
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 06 September 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.978154
This article is part of the Research Topic Case Reports in Atherosclerosis and Vascular Medicine: 2022 View all 10 articles
A 37-year-old Chinese man was admitted to the department of cardiology of the First Hospital of Jilin University for intermittent palpitation for 9 months, aggravating with chest pain for 3 days. After several examinations, he was diagnosed with giant left ventricular fistula of the diagonal branch of the left coronary artery. After routine treatment, which included improving circulation and administration of dual antiplatelet as well as hypolipidemic drugs among others, the patient’s symptoms did not improve. The fistula was too big for transcatheter occlusion to be performed. A multi-disciplinary suggestion was that the patient be subjected to “surgical closure treatment”; however, for personal reasons, he refused the operation. After discharge, oral beta-blockers were prescribed for the patient. Incidences of congenital coronary arterial fistula in congenital cardiovascular disease are rare, and incidences of the giant fistula being located in the left heart system are even rarer. We report an adult male with a giant left anterior descending diagonal coronary artery left ventricular fistula and show various accessory examination results. Non-invasive ultrasonic cardiography was the first diagnostic option for the disease and pre-admission evaluation. Auxiliary diagnosis and exclusion value of cardiovascular magnetic resonance (CMR) were revealed for the first time. Invasive coronary angiography (ICA) was demonstrated to be the gold standard method again and it was also found that computed tomography angiography (CTA) might be used instead of ICA for determining the exact relationships among anatomic structures. Furthermore, we performed a literature review on the diagnosis and treatment of patients with this condition.
Coronary artery fistula (CAF) refers to an abnormal coronary artery that bypasses the myocardial capillary network and terminates into any cardiac lumen or large vessel. It is characterized depending on the number, origin, course, termination, and presence of an aneurysm or stenotic lesion (1). It is a very rare coronary artery anomaly whose prevalence in the general population is estimated to be 0.002% (2). Even very small CAFs in children require close attention as they may develop with age (3). Our case was an adult male with intermittent palpitation and chest pains due to the left coronary artery diagonal branch left ventricular fistula.
A 37-year-old Chinese man presenting with untreated palpitation, nausea, and fatigue for 9 months and with worsening palpitation symptoms accompanied by precordial pain was admitted to our hospital. The pain radiated to both shoulders, lasted about 30 min, and improved by itself. The patient used to be physically healthy and had no family history of genetically related diseases, history of trauma and surgery, and no record of drinking. He had a history of smoking for more than 10 years, 1 pack a day, which he never quit until hospitalization. Physical examination revealed: temperature, 36.2°C; pulse, 93 beats/min; breathing, 18 times/min, and blood pressure, 132/78 mmHg. The rest of physical examination did not show any obvious abnormalities. The primary laboratory data are shown in Table 1 according to the time line of the patient’s admission. The patient’s electrocardiogram on admission was normal (Figure 1). Ultrasonic cardiography (UCG) showed left ventricular ectasia (Figure 2). The patient underwent computed tomography angiography (CTA) examination, which suggested a diagonal branch of coronary artery-left ventricular fistula (Figure 3), and was admitted to the cardiology department.
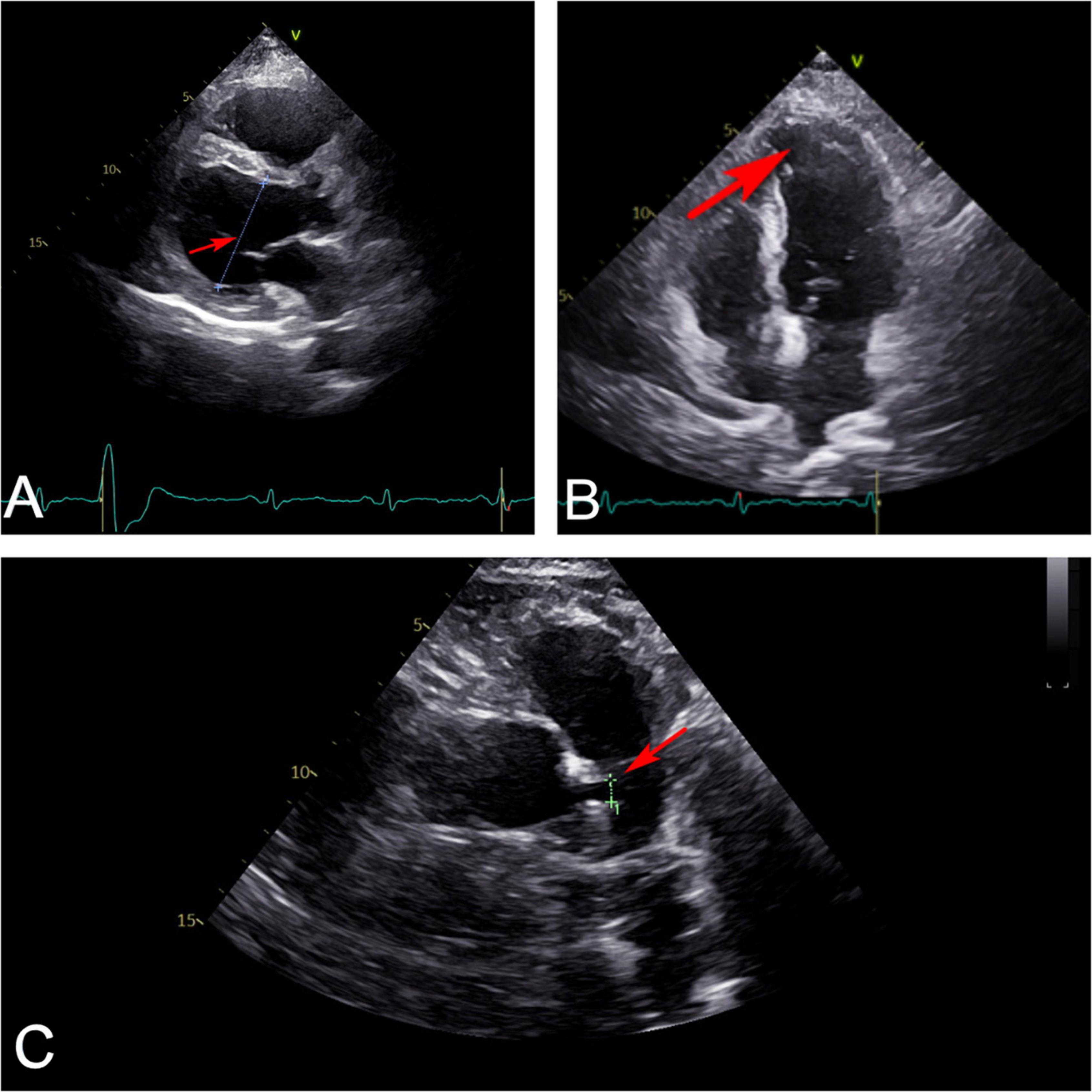
Figure 2. Ultrasonic cardiography showed that (A) left ventricle (red arrow) slightly enlarged from the parasternal long axis section view. (B) Apex of left ventricle (red arrow) bulged slightly outward from four-chamber view. (C) Left main coronary artery (red arrow) widened from random view.
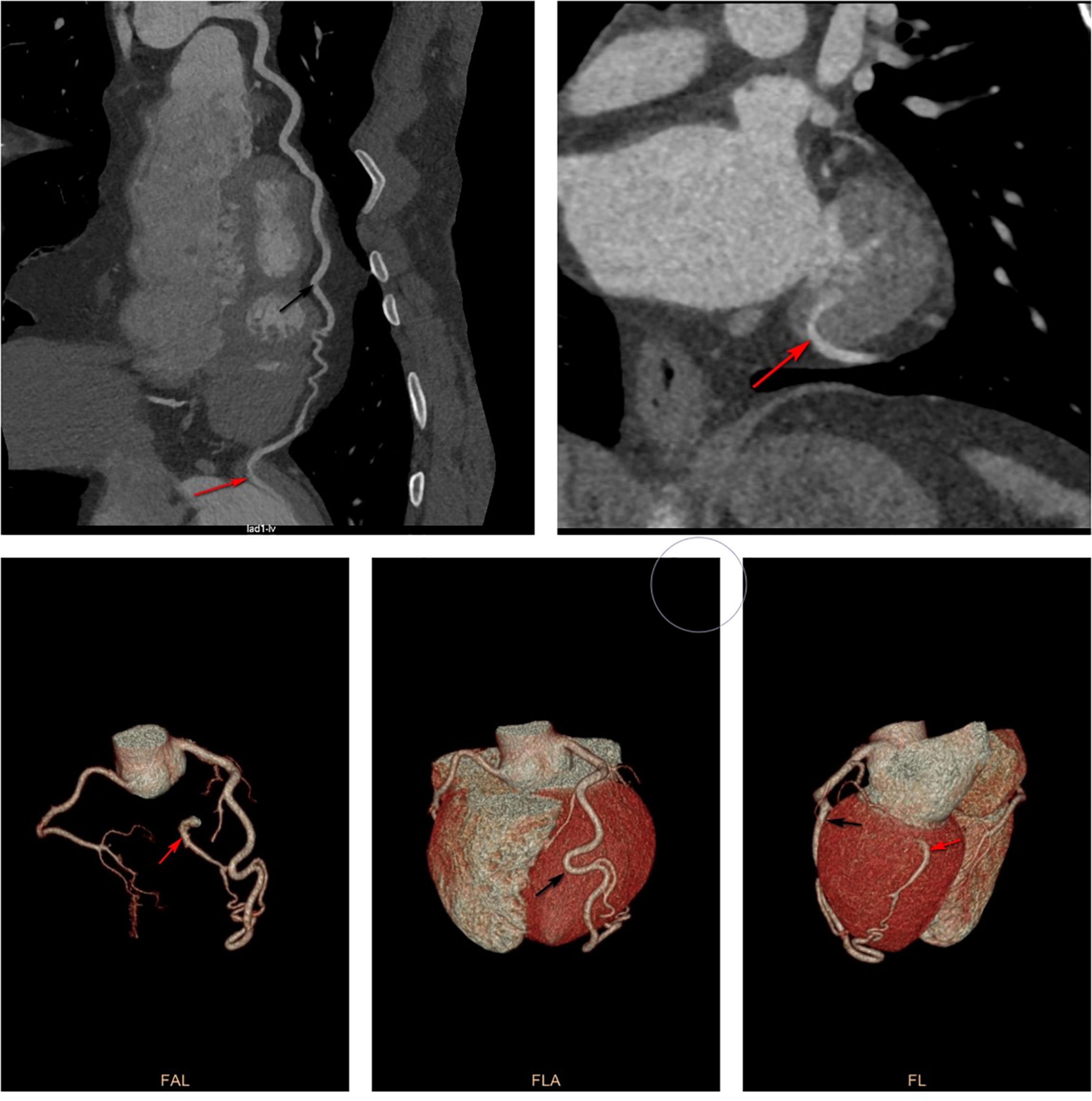
Figure 3. Computed tomography angiography showed that the diagonal branch of the left coronary artery was twisted, lengthened, expanded, extended along the left heart margin, and its distal end penetrated the myocardium from the basal segment of the left ventricular posterior edge into the left ventricle. Black arrow shows the thick diagonal branch; red arrow shows coronary artery-left ventricular fistula.
After admission, cardiovascular magnetic resonance (CMR) imaging and invasive coronary angiography (ICA) were performed. CMR of the heart showed suspicious fistula at the base of the inferior lateral wall (Figure 4) while ICA showed similar findings as CTA (Figure 5).
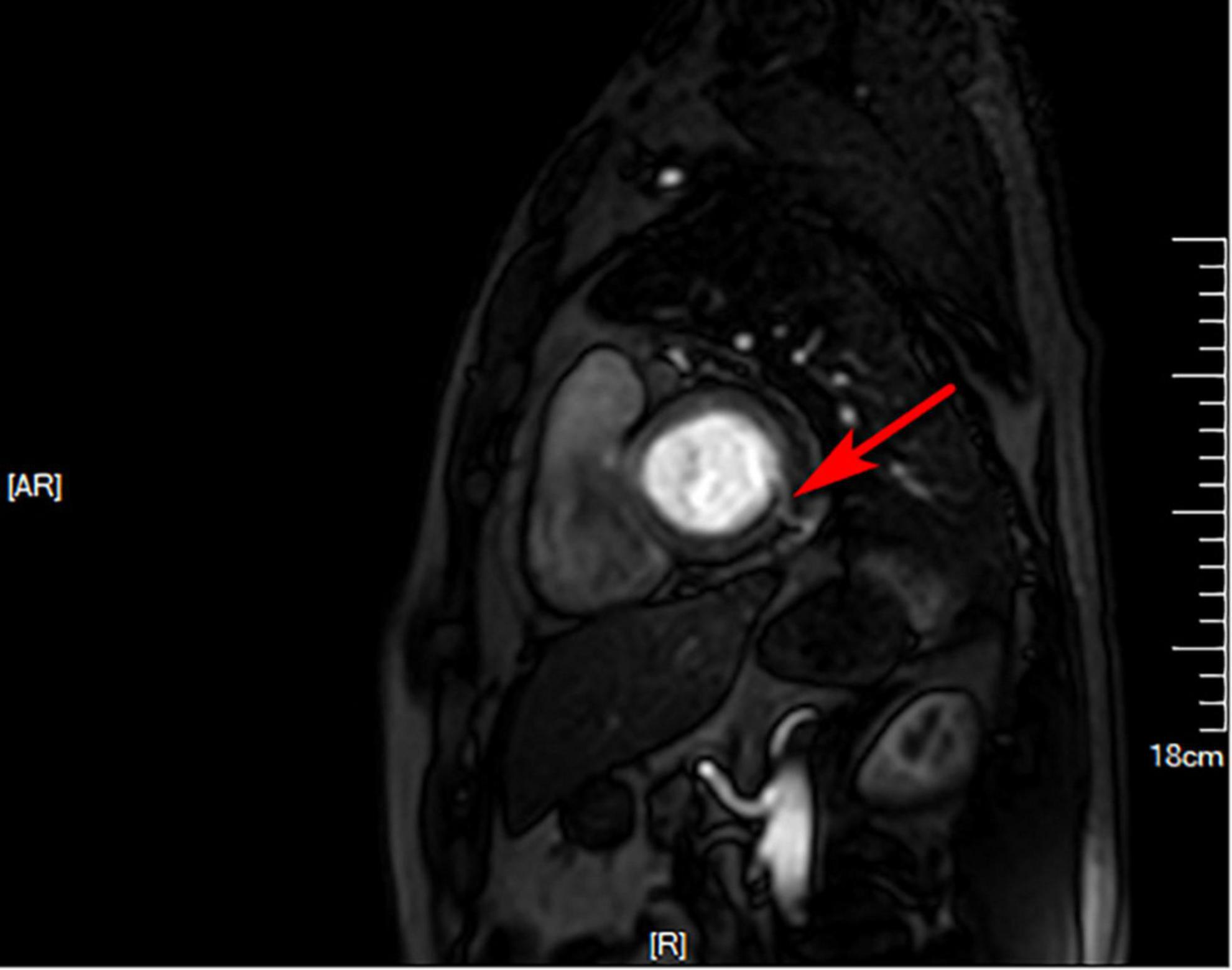
Figure 4. Cardiovascular magnetic resonance. Suspicious fistula at the base of the inferior lateral wall (red arrow) was seen from the left ventricular short axis at 4 o’clock direction.
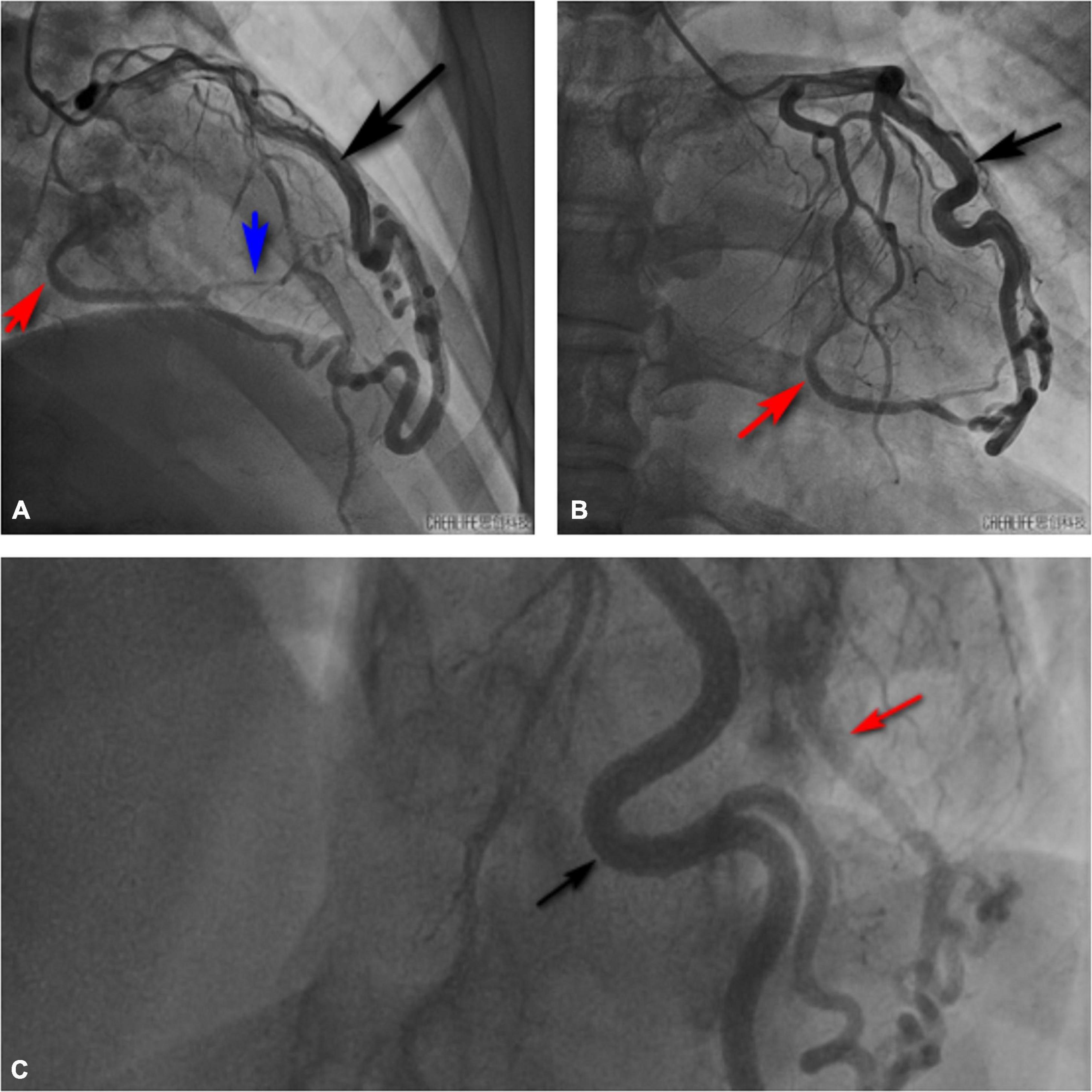
Figure 5. Invasive coronary angiography also showed that the diagonal branch of the left coronary artery was twisted, lengthened, expanded, extended along the left heart margin, and its distal end penetrated the myocardium from the basal segment of the left ventricular posterior edge into the left ventricle. Black arrow shows the thick diagonal branch; red arrow shows the coronary artery-left ventricular fistula; blue arrow shows the branch of coronary artery fistula that supplies the left ventricular posterior wall myocardium. (A) Right cranial view. (B) Anteroposterior view. (C) Left cranial view.
Treatment plans were: after admission, the patient was treated with papaverine, 120 mg, one time a day (QD), intravenous (I.V.); nicotinamide, 400 mg, QD, I.V.; shensongyangxin, 0.8 g, three times a day (TID), by mouth (P.O.); atorvastatin, 20 mg, QD, P.O.; aspirin, 100 mg, QD, P.O. and clopidogrel, 75 mg, QD, P.O. After treatment, there was no obvious improvement in patients’ symptoms. A multi-disciplinary team suggested “surgical closure treatment” under general anesthesia. However, for personal reasons, he refused the operation. After discharge, the patient was prescribed oral beta-blockers.
Clinically, CAF is a rare cardiac abnormality that should always be considered during differential diagnosis of chest pain and dyspnea, particularly in patients without significant risk factors for acquired heart disease. The etiologies and pathophysiological mechanism of CAF have not been fully established. However, it has been hypothesized that when there is no closure between the trabeculae connecting the coronary arteries, veins, and ventricles, a persistent sinus trabeculation may develop into CAF. As the flow increases, there is a significant increase in coronary branches proximal to the shunt site (4). Due to its hemodynamic consequences or complications, it is associated with various symptoms (5).
In the proximal segments of coronary arteries, CAFs are more likely to form aneurysms, which shows the significance of early diagnosis as early treatment can prevent rupture (3). The diagnostic methods, their advantages, and disadvantages in CAF are summarized in Table 2. Various non-invasive techniques, such as CTA, play a vital role in the diagnosis of these vascular anomalies. The CTA approach is excellent at revealing the origin, course, size, and termination site of CAF as well as its relationship with adjacent anatomic structures (6). We showed non-invasive UCG as the first diagnostic tool and the pre-admission evaluation value for the disease. The auxiliary diagnostic and exclusion values of CMR were assessed for the first time, and the gold standard value of ICA for determining the origin and course of coronary fistula was proven. In this study, CTA showed similar results to ICA. ICA is the commonly used tool, but it is invasive. CTA might be an alternative method for determining the exact relationships among anatomic structures, because of its excellent spatial resolution.
When considering clinical treatment indications and options for CAF, an accurate assessment of the clinical presentation and morphology, including anatomic origin and course, drainage site, as well as possible aneurysm is necessary (1). The American College of Cardiology and American Heart Association guidelines for managing congenital heart disease (CHD) in adults (2008) emphasizes that large CAFs should be closed after their course has been determined, regardless of symptoms (Class I, Level of Evidence: C); small or medium-sized fistulas should be closed if the patient presents with symptoms such as myocardial ischemia, arrhythmias, ventricular dilatation, or dysfunction of unknown origin, or if the fistula is complicated with endocarditis (Class I, Level of Evidence: C); Patients with small asymptomatic fistulas should not be treated but managed by clinical follow-up, including UCG every 3–5 years (Class III, Level of Evidence: C) (22). Symptomatic patients and those with large diameter CAF, whether symptomatic or not, should have their fistulas closed surgically or with transcatheter closure (5). There is consensus regarding the surgical treatment of patients with symptomatic CAF (23). Intracardiac surgical closure of CAF is appropriate for patients with late-onset, large fistulas, coronary arteries with aneurysms, and those who are not candidates for transcatheter treatment (23). Surgical or transcatheter treatments are linked to many risks and operative complications, such as procedural ST-T changes, and postoperative fever (5). Long-term follow-up is required to assess the effectiveness of management, recurrence, and late outcomes (23). Untreated large fistulas might lead to congestive heart failure and premature coronary arterial disease in affected vessels (24). There is no consensus regarding treating asymptomatic adult patients without significant shunts to prevent fistula-related complications (1). Although most patients with such anomalies are asymptomatic, early treatment is recommended to prevent the onset of complications, such as ventricular wall tumor, heart valve disease, cardiomyopathy, and infective endocarditis (25). Armsby et al. (26) performed transcatheter occlusion in 33 of 39 asymptomatic patients with a typical murmur and reported that all patients who accepted interventional therapy had good long-term prognostic outcomes. Researchers are still investigating suitable drugs for the disease. According to Karazisi et al. (5), antiplatelet or warfarin therapies should be considered, especially in coronary artery dilatation. For patients subjected to interventional operation, anticoagulation should be administered after operation. There are various drugs for different symptoms, such as drugs (beta-blockers or calcium channel blockers) for angina and those for treating high-risk factors (hyperlipidemia, hypertension, and diabetes among others). However, these recommendations are mostly empiric (5). Lifelong follow-up is always necessary to ensure that patients with CAF have no disease progression or further cardiac complications. In addition, the risk of infective endocarditis in those patients is also higher than that of ordinary people (11). At present, the patient demanded for conservative treatment and was informed of the above risk. He was also advised to receive regular UCG examination. Most cases of CAF are congenital. CHD is associated with many genes, such as chromatin modifiers, cilia, cilia transduction cell signaling, and maternal factors (27). Cilia and chromatin modifiers may drive the complex genetics of CHD (27). There are many hypotheses regarding the congenital etiologies of CAF. However, the specific molecular mechanisms underlying CAF pathogenesis have not been fully established. Targeted or causative therapies should be investigated through genomics, particularly the study of genes and receptors.
All of the above-mentioned diagnostic methods and treatment options have their merits and demerits. The best diagnostic and treatment plans should be selected based on patient condition and hospital facilities. UCG could be used for preliminary disease screening, CTA might be used instead of ICA for determining the exact relationships with anatomic structures, whereas CMR can be used to exclude other diseases hence help in the diagnosis. In terms of treatment plans, studies should aim at assessing various treatments to inform on the accurate treatment of CAF.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the First Hospital of Jilin University Ethics Committee. The ethics committee waived the requirement of written informed consent for participation.
JW conceived the idea and conceptualized the case. JW and QW collected the data. JW and HZ analyzed the data and drafted the manuscript. QT and QW reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (No. 82070362).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Frestad D, Helqvist S, Helvind M, Kofoed K. Giant aneurysm in a left coronary artery fistula: diagnostic cardiovascular imaging and treatment considerations. BMJ Case Rep. (2013) 2013:bcr2013008853. doi: 10.1136/bcr-2013-008853
2. Zenooz NA, Habibi R, Mammen L, Finn JP, Gilkeson RC. Coronary artery fistulas: CT findings. Radiographics. (2009) 29:781–9. doi: 10.1148/rg.293085120
3. Vaidya YP, Green GR. Coronary artery fistula. J Card Surg. (2019) 34:1608–16. doi: 10.1111/jocs.14267
4. Geller CM, Dimitrova KR, Hoffman DM, Tranbaugh RF. Congenital coronary artery fistulae: a rare cause of heart failure in adults. J Cardiothorac Surg. (2014) 9:87. doi: 10.1186/1749-8090-9-87
5. Karazisi C, Eriksson P, Dellborg M. Coronary artery fistulas: case series and literature review. Cardiology. (2017) 136:93–101. doi: 10.1159/000447445
6. Detorakis EE, Foukarakis E, Karavolias G, Dermitzakis A. Cardiovascular magnetic resonance and computed tomography in the evaluation of aneurysmal coronary-cameral fistula. J Radiol Case Rep. (2015) 9:10–21. doi: 10.3941/jrcr.v9i7.2305
7. Shakudo M, Yoshikawa J, Yoshida K, Yamaura Y. Noninvasive diagnosis of coronary artery fistula by Doppler color flow mapping. J Am Coll Cardiol. (1989) 13:1572–7. doi: 10.1016/0735-1097(89)90351-3
8. Velvis H, Schmidt KG, Silverman NH, Turley K. Diagnosis of coronary artery fistula by two-dimensional echocardiography, pulsed Doppler ultrasound and color flow imaging. J Am Coll Cardiol. (1989) 14:968–76. doi: 10.1016/0735-1097(89)90474-9
9. Dawn B, Talley JD, Prince CR, Hoque A, Morris GT, Xenopoulos NP, et al. Two-dimensional and Doppler transesophageal echocardiographic delineation and flow characterization of anomalous coronary arteries in adults. J Am Soc Echocardiogr. (2003) 16:1274–86. doi: 10.1067/S0894-7317(03)00554-6
10. Iida R, Yamamoto T, Suzuki T, Saeki S, Ogawa S. The usefulness of intraoperative transesophageal echocardiography to identify the site of drainage of coronary artery fistula. Anesth Analg. (2005) 101:330–1. doi: 10.1213/01
11. Buccheri D, Chirco PR, Geraci S, Caramanno G, Cortese B. coronary artery fistulae: anatomy, diagnosis and management strategies. Heart Lung Circ. (2018) 27:940–51. doi: 10.1016/j.hlc.2017.07.014
12. Danad I, Raijmakers PG, Knaapen P. Diagnosing coronary artery disease with hybrid PET/CT: it takes two to tango. J Nucl Cardiol. (2013) 20:874–90. doi: 10.1007/s12350-013-9753-8
13. Schmitt R, Froehner S, Brunn J, Wagner M, Brunner H, Cherevatyy O, et al. Congenital anomalies of the coronary arteries: imaging with contrast-enhanced, multi-detector computed tomography. Eur Radiol. (2005) 15:1110–21. doi: 10.1007/s00330-005-2707-z
14. Chang DS, Lee MH, Lee HY, Barack BM. MDCT of left anterior descending coronary artery to main pulmonary artery fistula. AJR Am J Roentgenol. (2005) 185:1258–60. doi: 10.2214/AJR.04.1415
15. Knaapen P. Computed Tomography to Replace Invasive Coronary Angiography? Circ Cardiovasc Imaging. (2019) 12:e008710. doi: 10.1161/CIRCIMAGING
16. Kilner PJ, Gatehouse PD, Firmin DN. Flow measurement by magnetic resonance: a unique asset worth optimising. J Cardiovasc Magn Reson. (2007) 9:723–8. doi: 10.1080/10976640701465090
17. Mitsutake R, Miura S-I, Shiga Y, Iwata A, Saku K. Coronary-pulmonary artery fistula with anomalous vessels arising from the right coronary sinus detected by 64-MDCT. Intern Med. (2009) 48:1893–6. doi: 10.2169/internalmedicine.48.2586
18. Jagia P, Goswami KC, Sharma S, Gulati GS. 16-MDCT in the evaluation of coronary cameral fistula. AJR Am J Roentgenol. (2006) 187:W227–8. doi: 10.2214/AJR.05.1586
19. Said SAM, Lam J, van der Werf T. Solitary coronary artery fistulas: a congenital anomaly in children and adults. A contemporary review. Congenit Heart Dis. (2006) 1:63–76. doi: 10.1111/j.1747-0803.2006.00012.x
20. Shabestari AA, Akhlaghpoor S, Fatehi M. Findings of bilateral coronary to pulmonary artery fistula in 64-multislice computed tomographic angiography: correlation with catheter angiography. J Comput Assist Tomogr. (2008) 32:271–3. doi: 10.1097/RCT.0b013e3180683bbe
21. Jamil G, Khan A, Malik A, Qureshi A. Aneurysmal coronary cameral fistula. BMJ Case Rep. (2013) 2013:bcr2013008649. doi: 10.1136/bcr-2013-008649
22. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American college of cardiology/american heart association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). developed in collaboration with the American society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. (2008) 52:e143–263. doi: 10.1016/j.jacc.2008.10.001
23. Jha NK, Kiraly L, Shah N, Al Mulla A, Mora B. Congenital aneurysmal right coronary artery with a fistula to the left atrium in an adult. J Cardiothorac Surg. (2019) 14:33. doi: 10.1186/s13019-019-0854-6
24. Latson LA. Coronary artery fistulas: how to manage them. Catheter Cardiovasc Interv. (2007) 70:110–6. doi: 10.1002/ccd.21125
25. Tirilomis T, Aleksic I, Busch T, Zenker D, Ruschewski W, Dalichau H. Congenital coronary artery fistulas in adults: surgical treatment and outcome. Int J Cardiol. (2005) 98:57–9. doi: 10.1016/j.ijcard
26. Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE. Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J Am Coll Cardiol. (2002) 39:1026–32. doi: 10.1016/s0735-1097(02)01742-4
Keywords: congenital cardiovascular disease, coronary artery fistula, diagnosis, review, case
Citation: Wang J, Zhang H, Tong Q and Wang Q (2022) Giant left coronary artery diagonal branch left ventricular fistula: A case report and review of literature. Front. Cardiovasc. Med. 9:978154. doi: 10.3389/fcvm.2022.978154
Received: 25 June 2022; Accepted: 15 August 2022;
Published: 06 September 2022.
Edited by:
Masanori Aikawa, Brigham and Women’s Hospital and Harvard Medical School, United StatesReviewed by:
Shu-Shui Wang, Guangdong Academy of Medical Sciences, ChinaCopyright © 2022 Wang, Zhang, Tong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Tong, dG9uZ3FpYW5Aamx1LmVkdS5jbg==; Quanwei Wang, d2FuZ3F1YW53ZWkyMTZAamx1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.