exposed to ionizing radiation, which is particularly unpopular among young subjects, especially women. This technique is more time-consuming and is also limited by its relatively high cost (64). In contrast, FGF23 concentrations are easily obtainable from the patients’ serum at a low cost, which could prove particularly valuable in emergency situations. Therefore, FGF23 can be used as an early and complementary predictor of adverse cardiac events.
Additionally, FGF23 may potentially predict the prognosis of cardiovascular diseases. Song et al. (65) have reported that FGF23 can independently predict the risk of in-stent restenosis in coronary heart disease patients who underwent PCI with a drug-eluting stent. Cornelissen et al. (66) showed similar accuracy in prognosis estimation between assessing FGF23 levels and the well-established Seattle Heart Failure (SHF) model in patients hospitalized for acute HF. Further understanding of the molecular mechanisms of FGF23 in the cardiovascular system will assist in developing and implementing new therapeutic strategies and prognosis estimation.
This review only included prospective studies, avoiding recall bias. Most of the included studies had a large sample size and had adjusted for potential confounding factors, such as age, gender, race, smoking, BMI, and basic disease histories. Nevertheless, the limitations of this study should be acknowledged. Firstly, our results were based on observational studies and a causal relationship cannot be confirmed. The residual confounding factors and the unmeasured factors could not be ruled out completely due to the inherent nature of observational research. Secondly, the majority of the included studies were performed in the United States or Europe, and the applicability of our findings to the Asian population requires further research. Finally, some studies may have included CKD patients in the general population, affecting the reliability of our results.
Overall, the increased serum FGF23 levels were associated with increased risks of CVDs and mortality in general populations. There was a potentially non-linear relationship between FGF23 and stroke, HF and all-cause mortality, whereas a potentially linear relationship between FGF23 and cardiovascular mortality was observed. Additional studies are needed to clarify the mechanism between FGF23 and CVDs in the general population. The clinical application of FGF23 levels to predict CVD risk requires further research.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
XL and PY were responsible for the entire project and revised the draft. PX, YZ, and ML performed the data extraction and statistical analysis, drafted the first version of the manuscript, and interpreted the data. All authors participated in the interpretation of the results and prepared the final version of the manuscript.
This work was supported by a grant from the Natural Science Foundation of Jiangxi Province (Nos. 20192ACBL21037, 202004BCJL23049, and 202002BAB21 6022 to JZ), the National Natural Science Foundation of China (Nos. 82160371 to JZ, 82100869 to PY, 21866019 to JM, and 82100347 to XL), the China Postdoctoral Science Foundation (No. 2021M703724 to XL), the Natural Science Foundation of Guangdong Province (No. 2022A1515010582 to XL), and the Science and Technology Projects in Guangzhou (202102010007 to JW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.989574/full#supplementary-material
1. Laslett LJ, Alagona P Jr., Clark BA III, Drozda JP Jr., Saldivar F, Wilson SR, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. (2012) 60(25 Suppl):S1–49. doi: 10.1016/j.jacc.2012.11.002
2. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16:203–12. doi: 10.1038/s41569-018-0119-4
3. Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. (2012) 318:1040–8. doi: 10.1016/j.yexcr.2012.02.027
4. Negri AL. Fibroblast growth factor 23: associations with cardiovascular disease and mortality in chronic kidney disease. Int Urol Nephrol. (2014) 46:9–17. doi: 10.1007/s11255-012-0370-2
5. Musgrove J, Wolf M. Regulation and effects of FGF23 in chronic kidney disease. Annu Rev Physiol. (2020) 82:365–90. doi: 10.1146/annurev-physiol-021119-034650
6. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. (2012) 82:737–47. doi: 10.1038/ki.2012.176
7. Wahl P, Wolf M. FGF23 in chronic kidney disease. Adv Exp Med Biol. (2012) 728:107–25. doi: 10.1007/978-1-4614-0887-1_8
8. Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. (2011) 79:1370–8. doi: 10.1038/ki.2011.47
9. Bansal N. Evolution of cardiovascular disease during the transition to end-stage renal disease. Semin Nephrol. (2017) 37:120–31. doi: 10.1016/j.semnephrol.2016.12.002
10. Gao S, Xu J, Zhang S, Jin J. Meta-analysis of the association between fibroblast growth factor 23 and mortality and cardiovascular events in hemodialysis patients. Blood Purif. (2019) 47(Suppl 1):24–30. doi: 10.1159/000496220
11. Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, et al. Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: a meta-analysis. J Am Soc Nephrol. (2018) 29:2015–27. doi: 10.1681/ASN.2017121334
12. Qin Z, Liu X, Song M, Zhou Q, Yu J, Zhou B, et al. Fibroblast growth factor 23 as a predictor of cardiovascular and all-cause mortality in prospective studies. Atherosclerosis. (2017) 261:1–11. doi: 10.1016/j.atherosclerosis.2017.03.042
13. Xue C, Yang B, Zhou C, Dai B, Liu Y, Mao Z, et al. Fibroblast growth factor 23 predicts all-cause mortality in a dose-response fashion in pre-dialysis patients with chronic kidney disease. Am J Nephrol. (2017) 45:149–59. doi: 10.1159/000454959
14. Umer A, Kelley GA, Cottrell LE, Giacobbi P Jr., Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. (2017) 17:683. doi: 10.1186/s12889-017-4691-z
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
17. Chan GC, Divers J, Russell GB, Langefeld CD, Wagenknecht LE, Hsu FC, et al. FGF23 concentration and APOL1 genotype are novel predictors of mortality in African Americans with type 2 diabetes. Diabetes Care. (2018) 41:178–86. doi: 10.2337/dc17-0820
18. Xu C, Doi SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. (2018) 16:138–44. doi: 10.1097/XEB.0000000000000132
19. Xu C, Thabane L, Liu T, Borhan A, Sun X. Flexible piecewise linear model for investigating dose-response relationship in meta-analysis: methodology, examples, and comparison. J Evid Based Med. (2019) 12:63–8. doi: 10.1111/jebm.12339
20. Liu X, Guo L, Xiao K, Zhu W, Liu M, Wan R, et al. The obesity paradox for outcomes in atrial fibrillation: evidence from an exposure-effect analysis of prospective studies. Obes Rev. (2020) 21:e12970. doi: 10.1111/obr.12970
21. Xu C, Liu Y, Jia PL, Li L, Liu TZ, Cheng LL, et al. The methodological quality of dose-response meta-analyses needed substantial improvement: a cross-sectional survey and proposed recommendations. J Clin Epidemiol. (2019) 107:1–11. doi: 10.1016/j.jclinepi.2018.11.007
22. Larney S, Tran LT, Leung J, Santo T Jr., Santomauro D, Hickman M, et al. All-cause and cause-specific mortality among people using extramedical opioids: a systematic review and meta-analysis. JAMA Psychiatry. (2020) 77:493–502. doi: 10.1001/jamapsychiatry.2019.4170
23. Di Giuseppe R, Kühn T, Hirche F, Buijsse B, Dierkes J, Fritsche A, et al. Plasma fibroblast growth factor 23 and risk of cardiovascular disease: results from the EPIC-Germany case-cohort study. Eur J Epidemiol. (2015) 30:131–41. doi: 10.1007/s10654-014-9982-4
24. Ix JH, Katz R, Kestenbaum BR, De Boer IH, Chonchol M, Mukamal KJ, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. (2012) 60:200–7. doi: 10.1161/circ.125.suppl_10.AP241
25. Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the heart and soul study. Ann Internal Med. (2010) 152:640–8. doi: 10.7326/0003-4819-152-10-201005180-00004
26. Sharma S, Katz R, Bullen AL, Chaves PHM, De Leeuw PW, Kroon AA, et al. Intact and c-terminal FGF23 assays-do kidney function, inflammation, and low iron influence relationships with outcomes? J Clin Endocrinol Metab. (2020) 105:e4875–85. doi: 10.1210/clinem/dgaa665
27. Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. (2014) 7:409–17. doi: 10.1161/CIRCHEARTFAILURE.113.000952
28. Panwar B, Jenny NS, Howard VJ, Wadley VG, Muntner P, Kissela BM, et al. Fibroblast growth factor 23 and risk of incident stroke in community-living adults. Stroke. (2015) 46:322–8. doi: 10.1161/STROKEAHA.114.007489
29. Wright CB, Dong C, Stark M, Silverberg S, Rundek T, Elkind MSV, et al. Plasma FGF23 and the risk of stroke: the northern manhattan study (NOMAS). Neurology. (2014) 82:1700–6. doi: 10.1212/WNL.0000000000000410
30. Paul S, Wong M, Akhabue E, Mehta RC, Kramer H, Isakova T, et al. Fibroblast growth factor 23 and incident cardiovascular disease and mortality in middle-aged adults. J Am Heart Assoc. (2021) 10:e020196. doi: 10.1161/JAHA.120.020196
31. Almahmoud MF, Soliman EZ, Bertoni AG, Kestenbaum B, Katz R, Lima JAC, et al. Fibroblast growth factor-23 and heart failure with reduced versus preserved ejection fraction: MESA. J Am Heart Assoc. (2018) 7:e008334. doi: 10.1161/JAHA.117.008334
32. Di Giuseppe RD, Buijsse B, Hirche F, Wirth J, Arregui M, Westphal S, et al. Plasma fibroblast growth factor 23, parathyroid hormone, 25-hydroxyvitamin D3, and risk of heart failure: a prospective, case-cohort study. J Clin Endocrinol Metab. (2014) 99:947–55. doi: 10.1210/jc.2013-2963
33. Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the atherosclerosis risk in communities study. J Am Heart Assoc. (2014) 3:e000936. doi: 10.1161/JAHA.114.000936
34. Robinson-Cohen C, Shlipak M, Sarnak M, Katz R, Peralta C, Young B, et al. Impact of race on the association of mineral metabolism with heart failure: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metabol. (2020) 105:e1144–51. doi: 10.1210/clinem/dgz218
35. Binnenmars SH, Hoogslag GE, Yeung SMH, Brouwers FP, Bakker SJL, van Gilst WH, et al. Fibroblast growth factor 23 and risk of new onset heart failure with preserved or reduced ejection fraction: the PREVEND Study. J Am Heart Assoc. (2022) 11:e024952. doi: 10.1161/JAHA.121.024952
36. Ärnlöv J, Carlsson AC, Sundstrom J, Ingelsson E, Larsson A, Lind L, et al. Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin J Am Soc Nephrol. (2013) 8:781–6. doi: 10.2215/CJN.09570912
37. Haring R, Enserro D, Xanthakis V, Mitchell GF, Benjamin EJ, Hamburg NM, et al. Plasma fibroblast growth factor 23: clinical correlates and association with cardiovascular disease and mortality in the framingham heart study. J Am Heart Assoc. (2016) 5:e003486. doi: 10.1161/JAHA.116.003486
38. Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, et al. Fibroblast growth factor-23 and incident atrial fibrillation: the multi-ethnic study of atherosclerosis (MESA) and the cardiovascular health study (CHS). Circulation. (2014) 130:298–307. doi: 10.1161/CIRCULATIONAHA.113.005499
39. Alonso A, Misialek JR, Eckfeldt JH, Selvin E, Coresh J, Chen LY, et al. Circulating fibroblast growth factor-23 and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. (2014) 3:e001082. doi: 10.1161/JAHA.114.001082
40. Akhabue E, Montag S, Reis JP, Pool LR, Mehta R, Yancy CW, et al. FGF23 (Fibroblast Growth Factor-23) and incident hypertension in young and middle-aged adults: the CARDIA study. Hypertension. (2018) 72:70–6. doi: 10.1161/HYPERTENSIONAHA.118.11060
41. Fyfe-Johnson AL, Alonso A, Selvin E, Bower JK, Pankow JS, Agarwal SK, et al. Serum fibroblast growth factor-23 and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. J Hypertension. (2016) 34:1266–72. doi: 10.1097/HJH.0000000000000936
42. Drew DA, Katz R, Kritchevsky S, Ix JH, Shlipak MG, Newman AB, et al. Fibroblast growth factor 23 and blood pressure in older adults: the health, aging, and body composition study. Hypertension. (2020) 76:236–43. doi: 10.1161/HYPERTENSIONAHA.120.14703
43. Panwar B, Judd SE, Wadley VG, Jenny NS, Howard VJ, Safford MM, et al. Association of fibroblast growth factor 23 with risk of incident coronary heart disease in community-living adults. JAMA Cardiol. (2018) 3:318–25. doi: 10.1001/jamacardio.2018.0139
44. Jovanovich A, Ix JH, Gottdiener J, McFann K, Katz R, Kestenbaum B, et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. (2013) 231:114–9. doi: 10.1016/j.atherosclerosis.2013.09.002
45. Deo R, Katz R, De Boer IH, Sotoodehnia N, Kestenbaum B, Mukamal KJ, et al. Fibroblast growth factor 23 and sudden versus non-sudden cardiac death: the cardiovascular health study. Am J Kidney Dis. (2015) 66:40–6. doi: 10.1053/j.ajkd.2014.10.025
46. Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. (2013) 83:160–6. doi: 10.1038/ki.2012.327
47. Brandenburg VM, Kleber ME, Vervloet MG, Tomaschitz A, Pilz S, Stojakovic T, et al. Fibroblast growth factor 23 (FGF23) and mortality: the ludwigshafen risk and cardiovascular health study. Atherosclerosis. (2014) 237:53–9. doi: 10.1016/j.atherosclerosis.2014.08.037
48. Sharma S, Katz R, Dubin RF, Drew DA, Gutierrez OM, Shlipak MG, et al. FGF23 and cause-specific mortality in community-living individuals—the health, aging, and body composition study. J Am Geriatr Soc. (2021) 69:711–7. doi: 10.1111/jgs.16910
49. Souma N, Isakova T, Lipiszko D, Sacco RL, Elkind MSV, DeRosa JT, et al. Fibroblast growth factor 23 and cause-specific mortality in the general population: the northern Manhattan study. J Clin Endocrinol Metab. (2016) 101:3779–86. doi: 10.1210/jc.2016-2215
50. Westerberg PA, Tivesten Å, Karlsson MK, Mellström D, Orwoll E, Ohlsson C, et al. Fibroblast growth factor 23, mineral metabolism and mortality among elderly men (Swedish MrOs). BMC Nephrol. (2013) 14:85. doi: 10.1186/1471-2369-14-85
51. De Jong MA, Eisenga MF, Van Ballegooijen AJ, Beulens JWJ, Vervloet MG, Navis G, et al. Fibroblast growth factor 23 and new-onset chronic kidney disease in the general population: the prevention of renal and vascular endstage disease (PREVEND) study. Nephrol Dialysis Transpl. (2021) 36:121–8. doi: 10.1093/ndt/gfz266
52. Singh GM, Danaei G, Pelizzari PM, Lin JK, Cowan MJ, Stevens GA, et al. The age associations of blood pressure, cholesterol, and glucose: analysis of health examination surveys from international populations. Circulation. (2012) 125:2204–11. doi: 10.1161/CIRCULATIONAHA.111.058834
53. Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. (2018) 123:825–48. doi: 10.1161/CIRCRESAHA.118.312563
54. Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. (2015) 26:1290–302. doi: 10.1681/ASN.2014050465
55. Marçais C, Maucort-Boulch D, Drai J, Dantony E, Carlier MC, Blond E, et al. Circulating klotho associates with cardiovascular morbidity and mortality during hemodialysis. J Clin Endocrinol Metab. (2017) 102:3154–61. doi: 10.1210/jc.2017-00104
56. Vázquez-Sánchez S, Poveda J, Navarro-García JA, González-Lafuente L, Rodríguez-Sánchez E, Ruilope LM, et al. An Overview of FGF-23 as a novel candidate biomarker of cardiovascular risk. Front Physiol. (2021) 12:632260. doi: 10.3389/fphys.2021.632260
57. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Investig. (2011) 121:4393–408. doi: 10.1172/JCI46122
58. Ding HY, Ma HX. Significant roles of anti-aging protein klotho and fibroblast growth factor23 in cardiovascular disease. J Geriatr Cardiol. (2015) 12:439–47.
59. Leifheit-Nestler M, Haffner D. Paracrine effects of FGF23 on the heart. Front Endocrinol. (2018) 9:278. doi: 10.3389/fendo.2018.00278
61. Law JP, Price AM, Pickup L, Radhakrishnan A, Weston C, Jones AM, et al. Clinical potential of targeting fibroblast growth factor-23 and αklotho in the treatment of uremic cardiomyopathy. J Am Heart Assoc. (2020) 9:e016041. doi: 10.1161/JAHA.120.016041
62. Lu X, Hu MC. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis. (2017) 3:15–23. doi: 10.1159/000452880
63. Murphy TP, Dhangana R, Pencina MJ, D’Agostino RB Sr. Ankle-brachial index and cardiovascular risk prediction: an analysis of 11,594 individuals with 10-year follow-up. Atherosclerosis. (2012) 220:160–7. doi: 10.1016/j.atherosclerosis.2011.10.037
64. Faggiano A, Santangelo G, Carugo S, Pressman G, Picano E, Faggiano P. Cardiovascular calcification as a marker of increased cardiovascular risk and a surrogate for subclinical atherosclerosis: role of echocardiography. J Clin Med. (2021) 10:1668. doi: 10.3390/jcm10081668
65. Song T, Fu Y, Wang Y, Li W, Zhao J, Wang X, et al. FGF-23 correlates with endocrine and metabolism dysregulation, worse cardiac and renal function, inflammation level, stenosis degree, and independently predicts in-stent restenosis risk in coronary heart disease patients underwent drug-eluting-stent PCI. BMC Cardiovasc Disord. (2021) 21:24. doi: 10.1186/s12872-020-01839-w
Keywords: FGF23, cardiovascular diseases, myocardial infarction, stroke, heart failure, mortality, meta-analysis
Citation: Liu M, Xia P, Tan Z, Song T, Mei K, Wang J, Ma J, Jiang Y, Zhang J, Zhao Y, Yu P and Liu X (2022) Fibroblast growth factor-23 and the risk of cardiovascular diseases and mortality in the general population: A systematic review and dose-response meta-analysis. Front. Cardiovasc. Med. 9:989574. doi: 10.3389/fcvm.2022.989574
Received: 08 July 2022; Accepted: 06 October 2022;
Published: 03 November 2022.
Edited by:
Toshiaki Nakano, Kyushu University, JapanReviewed by:
Peng-Yu Zhong, Nanchong Central Hospital, ChinaCopyright © 2022 Liu, Xia, Tan, Song, Mei, Wang, Ma, Jiang, Zhang, Zhao, Yu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujie Zhao, MTg0MjMxODkyQHFxLmNvbQ==; Peng Yu, eXVwZW5nX2p4bmRlZnlAMTYzLmNvbQ==; Xiao Liu, bGl1eDU4N0BtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.