
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 29 November 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.983308
This article is part of the Research Topic Insights in Heart Valve Disease: 2022 View all 10 articles
 Francesca Graziani1†
Francesca Graziani1† Giulia Iannaccone2†
Giulia Iannaccone2† Maria Chiara Meucci1
Maria Chiara Meucci1 Rosa Lillo1,2
Rosa Lillo1,2 Angelica Bibiana Delogu2,3
Angelica Bibiana Delogu2,3 Maria Grandinetti1
Maria Grandinetti1 Gianluigi Perri4
Gianluigi Perri4 Lorenzo Galletti4
Lorenzo Galletti4 Antonio Amodeo4
Antonio Amodeo4 Gianfranco Butera5
Gianfranco Butera5 Aurelio Secinaro6
Aurelio Secinaro6 Antonella Lombardo1,2
Antonella Lombardo1,2 Gaetano Antonio Lanza1,2
Gaetano Antonio Lanza1,2 Francesco Burzotta1,2*
Francesco Burzotta1,2* Filippo Crea1,2
Filippo Crea1,2 Massimo Massetti1,2
Massimo Massetti1,2Background: The clinical impact of valvular heart disease (VHD) in adult congenital heart disease (ACHD) patients is unascertained. Aim of our study was to assess the prevalence and clinical impact of severe VHD (S-VHD) in a real-world contemporary cohort of ACHD patients.
Materials and methods: Consecutive patients followed-up at our ACHD Outpatient Clinic from September 2014 to February 2021 were enrolled. Clinical characteristics and echocardiographic data were prospectively entered into a digitalized medical records database. VHD at the first evaluation was assessed and graded according to VHD guidelines. Clinical data at follow-up were collected. The study endpoint was the occurrence of cardiac mortality and/or unplanned cardiac hospitalization during follow-up.
Results: A total of 390 patients (median age 34 years, 49% males) were included and S-VHD was present in 101 (25.9%) patients. Over a median follow-up time of 26 months (IQR: 12–48), the study composite endpoint occurred in 76 patients (19.5%). The cumulative endpoint-free survival was significantly lower in patients with S-VHD vs. patients with non-severe VHD (Log rank p < 0.001). At multivariable analysis, age and atrial fibrillation at first visit (p = 0.029 and p = 0.006 respectively), lower %Sat O2, higher NYHA class (p = 0.005 for both), lower LVEF (p = 0.008), and S-VHD (p = 0.015) were independently associated to the study endpoint. The likelihood ratio test demonstrated that S-VHD added significant prognostic value (p = 0.017) to a multivariate model including age, severe CHD, atrial fibrillation, %Sat O2, NYHA, LVEF, and right ventricle systolic pressure > 45 mmHg.
Conclusion: In ACHD patients, the presence of S-VHD is independently associated with the occurrence of cardiovascular mortality and hospitalization. The prognostic value of S-VHD is incremental above other established prognostic markers.
The improvement of neonatal and pediatric cardiac care have led to the progressive increase of children with congenital heart disease (CHD) surviving to late adulthood (1), with a significant increase in the healthcare burden worldwide (2). Most adults with CHD (ACHD) retain a lifelong risk of cardiovascular complications, which is related both to the original defects and the possible sequelae of the cardiac surgery performed in childhood. Consequently, the risk of hospitalizations and mortality in ACHD patients remains higher than that of the general population (3, 4). Arrhythmias, heart failure (HF) and need for interventions on valvular heart diseases (VHD) are often part of the ACHD clinical history (5). Notably, in the large spectrum of ACHD, VHD are frequently encountered as primitive congenital lesions, post-surgery sequelae or as acquired new lesions (5).
Although the presence of VHD in ACHD represents a clinical challenge, most data on their relevance come from registries (6) and surveys (7) where the characterization of VHD was limited and their prognostic impact was not ascertained.
In the present study, we assessed the prevalence and clinical impact of severe VHD (S-VHD) in a real-world contemporary cohort of ACHD patients.
This is a single center observational clinical study reporting data that have been prospectively collected within the framework of clinical pathway dedicated to ACHD patients at Fondazione Policlinico Universitario “A. Gemelli,” which is a surgical/interventional tertiary Center and represents a national referral for ACHD patients and for heart valve disease.
For the present study, all patients evaluated in our ACHD outpatient clinic from September 2014 to February 2021 were screened. Patients with a patent foramen ovale, cardiomyopathies and congenital arrhythmias without any structural abnormalities were excluded. CHD distribution across patients population is depicted in Supplementary Table 1. Among the remaining ACHD patients, those who were treated by other Institutions and referred to our center just for a single consultation were also excluded.
Clinical, imaging and operation details were prospectively entered into a digitalized medical records database dedicated to cardiovascular patients (SI-cardio, GESI, Rome, Italy). From the above-mentioned database, we obtained the report of the first clinical outpatient evaluation that included complete medical history, vital signs, electrocardiogram (ECG), complete echocardiography. According to our institutional clinical pathway dedicated to ACHD patients (8), clinical assessment, ECG reading, and echocardiograms are directly performed and reported from experienced cardiologists specialized in the imaging and care of this patients’ population (FG, AD, and RL) and all patients that require an intervention are discussed in Heart Team, as previously described (9).
Comphrensive echocardiography was performed using commercially available ultrasound systems (Toshiba Artida, Tokyo, Japan; Philips Epiq 7, Amsterdam, Netherlands) equipped with 3.5 MHz or M5S transducers as previously reported (10) and was also used to record the presence and degree of VHD.
For each enrolled patient the clinical records were revised to extract the following data:
- Clinical findings: age, gender, CHD diagnosis at birth, number of cardiac interventions performed before our first evaluation, age at cardiac intervention(s), presence of genetic syndrome, severity of the CHD lesion (assessed according to the classification proposed in the latest ESC Guidelines) (11), previous pacemaker or implantable cardioverter defibrillator (PM/ICD) implantation, O2 saturation at rest (%Sat O2), New York Heart Association (NYHA) functional class, symptoms.
- ECG findings: rhythm; PR interval (msec); presence of right or left bundle branch block; QRS interval (msec).
- Echocardiographic findings: left ventricle ejection fraction (LVEF) assessed with biplane Simpson’s method (applied also for the evaluation of the systemic right ventricular function and the systolic function of single ventricle physiology patients), right ventricle systolic function expressed by tricuspid annular plane systolic excursion (TAPSE), parameters of diastolic function (E/A; E/e’, left atrium volume index, LAVi), right ventricle systolic pressure (RVSP), degree of valvular stenosis or regurgitation as defined by a multiparametric approach according to the current best practices recommended for VHD patients (12–15).
All forms of VHD were included: primary valvular disease (bicuspid aortic valve, Ebstein’s anomaly, etc.), valvular lesions secondary to the sequelae of the intervention performed for CHD (pulmonary regurgitation from repaired Tetralogy of Fallot, valvular insufficiency from previous valvulotomy etc.) as well as functional VHD (atrioventricular valve regurgitation from systemic right ventricle, univentricular heart or Fontan repair etc.) and percutaneous/surgical prosthesis dysfunction (11).
Severe valvular heart disease (S-VHD) was defined as the presence of at least one valve with severe dysfunction according to the latest guidelines. The grading of the severity of VHD is reported in Supplementary Table 2. Multiple VHD was defined as the presence of more than one valve with severe lesion. Severe VHD distribution according to the etiology has been depicted in Supplementary Table 3.
Clinical data at follow-up were collected through the medical records of the last evaluation at our ACHD Outpatient Clinic, or at the last hospital admission or by phone contact.
The endpoint of the study was the composite of cardiac death and/or cardiac hospitalization. Cardiac death was defined as any death without clear non-cardiac cause. Cardiac hospitalization was defined as any hospitalization due to heart failure and/or arrhythmias.
The occurrence of major arrhytmias not requiring hospitalization as well as the rate of cardiac interventions (percutaneous or surgical) were also recorded.
The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Ethical Committee (protocol number 4742).
Continuous variables normally distributed are presented as mean ± standard deviation whereas non-normally distributed data are presented as median and interquartile range (IQR). Categorical variables are expressed as frequencies and percentages.
The comparison of baseline characteristics between patients with S-VHD (no severe VHD: NS-VHD) was performed by the unpaired Student’s T test (for normally distributed continuous variables), Mann–Whitney U test (for non-normally distributed continuous variables) and Chi-square test or Fisher’s exact test, as appropriate (for categorical variables).
Cumulative event-free survival rates for the population, stratified by the presence of severe VHD, were calculated using the Kaplan–Meier method. The log-rank test was used to compare the two groups. Cox proportional hazards regression analysis was used to assess the association between clinical and echocardiographic parameters with the composite study endpoint. Exposure to percutaneous or surgical interventions was included in the analysis as a binary time-dependent term. The hazard ratio (HR) and 95% confidence intervals (CIs) were calculated for each variable. The proportional hazards assumption was verified based on Schoenfeld residuals. The entry criteria for the multivariate regression analysis were a significant association in univariate analysis (p < 0.05) and an amount of missing values that did not exceed 5% of the total study population. A minimum tolerance level of 0.5 was established to avoid multicollinearity between covariates. Moreover, to investigate the incremental prognostic value of S-VHD on the top of variables included in the multivariate analysis, the likelihood ratio test for nested models was performed. The change in global Chi-square was calculated and reported.
As secondary analysis, the occurrence of cardiovascular mortality and cardiovascular hospitalization under medical management (censoring patients at the time of interventions) was also performed, using Cox proportional hazard regression analysis.
All tests were two-sided and p-values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) and R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
During the study period, 596 patients were evaluated in our ACHD Outpatient Clinic. After exclusion of patients with patent foramen ovale, cardiomyopathies or congenital arrhythmias, and of those who had a single consultation, 422 ACHD patients were eligible for the study. Out of them, 32 patients had a follow-up time < 12 months and were excluded from the present study. Thus, a total of 390 patients constituted the study population. The flow chart of the study is graphically summarized in Figure 1.
The clinical characteristics of the study population are reported in Table 1. Overall, median age was 34 years (range 26–46) and 189 (49%) patients were male. Most patients (n = 256, 66%) underwent corrective or palliative procedure during childhood and 30 (8%) had a PM/ICD implantation. At our first evaluation, 146 (37%) patients presented with NYHA functional class > II and 22 (6%) had atrial flutter/atrial fibrillation (Af/Afib).
Nearby half of the population had moderate CHD (191 patients, 49%) and CHD was graded as severe in 40 patients (10%). Supplementary Figure 1 shows the distribution of severe CHD.
Severe valvular heart disease (S-VHD) was present in 101 patients (25.9%). The pulmonary valve was the valve more frequently affected by severe stenosis and/or regurgitation (n = 42, 40.5%), while the single most common valve lesion was severe tricuspid regurgitation (n = 31, 30.7%). The distribution of severe valvular lesions is illustrated in Figure 2. Among patients with S-VHD, 36 underwent a surgical procedure and 6 patients a percutaneous intervention.
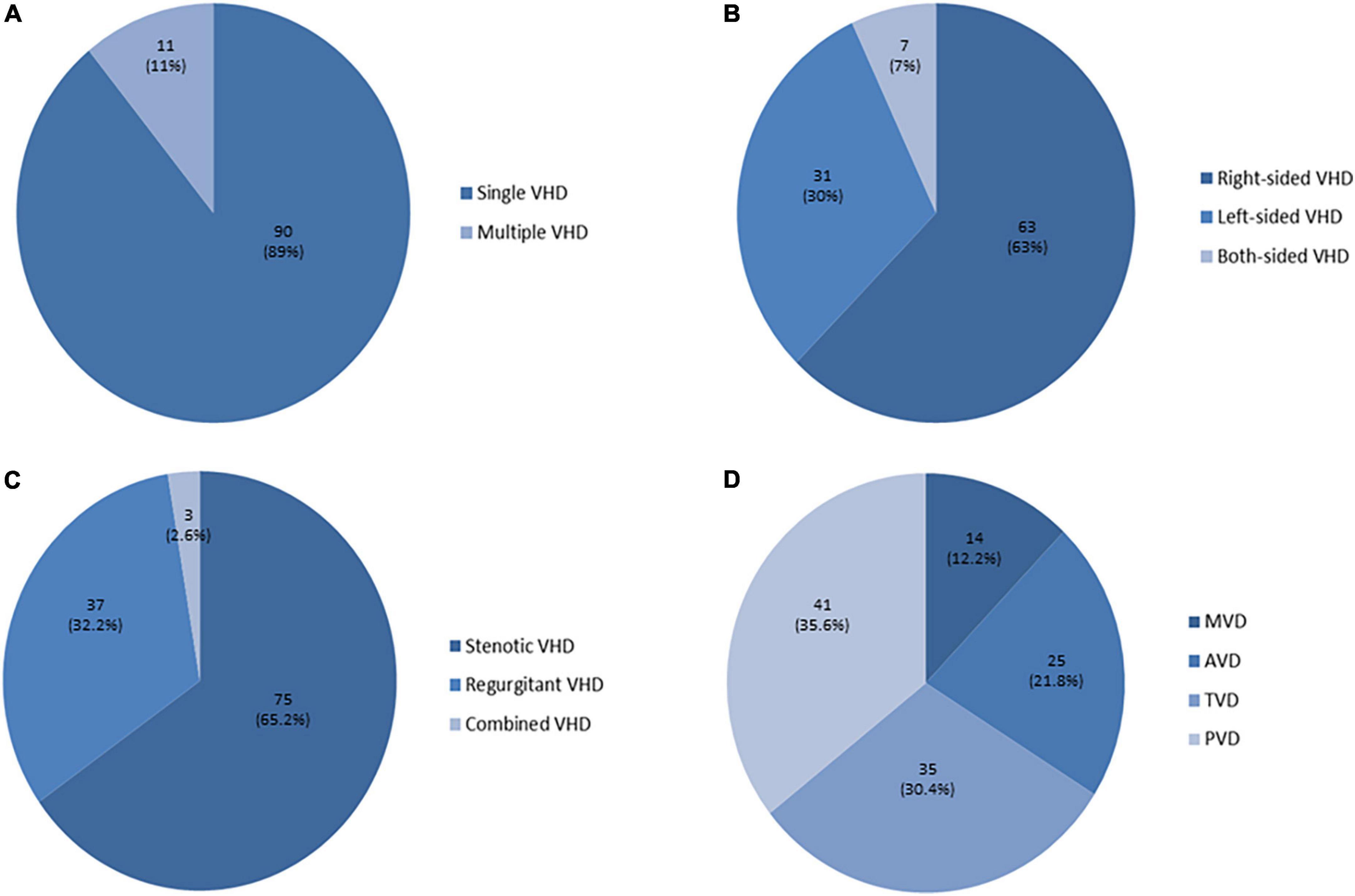
Figure 2. Distribution of severe heart valve lesions. (A) Among patients with severe valvular heart disease (S-VHD), 90 (89%) had single S-VHD (= 1 valve with severe dysfunction), while 11 (11%) had multiple S-VHD (≥ 2 valves with severe lesions). (B) Right-sided S-VHD (63 patients, 63%) were prevalent as compared to left-sided S-VHD (31 patients, 30%), while in few cases both-sided S-VHD were detected (7 patients, 7%). (C) When considering patients with multiple VHD (= more than one valve with severe dysfunction in the same patient), a total of 115 severe valvular dysfunctions were detected among the 101 patients with S-VHD. Severe regurgitant lesions were prevalent (75) as compared to severe stenotic lesions (37), while in three cases severe combined VHD was present (2 patients with severe pulmonary valve steno-insufficiency and 1 with severe mitral valve steno-insufficiency). (D) The pulmonary valve was the most frequently affected by severe stenosis and/or regurgitation (S-PVD, n = 41), followed by the tricuspid valve (S-TVD, n = 35). Severe aortic valve (S–AV) and mitral valve (S–MV) stenosis and/or regurgitation were less common (25 and 14 respectively).
Table 2 shows the comparison between patents with S-VHD and those with NS-VHD. Age at first visit was significantly higher in S-VHD patients (p < 0.001), who also were more likely to have a moderate or severe underlying CHD (p < 0.001) and to have undergone corrective or palliative surgery in pediatric age (p = 0.014). S-VHD patients had statistically significant lower %Sat O2 (p < 0.011) and were more often in NYHA functional class > II (p < 0.001).
At ECG, S-VHD patients presented more frequently with Af/Afib rhythm at first evaluation (p < 0.001) and had longer QRS duration (p < 0.001) as compared to NS-VHD patients.
At echocardiography, patients with S-VHD had lower LVEF (p = 0.027), lower TAPSE (p < 0.006), larger LAVi (p < 0.001), and increased RVSP (p < 0.001) as compared to NS-VHD patients.
Over a median follow-up time of 26 months (IQR: 12–48), the study endpoint occurred in a total of 76 patients (19.5%). Cardiovascular death occurred in 8 patients with 2 cases of sudden cardiac death. A cardiac intervention was performed in 78 patients (median time to intervention being 10 months, range 4–29). Patients with S-VHD underwent more frequently percutaneous or surgical intervention, as compared with NS-VHD (41.6 vs. 12.5%, p < 0.001).
Arrhythmias occurred more often in patients with S-VHD than NS-VHD (32.7 vs. 15.2%, p < 0.001).
The cumulative endpoint-free survival was significantly lower in patients with S-VHD vs. NS-VHD [Figure 3, 59 vs. 83% (Log rank p < 0.001)]. Supplementary Table 4 summarizes the prevalence of the single endpoints in the overall population as well as in S-VHD and NS-VHD groups.
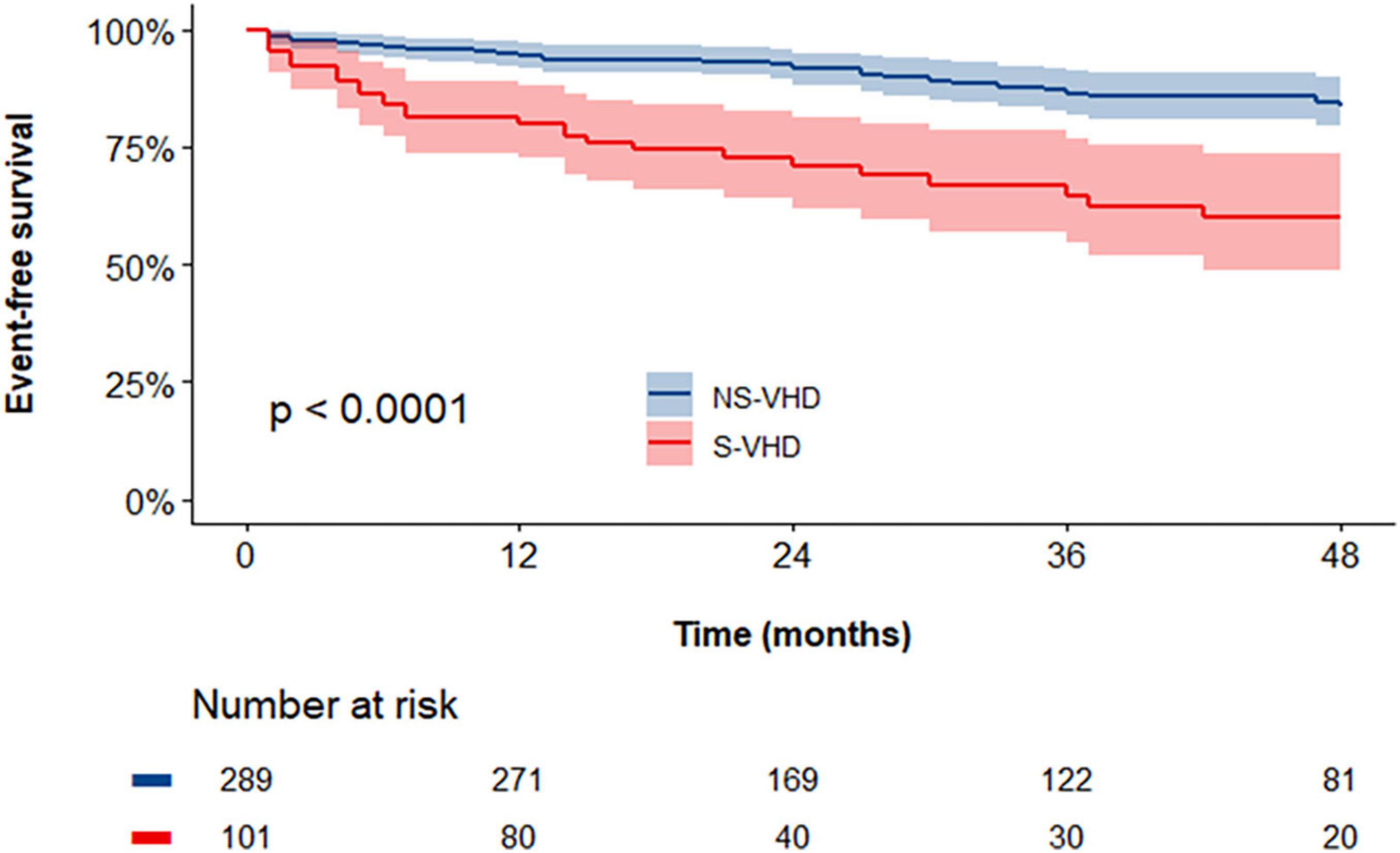
Figure 3. Kaplan–Meier curves assessing the cumulative event-free survival rates in severe valvular heart disease (S-VHD) and NS-VHD populations. S-VHD, severe valvular heart disease; NS-VHD, non-severe valvular heart disease.
At univariable Cox regression analysis (Table 3), S-VHD was significantly associated with an increased risk of the study endpoint [3.483 (2.086–5.816); p < 0.001]. In addition, age, severe CHD, Af/Afib at first visit, %Sat O2, NYHA class ≥ II, LVEF, TAPSE and increased RVSP (> 45 mmHg) were also associated with the study endpoint. On multivariable analysis (Table 3), the association between S-VHD and the study endpoint remained significant [HR: 1.925 (1.133–3.271); p = 0.015], after adjustment for age, severe CHD, Af/Afib at first visit, %Sat O2, NYHA class ≥ II, LVEF and increased RVSP. Notably, the likelihood ratio test demonstrated an incremental prognostic value by incorporating S-VHD in the multivariable model (changes in X2 = 5.70; p = 0.017) (Supplementary Figure 2).
The association of each subgroup of S-VHD (defined according to the affected valve and the type of valvular lesion), with outcomes is reported in Supplementary Figure 3. Each type of S-VHD was individually associated with the endpoint. After adjustment for significant clinical and echocardiographic variables (age, severe CHD, Af/Afib, NYHA ≥ II, Sat O2, LVEF and RVSP > 45 mmHg), isolated S-VHD (p = 0.049), left-sided VHD (p = 0.007), and regurgitant S-VHD (p = 0.016) retained their independent association with outcomes.
There was no significant difference in adverse outcomes occurrence between S-VHD patients who received corrective intervention during follow-up and those who did not [14 (33%) vs. 23 (39%), respectively, p = 0.56]. However, in the subgroup of the S-VHD population that underwent surgical/percutaneous procedure, most of the events [10, (71%)] occurred before the intervention.
To further confirm the prognostic value of S-VHD on the clinical evolution, the occurrence of cardiac mortality or cardiac hospitalization without cardiac operations was examined. After a median follow-up of 23 months (range: 9–44), 68 events occurred. At univariable Cox regression analysis, S-VHD was significantly associated with outcomes in patients medically treated [HR: 3.473 (2.144–5.627); p < 0.001]. The adjustment for significant clinical and echocardiographic variables (age, severe CHD, Af/Afib, NYHA class ≥ II, LVEF and increased RVSP) did not affect the powerful association of S-VHD with the occurrence of the composite endpoint under medical management [HR: 1.920 (1.093–3.373); p = 0.023].
The Figure 4 is an illustrative example of four patients included in our study, coupled according to the ACHD.
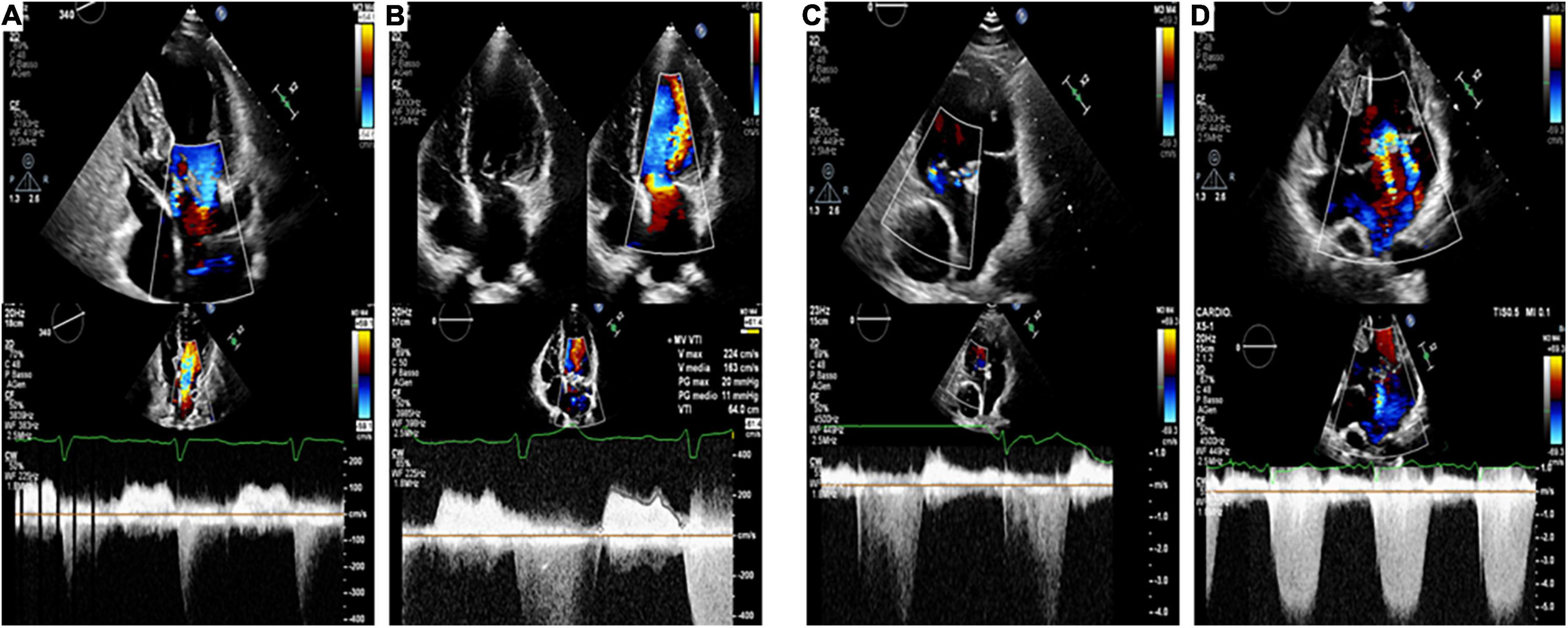
Figure 4. Echocardiographic images (Top: apical 4-chamber views, Bottom: CW Doppler through mitral valve and systemic AV valve) of four patients included in our study, coupled according to the ACHD [(A,B): repaired partial atrioventricular septal defect; (C,D): Fontan circulation], with (Panel B,D) and without (Panel A,C) associated S-VHD. This is illustrative of our findings, since patients (B–D) experienced hospitalization for heart failure. while patients (A–C) had an unremarkable clinical course.
The major and novel finding of our study in a contemporary real-world ACHD population is that the presence of severe VHD impacts prognosis, being independently associated with the risk of cardiac mortality and hospitalization. This highlights the need for careful VHD characterization in ACHD outpatient clinics and call for improvement in their management.
The management of patients with ACHD represents an expanding clinical field, with constantly increasing numbers, mainly due to the success of cardiac surgery and interventions in children (16).
These patients, however, cannot be considered cured, as most of them will suffer the long-term sequelae of their birth defect and/or the surgical interventions performed in childhood, which can take decades to manifest (17). The clinical complexity of the ACHD population, indeed, is increasing over time (1, 4, 18), with HF and arrhythmias representing the major causes of death or re-admission for these patients during adult life (18–20) and VHD are commonly found and frequently require an intervention (21, 22). To date, however, most data come from registries or surveys (based on large administrative databases, general electronic health records and death certificates) that cannot provide detailed information about important clinical features regarding baseline disease severity characterization and outcome measures. More importantly, these are frequently descriptive analyses in which factors often associated to ACHD, as the presence of VHD, are not evaluated as covariates. All these limits make the real burden of VHD on ACHD difficult to ascertain.
In this context, the major strength of our study is that we carefully collected clinical, ECG and echocardiographic data in a contemporary real-world population of ACHD patients followed at our dedicated outpatient clinic, with the specific aim to assess the role of severe VHD on prognosis. We found that half of the population had a VHD at least moderate at the first evaluation in our center. This is of particular importance, as moderate valve dysfunction has been shown to be detrimental in other clinical settings (23) as well as in ACHD (24). Moreover, the median age of our population at first visit was 34 years, thus the chance of progression of the VHD is expected to be high. As much as a quarter of patients presented at their first evaluation in our dedicated adult outpatient clinic with a severe valve dysfunction, that is expected to determine a major impact on the overall CHD hemodynamics. These patients were older, presented with more advanced NYHA functional class and more often had an underlying moderate or severe CHD, in line with recent studies reporting the increasing complexity of ACHD patients (1, 4–17). Moreover, patients with severe VHD were more prone to develop arrhythmias and more often required interventions/reinterventions, confirming previous observations (25–27).
The major finding of our study is that severe VHD at presentation was independently associated with an adverse outcome during the clinical course. S-VHD adds strong prognostic value to the multivariate model including significant clinical and echocardiographic variables. In particular, S-VHD had an independent prognostic impact on outcome while severe CHD did not. This is worth noting, since most data suggest that cardiovascular death and complications are associated with the severity of underlying CHD (21, 28, 29). A study (21) evaluating the trends in hospitalizations for ACHD (2003–2012), stratified into simple and complex disorder, showed that HF, respiratory disorders, and arrhythmias were the top three reasons for admission among patients with complex ACHD while VHD was among the three top causes for admission among patients with simple ACHD without atrial septal defect/patent foramen ovale.
However, these studies did not stratify patients for the degree of VHD while our study showed the relevance of the severity of the valvular heart lesion on ACHD outcome. This data is of particular importance as most studies reporting on VHD in CHD patients comes from national registries and surveys with the valve dysfunction often reported as a dichotomous variable and no mention on the type and degree of valvular lesion, or the affected valve or the underlying etiology. Of note, our study reveals the striking difference between the typical scenario of adult cardiology, in which VHD poses a significant burden dominated by far by aortic stenosis and mitral regurgitation (30) and ACHD valvular abnormalities. In fact, ACHD comprise a wide spectrum, with extremely heterogeneous anatomy, physiology and surgical history, including systemic right ventricles, systemic single ventricle and Fontan palliation. Due to this complexity, ACHD-related VHD are difficult to categorize as primary, post-surgical or functional lesions. Indeed, on an individual basis, patients often fall in more than one category. Thus, whether there is a different prognostic role for primitive vs. post-surgical or functional VHD cannot be deduced from our study.
In this context, right-sided valvular lesions were prevalent in our study but left-sided VHD (including all systemic atrio-ventricular valves) retained their independent association with outcomes. These findings highlight the importance of considering valvular heart lesions in ACHD according to the functional, rather than the anatomical classification.
Some limitations of the present study should be acknowledged. Firstly, we included a wide spectrum of congenital heart diseases patients with heterogeneous valvular lesions. However, this is a problem commonly encountered in studies on ACHD, considering that most CHD are rare diseases. In addition, this is an observational study conducted in a tertiary referral center for ACHD and VHD, thus our population could not be representative of average ACHD patients. Moreover, we did not systematically collect cardiac magnetic resonance (CMR) data that is the gold standard for the assessment of most ACHD patients. However, the presence and degree of VHD was carefully established according to the most recent echocardiography guidelines and echo studies were personally performed by experienced team in the imaging and care of VHD and ACHD. Moreover we did perform CMR in every patient with pulmonary regurgitation to quantify its severity by means of regurgitant fraction. It is also worth noting that echocardiography is still the gold standard for the assessment of valvular stenosis.
Our study shows that the occurrence of S-VHD in ACHD patients is a major threat, being a significant independent predictor of hospitalization or death. The prognostic value of severe valve dysfunction is incremental above other established prognostic markers and consistent independently from CHD severity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethic Committee Fondazione Policlinico Universitario Agostino Gemelli, n°4742. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
FG, GI, MCM, and RL: study design, data collection, and statistical analysis. All authors contributed to the drafting and revision of the manuscript, to data analysis improvement and discussion development.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.983308/full#supplementary-material
1. Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. (2010) 56:1149–57. doi: 10.1016/j.jacc.2010.03.085
2. Baumgartner H. Geriatric congenital heart disease: a new challenge in the care of adults with congenital heart disease? Eur Heart J. (2014) 35:683–5. doi: 10.1093/eurheartj/eht358
3. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. (2016) 134:101–9. doi: 10.1161/CIRCULATIONAHA.115.019307
4. Diller GP, Kempny A, Alonso-Gonzalez R, Swan L, Uebing A, Li W, et al. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation. (2015) 132:2118–25. doi: 10.1161/CIRCULATIONAHA.115.017202
5. Holst KA, Said SM, Nelson TJ, Cannon BC, Dearani JA. Current interventional and surgical management of congenital heart disease: specific focus on valvular disease and cardiac arrhythmias. Circ Res. (2017) 120:1027–44. doi: 10.1161/CIRCRESAHA.117.309186
6. Tobler D, Schwerzmann M, Bouchardy J, Engel R, Stambach D, Attenhofer Jost C, et al. On behalf of sacher. swiss adult congenital heart disease registry (SACHER) - rationale, design and first results. Swiss Med Wkly. (2017) 147:w14519. doi: 10.4414/smw.2017.14519
7. Lin YS, Liu PH, Wu LS, Chen YM, Chang CJ, Chu PH. Major adverse cardiovascular events in adult congenital heart disease: a population-based follow-up study from Taiwan. BMC Cardiovasc Disord. (2014) 14:38. doi: 10.1186/1471-2261-14-38
8. Graziani F, Delogu AB. Evaluation of adults with congenital heart disease. World J Pediatr Congenit Heart Surg. (2016) 7:185–91. doi: 10.1177/2150135115623285
9. Burzotta F, Graziani F, Trani C, Aurigemma C, Bruno P, Lombardo A, et al. Clinical impact of heart team decisions for patients with complex valvular heart disease: a large, single-center experience. J Am Heart Assoc. (2022) 11:e024404. doi: 10.1161/JAHA.121.024404
10. Panaioli E, Birritella L, Graziani F, Lillo R, Grandinetti M, Di Molfetta A, et al. Right ventricle-pulmonary artery coupling in repaired tetralogy of Fallot with pulmonary regurgitation: clinical implications. Arch Cardiovasc Dis. (2022) 115:67–77. doi: 10.1016/j.acvd.2021.12.006
11. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. (2021) 42:563–645. doi: 10.1093/eurheartj/ehaa554
12. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. (2017) 30:303–71. doi: 10.1016/j.echo.2017.01.007
13. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. (2009) 22:1–23. doi: 10.1016/j.echo.2008.11.029
14. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. (2017) 18:254–75. doi: 10.1093/ehjci/jew335
15. Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2016) 17:589–90. doi: 10.1093/ehjci/jew025
16. Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. (2010) 122:2264–72. doi: 10.1161/CIRCULATIONAHA.110.946343
17. Gilljam T, Mandalenakis Z, Dellborg M, Lappas G, Eriksson P, Skoglund K, et al. Development of heart failure in young patients with congenital heart disease: a nation-wide cohort study. Open Hear. (2019) 6:e000858. doi: 10.1136/openhrt-2018-000858
18. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. (2009) 54:460–7. doi: 10.1016/j.jacc.2009.04.037
19. Burchill LJ, Gao L, Kovacs AH, Opotowsky AR, Maxwell BG, Minnier J, et al. Hospitalization trends and health resource use for adult congenital heart disease-related heart failure. J Am Heart Assoc. (2018) 7:e008775. doi: 10.1161/JAHA.118.008775
20. Hernández-Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, et al. ESC Scientific Document Group. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace. (2018) 20:1719–53. doi: 10.1093/europace/eux380
21. Agarwal S, Sud K, Menon V. Nationwide hospitalization trends in adult congenital heart disease across 2003-2012. J Am Heart Assoc. (2016) 5:e002330. doi: 10.1161/JAHA.115.002330
22. Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Tchervenkov C, Martucci G, et al. Valvular operations in patients with congenital heart disease: increasing rates from 1988 to 2005. Ann Thorac Surg. (2010) 90:1563–9. doi: 10.1016/j.athoracsur.2010.07.017
23. Rahhab Z, El Faquir N, Tchetche D, Delgado V, Kodali S, Mara Vollema E, et al. Expanding the indications for transcatheter aortic valve implantation. Nat Rev Cardiol. (2020) 17:75–84. doi: 10.1038/s41569-019-0254-6
24. Van De Bruaene A, Hickey EJ, Kovacs AH, Crean AM, Wald RM, Silversides CK, et al. Phenotype, management and predictors of outcome in a large cohort of adult congenital heart disease patients with heart failure. Int J Cardiol. (2018) 252:80–7. doi: 10.1016/j.ijcard.2017.10.086
25. Holst KA, Dearani JA, Burkhart HM, Connolly HM, Warnes CA, Li Z, et al. Risk factors and early outcomes of multiple reoperations in adults with congenital heart disease. Ann Thorac Surg. (2011) 92:122–8. doi: 10.1016/j.athoracsur.2011.03.102
26. Holst KA, Dearani JA, Burkhart HM, Connolly HM, Warnes CA, Li Z, et al. Reoperative multivalve surgery in adult congenital heart disease. Ann Thorac Surg. (2013) 95:1383–9. doi: 10.1016/j.athoracsur.2012.12.009
27. Koyak Z, Achterbergh RC, de Groot JR, Berger F, Koolbergen DR, Bouma BJ, et al. Postoperative arrhythmias in adults with congenital heart disease: incidence and risk factors. Int J Cardiol. (2013) 169:139–44. doi: 10.1016/j.ijcard.2013.08.087
28. Arnaert S, De Meester P, Troost E, Droogne W, Van Aelst L, Van Cleemput J, et al. Heart failure related to adult congenital heart disease: prevalence. outcome and risk factors. ESC Heart Fail. (2021) 8:2940–50. doi: 10.1002/ehf2.13378
29. Berdat PA, Immer F, Pfammatter JP, Carrel T. Reoperations in adults with congenital heart disease: analysis of early outcome. Int J Cardiol. (2004) 93:239–45. doi: 10.1016/j.ijcard.2003.04.005
Keywords: valvular heart disease, adult congenital heart disease (ACHD), prognosis, hospitalization, mortality
Citation: Graziani F, Iannaccone G, Meucci MC, Lillo R, Delogu AB, Grandinetti M, Perri G, Galletti L, Amodeo A, Butera G, Secinaro A, Lombardo A, Lanza GA, Burzotta F, Crea F and Massetti M (2022) Impact of severe valvular heart disease in adult congenital heart disease patients. Front. Cardiovasc. Med. 9:983308. doi: 10.3389/fcvm.2022.983308
Received: 30 June 2022; Accepted: 10 November 2022;
Published: 29 November 2022.
Edited by:
Alan R. Morrison, Brown University, United StatesReviewed by:
Anastasia D. Egorova, Leiden University Medical Center, NetherlandsCopyright © 2022 Graziani, Iannaccone, Meucci, Lillo, Delogu, Grandinetti, Perri, Galletti, Amodeo, Butera, Secinaro, Lombardo, Lanza, Burzotta, Crea and Massetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Burzotta, ZnJhbmNlc2NvLmJ1cnpvdHRhQHBvbGljbGluaWNvZ2VtZWxsaS5pdA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.